14 July 2020: Original Paper
Pretreatment with a Phosphodiesterase-3 Inhibitor, Milrinone, Reduces Hepatic Ischemia-Reperfusion Injury, Minimizing Pericentral Zone-Based Liver and Small Intestinal Injury in Rats
Shinichi Nakanuma1ABCDEFG*, Hidehiro Tajima1AC, Hiroyuki Takamura1ACG, Seisho Sakai1A, Ryosuke Gabata1B, Mitsuyoshi Okazaki1B, Hiroyuki Shinbashi1B, Yoshinao Ohbatake1B, Isamu Makino1B, Hironori Hayashi2A, Tomoharu Miyashita1AC, Sachio Fushida1A, Tetsuo Ohta1ACEDOI: 10.12659/AOT.922306
Ann Transplant 2020; 25:e922306
Abstract
BACKGROUND: Severe pericentral zone (zone 3)-based liver injury (LI) may become intractable, with allograft dysfunction after liver transplantation. The phosphodiesterase-3 inhibitor, milrinone, has been reported to attenuate hepatic ischemia–reperfusion injury (IRI). This study clarified how hepatic IRI involved zone 3-based LI, in which zone milrinone was effective, and whether milrinone could improve small intestinal injury (SII) with hepatic IRI.
MATERIAL AND METHODS: Rats were divided into sham, ischemia–reperfusion (IR), or IR+milrinone groups (n=13 per group). Milrinone was administered intraportally via intrasplenic injection, and whole hepatic ischemia was induced for 30 min. Five hours after reperfusion, serum chemistry and histopathological findings were compared. Expression of CD34 for the detection of altered sinusoidal endothelium as sinusoidal capillarization and cleaved caspase-3 as an apoptosis marker were analyzed via immunohistochemistry. Survival rates were examined after 45 min of whole hepatic ischemia.
RESULTS: Serum aspartate aminotransferase and direct bilirubin levels were significantly decreased in the IR+milrinone group compared with those of the IR group. The degree of LI, sinusoidal capillarization and apoptosis at zone 3 in the IR group was significantly increased compared with those at the periportal zone (zone 1). These findings at zone 3 in the IR group were improved in the IR+milrinone group. SII with villus congestion and apoptosis in the IR group was significantly attenuated in the IR+milrinone group. The 7-day survival rate was significantly elevated in the IR+milrinone group as compared with that of the IR group.
CONCLUSIONS: A hepatic IRI model caused zone 3-based LI and SII, which were attenuated by intraportal administration of milrinone.
Keywords: Intestine, Small, Liver, Milrinone, Phosphodiesterase 3 Inhibitors, Ischemia, Ischemic Preconditioning, Rats, Wistar
Background
Various mechanisms can cause graft function to deteriorate in the early phase after liver transplantation (LT). We previously reported a liver transplant recipient who experienced allograft dysfunction caused by severe pericentral zone (zone 3)-based liver injury (LI) progressing from central perivenulitis (CP) to centrilobular fibrosis with sinusoidal obstruction syndrome (SOS) or veno-occlusive disease (VOD), resulting in graft loss [1,2]. Although SOS and VOD are uncommon after LT [3], CP is commonly observed as mild or moderate zone 3-based LI after LT [4]. CP has multiple causes, including ischemia–reperfusion injury (IRI), drugs, viral hepatitis, autoimmune hepatitis, and rejection [5], thus making it difficult to ascertain the cause of CP and select an effective treatment.
Hepatic IRI is an inevitable complication of LT, which also occurs with trauma and shock and during liver surgery with temporary clamping of the hepatoduodenal ligament (HDL), known as the Pringle maneuver, to minimize blood loss [6]. Previous experimental reports demonstrated that LI with hepatic IRI often occurs mainly in zone 3 [7–9]. However, the underlying mechanism of zone 3-based LI induced by hepatic IRI remains unclear. We hypothesized that severe hepatic IRI in the early phase after LT was associated with induction and development of refractory zone 3-based LI. Therefore, clarifying the mechanism of zone 3-based LI induced by hepatic IRI may help improve outcomes in the early phase after LT.
Drip infusion of phosphodiesterase-3 (PDE3) inhibitors, such as milrinone and amrinone, have been used clinically to treat heart failure [10,11]. PDE3 inhibitors inhibit type 3 phosphodiesterase, which converts 3′,5′-cyclic adenosine monophosphate (cAMP) to adenosine monophosphate, which increases the intracellular cAMP concentration and cAMP-dependent protein kinase (protein kinase A) activation, exerting positive inotropic and vasodilatory effects [12,13]. PDE3 inhibitors also produce inhibitory signals to cAMP in platelets to inhibit platelet function [14,15]. Therefore, PDE3 inhibitors possess platelet anti-aggregating effects. Moreover, a previous study found that PDE3 inhibitors enhanced the release of nitric oxide (NO) from endothelial cells and induced vasodilation [16]. Previous reports demonstrated that drip infusion of PDE3 inhibitors attenuated hepatic IRI in animal models [17–24]. Increased blood flow to hepatic tissues, endothelial protective effects, platelet anti-aggregating effects, anti-inflammatory actions, increased bile output, and improved survival rates were reported to be benefits of PDE3 inhibitors on hepatic IRI [18,19,21,22]. However, few experimental studies have assessed the regional specificity of the pharmacological suppressive effects of PDE3 inhibitors on LI with hepatic IRI.
Previous evidence has shown that hepatic IRI damages other organs such as the lungs, heart, kidneys, and small intestine [25–31]. In clinical practice, the Pringle maneuver causes congestion in the small intestine induced by HDL clamping. Severe small intestinal congestion usually occurs during the anhepatic period of LT surgery. Pathologically, previous experimental reports have demonstrated that hepatic IRI and an animal model of LT presented small intestinal injury (SII), including mucosal detachment and intestinal barrier disturbances, resulting in increased enterotoxin absorption and bacterial translocation (BT) [32–34]. Additionally, most candidate recipients for LT have preoperative complications such as edema and mucosal atrophy in the small intestine due to portal hypertension or malnutrition. Bacteremia occurs at a high rate after LT in adults and induces a higher mortality rate in those who develop it. Moreover, some intestinal bacteria were included as etiological organisms [35]. Therefore, some recipients with bacteremia may experience BT induced by SII. The pathophysiology of SII with hepatic IRI should be studied to determine a method of attenuation. Most reports on the attenuating effects of PDE3 inhibitors on hepatic IRI focused on LI; however, few reports have assessed SII.
This study was conducted to clarify the lobular distribution of LI with hepatic IRI and the regional specificity of the attenuating effects of the PDE3 inhibitor milrinone. We also evaluated the mechanism of SII with hepatic IRI and examined the pharmacological effects of milrinone on SII.
Material and Methods
REAGENTS:
Milrinone, a PDE3 inhibitor, was purchased from FUJIFILM Wako Pure Chemical Corporation (Osaka, Japan) and dissolved in distilled water with equimolar lactic acid. The milrinone dose (50 μg/kg) used in this study corresponds to that applied clinically as a loading dose. This dose was also used intravenously in a rat model of hepatic IRI as in previous experimental reports, which showed that the dose did not affect systemic hemodynamics [21,22,24].
ANIMALS:
Eight-week-old male Wistar rats weighing 250–300 g (Charles River Laboratories, Kanagawa, Japan) were maintained under a 12-h light/dark cycle in the Animal Experiment Laboratory of Kanazawa University. The rats were allowed free access to water and standard solid feed. The experiments and procedures were approved by the Animal Care and Use Committee of the University of Kanazawa (AP-173871; Kanazawa, Japan).
EXPERIMENTAL PROTOCOL AND PROCEDURES:
Figure 1A shows the experimental protocol. Before the experimental surgery, the rats were fasted for 24 h but had free access to water. Inhalation anesthesia using 1.5% isoflurane with an artificial respirator (KN-1071 NARCOBIT TypeII; Natsume Seisakusho Co., Tokyo, Japan) was used in all surgical procedures. After injecting 100 IU/kg of heparin sodium, a midline incision was performed to expose the liver. The rats were randomly divided into the sham, ischemia-reperfusion (IR), or IR+milrinone groups (n=13 per group). Either milrinone or saline was intraportally administered via intrasplenic injection. In the IR+milrinone group, milrinone (50 μg/kg) was injected into the spleen 5 min before whole hepatic ischemia. Intrasplenic injection was performed only once at the inferior pole of the spleen (Figure 1B) as an alternative route for continuous intraportal administration without portal vein injury [36–38]. In the IR and sham groups, saline was injected into the spleen. The insertion site of the splenic injection was cauterized via electric cautery (Gemini Cautery System; CellPoint Scientific Inc., Gaithersburg, MD) to stop the bleeding (Figure 1B). The HDL was clamped with a hemostasis clip (KN-353 AL-1; Natsume Seisakusho Co., Tokyo, Japan). After 30 min of total hepatic ischemia, the clip was removed to initiate hepatic reperfusion, and the abdominal cavity was closed with a 4-0 silk suture. Immediately after operation, the rats were allowed free access to water and standard solid feed. In sham groups, the rats underwent the same operation as the other animals; however, the HDL was not clamped. Five hours after the reperfusion or sham operation, the rats were killed to collect blood samples and liver and small intestinal specimens.
BIOCHEMICAL ANALYSIS AND PLATELET COUNTS:
Plasma samples were immediately separated from the blood samples by centrifugation. The plasma samples were stored at −80°C. Serum concentrations of aspartate aminotransferase (AST), alanine aminotransferase (ALT), total bilirubin (T-Bil) direct bilirubin (D-Bil), and lactate dehydrogenase (LDH) as standard indicators of hepatocellular injury, and hyaluronic acid (HA), as a marker of injury to hepatic sinusoidal endothelial cells (SECs), were measured from the same samples by SRL, Inc. (Tokyo, Japan). Hepatic SECs are the main location of HA clearance; therefore, SEC damage increases serum HA levels [39]. Platelet count was evaluated by an automated blood cell counter (Celltac α MEK-6458; Nihon Kohden, Tokyo, Japan) using 500 μL of sampled blood.
HISTOLOGICAL ANALYSIS AND IMMUNOHISTOCHEMISTRY:
Liver tissue was sampled from the left lateral lobe, and small intestinal tissue was sampled at full thickness from the distal ileum (10 cm from the ileocecal valve). The intestinal lumen was carefully cannulated and gently washed with 10% formalin before fixing. Liver and small intestinal tissues were fixed in 10% neutral buffered formalin, processed, embedded in paraffin, and sliced serially into 4-μm sections. Slides were stained with hematoxylin and eosin (H&E). Additional slides were deparaffinized in xylene and rehydrated for immunohistochemical analysis using the avidin-biotin peroxidase complex method. Anti-CD34 antibody (1: 100; ab81289; Abcam, Tokyo, Japan) was used to assess alterations in the sinusoidal endothelium as sinusoidal capillarization. Apoptosis was assessed using anti-cleaved caspase-3 antibody (1: 1000; 9664; Cell Signaling Technology, Danvers, MA). Anti-single-stranded deoxyribonucleic acid (ss-DNA) antibody (1: 50; Immuno-Biological Laboratories, Fujioka, Gunma, Japan) was used as a marker of apoptotic DNA fragmentation. Tissue sections were examined by an independent investigator.
LIVER TISSUES: Distribution of hepatic IRI in liver tissues was categorized using the Rappaport classification [40]. Zone 1 is the periportal zone and zone 3 is the pericentral zone. Zone 2 is the region between zones 1 and 3. The hepatic IRI severity in each zone was scored from 0 to 4 on the basis of the degrees of congestion, hepatocyte vacuolization, and necrosis, according to Suzuki’s criteria [41]. In the immunohistochemical analysis of CD34, the areas with hepatic SEC staining were graded using a subjective grading scale from 0 to 3 as follows: 0 (none), 1 (weak), 2 (moderate), and 3 (strong). In the immunohistochemical analysis of cleaved caspase-3, the cleaved caspase-3-positive hepatocytes per field were counted. The scoring was done at 10 random fields (magnification, ×200) of each zone and averaged for each zone per slide.
SMALL INTESTINAL TISSUES: Mucosal injury, villus congestion, villus height, and mucosal thickness in the small intestine were quantitatively analyzed. Mucosal injury was graded using the Park score [42] from 0 to 8. Villus congestion was graded using a subjective grading scale from 0 to 4 as follows: 0 (no congestion), 1 (mild), 2 (partial), 3 (entire), and 4 (entire/severe). The scoring was done at 10 random fields (magnification, ×100) per slide and averaged per slide. In the immunohistochemical analysis of cleaved caspase-3 and ss-DNA, the number of cells that stained strongly positive per field was calculated from 10 random fields (magnification, ×400) per slide and averaged.
SURVIVAL ANALYSIS:
A previous report showed that 30 min of whole hepatic ischemia was not life threatening, and the rats lived after hepatic IR [39]. Therefore, we used a hepatic IRI model with 45 min of total hepatic ischemia to analyze survival. The survival rate was calculated from the number of animals living longer than 7 days postoperatively. In the survival study, the rats were divided into the IR (n=13) and IR+milrinone (n=13) groups.
STATISTICAL ANALYSIS:
Data are presented as the mean±standard deviation. Statistical analyses were performed by Student’s
Results
MACROSCOPIC FINDINGS:
Before the liver ischemia, macroscopic findings for both the liver and small intestine showed no abnormalities. After 30 min of ischemia, the liver surface appeared ischemic because of the blocked inflow of blood to the liver. The small intestine became distended, congested, and dark red because of the obstructed outflow of blood (Figure 2A). Five hours after reperfusion, the liver surfaces of the IR and IR+milrinone groups appeared brown and slightly congested. The small intestines of the IR group remained congested and slightly distended, whereas these effects on the small intestines were attenuated in the IR+milrinone group (Figure 2B).
BIOCHEMISTRY AND PLATELET COUNTS:
Table 1 shows the serum biochemistry test and platelet count results. Serum AST, ALT, T-Bil, D-Bil, and LDH, as standard indicators of hepatocellular injury, were significantly elevated in the IR group compared with those of the sham group (P<0.001, P<0.001, P<0.001, P<0.001, and P=0.022, respectively). Serum HA, a marker of hepatic SEC injury, was significantly elevated in the IR group compared with that of the sham group (P<0.001). Serum AST and D-Bil levels were significantly lower in the IR+milrinone group than in the IR group (P=0.016 and P=0.007, respectively). Platelet counts were evaluated to determine milrinone’s anti-aggregating effect on platelet count kinetics in hepatic IRI. Platelet counts were significantly decreased in the IR group as compared with those of the sham group (P=0.035). Platelets counts were significantly higher in the IR+milrinone group than in the IR group (P=0.022).
SURVIVAL RATE:
After 45 min of whole hepatic ischemia, 6 of the 13 rats in the IR group died within 24 h after surgery. The 7-day survival rate of the IR group was 53.8%. Twelve of the 13 rats in the IR+milrinone group survived, and the 7-day survival rate was increased significantly to 92.3% (P=0.03; Figure 9).
Discussion
Previous reports stated that zone 3 had the highest distribution of necrosis induced by hepatic IRI in the liver parenchyma of a rat LT model [8,9]. In our hepatic IRI model, zone 3 also showed the highest distribution of necrosis compared with zones 1 and 2. Hepatocyte vacuolization was also more significant in zone 3 than in zone 1. Immunohistochemical staining revealed that zone 3 contained significantly more hepatocytes expressing cleaved caspase-3, a marker of apoptosis, than did zone 1. Under normal conditions, zone 3 is relatively hypoxic compared with zone 1 [43,44]. Therefore, the oxygen concentration in zone 3 may be lower during hepatic ischemia. Additionally, a previous experimental report revealed that hepatocytes cultured at the oxygen levels of zone 3 were more susceptible to anoxia-induced cell damage than were hepatocytes cultured at zone 1 oxygen levels because of increased membrane blebbing and loss of mitochondrial membrane potential [44]. Moreover, hepatocytes cultured at zone 3 oxygen levels were less able to recover after reoxygenation [44]. Hepatocyte vacuolization, necrosis, and apoptosis developed mainly in zone 3 compared with zone 1 because worsening hepatic ischemia-induced hypoxemia may strongly affect the hepatocytes in zone 3.
Sinusoidal endothelial injury tended to occur in zone 3 because intercellular adhesion molecule-1 expression induced by hepatic IRI was detected at the sinusoidal endothelium mainly in zone 3 [19]. Our hepatic IRI model also revealed that serum HA levels, which indicated the degree of SEC injury, increased significantly. In addition, immunohistochemical staining revealed significantly stronger expression of CD34, a marker of sinusoidal capillarization, in zone 3 than in zone 1 of the IR group. Under normal conditions, SECs have fenestrations, which facilitate transport of fluids, solutes, oxygen, and particles from the sinusoidal lumen to the space of Disse and hepatocytes [45]. Alterations in the SECs, such as loss of fenestrations and deposition of a basement membrane, are called sinusoidal capillarization. Sinusoidal capillarization impedes SEC function via the fenestrations [46]. CD34 is an intercellular adhesion protein often used to detect the presence of sinusoidal capillarization [47–50]. Previous reports revealed that CD34 expression occurred simultaneously with SEC injury, hepatocyte apoptosis, and liver functional impairment [47,49,50]. In our hepatic IRI model, sinusoidal capillarization occurred mainly in zone 3 and may have affected hepatic parenchymal injury, including vacuolization, necrosis, and apoptosis, which developed in zone 3 compared with zone 1.
Endothelial nitric oxide synthase (eNOS) mediated the production of NO, a vasodilator [51]. eNOS in SECs plays an important physiological role in decreasing portal pressure, attenuating hepatic IRI injury, and improving hepatic microcirculation [51]. Previous reports revealed that NO levels and eNOS function were markedly reduced in LT and hepatic IRI models because of deteriorated SEC function [17,52–54], consequently leading to hepatic microcirculatory failure [55]. In our hepatic IRI model, congestion occurred mainly in zone 3 compared with zone 1. One possible mechanism for this may be associated with reduced red blood cell velocity induced by deteriorated SEC function. Regarding the congestion at zone 3, we previously used a VOD model, characterized by severe congestion and SEC injury in zone 3, to demonstrate that extravasated platelet aggregation (EPA) occurred after sinusoidal endothelial denuding, and platelet activation resulted in the release of PAI-1 [49,50]. PAI-1 negatively regulates hepatocyte proliferation by inhibiting urokinase-type plasminogen activator, which activates hepatocyte growth factor [56,57]. In our hepatic IRI model, approximately 60% of rats in the IR group showed congestion in zone 3. Liver regeneration may be suppressed because of EPA in these rats, and further investigation is required.
In previous studies of hepatic IRI models, milrinone and amrinone improved hepatic circulation, inducing increased cAMP concentrations and NO production [17,19,21]. In our hepatic IRI model, morphologically, the attenuation effect of milrinone administration was observed in the necrosis and total Suzuki’s scores in both zones 1 and 3, and congestion in zone 3. Immunohistochemical staining revealed that administering milrinone attenuated CD34 expression in the sinusoidal endothelium and decreased the cleaved caspase 3-positive hepatocytes in zone 3. We suggest that the endothelial protective effects of milrinone inhibited the sinusoidal capillarization associated with SEC injury, which consequently maintained the SEC function in reducing hepatocyte apoptosis in zone 3 compared with that in zone 1. We also speculated that milrinone improved SEC injury following hepatic circulation during reperfusion, consequently decreasing congestion. These attenuating effects on LI might be reflected in the significant decrease in hepatocellular injury markers shown in the serum biochemistry results for the IR+milrinone group.
SII following hepatic IRI with total hepatic ischemia is considered a congestive injury affected by obstructing the outflow of blood from the small intestines. Therefore, previous experimental and clinical reports demonstrated that using a shunt to attenuate small intestinal congestion improved the outcomes of hepatobiliary–pancreatic surgery and animal models of hepatic IRI [58–60]. The efficacy of the temporary portocaval shunt for avoiding small intestinal congestion has been recognized in LT [58]. An antithrombogenic Anthron® bypass tube was placed between the superior mesenteric and femoral veins to reduce small intestinal congestion in a pancreaticoduodenectomy for a tumor invading the portal vein during portal vein resection and reconstruction [59]. Portosystemic shunting in an animal model attenuated hepatic IRI after 30 min of total hepatic ischemia [60]. Thus, suppressing small intestinal congestion is a simple but effective method for treating hepatic IRI. Our hepatic IRI model revealed mucosal injury, severe villus congestion, and atrophy. In addition, immunohistochemistry results revealed that expressions of both cleaved caspase-3 and ss-DNA as apoptosis markers occurred mainly at the lamina propria. Previous reports also demonstrated that apoptosis occurred with SII following hepatic IRI with total hepatic ischemia [29,31]. However, the location where apoptosis localizes in the villi is unclear. In our model, most SII occurred at the lamina propria, but we could not identify which cells in the lamina propria were primarily damaged. Further studies are needed to elucidate the mechanisms of these findings.
Administering milrinone significantly improved mucosal injury, villus congestion, and apoptosis in the small intestine. Milrinone attenuated the congestion in both the liver parenchyma and the small intestine. These data may indicate that portal vein pressure was decreased because of improved hepatic circulation from milrinone during reperfusion, which attenuated the small intestinal congestion. Moreover, this improved outflow circulation in the small intestine may also attenuate the mucosal injury. Our data indicated that administering milrinone attenuated both the LI and SII induced by hepatic IRI.
Some studies have focused on the pathophysiology of platelets in hepatic IRI. In experimental settings, hepatic IRI with shorter hepatic ischemia times revealed that most adherent platelets were detected in zones 1 and 2 [61]. In the severe model, platelets were also aggregated after 45 min of hepatic ischemia and were considered to be involved in LT and SEC injury development [19]. However, the regional specificity of the platelet aggregation in the severe hepatic IRI model was not fully assessed. In clinical settings, allograft tissue from a recipient of a diseased donor LT with extended cold ischemic time showed that the activated platelets were aggregated mainly in zone 3 [1]. In addition, this recipient presented primary graft dysfunction and severe thrombocytopenia. In our hepatic IRI model, platelet counts decreased 5 h after reperfusion in hepatic IRI with 30 min of hepatic ischemia. This might indicate that platelets were consumed in the liver parenchyma as adherent platelets or platelet aggregation resulted in decreased platelet counts, which was significantly attenuated in the IR+milrinone group. Amrinone and milrinone possess platelet antiaggregating effects [14,15,19]. Therefore, milrinone might suppress platelet aggregation in LI.
For LT in rats, warm whole hepatic ischemia for 30 min was not life threatening, and the rats lived after LT [39]. However, increasing the hepatic ischemic time to 45 min in the hepatic IRI model with HDL clamping decreased the 7-day survival rate to 70% [62]. A previous report demonstrated the outcome of administering milrinone on the survival rate in a hepatic IRI model [21]. In the hepatic IRI model with 60 min of ischemia in the left lateral liver lobe, pretreatment with milrinone improved the 7-day survival rate from 66.6% to 94.4% [21]. A hepatic IRI model with partial hepatic ischemia showed minimal small intestinal congestion and injury because most portal vein flow was preserved. In the hepatic IRI model with total hepatic ischemia, our study and previous reports indicated SII in addition to LI [29–31]. Therefore, in our study, the 7-day survival rate of the hepatic IRI model after 45 min of total hepatic ischemia worsened to 53.8%. Although pretreatment with milrinone was used in a hepatic IRI model with total hepatic ischemia, the survival rate improved to 92.3%. Our study indicated that administering milrinone synergistically attenuated both the LI and SII. These pharmaceutical benefits might contribute to improving the survival rate of animals with hepatic IRI with total hepatic ischemia.
The terminal half-life of milrinone via infusion is 2–7 h [12]. Therefore, administering milrinone as a pretreatment for hepatic IRI presented a pharmaceutical effect that continued during the ischemia and after reperfusion. Previous reports demonstrated that both pre- and posttreatment with milrinone attenuated hepatic IRI [18,21,22,24]. In this study, we selected milrinone administration as a pretreatment for hepatic IRI because we thought that the prophylactic administration before the development of LI was important.
Pretreatment with milrinone in the hepatic IRI model via direct injection into the portal vein involved a risk of abdominal bleeding from the insertion site after HDL clamping because of increased portal vein pressure. Hemostasis at the insertion site using sutures or electric cautery also carried a risk of portal vein stenosis or portal thrombosis. Therefore, in this study, milrinone was administered through the splenic vein to the portal vein via intrasplenic injection. Intrasplenic injection was used experimentally and clinically as an alternative route for continuous intraportal administration without portal vein injury [36–38,63]. This procedure helped ensure a stable supply of milrinone pretreatment directly to the liver and helped maintain stability of the experimental model. In addition, we cauterized the insertion site of the intrasplenic injection on the capsule using electric cautery to stop the bleeding.
Conclusions
Our data suggested that LI induced by hepatic IRI with total hepatic ischemia by HDL clamping occurred significantly in zone 3. Therefore, hepatic IRI may be partially associated with induction and development of severe zone 3-based LI in the early phase after LT. Moreover, SII also occurred with the hepatic IRI. Milrinone pretreatment via intrasplenic injection for hepatic IRI attenuated LI and SII, and consequently improved the survival rates. This study showed that intraportal administration of milrinone may be a useful preemptive treatment for hepatic IRI.
Figures
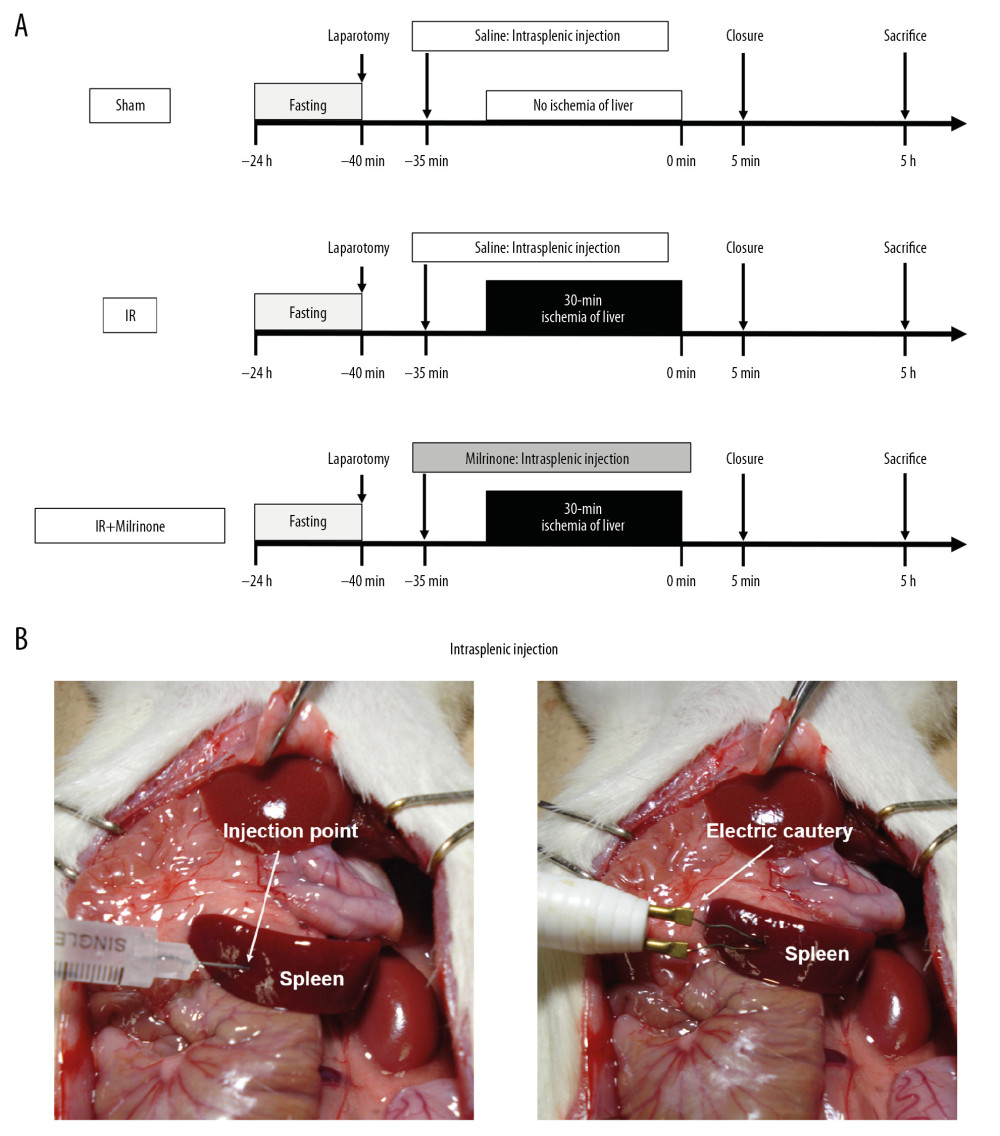 Figure 1. Experimental protocol: In the ischemia-reperfusion (IR)+milrinone group, milrinone was injected into the spleen before whole hepatic ischemia. In the IR and sham groups, saline was injected into the spleen. After 30 min of whole hepatic ischemia, hepatic reperfusion was initiated. Five hours after the reperfusion or sham operation, the rats were killed to collect blood samples and liver and small intestinal specimens (A). Either milrinone or saline was intraportally administered via intrasplenic injection. The insertion site of the splenic injection was cauterized via electric cautery to stop the bleeding (B).
Figure 1. Experimental protocol: In the ischemia-reperfusion (IR)+milrinone group, milrinone was injected into the spleen before whole hepatic ischemia. In the IR and sham groups, saline was injected into the spleen. After 30 min of whole hepatic ischemia, hepatic reperfusion was initiated. Five hours after the reperfusion or sham operation, the rats were killed to collect blood samples and liver and small intestinal specimens (A). Either milrinone or saline was intraportally administered via intrasplenic injection. The insertion site of the splenic injection was cauterized via electric cautery to stop the bleeding (B). 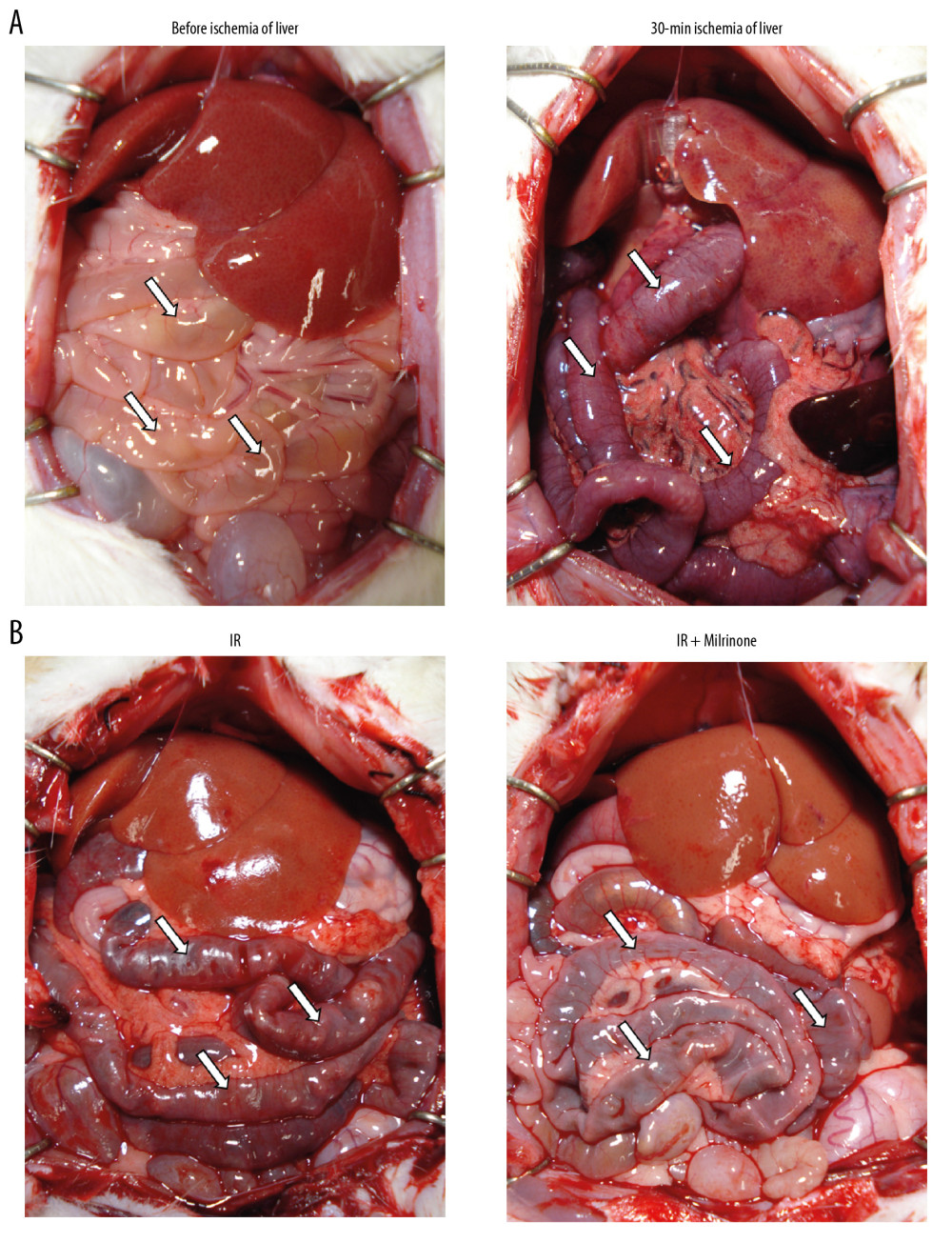 Figure 2. Macroscopic findings: Before ischemia of the liver, both the liver and small intestine showed no abnormalities. After 30 min of ischemia of the liver, the liver surfaces appeared ischemic and the small intestines became distended, congested, and dark red (A). Five hours after reperfusion, the liver surfaces of the ischemia–reperfusion (IR) and IR+milrinone groups appeared brown and slightly congested. The small intestines of the IR group remained congested and slightly distended. These findings in the small intestines were attenuated in the IR+milrinone group (B). White arrows, small intestine.
Figure 2. Macroscopic findings: Before ischemia of the liver, both the liver and small intestine showed no abnormalities. After 30 min of ischemia of the liver, the liver surfaces appeared ischemic and the small intestines became distended, congested, and dark red (A). Five hours after reperfusion, the liver surfaces of the ischemia–reperfusion (IR) and IR+milrinone groups appeared brown and slightly congested. The small intestines of the IR group remained congested and slightly distended. These findings in the small intestines were attenuated in the IR+milrinone group (B). White arrows, small intestine. 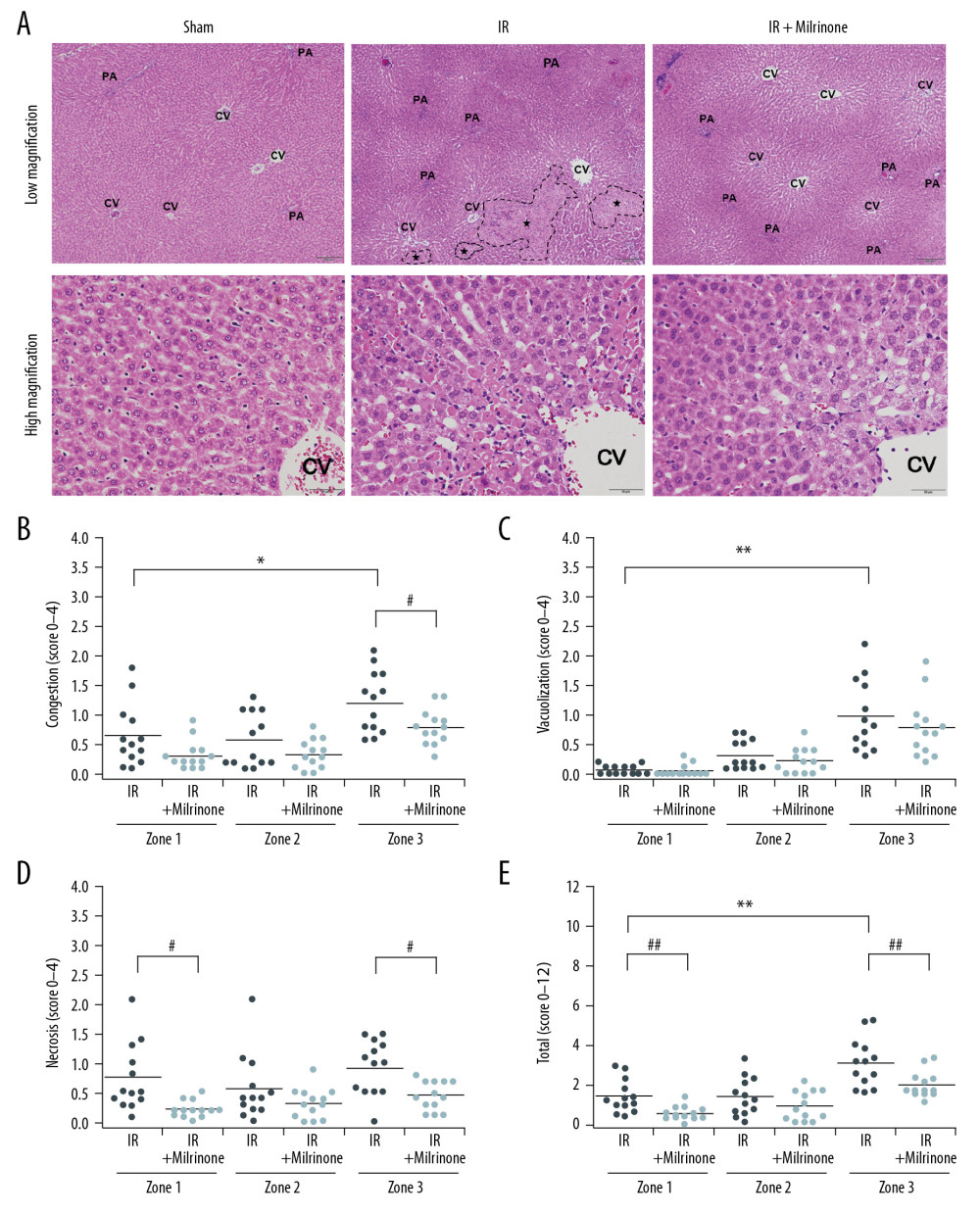 Figure 3. Histological changes of liver parenchyma related to hepatic ischemia-reperfusion (IR) injury: Representative hematoxylin and eosin staining sections of the sham, IR, and IR+milrinone groups (A). The livers of the IR group revealed histological changes characterized by hepatocellular vacuoles and focal necrosis (*), primarily in pericentral zone (zone 3) at low magnification. At high magnification, congestion, single cell necrosis of hepatocytes, and hepatocytes with vacuoles and swelling were observed around the central vein. Additionally, the sinusoidal structures were collapsed. In contrast, in the livers of the IR+milrinone group, focal necrosis of hepatocytes was markedly attenuated, and the lobular architecture was preserved. However, hepatocellular vacuoles were recognizable at zone 3 at low magnification. High magnification revealed the hepatocytes with vacuoles and swelling but little necrosis. The sinusoidal structure was almost maintained, and congestion was mild. PA – portal area; CV – central vein. Quantification of liver parenchyma injury with hepatic IR injury using Suzuki’s criteria at 5 h after reperfusion (B–E). The congestion score revealed that the zone 3 score in the IR group was significantly elevated as compared with that of the periportal zone (zone 1). The zone 3 score in the IR+milrinone group was significantly attenuated compared with that of the IR group (B). The hepatocyte vacuolization score revealed that the zone 3 score in the IR group was significantly higher than that of zone 1 (C). The necrosis scores revealed that the scores at zones 1 and 3 of the IR+milrinone group were significantly improved compared with those at the same zones in the IR group (D). The total scores revealed that the zone 3 score in the IR group was significantly higher than that of zone 1. The zones 1 and 3 scores of the IR+milrinone group were significantly attenuated as compared with those at the same zone in the IR group (E). * Significantly (P<0.01) different from zone 1 of the IR group. ** Significantly (P<0.001) different from zone 1 of the IR group. # Significantly (P<0.05) different from the IR group of the same zone. ## Significantly (P<0.01) different from the IR group of the same zone.
Figure 3. Histological changes of liver parenchyma related to hepatic ischemia-reperfusion (IR) injury: Representative hematoxylin and eosin staining sections of the sham, IR, and IR+milrinone groups (A). The livers of the IR group revealed histological changes characterized by hepatocellular vacuoles and focal necrosis (*), primarily in pericentral zone (zone 3) at low magnification. At high magnification, congestion, single cell necrosis of hepatocytes, and hepatocytes with vacuoles and swelling were observed around the central vein. Additionally, the sinusoidal structures were collapsed. In contrast, in the livers of the IR+milrinone group, focal necrosis of hepatocytes was markedly attenuated, and the lobular architecture was preserved. However, hepatocellular vacuoles were recognizable at zone 3 at low magnification. High magnification revealed the hepatocytes with vacuoles and swelling but little necrosis. The sinusoidal structure was almost maintained, and congestion was mild. PA – portal area; CV – central vein. Quantification of liver parenchyma injury with hepatic IR injury using Suzuki’s criteria at 5 h after reperfusion (B–E). The congestion score revealed that the zone 3 score in the IR group was significantly elevated as compared with that of the periportal zone (zone 1). The zone 3 score in the IR+milrinone group was significantly attenuated compared with that of the IR group (B). The hepatocyte vacuolization score revealed that the zone 3 score in the IR group was significantly higher than that of zone 1 (C). The necrosis scores revealed that the scores at zones 1 and 3 of the IR+milrinone group were significantly improved compared with those at the same zones in the IR group (D). The total scores revealed that the zone 3 score in the IR group was significantly higher than that of zone 1. The zones 1 and 3 scores of the IR+milrinone group were significantly attenuated as compared with those at the same zone in the IR group (E). * Significantly (P<0.01) different from zone 1 of the IR group. ** Significantly (P<0.001) different from zone 1 of the IR group. # Significantly (P<0.05) different from the IR group of the same zone. ## Significantly (P<0.01) different from the IR group of the same zone. 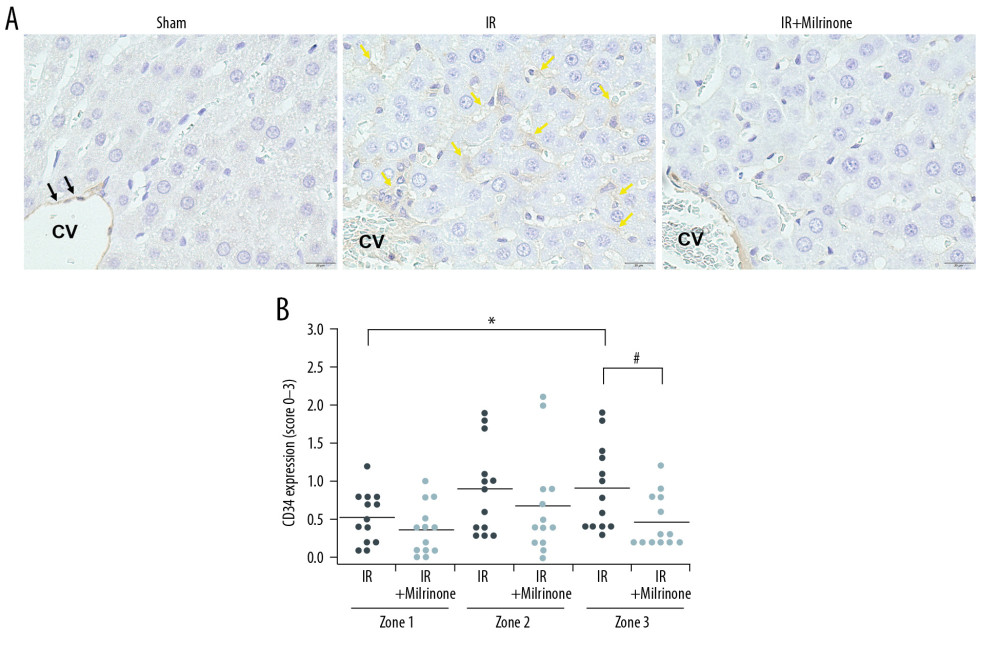 Figure 4. Assessment of CD34 expression in the liver parenchyma: CD34 was expressed at the central vein (CV) vascular endothelium (black arrows), but almost no CD34 was expressed around the CV in the sham group. CD34 expression was detected at the sinusoidal endothelium as slightly thickened endothelium along the sinusoid (yellow arrows) in the pericentral zone (zone 3) of the ischemia–reperfusion (IR) group. In the IR+milrinone group, CD34 expression was attenuated and detected as sinusoidal endothelial cells lining the sinusoid (A). The IR group presented significantly elevated zone 3 scores compared with those of the periportal zone (zone 1). The zone 3 score was significantly attenuated in the IR+milrinone group compared with that of the IR group (B). * Significantly different (P<0.05) from zone 1 of the IR group. # Significantly different (P<0.05) from the IR group of the same zone.
Figure 4. Assessment of CD34 expression in the liver parenchyma: CD34 was expressed at the central vein (CV) vascular endothelium (black arrows), but almost no CD34 was expressed around the CV in the sham group. CD34 expression was detected at the sinusoidal endothelium as slightly thickened endothelium along the sinusoid (yellow arrows) in the pericentral zone (zone 3) of the ischemia–reperfusion (IR) group. In the IR+milrinone group, CD34 expression was attenuated and detected as sinusoidal endothelial cells lining the sinusoid (A). The IR group presented significantly elevated zone 3 scores compared with those of the periportal zone (zone 1). The zone 3 score was significantly attenuated in the IR+milrinone group compared with that of the IR group (B). * Significantly different (P<0.05) from zone 1 of the IR group. # Significantly different (P<0.05) from the IR group of the same zone. 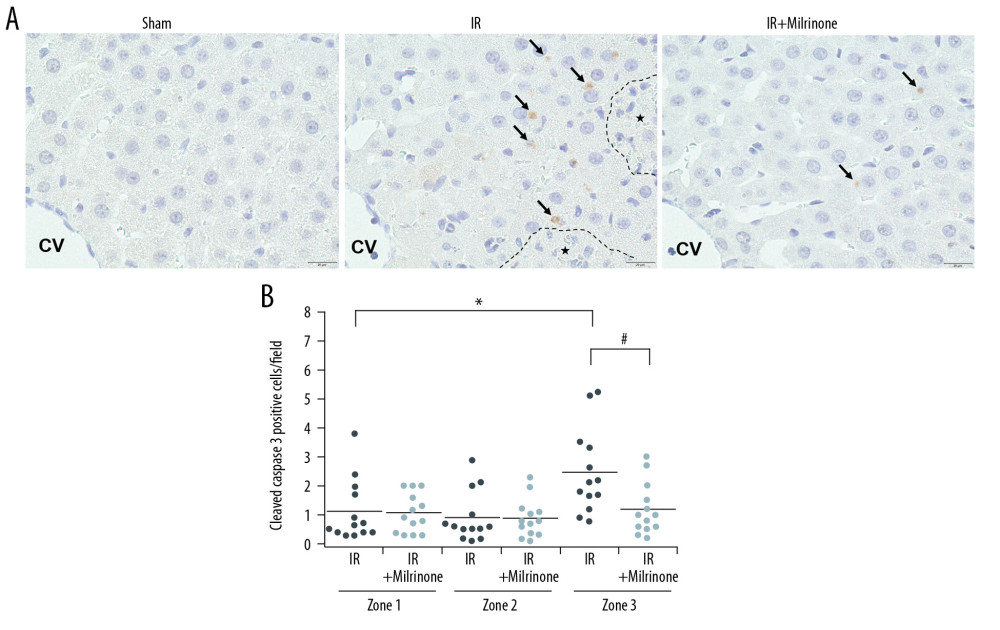 Figure 5. Assessment of cleaved caspase-3 expression in the liver parenchyma: The sham group had no cleaved caspase-3-positive cells in the hepatic parenchyma. In the ischemia-reperfusion (IR) group, cleaved caspase-3 was expressed in the cytoplasm of the hepatocytes (black arrows) around the necrotic area (*) in the pericentral zone (zone 3). The IR+milrinone group had fewer cleaved caspase 3-positive hepatocytes (A). The IR group had significantly more cleaved caspase-3-positive hepatocytes in zone 3 than in the periportal zone (zone 1). The IR+milrinone group had significantly fewer cleaved caspase-3-positive hepatocytes in zone 3 than did the IR group (B). * Significantly different (P<0.05) from zone 1 of the IR group. # Significantly different (P<0.05) from the IR group of the same zone. CV – central vein.
Figure 5. Assessment of cleaved caspase-3 expression in the liver parenchyma: The sham group had no cleaved caspase-3-positive cells in the hepatic parenchyma. In the ischemia-reperfusion (IR) group, cleaved caspase-3 was expressed in the cytoplasm of the hepatocytes (black arrows) around the necrotic area (*) in the pericentral zone (zone 3). The IR+milrinone group had fewer cleaved caspase 3-positive hepatocytes (A). The IR group had significantly more cleaved caspase-3-positive hepatocytes in zone 3 than in the periportal zone (zone 1). The IR+milrinone group had significantly fewer cleaved caspase-3-positive hepatocytes in zone 3 than did the IR group (B). * Significantly different (P<0.05) from zone 1 of the IR group. # Significantly different (P<0.05) from the IR group of the same zone. CV – central vein. 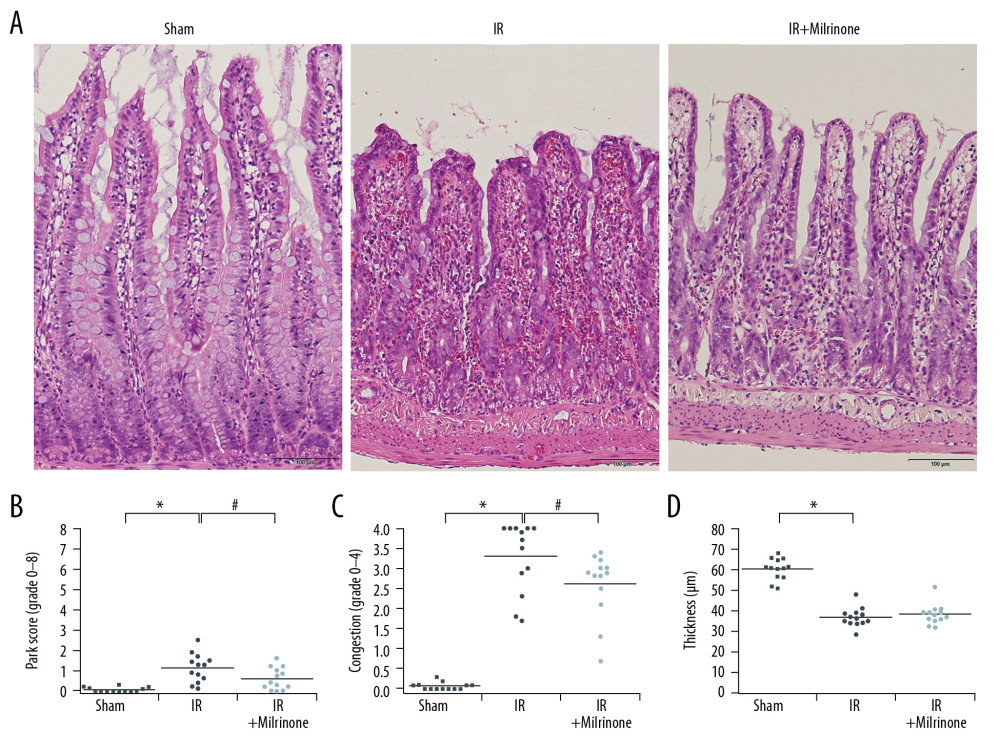 Figure 6. Histological changes of small intestine related to hepatic ischemia-reperfusion (IR) injury: In the IR group, the ilea showed severe congestion in the lamina propria with shortened villi. In the IR+milrinone group ilea, the villus architecture was preserved, and the congestion was decreased (A). The Park and congestion scores in the villi of the IR group were significantly higher than those of the sham group. These villus scores of the IR+milrinone group were significantly decreased as compared with those of the IR group. The IR group villi were lower than those of the sham group (B–D). * Significantly (P<0.001) different from the sham group. # Significantly (P<0.05) different from the IR group.
Figure 6. Histological changes of small intestine related to hepatic ischemia-reperfusion (IR) injury: In the IR group, the ilea showed severe congestion in the lamina propria with shortened villi. In the IR+milrinone group ilea, the villus architecture was preserved, and the congestion was decreased (A). The Park and congestion scores in the villi of the IR group were significantly higher than those of the sham group. These villus scores of the IR+milrinone group were significantly decreased as compared with those of the IR group. The IR group villi were lower than those of the sham group (B–D). * Significantly (P<0.001) different from the sham group. # Significantly (P<0.05) different from the IR group. 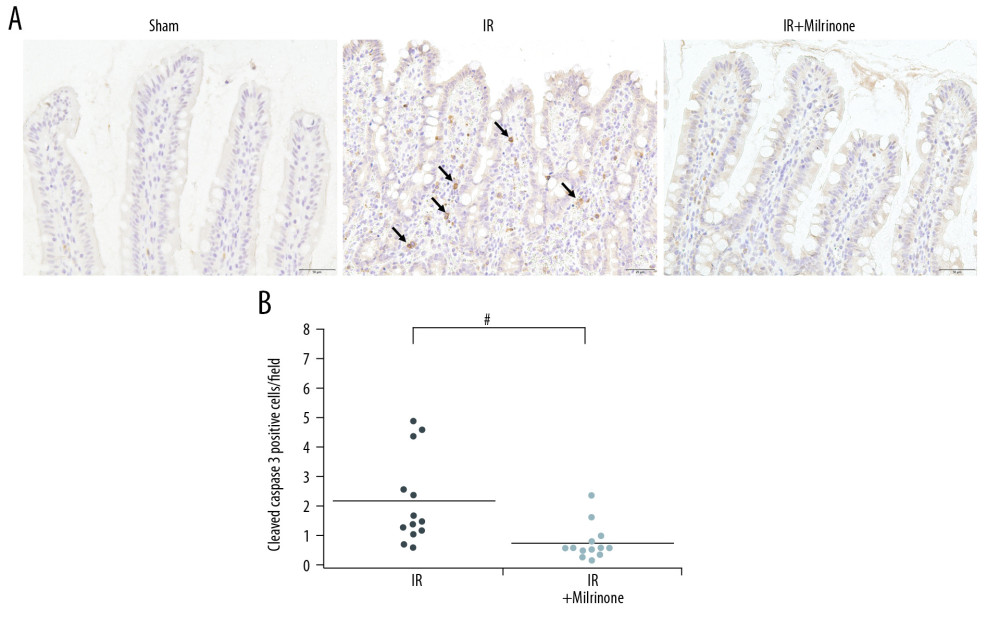 Figure 7. Assessment of cleaved caspase-3 expression in the small intestine: The sham group ilea contained no cleaved caspase-3-positive cells. In the ischemia–reperfusion (IR) group, some cells were strongly positive for cleaved caspase-3 expression (black arrows), mainly at the lamina propria, with congestion. The IR+milrinone group ilea had fewer of these cells (A). The small intestines of the IR+milrinone group had significantly fewer of these cells than did the IR group (B). # Significantly different (P<0.01) from the IR group.
Figure 7. Assessment of cleaved caspase-3 expression in the small intestine: The sham group ilea contained no cleaved caspase-3-positive cells. In the ischemia–reperfusion (IR) group, some cells were strongly positive for cleaved caspase-3 expression (black arrows), mainly at the lamina propria, with congestion. The IR+milrinone group ilea had fewer of these cells (A). The small intestines of the IR+milrinone group had significantly fewer of these cells than did the IR group (B). # Significantly different (P<0.01) from the IR group. 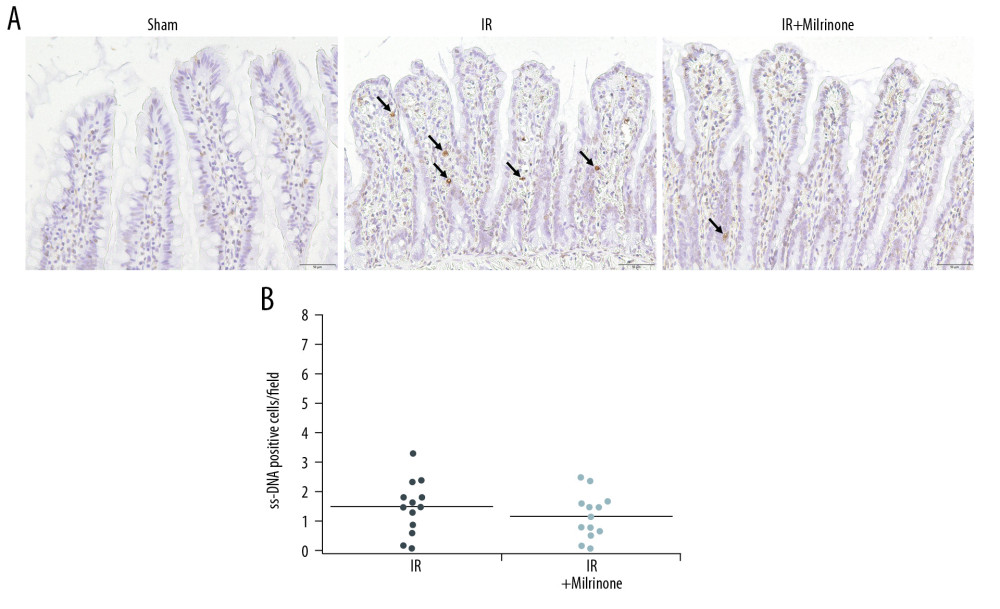 Figure 8. Assessment of single-stranded deoxyribonucleic acid (ss-DNA) expression in the small intestine: The sham group ilea contained no strongly stained ss-DNA-positive cells. The ischemia-reperfusion (IR) group showed some cells that were strongly positive for ss-DNA labeling (black arrows), mainly at the lamina propria, with congestion. Localization of the ss-DNA expression was similar to that of the cleaved caspase-3 expression (A). The IR+milrinone group had fewer of these cells in the small intestines than did the IR group, but the difference was not significant (B).
Figure 8. Assessment of single-stranded deoxyribonucleic acid (ss-DNA) expression in the small intestine: The sham group ilea contained no strongly stained ss-DNA-positive cells. The ischemia-reperfusion (IR) group showed some cells that were strongly positive for ss-DNA labeling (black arrows), mainly at the lamina propria, with congestion. Localization of the ss-DNA expression was similar to that of the cleaved caspase-3 expression (A). The IR+milrinone group had fewer of these cells in the small intestines than did the IR group, but the difference was not significant (B). ![Survival analysis (hepatic ischemia-reperfusion [IR] injury model with 45-min whole hepatic ischemia): Six of the 13 rats in the IR group died within 24 h after surgery. The 7-day survival rate of the IR group was 53.8%. Twelve of the 13 rats in the IR+milrinone group survived, and the 7-day survival rate was increased significantly to 92.3% (P=0.03).](https://jours.isi-science.com/imageXml.php?i=anntransplant-25-e922306-g009.jpg&idArt=922306&w=1000) Figure 9. Survival analysis (hepatic ischemia-reperfusion [IR] injury model with 45-min whole hepatic ischemia): Six of the 13 rats in the IR group died within 24 h after surgery. The 7-day survival rate of the IR group was 53.8%. Twelve of the 13 rats in the IR+milrinone group survived, and the 7-day survival rate was increased significantly to 92.3% (P=0.03).
Figure 9. Survival analysis (hepatic ischemia-reperfusion [IR] injury model with 45-min whole hepatic ischemia): Six of the 13 rats in the IR group died within 24 h after surgery. The 7-day survival rate of the IR group was 53.8%. Twelve of the 13 rats in the IR+milrinone group survived, and the 7-day survival rate was increased significantly to 92.3% (P=0.03). References
1. Takamura H, Nakanuma S, Hayashi H, Severe veno-occlusive disease/sinusoidal obstruction syndrome after deceased-donor and living-donor liver transplantation: Transplant Proc, 2014; 46; 3523-35
2. Nakanuma S, Miyashita T, Hayashi H, Extravasated platelet aggregation in liver zone 3 may correlate with the progression of sinusoidal obstruction syndrome following living donor liver transplantation: A case report: Exp Ther Med, 2015; 9; 1119-24
3. Sebagh M, Debette M, Samuel D, ‘Silent’ presentation of veno-occlusive disease after liver transplantation as part of the process of cellular rejection with endothelial predilection: Hepatology, 1999; 30; 1144-50
4. Demetris AJ, Adeyi O, Bellamy COBanff Working Group, Liver biopsy interpretation for causes of late liver allograft dysfunction: Hepatology, 2006; 44; 489-501
5. Burt AD, Ferrell LD, Portmann BC: MacSween’s pathology of the liver, 2011; 863-67, London, Churchill Livingstone Elsevier
6. Kim YI, Chung HJ, Song KE, Evaluation of a protease inhibitor in the prevention of ischemia and reperfusion injury in hepatectomy under intermittent Pringle maneuver: Am J Surg, 2006; 191; 72-76
7. Branum GD, Selim N, Liu X, Ischaemia and reperfusion injury of rat liver increases expression of glutathione S-transferase A1/A2 in zone 3 of the hepatic lobule: Biochem J, 1998; 330; 73-79
8. Shimizu H, Miyazaki M, Ito H, Mechanism of cold ischemia-reperfusion-induced graft injury after orthotopic liver transplantation in rats: Hepatogastroenterology, 2001; 48; 216-19
9. Kern H, Bald C, Brill T, The influence of retrograde reperfusion on the ischaemia-/reperfusion injury after liver transplantation in the rat: Int J Exp Pathol, 2008; 89; 433-37
10. Niemann JY, Garner D, Khaleeli E, Lewis RJ, Milrinone facilitates resuscitation from cardiac arrest and attenuate postresuscitation myocardial dysfunction: Circulation, 2003; 108; 3031-35
11. Jebeli M, Ghazinoor M, Mandegar MH, Effect of milrinone on short-term outcome of patients with myocardial dysfunction undergoing coronary artery bypass graft: A randomized controlled trial: Cardiol J, 2010; 17; 73-78
12. Skoyles JR, Sherry KM, Pharmacology, mechanisms of action and uses of selective phosphodiesterase inhibitors: Br J Anaesth, 1992; 68; 293-302
13. Polson JB, Strada SJ, Cyclic nucleotide phosphodiesterases and vascular smooth muscle: Annu Rev Pharmacol Toxicol, 1996; 36; 403-27
14. Anfossi G, Massucco P, Piretto V, Interplay between milrinone and adenosine in the inhibition of human platelet response: Gen Pharmacol, 1996; 27; 1149-54
15. Sly MK, Eberhart RC, Prager MD, Anti-platelet action of nitric oxide and selective phosphodiesterase inhibitors: Shock, 1997; 8; 115-18
16. Mori K, Takeuchi S, Moritoki H, Endothelium-dependent relaxation of rat thoracic aorta by amrinone-induced nitric oxide release: Eur Heart J, 1996; 17; 308-16
17. Katsuragi K, Takemura S, Minamiyama Y, Combined use of adenosine and amrinone inhibits reperfusion injury of rat liver: Pathophysiology, 2001; 8; 29-34
18. Ikegami T, Nishizaki T, Hiroshige S, Experimental study of a type 3 phosphodiesterase inhibitor on liver graft function: Br J Surg, 2001; 88; 59-64
19. Kobayashi T, Sugawara Y, Ohkubo T, Effects of amrinone on hepatic ischemia-reperfusion injury in rats: J Hepatol, 2002; 37; 31-38
20. Ishikawa H, Jin MB, Ogata T, Role of cyclic nucleotides in ischemia and reperfusion injury of canine livers: Transplantation, 2002; 73; 1041-48
21. Kume M, Banafsche R, Yamamoto Y, Dynamic changes of post-ischemic hepatic microcirculation improved by a pre-treatment of phosphodiesterase-3 inhibitor, milrinone: J Surg Res, 2006; 136; 209-18
22. Satoh K, Kume M, Abe Y, Implication of protein kinase A for a hepato-protective mechanism of milrinone pretreatment: J Surg Res, 2009; 155; 32-39
23. Kucuk C, Akcan A, Akyýldýz H, Effects of amrinone in an experimental model of hepatic ischemia-reperfusion injury: J Surg Res, 2009; 151; 74-79
24. Toyoda T, Tosaka S, Tosaka R, Milrinone-induced postconditioning reduces hepatic ischemia-reperfusion injury in rats: The roles of phosphatidylinositol 3-kinase and nitric oxide: J Surg Res, 2014; 186; 446-51
25. Peralta C, Perales JC, Bartrons R, The combination of ischemic preconditioning and liver Bcl-2 overexpression is a suitable strategy to prevent liver and lung damage after hepatic ischemia-reperfusion: Am J Pathol, 2002; 160; 2111-22
26. Nielsen VG, Tan S, Baird MS, Xanthine oxidase mediates myocardial injury after hepatoenteric ischemia-reperfusion: Crit Care Med, 1997; 25; 1044-50
27. Suzuki S, Serizawa A, Sakaguchi T, The roles of platelet-activating factor and endothelin-1 in renal damage after total hepatic ischemia and reperfusion: Transplantation, 2000; 69; 2267-73
28. Leister I, Sydow J, Stojanovic T, Impact of vasoactive intestinal polypeptide and gastrin-releasing peptide on small bowel microcirculation and mucosal injury after hepatic ischemia/reperfusion in rats: Int J Colorectal Dis, 2005; 20; 42-48
29. Jiang H, Meng F, Li W, Splenectomy ameliorates acute multiple organ damage induced by liver warm ischemia reperfusion in rats: Surgery, 2007; 141; 32-40
30. Yildiz F, Terzi A, Coban S, Purified micronized flavonoid fraction ameliorates the injury of spleen and ileum secondary to hepatic ischemia-reperfusion in rats: Dig Dis Sci, 2010; 55; 2237-43
31. Lauz Medeiros SH, de Oliveira Menezes A, Zogbi L, N-Acetylcysteine use in hepatic ischemia/reperfusion in rats minimizing bowel injury: Transplant Proc, 2016; 48; 2371-74
32. Simic M, Vukovic R, Fabri M, Damage in the hemato-enteral barrier in experimental liver transplantation: Acta Chir Iugosl, 1990; 37; 75-78
33. Liu P, Lu X, Han M, Influence of portal triad clamping on the intestine in pigs: Hunan Yi Ke Da Xue Xue Bao, 1998; 23; 246-48 [in Chinese]
34. Xie Y, Luo Z, Li Z, Structural shifts of fecal microbial communities in rats with acute rejection after liver transplantation: Microb Ecol, 2012; 64; 546-54
35. Iida T, Kaido T, Yagi S, Posttransplant bacteremia in adult living donor liver transplant recipients: Liver Transpl, 2010; 16; 1379-85
36. Nomi S, Naito K, Kahan BD, Pellis NR, Intrasplenic administration of interleukin-2 to potentiate specific chemoimmunotherapy in tumor-bearing mice: Cancer Res, 1986; 46; 5606-10
37. Matsumata T, Kanematsu T, Sonoda T, Acute liver failure in rats inhibited by intrasplenic administration of OK-432: J Surg Res, 1986; 40; 43-48
38. Kanoh K, Shimura T, Suzuki H, Antitumor effect of a splenic injection of 5-fluorouracil on metastatic liver cancer in mice: J Pharmacol Exp Ther, 2004; 308; 168-74
39. Okumura S, Uemura T, Zhao X, Liver graft preservation using perfluorocarbon improves the outcomes of simulated donation after cardiac death liver transplantation in rats: Liver Transpl, 2017; 23; 1171-85
40. Rappaport AM, Borowy ZJ, Lougheed WM, Lotto WN, Subdivision of hexagonal liver lobules into a structural and functional unit; Role in hepatic physiology and pathology: Anat Rec, 1954; 119; 11-33
41. Suzuki S, Toledo-Pereyra LH, Rodriguez FJ, Cejalvo D, Neutrophil infiltration as an important factor in liver ischemia and reperfusion injury. Modulating effects of FK506 and cyclosporine: Transplantation, 1993; 55; 1265-72
42. Park PO, Haglund U, Bulkley GB, Fält K, The sequence of development of intestinal tissue injury after strangulation ischemia and reperfusion: Surgery, 1990; 107; 574-80
43. Jungermann K, Kietzmann T, Oxygen: Modulator of metabolic zonation and disease of the liver: Hepatology, 2000; 31; 255-60
44. Broughan TA, Naukam R, Tan C, Effects of hepatic zonal oxygen levels on hepatocyte stress responses: J Surg Res, 2008; 145; 150-60
45. Wisse E, De Zanger RB, Charels K, The liver sieve: Considerations concerning the structure and function of endothelial fenestrae, the sinusoidal wall and the space of Disse: Hepatology, 1985; 5; 683-92
46. Mori T, Okanoue T, Sawa Y, Defenestration of the sinusoidal endothelial cell in a rat model of cirrhosis: Hepatology, 1993; 17; 891-97
47. Narita M, Oussoultzoglou E, Chenard M-P, Liver injury due to chemotherapy-induced sinusoidal obstruction syndrome is associated with sinusoidal capillarization: Ann Surg Oncol, 2012; 19; 2230-37
48. Guffroy M, Falahatpisheh H, Biddle K, Liver microvascular injury and thrombocytopenia of antibody-calicheamicin conjugates in cynomolgus monkeys – Mechanism and monitoring: Clin Cancer Res, 2017; 23; 1760-70
49. Hirata M, Tajima H, Miyashita T, Extravasated platelet aggregation in the livers of rats with drug-induced hepatic sinusoidal obstruction syndrome: Mol Med Rep, 2017; 15; 3147-52
50. Miyata T, Tajima H, Hirata M, Phosphodiesterase III inhibitor attenuates rat sinusoidal obstruction syndrome through inhibition of platelet aggregation in Disse’s space: J Gastroenterol Hepatol, 2018; 33; 950-57
51. Liu S, Rockey DC, Cicletanine stimulates eNOS phosphorylation and NO production via Akt and MAP kinase/Erk signaling in sinusoidal endothelial cells: Am J Physiol Gastrointest Liver Physiol, 2013; 305; 163-71
52. Koeppel TA, Thies JC, Schemmer P, Inhibition of nitric oxide synthesis in ischemia/reperfusion of the rat liver is followed by impairment of hepatic microvascular blood flow: J Hepatol, 1997; 27; 163-69
53. Miyashita T, Nakanuma S, Ahmed AK, Ischemia reperfusion-facilitated sinusoidal endothelial cell injury in liver transplantation and the resulting impact of extravasated platelet aggregation: Eur Surg, 2016; 48; 92-98
54. Ito T, Kuriyama N, Kato H, Sinusoidal protection by sphingosine-1-phosphate receptor 1 agonist in liver ischemia-reperfusion injury: J Surg Res, 2018; 222; 139-52
55. Serracino-Inglott F, Habib NA, Mathie RT, Hepatic ischemia reperfusion injury: Am J Surg, 2001; 181; 160-66
56. Mars WM, Zarnegar R, Michalopoulos GK, Activation of hepatocyte growth factor by the plasminogen activators uPA and tPA: Am J Pathol, 1993; 143; 949-58
57. Watanabe K, Togo S, Takahashi T, PAI-1 plays an important role in liver failure after excessive hepatectomy in the rat: J Surg Res, 2007; 143; 13-19
58. de Cenarruzabeitia IL, Lázaro JL, Bilbao I, Balsells J, Portocaval shunt throughout anhepatic phase in orthotopic liver transplantation for cirrhotic patients: Transplant Proc, 2007; 39; 2280-84
59. Nakao A, Nonami T, Harada A, Portal vein resection with a new antithrombogenic catheter: Surgery, 1990; 108; 913-18
60. Bedirli A, Sakrak O, Soyuer I, Muhtaroglu S, Portosystemic shunt prevents apoptosis in rat intestinal mucosa caused by total hepatic ischemia: Eur Surg Res, 2004; 36; 293-99
61. Nakano Y, Kondo T, Matsuo R: J Surg Res, 2008; 149; 192-98
62. Kuroda S, Tashiro H, Igarashi Y, Rho inhibitor prevents ischemia-reperfusion injury in rat steatotic liver: J Hepatol, 2012; 56; 146-52
63. Okuno K, Ohnishi H, Koh K, Clinical trials of intrasplenic arterial infusion of interleukin-2 (IS-IL-2) to patients with advanced cancer: Biotherapy, 1992; 4; 257-65
Figures
 Figure 1. Experimental protocol: In the ischemia-reperfusion (IR)+milrinone group, milrinone was injected into the spleen before whole hepatic ischemia. In the IR and sham groups, saline was injected into the spleen. After 30 min of whole hepatic ischemia, hepatic reperfusion was initiated. Five hours after the reperfusion or sham operation, the rats were killed to collect blood samples and liver and small intestinal specimens (A). Either milrinone or saline was intraportally administered via intrasplenic injection. The insertion site of the splenic injection was cauterized via electric cautery to stop the bleeding (B).
Figure 1. Experimental protocol: In the ischemia-reperfusion (IR)+milrinone group, milrinone was injected into the spleen before whole hepatic ischemia. In the IR and sham groups, saline was injected into the spleen. After 30 min of whole hepatic ischemia, hepatic reperfusion was initiated. Five hours after the reperfusion or sham operation, the rats were killed to collect blood samples and liver and small intestinal specimens (A). Either milrinone or saline was intraportally administered via intrasplenic injection. The insertion site of the splenic injection was cauterized via electric cautery to stop the bleeding (B). Figure 2. Macroscopic findings: Before ischemia of the liver, both the liver and small intestine showed no abnormalities. After 30 min of ischemia of the liver, the liver surfaces appeared ischemic and the small intestines became distended, congested, and dark red (A). Five hours after reperfusion, the liver surfaces of the ischemia–reperfusion (IR) and IR+milrinone groups appeared brown and slightly congested. The small intestines of the IR group remained congested and slightly distended. These findings in the small intestines were attenuated in the IR+milrinone group (B). White arrows, small intestine.
Figure 2. Macroscopic findings: Before ischemia of the liver, both the liver and small intestine showed no abnormalities. After 30 min of ischemia of the liver, the liver surfaces appeared ischemic and the small intestines became distended, congested, and dark red (A). Five hours after reperfusion, the liver surfaces of the ischemia–reperfusion (IR) and IR+milrinone groups appeared brown and slightly congested. The small intestines of the IR group remained congested and slightly distended. These findings in the small intestines were attenuated in the IR+milrinone group (B). White arrows, small intestine. Figure 3. Histological changes of liver parenchyma related to hepatic ischemia-reperfusion (IR) injury: Representative hematoxylin and eosin staining sections of the sham, IR, and IR+milrinone groups (A). The livers of the IR group revealed histological changes characterized by hepatocellular vacuoles and focal necrosis (*), primarily in pericentral zone (zone 3) at low magnification. At high magnification, congestion, single cell necrosis of hepatocytes, and hepatocytes with vacuoles and swelling were observed around the central vein. Additionally, the sinusoidal structures were collapsed. In contrast, in the livers of the IR+milrinone group, focal necrosis of hepatocytes was markedly attenuated, and the lobular architecture was preserved. However, hepatocellular vacuoles were recognizable at zone 3 at low magnification. High magnification revealed the hepatocytes with vacuoles and swelling but little necrosis. The sinusoidal structure was almost maintained, and congestion was mild. PA – portal area; CV – central vein. Quantification of liver parenchyma injury with hepatic IR injury using Suzuki’s criteria at 5 h after reperfusion (B–E). The congestion score revealed that the zone 3 score in the IR group was significantly elevated as compared with that of the periportal zone (zone 1). The zone 3 score in the IR+milrinone group was significantly attenuated compared with that of the IR group (B). The hepatocyte vacuolization score revealed that the zone 3 score in the IR group was significantly higher than that of zone 1 (C). The necrosis scores revealed that the scores at zones 1 and 3 of the IR+milrinone group were significantly improved compared with those at the same zones in the IR group (D). The total scores revealed that the zone 3 score in the IR group was significantly higher than that of zone 1. The zones 1 and 3 scores of the IR+milrinone group were significantly attenuated as compared with those at the same zone in the IR group (E). * Significantly (P<0.01) different from zone 1 of the IR group. ** Significantly (P<0.001) different from zone 1 of the IR group. # Significantly (P<0.05) different from the IR group of the same zone. ## Significantly (P<0.01) different from the IR group of the same zone.
Figure 3. Histological changes of liver parenchyma related to hepatic ischemia-reperfusion (IR) injury: Representative hematoxylin and eosin staining sections of the sham, IR, and IR+milrinone groups (A). The livers of the IR group revealed histological changes characterized by hepatocellular vacuoles and focal necrosis (*), primarily in pericentral zone (zone 3) at low magnification. At high magnification, congestion, single cell necrosis of hepatocytes, and hepatocytes with vacuoles and swelling were observed around the central vein. Additionally, the sinusoidal structures were collapsed. In contrast, in the livers of the IR+milrinone group, focal necrosis of hepatocytes was markedly attenuated, and the lobular architecture was preserved. However, hepatocellular vacuoles were recognizable at zone 3 at low magnification. High magnification revealed the hepatocytes with vacuoles and swelling but little necrosis. The sinusoidal structure was almost maintained, and congestion was mild. PA – portal area; CV – central vein. Quantification of liver parenchyma injury with hepatic IR injury using Suzuki’s criteria at 5 h after reperfusion (B–E). The congestion score revealed that the zone 3 score in the IR group was significantly elevated as compared with that of the periportal zone (zone 1). The zone 3 score in the IR+milrinone group was significantly attenuated compared with that of the IR group (B). The hepatocyte vacuolization score revealed that the zone 3 score in the IR group was significantly higher than that of zone 1 (C). The necrosis scores revealed that the scores at zones 1 and 3 of the IR+milrinone group were significantly improved compared with those at the same zones in the IR group (D). The total scores revealed that the zone 3 score in the IR group was significantly higher than that of zone 1. The zones 1 and 3 scores of the IR+milrinone group were significantly attenuated as compared with those at the same zone in the IR group (E). * Significantly (P<0.01) different from zone 1 of the IR group. ** Significantly (P<0.001) different from zone 1 of the IR group. # Significantly (P<0.05) different from the IR group of the same zone. ## Significantly (P<0.01) different from the IR group of the same zone. Figure 4. Assessment of CD34 expression in the liver parenchyma: CD34 was expressed at the central vein (CV) vascular endothelium (black arrows), but almost no CD34 was expressed around the CV in the sham group. CD34 expression was detected at the sinusoidal endothelium as slightly thickened endothelium along the sinusoid (yellow arrows) in the pericentral zone (zone 3) of the ischemia–reperfusion (IR) group. In the IR+milrinone group, CD34 expression was attenuated and detected as sinusoidal endothelial cells lining the sinusoid (A). The IR group presented significantly elevated zone 3 scores compared with those of the periportal zone (zone 1). The zone 3 score was significantly attenuated in the IR+milrinone group compared with that of the IR group (B). * Significantly different (P<0.05) from zone 1 of the IR group. # Significantly different (P<0.05) from the IR group of the same zone.
Figure 4. Assessment of CD34 expression in the liver parenchyma: CD34 was expressed at the central vein (CV) vascular endothelium (black arrows), but almost no CD34 was expressed around the CV in the sham group. CD34 expression was detected at the sinusoidal endothelium as slightly thickened endothelium along the sinusoid (yellow arrows) in the pericentral zone (zone 3) of the ischemia–reperfusion (IR) group. In the IR+milrinone group, CD34 expression was attenuated and detected as sinusoidal endothelial cells lining the sinusoid (A). The IR group presented significantly elevated zone 3 scores compared with those of the periportal zone (zone 1). The zone 3 score was significantly attenuated in the IR+milrinone group compared with that of the IR group (B). * Significantly different (P<0.05) from zone 1 of the IR group. # Significantly different (P<0.05) from the IR group of the same zone. Figure 5. Assessment of cleaved caspase-3 expression in the liver parenchyma: The sham group had no cleaved caspase-3-positive cells in the hepatic parenchyma. In the ischemia-reperfusion (IR) group, cleaved caspase-3 was expressed in the cytoplasm of the hepatocytes (black arrows) around the necrotic area (*) in the pericentral zone (zone 3). The IR+milrinone group had fewer cleaved caspase 3-positive hepatocytes (A). The IR group had significantly more cleaved caspase-3-positive hepatocytes in zone 3 than in the periportal zone (zone 1). The IR+milrinone group had significantly fewer cleaved caspase-3-positive hepatocytes in zone 3 than did the IR group (B). * Significantly different (P<0.05) from zone 1 of the IR group. # Significantly different (P<0.05) from the IR group of the same zone. CV – central vein.
Figure 5. Assessment of cleaved caspase-3 expression in the liver parenchyma: The sham group had no cleaved caspase-3-positive cells in the hepatic parenchyma. In the ischemia-reperfusion (IR) group, cleaved caspase-3 was expressed in the cytoplasm of the hepatocytes (black arrows) around the necrotic area (*) in the pericentral zone (zone 3). The IR+milrinone group had fewer cleaved caspase 3-positive hepatocytes (A). The IR group had significantly more cleaved caspase-3-positive hepatocytes in zone 3 than in the periportal zone (zone 1). The IR+milrinone group had significantly fewer cleaved caspase-3-positive hepatocytes in zone 3 than did the IR group (B). * Significantly different (P<0.05) from zone 1 of the IR group. # Significantly different (P<0.05) from the IR group of the same zone. CV – central vein. Figure 6. Histological changes of small intestine related to hepatic ischemia-reperfusion (IR) injury: In the IR group, the ilea showed severe congestion in the lamina propria with shortened villi. In the IR+milrinone group ilea, the villus architecture was preserved, and the congestion was decreased (A). The Park and congestion scores in the villi of the IR group were significantly higher than those of the sham group. These villus scores of the IR+milrinone group were significantly decreased as compared with those of the IR group. The IR group villi were lower than those of the sham group (B–D). * Significantly (P<0.001) different from the sham group. # Significantly (P<0.05) different from the IR group.
Figure 6. Histological changes of small intestine related to hepatic ischemia-reperfusion (IR) injury: In the IR group, the ilea showed severe congestion in the lamina propria with shortened villi. In the IR+milrinone group ilea, the villus architecture was preserved, and the congestion was decreased (A). The Park and congestion scores in the villi of the IR group were significantly higher than those of the sham group. These villus scores of the IR+milrinone group were significantly decreased as compared with those of the IR group. The IR group villi were lower than those of the sham group (B–D). * Significantly (P<0.001) different from the sham group. # Significantly (P<0.05) different from the IR group. Figure 7. Assessment of cleaved caspase-3 expression in the small intestine: The sham group ilea contained no cleaved caspase-3-positive cells. In the ischemia–reperfusion (IR) group, some cells were strongly positive for cleaved caspase-3 expression (black arrows), mainly at the lamina propria, with congestion. The IR+milrinone group ilea had fewer of these cells (A). The small intestines of the IR+milrinone group had significantly fewer of these cells than did the IR group (B). # Significantly different (P<0.01) from the IR group.
Figure 7. Assessment of cleaved caspase-3 expression in the small intestine: The sham group ilea contained no cleaved caspase-3-positive cells. In the ischemia–reperfusion (IR) group, some cells were strongly positive for cleaved caspase-3 expression (black arrows), mainly at the lamina propria, with congestion. The IR+milrinone group ilea had fewer of these cells (A). The small intestines of the IR+milrinone group had significantly fewer of these cells than did the IR group (B). # Significantly different (P<0.01) from the IR group. Figure 8. Assessment of single-stranded deoxyribonucleic acid (ss-DNA) expression in the small intestine: The sham group ilea contained no strongly stained ss-DNA-positive cells. The ischemia-reperfusion (IR) group showed some cells that were strongly positive for ss-DNA labeling (black arrows), mainly at the lamina propria, with congestion. Localization of the ss-DNA expression was similar to that of the cleaved caspase-3 expression (A). The IR+milrinone group had fewer of these cells in the small intestines than did the IR group, but the difference was not significant (B).
Figure 8. Assessment of single-stranded deoxyribonucleic acid (ss-DNA) expression in the small intestine: The sham group ilea contained no strongly stained ss-DNA-positive cells. The ischemia-reperfusion (IR) group showed some cells that were strongly positive for ss-DNA labeling (black arrows), mainly at the lamina propria, with congestion. Localization of the ss-DNA expression was similar to that of the cleaved caspase-3 expression (A). The IR+milrinone group had fewer of these cells in the small intestines than did the IR group, but the difference was not significant (B).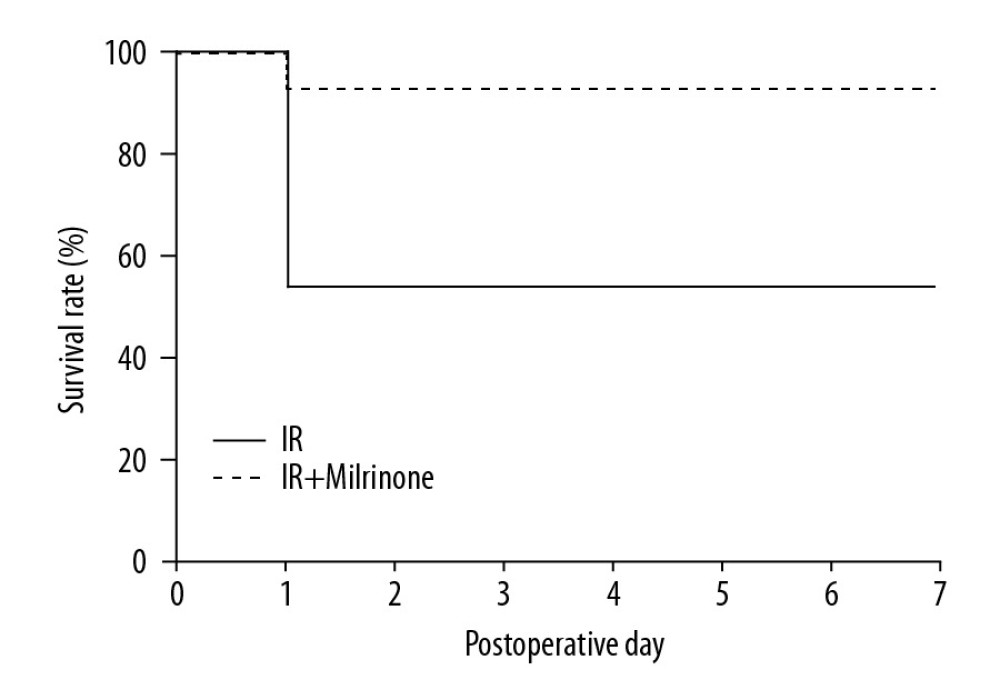 Figure 9. Survival analysis (hepatic ischemia-reperfusion [IR] injury model with 45-min whole hepatic ischemia): Six of the 13 rats in the IR group died within 24 h after surgery. The 7-day survival rate of the IR group was 53.8%. Twelve of the 13 rats in the IR+milrinone group survived, and the 7-day survival rate was increased significantly to 92.3% (P=0.03).
Figure 9. Survival analysis (hepatic ischemia-reperfusion [IR] injury model with 45-min whole hepatic ischemia): Six of the 13 rats in the IR group died within 24 h after surgery. The 7-day survival rate of the IR group was 53.8%. Twelve of the 13 rats in the IR+milrinone group survived, and the 7-day survival rate was increased significantly to 92.3% (P=0.03). Tables
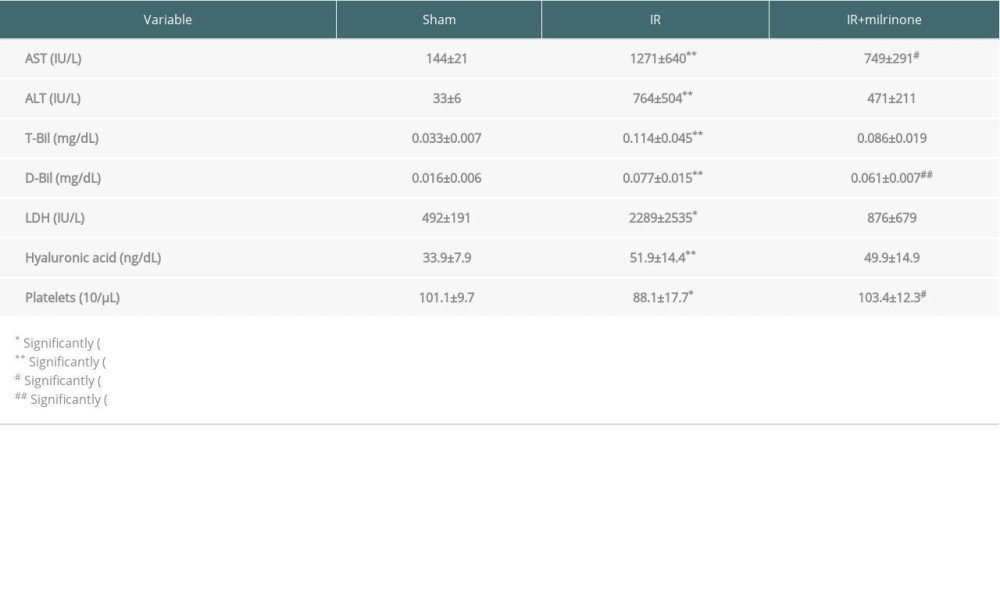 Table 1. Serum aspartate aminotransferase (AST), alanine aminotransferase (ALT), total bilirubin (T-Bil), direct bilirubin (D-Bil), lactate dehydrogenase (LDH), hyaluronic acid (HA) levels, and platelet counts 5 h after reperfusion: Serum AST, ALT, T-Bil, D-Bil, LDH, and HA were significantly elevated in the ischemia-reperfusion (IR) group compared with those of the sham group. Serum AST and D-Bil levels were significantly lower in the IR+milrinone group than in the IR group. Platelet counts were significantly decreased in the IR group as compared with those of the sham group. Platelet counts were significantly higher in the IR+milrinone group than in the IR group.
Table 1. Serum aspartate aminotransferase (AST), alanine aminotransferase (ALT), total bilirubin (T-Bil), direct bilirubin (D-Bil), lactate dehydrogenase (LDH), hyaluronic acid (HA) levels, and platelet counts 5 h after reperfusion: Serum AST, ALT, T-Bil, D-Bil, LDH, and HA were significantly elevated in the ischemia-reperfusion (IR) group compared with those of the sham group. Serum AST and D-Bil levels were significantly lower in the IR+milrinone group than in the IR group. Platelet counts were significantly decreased in the IR group as compared with those of the sham group. Platelet counts were significantly higher in the IR+milrinone group than in the IR group. Table 1. Serum aspartate aminotransferase (AST), alanine aminotransferase (ALT), total bilirubin (T-Bil), direct bilirubin (D-Bil), lactate dehydrogenase (LDH), hyaluronic acid (HA) levels, and platelet counts 5 h after reperfusion: Serum AST, ALT, T-Bil, D-Bil, LDH, and HA were significantly elevated in the ischemia-reperfusion (IR) group compared with those of the sham group. Serum AST and D-Bil levels were significantly lower in the IR+milrinone group than in the IR group. Platelet counts were significantly decreased in the IR group as compared with those of the sham group. Platelet counts were significantly higher in the IR+milrinone group than in the IR group.
Table 1. Serum aspartate aminotransferase (AST), alanine aminotransferase (ALT), total bilirubin (T-Bil), direct bilirubin (D-Bil), lactate dehydrogenase (LDH), hyaluronic acid (HA) levels, and platelet counts 5 h after reperfusion: Serum AST, ALT, T-Bil, D-Bil, LDH, and HA were significantly elevated in the ischemia-reperfusion (IR) group compared with those of the sham group. Serum AST and D-Bil levels were significantly lower in the IR+milrinone group than in the IR group. Platelet counts were significantly decreased in the IR group as compared with those of the sham group. Platelet counts were significantly higher in the IR+milrinone group than in the IR group. In Press
15 Mar 2024 : Review article
Approaches and Challenges in the Current Management of Cytomegalovirus in Transplant Recipients: Highlighti...Ann Transplant In Press; DOI: 10.12659/AOT.941185
18 Mar 2024 : Original article
Does Antibiotic Use Increase the Risk of Post-Transplantation Diabetes Mellitus? A Retrospective Study of R...Ann Transplant In Press; DOI: 10.12659/AOT.943282
20 Mar 2024 : Original article
Transplant Nephrectomy: A Comparative Study of Timing and Techniques in a Single InstitutionAnn Transplant In Press; DOI: 10.12659/AOT.942252
28 Mar 2024 : Original article
Association Between FEV₁ Decline Rate and Mortality in Long-Term Follow-Up of a 21-Patient Pilot Clinical T...Ann Transplant In Press; DOI: 10.12659/AOT.942823
Most Viewed Current Articles
05 Apr 2022 : Original article
Impact of Statins on Hepatocellular Carcinoma Recurrence After Living-Donor Liver TransplantationDOI :10.12659/AOT.935604
Ann Transplant 2022; 27:e935604
12 Jan 2022 : Original article
Risk Factors for Developing BK Virus-Associated Nephropathy: A Single-Center Retrospective Cohort Study of ...DOI :10.12659/AOT.934738
Ann Transplant 2022; 27:e934738
22 Nov 2022 : Original article
Long-Term Effects of Everolimus-Facilitated Tacrolimus Reduction in Living-Donor Liver Transplant Recipient...DOI :10.12659/AOT.937988
Ann Transplant 2022; 27:e937988
15 Mar 2022 : Case report
Combined Liver, Pancreas-Duodenum, and Kidney Transplantation for Patients with Hepatitis B Cirrhosis, Urem...DOI :10.12659/AOT.935860
Ann Transplant 2022; 27:e935860








