02 October 2020: Original Paper
Safe Long-Term Outcome After Kidney Donation in Older Donors: A Single-Center Experience
Antonia Schuster1ABCDEF*, Paula Franke1BCDE, Louisa Steines1BCDE, Daniel Zecher1BCDE, Christina Hackl2BCDE, Jens Werner2BCDE, Tobias Bergler1ABCDEF, Bernhard Banas1ABCDEDOI: 10.12659/AOT.924235
Ann Transplant 2020; 25:e924235
Abstract
BACKGROUND: Declining numbers of deceased donors and prolonged waiting time emphasize the importance of living kidney donation. Furthermore, because of the changing age structures with increasingly older recipients, the question of acceptance of older donors is becoming more relevant. However, sufficient long-term outcome data, especially for older donors – including histopathological analysis – are lacking. The aim of this study was to analyze the Regensburg Living Donor Cohort with regard to age <65 and ≥65 years, with a 10-year follow-up to identify attributable risk factors.
MATERIAL AND METHODS: All donors were analyzed for renal, cardiovascular, and pre-existing conditions at baseline and at follow-up. They were studied for predefined renal and additional end-points, eg cardiovascular ones and various stratifications such as estimated glomerular filtration rate (eGFR). Additionally, as a unique feature in such an analysis, a histopathological workup of pre-existing chronic lesions of the donated kidneys was added.
RESULTS: On average, donors in the group <65 years were 50 years old at the time of donation compared with 68 years in the older group. Creatinine at baseline was 0.8 mg/dl in both groups, corresponding to an eGFR of 96.8±12.8 ml/min (Chronic Kidney Disease Epidemiology Collaboration [CKD-EPI]) and 83.7±10.3 ml/min (CKD-EPI). In the follow-up, donors ≥65 years showed a statistically significantly worse eGFR and a greater eGFR decline, being accompanied by more pronounced chronic histopathological lesions, eg glomerulopathy, than the control group. However, this was largely constant over the entire observation period and no donor developed an end-stage renal disease or an eGFR below 30 ml/min.
CONCLUSIONS: To summarize, living kidney donation after an intensive screening is safe even for older donors; however, a precise aftercare to ensure balanced risk profile for living donors is mandatory.
Keywords: Age Factors, Albuminuria, Pathology, Renal Insufficiency, Chronic, Tissue and Organ Procurement, Glomerular Filtration Rate, Kidney, Kidney Transplantation, Living Donors, Nephrectomy, Risk Factors, Tissue and Organ Harvesting
Background
Renal transplantation is the most beneficial treatment option for patients with end-stage renal disease (ESRD), being associated with a better outcome for recipients in terms of reduction of cardiovascular events and mortality and an improved quality of life compared with ongoing dialysis [1–6]. Comparing the outcome regarding renal function, it was shown that patients receiving a living donor kidney showed better kidney function and a longer graft survival compared with recipients of a deceased-donor kidney [7].
However, such evaluations regarding the long-term outcome concerning renal, cardiovascular, and general end-points in a European collective are not considering the donors. Being faced with a declining number of deceased-donor organs in recent years, especially in Germany, living kidney donation has become increasingly important. In Germany, 2291 kidneys were transplanted in 2018, of which 27.8% (n=638) could be realized only by living kidney donation [8].
Previous studies have shown that living kidney donation is associated with a low perioperative risk with regard to operative complications and no aggravated risk concerning the long-term mortality was found [9,10]. However, it has been demonstrated that kidney donation increases the frequency of albuminuria and the risk of new onset of arterial hypertension [11,12]. The key question of to what extent the donation itself causes an increased risk for developing ESRD is of great importance in the evaluation of possible living kidney donors [13,14].
On the basis of their study of the frequency of ESRD after living kidney donation, Massie et al. designed an online tool to calculate the risk for ESRD after having donated a kidney [15]. In the course of donor selection and the discussion about suitable donors, the question of acceptance of older donors is becoming increasingly important. If one looks at age development in Germany, it can clearly be seen that the number of people over age 65 will increase from the present 19.3% up to 27.8% by 2050 [16]. Furthermore, people’s life expectancy continues to increase [17]. The question of what requirements an older donor has to meet is therefore of great interest.
Considering all available literature, there are few data addressing possible risk factors and consequences after living kidney donation in a European white donor population focusing on older donors. In particular, no histopathological analysis of donor biopsies is currently available. Additionally, only inconsistent data are available about which necessary requirements a living kidney donor in such a cohort should fulfill and which risk factors should be avoided. Thus, further investigations to optimize donor selection are mandatory.
The aim of this study was to analyze the Regensburg Living Donor Cohort with regard to age to identify risk factors worth noting for an older living donor. For this purpose, our living donor cohort was stratified by age <65 and ≥65 years in accordance with the Eurotransplant allocation regimen, where patients are transferred from regular allocation (Eurotransplant Kidney Allocation System) to Eurotransplant Senior Program by reaching the age of 65. We then characterized and evaluated both groups for renal (e.g. decline in renal function, need for dialysis initiation, etc.), cardiovascular (incidence of
Material and Methods
BASELINE CHARACTERISTICS:
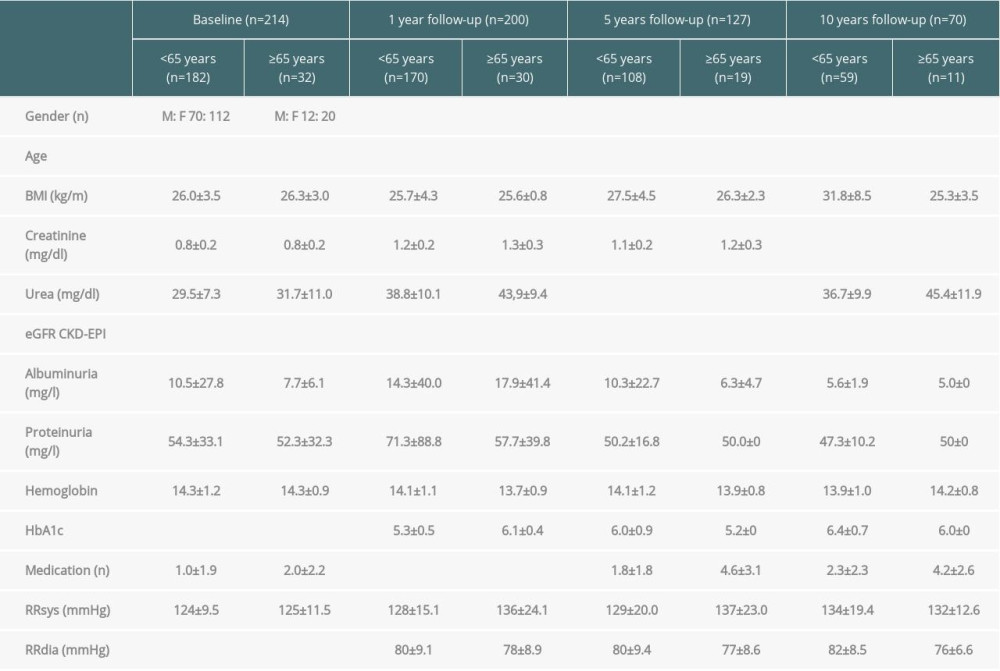
All donors who underwent a living kidney donation at the University Hospital Regensburg from 1 January 2001 to 31 July 2016 were included in the analysis (n=214) and stratified according to their age at baseline (<65 and ≥65 years). Baseline characteristics of donors including demographic, renal, cardiovascular, and other pre-existing conditions were analyzed. The clinical course of all donors was analyzed 1, 5, and 10 years after kidney donation. In total, 56 individual parameters per living kidney donor were included in the baseline and follow-up analysis (for detailed information see Table 1). Overall, the data of 87% of all donors who completed the whole observation period (10 years) were analyzed at the end of the follow-up. Results were compared with the abovementioned risk scores by Grams et al. [14] and Massie et al. [15]. All analyses were performed with approval of the local institutional review board.
RELEVANCE OF THE RESULTING ESTIMATED GLOMERULAR FILTRATION RATE (EGFR) AFTER KIDNEY DONATION:
We looked at the eGFR course of each kidney donor in the follow-up to identify attributable risk factors. For this purpose, the delta eGFR of each individual donor of 1 year after kidney donation in comparison with 5 and 10 years after donation was calculated. In a first analysis, the baseline data of all donors who showed an eGFR decline were compared with the data of donors with an eGFR increase 5 and 10 years after kidney donation. In a second analysis, the baseline data of all donors who had a disproportional eGFR loss (≥5 ml/min after 5 years; ≥10 ml/min after 10 years) were compared with a corresponding donor group with an eGFR increase ≥5 ml/min after 5 years and ≥10 ml/min after 10 years. Additionally, the percent change in eGFR over the observation period was analyzed in relation to age.
ANALYSIS OF THE DONOR-ATTRIBUTABLE HISTOPATHOLOGICAL LESIONS:
The kidney recipients at our center receive a biopsy as standard of care protocol after 14 days. We analyzed these 14-day biopsies for chronic histopathological lesions, being reliably assignable to the corresponding donor. Occurrence of glomerulopathy, IFTA, and arteriolopathy in accordance with donor age were of interest. In addition, we analyzed the baseline and follow-up parameters of patients with recognizable chronic histopathologic lesions in the 14-day biopsies compared with the cohort of donors without such chronic findings.
STATISTICAL ANALYSIS:
Continuous variables are presented as mean±standard deviation, whereas categorical data are shown as frequency distributions (
Results
BASELINE CHARACTERISTICS OF THE ENTIRE STUDY POPULATION:
In the observation period, 214 living kidney donations were performed at the University Hospital Regensburg. Donors (182) were younger than 65 years at the time of surgery and were compared with the 32 donors older than 65. In both groups more women (F) than men (M) donated a kidney (F: M 112: 70; F: M 20: 12). The mean donor age was 50±8.6 years compared with 68±3.4 years (p=2.3×10−25). Younger donors had a predonation body mass index (BMI) of 26±3.5 kg/m2, which was comparable with the BMI of older donors with 26.3±3.0 kg/m2 (p=0.6). The mean creatinine was 0.8±0.2 mg/dl in both groups (p=0.4), both corresponding to a different eGFR of 96.8±12.8 ml/min (CKD-EPI) and 83.7±10.3 ml/min (CKD-EPI) (p=1.2×10−7) (Table 1). There was no relevant albuminuria or proteinuria in the 24-h measurement (average albuminuria in 24-h urine was 10.5±27.8 mg/L (<65 years) vs. 7.7±6.1 mg/L (≥65 years) (p=0.7), proteinuria 54.3±33.1 mg/L (<65 years) vs. 52.3±32.3 mg/L (≥65 years) (p=0.8). The graphic progression of creatinine and eGFR (CKD-EPI) of both groups over the entire observation period can be seen in Figure 1.
Considering further laboratory parameters as markers for the cardiovascular risk profile, the donors in our collective showed normal glycated hemoglobin (HbA1c) of 5.5% (<65 years) and 5.7% (≥65 years) (
Furthermore, in the collective of elderly patients, we observed a significantly higher overall medication intake (baseline: n=1
In apparatus-based diagnostics, echocardiography showed normal ejection fraction (62% vs. 61%) and ambulatory 24-h blood pressure showed an average of 124/77 mmHg for younger donors and 125/73 mmHg for older donors (systolic measurement: p=0.4; diastolic measurement: p=0.008) (Table 1). One older female donor was treated for breast cancer 24 years before kidney donation.
The parameters significantly changed at baseline (age, C-reactive protein [CRP], HbA1c, total number of medications, antihypertensives, antidiabetic medications) were in a further step included in a multivariate analysis. The aim was to analyze the influence of these parameters on resulting eGFR. Age is the dominant factor for donor eGFR.
FOLLOW-UP DATA OF BOTH COHORTS:
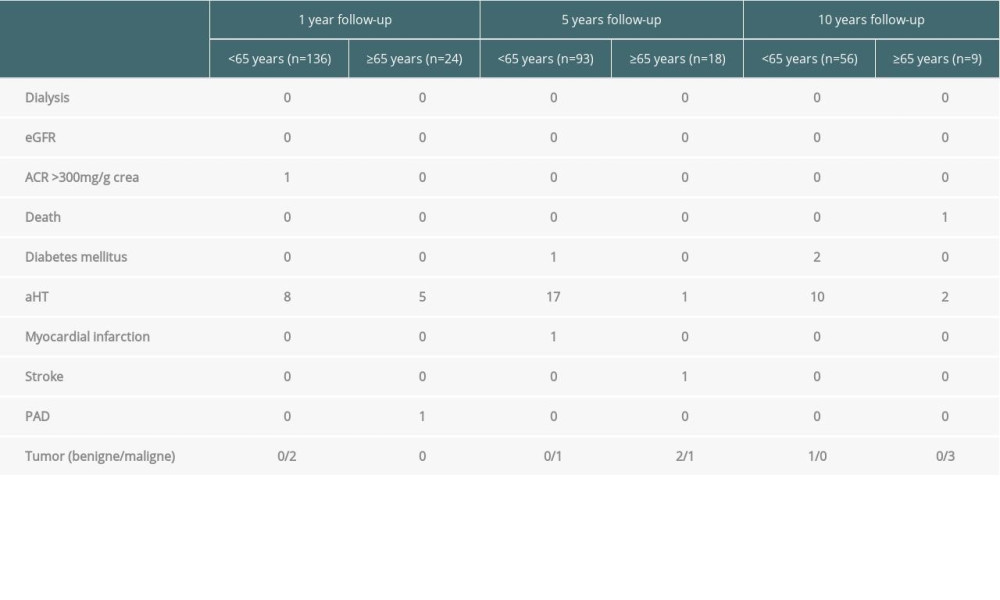
During follow-up, renal outcome parameters included the need for dialysis initiation, worsening of eGFR to less than 30 ml/min, albuminuria >300 mg/g creatinine, and death. Additionally, we analyzed the occurrence of de novo onset of arterial hypertension, diabetes mellitus, myocardial infarction, stroke, or peripheral artery disease, as well as the incidence of benign and malignant tumors. Data concerning the baseline and follow-up parameters are shown in Table 1.
Looking at the primary end-points in both groups, it was found that 1 donor from the older group died 10 years after kidney donation because of lung cancer. Need for dialysis initiation or deterioration of the eGFR (CKD-EPI) to <30 ml/min was not observed in either cohort. One younger donor showed an albuminuria >300 mg/g creatinine 1 year after donation.
In 26% of the younger donors and in 33% of the older donors, new-onset arterial hypertension occurred during the follow-up of 10 years. As at baseline, the older patients were noticed throughout the follow-up because of a significantly higher number of antihypertensives (1 year: n=1.6 vs. 0.6, p=2.3×10−5; 5 years: 2.1 vs. 0.8, p=6.0×10−6; 10 years: 2.4 vs. 1.2 medications, p=0.008). Looking at the measured blood pressure values, there is no statistically significant difference between the 2 groups. When analyzing the blood pressure course within the individual groups, however, the blood pressure values after donation deteriorated compared with the baseline time, which also reached the level of significance in both groups (<65 years: baseline vs. 1 year: p=0.002 [systolic] and 0.001 [diastolic]; baseline vs. 5 years: 0.007 [systolic] and 0.004 [diastolic]; baseline vs. 10 years: 8.6×10−7 and 7.9×10−5) (≥65 years: baseline vs. 1 year: p=0.02 and 0.008; baseline vs. 5 years: p=0.02 and 0.03). Only in the group of older people did we see a trend toward increased values compared with the baseline after 10 years, but without statistical significance (systolic: p=0.1, diastolic: p=0.1). Nevertheless, the values remain on average within the normotensive range. An overview of the outcome parameter of the entire study population can be found in Table 2.
To complement the analysis, we entered our donor data in the online tools developed by Massie et al. [15] to comparatively assess the risk of developing an ESRD. It was found that the estimated risk for ESRD in our entire cohort is within the described range. In their work, the risk of an ESRD is on average 34 cases/10 000 donors, whereby a pronounced variance was found. The estimation for our collective resulted in a 20-year risk that for men was 48/10 000 donors and for women in the range of 21/10 000 donors [15]. Looking at the calculation for patients older and younger than 65 years, the cohort of older men showed a 10-year risk of 14.9/10 000 donors compared with 8.2/10 000 donors in the younger group. The 10-year risk for women older than 65 years is 7/10 000 donors compared with 3.7/10 000 donors in the younger group.
CONSIDERATION OF THE EGFR:
For this purpose, the delta eGFR of each individual donor between the eGFR at 1 year in comparison with the eGFR at 5 and 10 years after donation was calculated. After 5 years, the delta eGFR varied between −19.4 ml/min and +28.5 ml/min. After 10 years, the delta eGFR varied between −23.7 ml/min and +23.3 ml/min. In this analysis we were able to identify age at donation as a prognostically relevant factor for the subsequent development of eGFR. Thus, both 5 and 10 years after donation, an eGFR increase was only observed in patients younger than 40 years (n=13;
ANALYSIS OF THE DONOR-ATTRIBUTABLE HISTOPATHOLOGICAL LESIONS:
Biopsies (201) were available from the abovementioned 214 living donors at 14±5 days. Looking at the analysis by age (≤65 years), 167 biopsies (<65 years) were compared with 34 biopsies (≥65 years). A difference with respect to the number of analyzed glomeruli could not be determined (9 vs. 9, p=0.78). However, older donors showed pronounced glomerulopathy (0.3 vs. 0.08, p=0.002). Significance was also achieved with respect to IFTA extent (0.2 vs. 0.05, p=0.01) and arteriolopathy (0.7 vs. 0.2, p=4.7×10−10) (Figure 2).
If we compare donors showing one of the 3 histological changes mentioned above with donors without any conspicuous histological changes, donors with chronic histopathological lesions are significantly more likely to take medication for the treatment of arterial hypertension. This observation can be demonstrated both at baseline and throughout the entire follow-up (baseline: 0.4
The parameters significantly changed at baseline (age, CRP, BMI, antihypertensives, total number of medications) in the group of patients with glomerulopathy were additionally analyzed by multivariate analysis. The aim was to analyze the influence of the parameters on the resulting eGFR. It turned out that older age (>60 years) could be seen as an influencing factor on the course of the eGFR.
Discussion
As living kidney donation – because of the declining numbers of deceased organ donors – is of prime importance, the identification of attributable risk factors associated with kidney donation is mandatory. Because of the aging of the recipients, the question of acceptance of older donors is relevant in the context of the discussion about potential donors. A meticulous selection and assessment of a potential donor is thus not only important in terms of the outcome for the recipient, but equally for the donor. In our study, we stratified 214 kidney living donors according to their age at baseline and analyzed the long-term outcome over the subsequent 10 years. Besides sole analysis of biological risk factors, an intensified histopathological analysis of chronic, donor-attributable lesions was performed. Over the entire observation period, no donor in both cohorts needed dialysis initiation, nor were there any donors with an eGFR <30 ml/min. Considering possible cardiovascular risk factors, the development of arterial hypertension after donation was demonstrated in 26% (<65) and 33% (≥65) respectively of the donors, but without impact on major cardiovascular end-points (myocardial infarction, etc.). The detected prevalence of arterial hypertension after kidney donation is comparable with the published guidelines, which state a prevalence for arterial hypertension in European countries of 30–45%, with a significant increase in older patients [18]. These data are confirmed by a Japanese analysis of kidney donors that found that older donors had a history of hypertension [19]. In our cohort the older donors also showed a more frequent use of antihypertensives both at baseline and during follow-up. Looking at the blood pressure values over time, higher blood pressure values were measured in the older cohort, whereby the values themselves were still within the normotensive range. Whereas in our cohort no cardiovascular effect associated with the donation could be demonstrated, Mjøen et al. found an increased overall mortality as well as an increased cardiovascular mortality in living kidney donors compared with a nondonating collective [9].
Addressing the extent of histopathological, donor-attributable chronic lesions, older age was a risk factor for increased glomerulo- and arteriolopathy and IFTA. As verified by multivariate analysis, age at donation was the most powerful risk factor for deterioration of renal function in our analysis, meaning that donors older than 65 years showed significantly inferior eGFR in the corresponding stratification over the entire follow-up. As well in the analysis of the eGFR slope, it could be shown that older donors more often had an eGFR decline – reliably proved by the detected histopathological lesions – whereas younger donors, especially younger than 40 years – without proven chronic lesions – had possibly even an eGFR increase. This observation is supported by the literature. Ibrahim et al. [20] were able to show that an eGFR <60 ml/min or <30 ml/min was associated with older donor age. In their study, a relationship between the deterioration of the eGFR and an increased systolic blood pressure as well as an increased BMI could also be found. Such associations have not been observed in our collective. As already described in the work of Chatzikyrkou et al., a larger eGFR decrease over time in the group of older donors could also be demonstrated in our collective [21]. For example, the older cohort showed an eGFR loss from baseline to 1 year after donation of 39% compared with 34% in the younger ones. After 10 years, this development was confirmed with an eGFR loss of 43% (≥65 years) to 29% (<65 years). Besides age, 24-h proteinuria at baseline and subsequent albuminuria and proteinuria were associated with more intensified eGFR decline. In their analysis of 211 living donors, Grupper et al. [22] were also able to demonstrate a more frequent occurrence of albuminuria after donation compared with a healthy control group. Despite the decrease in the eGFR and the histopathological changes found in the collective of donors older than 65 years, no relevant difference in the risk of developing ESRD could be found either in the Massie calculation [15] or in our cohort. Since no donor had ESRD in our donor population, the relevance of older age or sex could not be identified as an additional risk factor for worse renal end-points (eGFR <30 ml/min, dialysis). However, creatinine slope was relevantly affected by donor age. In contrast, when looking at the literature, Massie et al. [15] showed in their analysis of 133 824 living donors in the United States that an African American background, male sex, and younger age are considered risk factors for the development of ESRD in kidney donors. Men were more likely to develop ESRD, whereas older age was associated with increased ESRD risk only among the white population. An increased BMI or a blood relationship between donor and recipient were also identified as risk factors, which were also not seen in our analysis. When comparing the data of Massie et al. [15] with our collective, one must consider that a direct transfer has to be seen with caution, since the US collective of Massie et al. showed a significant proportion of African American donors (12.5%), which does not apply to our collective. The observations of Massie et al. are consistent with the work of Gibney et al. [23], who again identified male sex and an African American background as risk factors. In this work, however, an increased occurrence of ESRD was observed in donors younger than 35 years [23]. This stands in strict contradiction to our findings.
In their work, Grams et al. [14] analyzed a total of 7 cohort studies to identify risk factors for the development of ESRD in the general population. Sex, eGFR < 90 ml/min, albuminuria, the presence of arterial hypertension, nicotine consumption, and diabetes mellitus were highlighted as risk factors. As older donors were not affected by ESRD after donation, Grams et al. hypothesized that older donors should be considered as appropriate donors [14]. This conclusion is also supported by our analysis. However, one has to keep in mind that a decline in renal function was more likely to happen in older donors and those with pre-existing proteinuria, so that a combined presence of these 2 risk factors (donor age <65 years, pre-existing proteinuria) should be avoided. The results of the histopathological analysis also displayed more chronic lesions in the older donors. Detailed analysis of the overall risk profile of an older donor, taking into account the factors mentioned, is therefore unavoidable before a donation. The accumulation of several risk factors, eg older age, pre-existing arterial hypertension, and proteinuria, should be avoided. In addition, close monitoring of these patients after donation is urgently advisable. Although our study is the first that combines examinations of traditional risk factors with a histopathological workup, it has some important limitations. Because of its design as a monocentric evaluation targeting postdonation risk for donors, its findings cannot be generalized. According to our study concept, we evaluated the risk factors worth noting for white kidney donors being evaluated and follow-up in due consideration of our center strategy.
Conclusions
In summary, our data showed a decreased eGFR and a pronounced eGFR loss in older donors but with no relevant worsening over the 10-year follow-up period and with no worsening of renal function to eGFR levels of <30 ml/min or the need for dialysis initiation in any donor. Furthermore, no relevant increase in cardiovascular and renal end-points could be seen in either group. Although the acceptance of older donors seems appropriate, the decline in renal function accompanied by increased chronic lesions in histopathological workup of these donors demands precise workup given their risk profile. Thus, it can be concluded that a living kidney donation, after a meticulous donor screening and uninterrupted and precise follow-up, is safe.
Figures
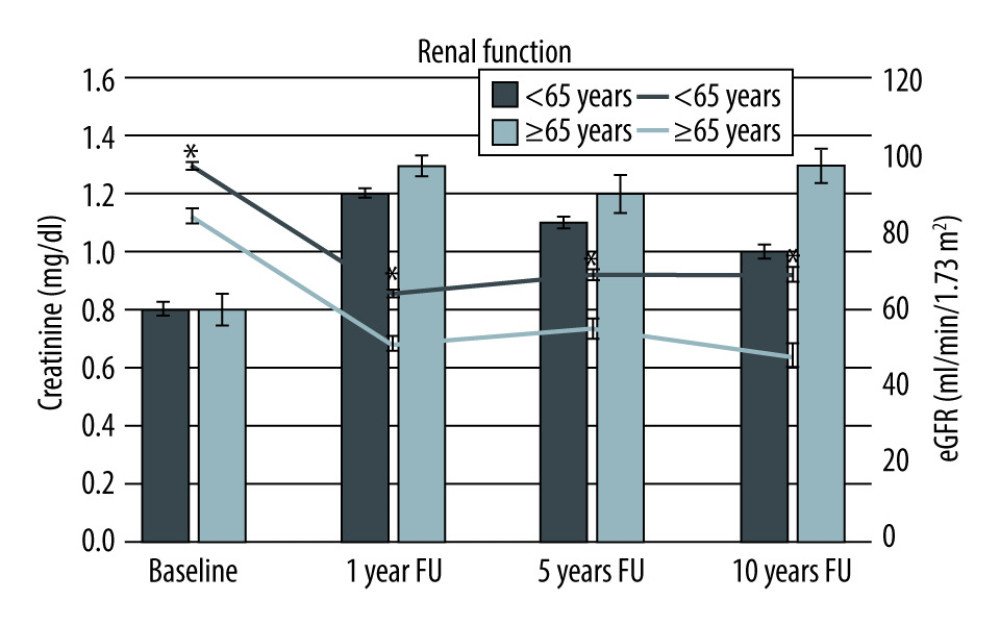 Figure 1. Course of creatinine and estimated glomerular filtration rate stratified by age at baseline and over the 10-year observation period. * p<0.05.
Figure 1. Course of creatinine and estimated glomerular filtration rate stratified by age at baseline and over the 10-year observation period. * p<0.05. 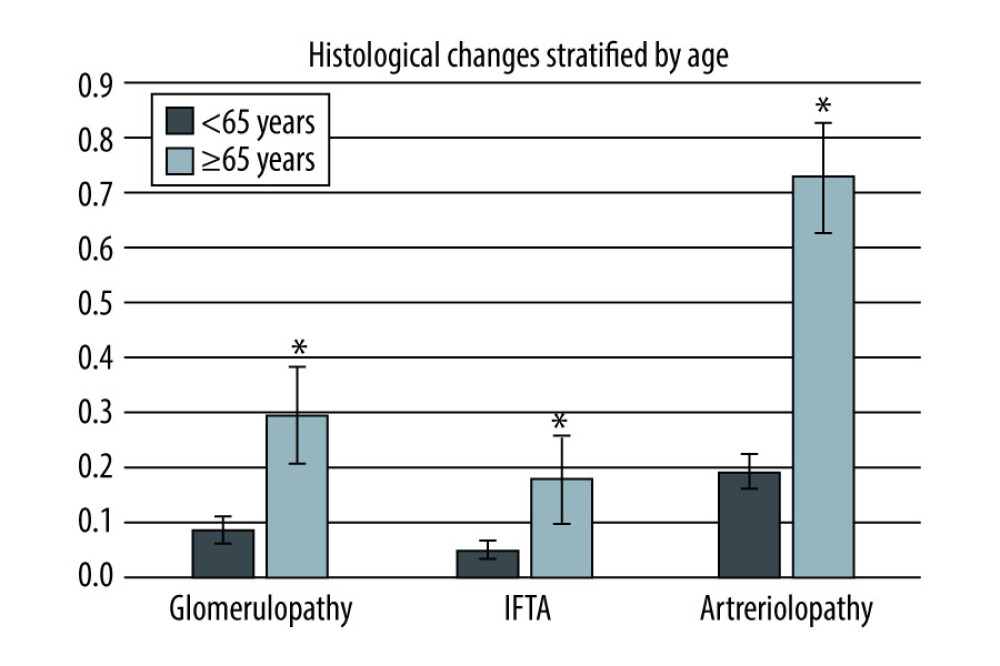 Figure 2. Comparison of different histological changes (glomerulopathy, interstitial fibrosis and tubular atrophy, arteriolopathy) in donors <65 or ≥65 years. * p<0.05.
Figure 2. Comparison of different histological changes (glomerulopathy, interstitial fibrosis and tubular atrophy, arteriolopathy) in donors <65 or ≥65 years. * p<0.05. References
1. Tonelli M, Wiebe N, Knoll G, Systematic review. Kidney transplantation compared with dialysis in clinically relevant outcomes: Am J Transplant, 2011; 11(10); 2093-109
2. Jassal SV, Krahn MD, Naglie G, Kidney transplantation in the elderly. A decision analysis: J Am Soc Nephrol, 2003; 14(1); 187-96
3. Muzaale AD, Massie AB, Wang M-C, Risk of end-stage renal disease following live kidney donation: J Am Med Assoc, 2014; 311(6); 579-86
4. Wolfe RA, Ashby VB, Milford EL, Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant: N Engl J Med, 1999; 341(23); 1725-30
5. Czyżewski L, Sańko-Resmer J, Wyzgał J, Kurowski A, Assessment of health-related quality of life of patients after kidney transplantation in comparison with hemodialysis and peritoneal dialysis: Ann Transplant, 2014; 19; 576-85
6. Purnell TS, Auguste P, Crews DC, Comparison of life participation activities among adults treated by hemodialysis, peritoneal dialysis, and kidney transplantation. A systematic review: Am J Kidney Dis, 2013; 62(5); 953-73
7. Hariharan S, Johnson CP, Bresnahan BA, Improved graft survival after renal transplantation in the United States, 1988 to 1996: N Engl J Med, 2000; 342(9); 605-12
8. Statistics.eurotransplant.org: 2053P_2017_kidney: 09.04.2018: Counting recipient transplants._DSO Jahresbericht, 2018
9. Mjøen G, Hallan S, Hartmann A, Long-term risks for kidney donors: Kidney Int, 2014; 86(1); 162-67
10. Segev DL, Muzaale AD, Caffo BS, Perioperative mortality and long-term survival following live kidney donation: J Am Med Assoc, 2010; 303(10); 959-66
11. Boudville N, Prasad GVR, Knoll GMN, Meta-analysis. Risk for hypertension in living kidney donors: Ann Intern Med, 2006; 145(3); 185-96
12. Garg AX, Muirhead N, Knoll G, Proteinuria and reduced kidney function in living kidney donors. A systematic review, meta-analysis, and meta-regression: Kidney Int, 2006; 70(10); 1801-10
13. Ibrahim HN, Foley R, Tan L, Long-term consequences of kidney donation: N Engl J Med, 2009; 360(5); 459-69
14. Grams ME, Sang Y, Levey AS, Kidney-failure risk projection for the living kidney-donor candidate: N Engl J Med, 2016; 374(5); 411-21
15. Massie AB, Muzaale AD, Luo X, Quantifying postdonation risk of ESRD in living kidney donors: J Am Soc Nephrol, 2017; 28(9); 2749-55
16. : Statistisches Bundesamt: 10., 11. und 12. koordinierte Bevölkerungsvoraus-berechnung, Bundeszentral für politische Bildung, 2012 [in German]www.bpb.de
17. : Statistisches Bundesamt: Ergebnisse der 14. Koordinierte Bevölkerungsvoraus-berechnung, Bundeszentral für politische Bildung, 2012 [in German]www.bpb.de
18. : Pocket Guidelines on Arterial Hypertension, German Hypertension League, Seite 7, 2014
19. Toyoda M, Yamanaga S, Kawabata C, Long-term safety of living kidney donors aged 60 and older: Transplant Proc, 2014; 46(2); 318-20
20. Ibrahim HN, Foley RN, Reule SA, Renal function profile in white kidney donors. The first 4 decades: J Am Soc Nephrol, 2016; 27(9); 2885-93
21. Chatzikyrkou C, Scurt FG, Clajus C, Predictors of outcomes of living kidney donation. Impact of sex, age and preexistent hypertension: Transplant Proc, 2019; 51(2); 396-404
22. Grupper A, Angel Y, Baruch A, Long term metabolic and renal outcomes of kidney donors compared to controls with excellent kidney function: BMC Nephrol, 2019; 20(1); 30
23. Gibney EM, Parikh CR, Garg AX, Age, gender, race, and associations with kidney failure following living kidney donation: Transplant Proc, 2008; 40(5); 1337-40
Figures
 Figure 1. Course of creatinine and estimated glomerular filtration rate stratified by age at baseline and over the 10-year observation period. * p<0.05.
Figure 1. Course of creatinine and estimated glomerular filtration rate stratified by age at baseline and over the 10-year observation period. * p<0.05. Figure 2. Comparison of different histological changes (glomerulopathy, interstitial fibrosis and tubular atrophy, arteriolopathy) in donors <65 or ≥65 years. * p<0.05.
Figure 2. Comparison of different histological changes (glomerulopathy, interstitial fibrosis and tubular atrophy, arteriolopathy) in donors <65 or ≥65 years. * p<0.05. In Press
15 Mar 2024 : Review article
Approaches and Challenges in the Current Management of Cytomegalovirus in Transplant Recipients: Highlighti...Ann Transplant In Press; DOI: 10.12659/AOT.941185
18 Mar 2024 : Original article
Does Antibiotic Use Increase the Risk of Post-Transplantation Diabetes Mellitus? A Retrospective Study of R...Ann Transplant In Press; DOI: 10.12659/AOT.943282
20 Mar 2024 : Original article
Transplant Nephrectomy: A Comparative Study of Timing and Techniques in a Single InstitutionAnn Transplant In Press; DOI: 10.12659/AOT.942252
28 Mar 2024 : Original article
Association Between FEV₁ Decline Rate and Mortality in Long-Term Follow-Up of a 21-Patient Pilot Clinical T...Ann Transplant In Press; DOI: 10.12659/AOT.942823
Most Viewed Current Articles
05 Apr 2022 : Original article
Impact of Statins on Hepatocellular Carcinoma Recurrence After Living-Donor Liver TransplantationDOI :10.12659/AOT.935604
Ann Transplant 2022; 27:e935604
12 Jan 2022 : Original article
Risk Factors for Developing BK Virus-Associated Nephropathy: A Single-Center Retrospective Cohort Study of ...DOI :10.12659/AOT.934738
Ann Transplant 2022; 27:e934738
22 Nov 2022 : Original article
Long-Term Effects of Everolimus-Facilitated Tacrolimus Reduction in Living-Donor Liver Transplant Recipient...DOI :10.12659/AOT.937988
Ann Transplant 2022; 27:e937988
15 Mar 2022 : Case report
Combined Liver, Pancreas-Duodenum, and Kidney Transplantation for Patients with Hepatitis B Cirrhosis, Urem...DOI :10.12659/AOT.935860
Ann Transplant 2022; 27:e935860