05 January 2021: Original Paper
Transplantation of Enterovirus 71 Virion Protein Particle Vaccine Protects Against Enterovirus 71 Infection in a Neonatal Mouse Model
Li Lei12BCDEF, Qing Li1BCDF, Shuhong Xu2BCF, Mingyang Tian2BCD, Xinghui Zheng1BCD, Yunxia Bi1BF, Bo Huang1ABDEG*DOI: 10.12659/AOT.924461
Ann Transplant 2021; 26:e924461
Abstract
BACKGROUND: Enterovirus 71 (EV71) is the pathogen most likely to cause HFMD in young children (1–5 years old). A small number of virion protein (VP) vaccine candidates are considered as the protective molecules in EV71 models. This study aimed to observe comprehensive immunogenicity for a promising EV71 vaccine depending on VP1 in neonatal mouse EV71 models.
MATERIAL AND METHODS: VP1 was isolated from patients and associated peptides were synthesized. EV71 particles were inactivated and mixed with Freund’s complete adjuvant to prepare peptide vaccines. An EV71 vaccine was administered to establish the mouse model and the mice were infected with EV71. Hematoxylin and eosin staining was used to examine inflammatory response in EV71-infected neonatal mice. A semi-quantitative reverse transcription-polymerase chain reaction assay was performed to evaluate the levels of EV71 virus in skeletal muscle, small intestines, and brain tissues.
RESULTS: Three peptides were selected from 20 VP1 peptides due to their exhibition of the highest immunogenicity. The peptide injection improved inflammation and decreased EV71 particle levels in muscle, small intestines, and brain tissues. The injection also decreased lesions in the small intestines of EV71-infected mice and protected brain tissues from the EV71 infection.
CONCLUSIONS: The present study confirmed the immuno-protective effects of VP1 vaccine transplantation in mice infected with EV71 virus. Our results provide valuable information that can be used in further studies investigating the specific mechanism of the anti-EV71 vaccine.
Keywords: Bacterial Vaccines, Enterovirus, Hand, Foot and Mouth Disease, Viral Structural Proteins, Animals, Newborn, Antibodies, Neutralizing, Antibodies, Viral, Enterovirus A, Human, Enterovirus Infections, Vaccines, Virus-Like Particle, Viral Vaccines, Virion
Background
Hand, foot, and mouth disease (HFMD) is a type of febrile disorder mainly affecting infants and young children (1–5 years old) [1–3]. HFMD is always characterized by ulcers on the hands, mouth, and/or feet [4]. Enterovirus 71 (EV71) is the pathogen most likely to cause HFMD, which also affects the central nervous system [5]. EV71 belongs to the
According to previous studies, virion proteins (VPs) are considered to be promising potential candidates for HFMD vaccines, as they are non-infectious particles [17]. Actually, VPs have been successfully used to develop vaccines against the hepatitis B virus and human papillomavirus (HPV) [18]. In recent years, HFMD vaccines were also designed by recombining the EV71 VPs, and it was demonstrated that these vaccines could neutralize the HFMD model [19]. In this investigation, the potential EV71 vaccine was established by employing VP1 as the antigen and evaluating the efficacy of the EV71 vaccine with a series of strategies, including etiological and pathological evaluation and lethal challenge analysis.
Material and Methods
PROTEIN EPITOPE IDENTIFICATION:
A total of 6 patients (male, n=3; female, n=3; age range, 1–5 years) with HFMD were recruited between January 2012 and December 2016 from the Third Affiliated Hospital of Zunyi Medical University (Zunyi, China). Blood samples were obtained and serum antibodies were examined using the EV71-IgG ELISA kit (cat. no. TW-Bio-E4381, R&D, MN, USA), based on the protocol of the manufacturer. ELISA was used to identify the best immunizing antigen for the EV71 vaccine using patients’ serum antibodies. In this study, the serum with the highest antibody titer was used to select the appropriate synthetic peptides after serum antibodies of the 6 samples were tested separately.
The present research was approved by the Ethics Committee of the Third Affiliated Hospital of Zunyi Medical University. All of the patients involved in this study provided written informed consent and approved this study.
EV71 ISOLATION AND PEPTIDE SYNTHESIS:
The human EV71 strain was isolated from the 6 patients using the following processes. 1) Sample pretreatment of throat swab: Put throat swab in virus preservation solution and stir 40 times to wash down the virus adhered to the swab and cells containing the virus. The preservation solution was then collected and centrifuged at 4°C and 10 000 rpm for 20 min. The supernatant was retained and filtered for sterilization. 2) Virus culture and isolation: Take 0.4 ml of virus supernatant, and inoculate it on the healthy human embryo rhabdomyoma (RD) cells with a full monolayer. The RD cells were cultured with 5% CO2 at 37°C. The growth and pathological effects of RD cells were observed by microscopy every day. When 80%–90% of RD cells demonstrated the pathological effect, cells and maintenance fluid were harvested and centrifuged and the supernatant was retained.
Then, the genome of EV71 was isolated using the TaKaRa MiniBest Viral RNA/DNA Extraction Kit (Cat. No. 9766) following the manufacturer’s protocol.
Using the above isolated EV71 genome, the amino acid (aa) sequence of EV71 was divided into 20 groups (20 aa/group except for the Peptide 20 group) (Table 1).
INACTIVATION OF EV71 PARTICLES:
Inactivated EV71 particles were prepared as described previously [20]. The inactivated EV71 particles were quantified by western blot analysis as previously described [21]. Briefly, total protein was extracted using Triton X-100 diluted in phenylmethylsulfonyl fluoride (Beyotime Biotech., Shanghai, China) at a final dosage of 100 μg/ml. The protein concentrations were quantified using a BCA kit (Sigma-Aldrich, St. Louis, MO, USA). Then, a total of 0.2 μg protein was added to each lane of 15% SDS-PAGE (Beyotime Biotech.). The SDS-PAGE produced protein in lanes was transferred onto the polyvinylidene difluoride membranes (PVDF, Ameresco, Inc., Framingham, MA, USA). Then, the PVDF membranes were blocked using 5% non-fat milk in PBS containing 0.05% Tween-20 (ShineGene, Molecular Biotech, Inc., Shanghai, China) for 1 h at 37˚C. The PVDF membranes were treated using the mouse anti-human EV71 antibody (1: 1000, Cat. No. ab169442) and mouse anti-human β-actin antibody (1: 2000, Cat. No. ab8224) at 4°C overnight. Subsequently, the PVDF membranes were treated using HRP-conjugated rabbit anti-mouse IgG (1: 1000, Cat. No. ab6728) at 37°C for 2 h. All of the above antibodies were purchased from Abcam Biotech. (Cambridge, MA, USA). Finally, the western blot bands were visualized with Pierce™ ECL Plus Western Blotting Substrate (Cat. No. 32132; Pierce, Waltham, MA, USA). The TCID50 was evaluated as described in a previous study [22].
PEPTIDE VACCINE PREPARATION:
The obtained peptides were dissolved in 0.9% NaCl and adjusted to a concentration of 1 mg/ml. Subsequently, the peptide vaccine solution was prepared following the 1: 1 (v/v) mixture of peptides and Freund’s complete adjuvant (Cat. No. P2036, Beyotime Biotech., Shanghai, China).
ESTABLISHMENT OF A MOUSE MODEL AND EV71 INFECTION:
We obtained 18 healthy 6–8-week-old Balb/C mice (female, n=12; male, n=6; weight range, 150–200 g) from HFK Bioscience Biotech. (Beijing, China). Mice were housed under the conditions of 24–25°C, 50–60% humidity, and a light/dark cycle of 12/12 h. The mice had free access to food and water. The mice were divided into 6 groups (2 females and 1 male in each group) to produce newborn mice (suckling mice). The mice in 3 of the 6 groups were administered synthesized peptide 2, synthesized peptide 4, and synthesized peptide 8, followed by mating and reproduction, to obtain the newborn mice (as the experimental groups). Then, the newborn mice were divided into a Peptide 2 group (n=4), a Peptide 4 group (n=4), and a Peptide 8 group (n=4) for subsequent experiments. The mice in the other 3 groups were not administered any peptides, were allowed to mate and reproduce, and the newborn mice were used as the control groups. The newborn mice were divided into a Newborn group (newborn mice without any treatment, n=4), a Newborn peptide group (newborn mice received peptide only), and a Newborn EV71 group (newborn mice received an EV71 infection).
The EV71 infection mouse model was established as described in a previous study [23], with a few modifications. According to the Reed-Muench method, the specific value or titer of EV71 was 10−4.5/0.1 ml. A total of 5×105 TCID50 EV71 particles were intraperitoneally injected into each newborn mouse. One day after EV71 infection, neonatal mice were killed and the skeletal muscle, small intestines, and brain tissues were extracted for testing. The peptides (0.1 ml/mouse) were intraperitoneally injected and re-administered 1 week later. Finally, the mice in each group were fed together and were able to mate freely.
All of the animal experiments or tests were approved by Ethics Committee of the Third Affiliated Hospital of Zunyi Medical University.
HE STAINING ASSAY:
The skeletal muscle, small intestines, and brain tissues were treated with 4% paraformaldehyde at 4°C for 24 h. Then, the tissues were embedded in paraffin (Beyotime Biotech.) and cut into sections 4 μm thick. Tissue slices were subsequently de-paraffinized as previously described [23]. The tissue sections were used for HE staining assay as previously described [24]. Briefly, the above tissue sections were stained with hematoxylin for 10 min, followed by eosin staining for 30 s. Both hematoxylin and eosin staining were conducted at room temperature. The stained sections were imaged with an inverted fluorescence microscope (Model IX70; Olympus, Tokyo, Japan) and analyzed using the Medical Image Analysis System (HMIAS22000; Wuhan Champion Image Technology Co., Ltd., Wuhan, China).
QUANTITATIVE REAL-TIME (QRT-PCR):
RNAs in the skeletal muscle tissues were extracted with 1 ml TRIzol reagent (Thermo Scientific Pierce, Rockford, IL, USA). Samples were centrifuged at 10 000 ×g for 5 min at 37°C and the supernatants were stored for subsequent experimentation. Subsequently, total RNA was precipitated using 1 ml isopropanol and washed twice in 70% ethanol. The RNA precipitations were dissolved in diethyl pyrocarbonate (Sigma-Aldrich; Merck KGaA) pre-treated water. RNAs were reverse-transcribed into the complementary DND (cDNA) using the Superscript III First-Strand Synthesis system (Western Biotech. Inc., Chongqing, China) following the manufacturer’s protocol. PCR assay was conducted to examine EV71 virus and β-actin mRNA expression. PCR assay was then conducted with a Takara SYBR Green PCR kit (Cat. No. DRR820A; Takara, Dalian, China) using T100 RT-PCR cycler (Bio-Rad., Hercules, CA, USA). The following listed primers were used for the amplifications:
The PCR assay underwent the following processes: Initial denaturation for 5 min at 95°C, then 30 cycles of 95°C for 30 s, 58°C for 45 s, and 72°C for 30 s. The final extension step was 72°C for 10 min. The DNA products were run on a 1.5% agarose gel containing ethidium bromide (Beyotime Biotech.). DNA bands were visualized using a gel imager and bands were quantified using LightCycler® 96 software (version 1.1.0.1320; Roche Applied Science, Penzberg, Germany) with β-actin as the internal reference gene.
STATISTICAL ANALYSIS:
Data are expressed as mean±standard deviation (SD) and were analyzed with professional SPSS software 19.0 (SPSS, Inc., Chicago, IL, USA). Tukey’s post hoc test-validated one-way analysis of variance (ANOVA) was conducted to analyze statistical differences among multiple groups.
Results
THREE PEPTIDES WERE SELECTED FROM VP1:
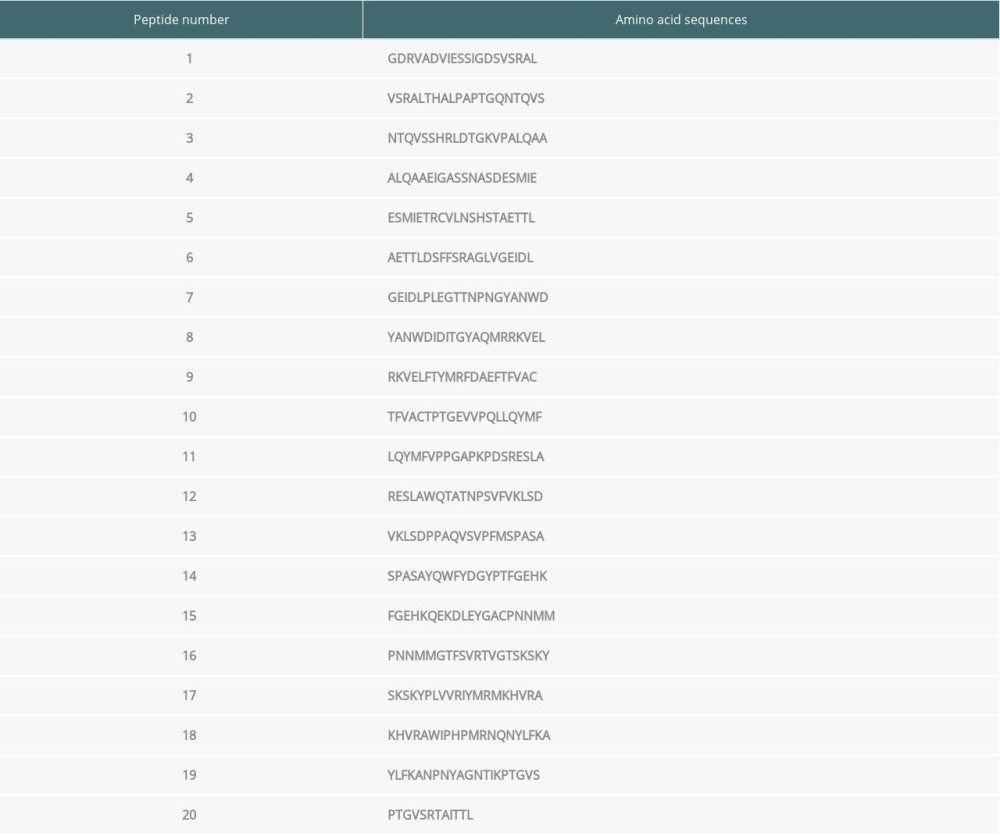
Serum samples from patients with high serum antibody concentrations (>35.71 ng/ml) were used for the current study. Among the 20 synthesized peptides truncated from the VP1 protein, ELISA assay revealed that the immunogenicity was stronger in peptide 2, peptide 4, and peptide 8 compared with the other peptides, and these 3 were used for immunizing mice (Figure 1A; P<0.05). Therefore, the above 3 peptide vaccine immune groups were named as Peptide 2, Peptide 4 and Peptide 8.
PEPTIDE INJECTIONS DECREASED EV71 PARTICLE LEVELS IN MUSCLE TISSUES:
The EV71 virus gene distribution in skeletal muscle, small intestines, and brain tissues were determined using semi-quantitative RT-PCR (Figure 1B). The semi-quantitative RT-PCR results also demonstrated that the EV71 virus levels in skeletal muscle, small intestines, and brain tissues of the Peptide 2 group, Peptide 4 group, and Peptide 8 group were remarkably lower than in the Newborn EV71 group (Figure 1C, all P<0.01).
PEPTIDE INJECTIONS AMELIORATED INFLAMMATION AND DECREASED EV71 PARTICLE LEVELS IN MUSCLE TISSUES:
The HE staining indicated that the Newborn EV71 group exhibited a severe inflammatory response with myo-necrosis, myo-degeneration, and muscle fiber atrophy (Figure 2). The muscle tissue samples in the Peptide 2, 4, and 8 groups appeared more defined and there was less inflammation compared with the Newborn EV71 group (Figure 2). The muscle condition in the Newborn peptide group was similar to that of the Newborn group.
PEPTIDE INJECTIONS DECREASED THE NUMBER OF LESIONS IN SMALL INTESTINES OF EV71-INFECTED MICE:
HE staining was also used to examine the lesions in the small intestines of the EV71-infected mice. The results indicated that there were fewer lesions in the Peptide 2 group, Peptide 4 group, and Peptide 8 group compared with that in the Newborn EV71 group (Figure 3).
PEPTIDES PROTECTED BRAIN TISSUES FROM EV71 INFECTIONS:
To investigate the effects of peptides on the central nerve system, the brain tissues were isolated, and HE staining was performed to observe the inflammatory response and lesions. The results demonstrated that EV71-infected newborn mice (without peptide injection) exhibited an inflammatory response and several lesions in the brain tissues (Figure 4). The Peptide 2 group, Peptide 4 group, and Peptide 8 group exhibited less inflammatory infiltration and fewer lesions compared with the Newborn EV71 group (Figure 4).
Discussion
Several enteroviruses are associated with the incidence of HFMD in clinical practice, including EV71, CVA16, and CVA6 [4–6]; therefore, it is necessary to discover a broadly applicable neutralizing vaccine. In fact, humans are considered to be the only natural host for the enteroviruses [5]. According to previous studies [25–27], most vaccine research is focused on EV71 virus and the newborn immuno-competent mouse to develop small-animal
The antibody response and the cellular immune response serve important roles in human immunity [30,31], which can trigger marked immune responses [31]. It is found that EV71 virus easily produces high levels of maternal antibody. The incidence rate of HFMD is lower in children under 1 years old, which may be related to the higher level of maternal antibody in newborns. The maternal antibody of mammals is mainly transmitted to newborn through the placenta and colostrum, so that it can provide immune protection. After newborn animals ingest colostrum, they can obtain maternal antibody immediately, and this improves the resistance of newborn animals. Therefore, when an animal model of vaccine immunization was established, mother mice were immunized by vaccine so that the offspring mice could obtain maternal antibody through placenta and milk. Therefore, the vaccine had an indirect immune-protective effect on the newborn mice. In summary, the adult mice were administered synthetic peptides and reproduced, providing the suckling mice used in this study.
In our study, among serum samples of 6 patients, the serum with the highest antibody titer was selected and the binding of serum and synthetic peptide was assessed by indirect ELISA. The highest binding rate of the synthetic peptide to EV71 IgG antibody was found. This synthetic peptide demonstrated the highest specificity; therefore, we used the synthesized peptide to immunize mice. The present study demonstrated that immunization with a VP1-synthesized vaccine could trigger an effective protective response to an EV71 infection in a small number of tissues in the mouse model, including the skeletal muscle, small intestines, and brain tissues. These findings were consistent with the effect of a number of other VP1-associated vaccines, including the HPV and influenza viruses [32,33].
A previous study reported that the muscle cells are the most important sites for EV71 proliferation in patients living in Fuyang, China [24]. EV71 is presumed to infect the CNS through peripheral nerves; therefore, CNS injury may also be responsible for the EV71-infected mouse mortalities [34]. The present study also performed a pathological analysis of the small intestines to observe the immune-protective effects
Conclusions
This study confirmed the immuno-protective effects of VP1 vaccine against EV71 infection in a mouse model and provides a valuable clue in the investigation of specific mechanisms of the anti-EV71 vaccine.
Figures
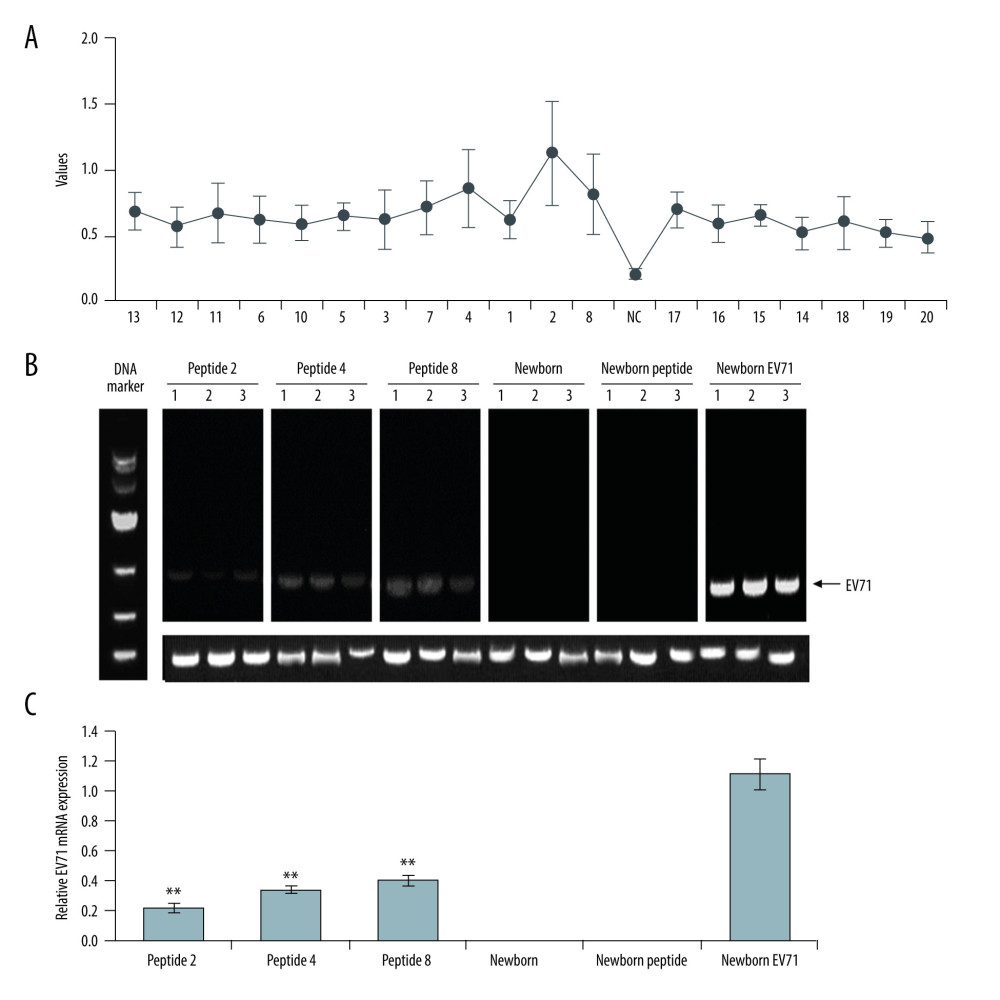 Figure 1. Peptide injections decrease EV71 particle levels in muscle, small intestines, and brain tissues. (A) ELISA immunogenicity analysis for the synthesized peptides (1–20). Lanes 1–20 represent the synthesized peptides. (B) cDNA bands for EV71 distribution in tissues. Lanes 1, 2, and 3 show the level of EV71 virus in skeletal muscle, small intestines, and brain tissues, respectively. (C) Quantification and statistical analysis of relative mRNA expression of the EV71 virus by a semi-quantitative RT-PCR assay. ** P<0.01 vs. the Newborn EV71 group. EV71 – enterovirus 71.
Figure 1. Peptide injections decrease EV71 particle levels in muscle, small intestines, and brain tissues. (A) ELISA immunogenicity analysis for the synthesized peptides (1–20). Lanes 1–20 represent the synthesized peptides. (B) cDNA bands for EV71 distribution in tissues. Lanes 1, 2, and 3 show the level of EV71 virus in skeletal muscle, small intestines, and brain tissues, respectively. (C) Quantification and statistical analysis of relative mRNA expression of the EV71 virus by a semi-quantitative RT-PCR assay. ** P<0.01 vs. the Newborn EV71 group. EV71 – enterovirus 71. 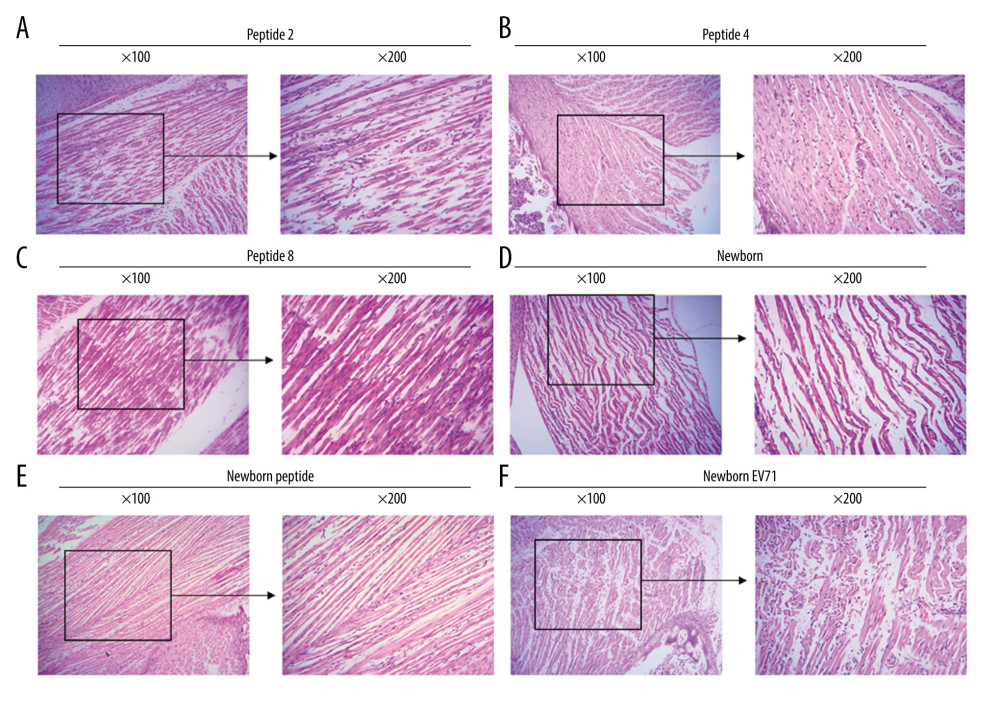 Figure 2. Peptide injections ameliorate inflammation. Hematoxylin and eosin staining of skeletal muscle tissues in the (A) Peptide 2, (B) Peptide 4, (C) Peptide 8, (D) Newborn, (E) Newborn peptide, and (F) Newborn EV71 groups. EV71 – enterovirus 71.
Figure 2. Peptide injections ameliorate inflammation. Hematoxylin and eosin staining of skeletal muscle tissues in the (A) Peptide 2, (B) Peptide 4, (C) Peptide 8, (D) Newborn, (E) Newborn peptide, and (F) Newborn EV71 groups. EV71 – enterovirus 71.  Figure 3. Peptide injections decrease the number of lesions in the small intestines of EV71-infected mice. Hematoxylin and eosin staining of small intestines tissues in the (A) Peptide 2, (B) Peptide 4, (C) Peptide 8, (D) Newborn, (E) Newborn peptide, and (F) Newborn EV71 groups. EV71 – enterovirus 71.
Figure 3. Peptide injections decrease the number of lesions in the small intestines of EV71-infected mice. Hematoxylin and eosin staining of small intestines tissues in the (A) Peptide 2, (B) Peptide 4, (C) Peptide 8, (D) Newborn, (E) Newborn peptide, and (F) Newborn EV71 groups. EV71 – enterovirus 71. 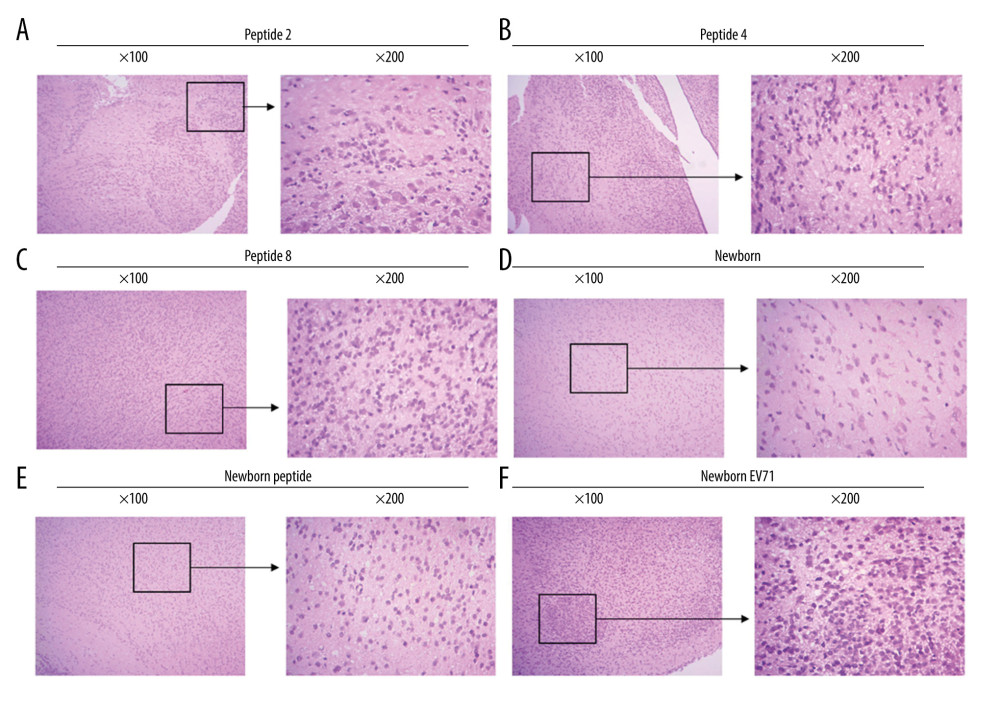 Figure 4. Peptides protect brain tissues from EV71 infections. Hematoxylin and eosin staining of brain tissues in the (A) Peptide 2, (B) Peptide 4, (C) Peptide 8, (D) Newborn, (E) Newborn peptide, and (F) Newborn EV71 groups. EV71 – enterovirus 71.
Figure 4. Peptides protect brain tissues from EV71 infections. Hematoxylin and eosin staining of brain tissues in the (A) Peptide 2, (B) Peptide 4, (C) Peptide 8, (D) Newborn, (E) Newborn peptide, and (F) Newborn EV71 groups. EV71 – enterovirus 71. References
1. Chen S, Yi K, Chen X, A simple scoring system for quick, accurate, and reliable early diagnosis of hand, foot, and mouth disease: Med Sci Monit, 2018; 24; 8627-38
2. Geoghegan JL, Tan le V, Kuhnert D, Phylodynamics of enterovirus A71-associated hand, foot, and mouth disease in Viet Nam: J Virol, 2015; 89; 8871-79
3. Wang Y, Zou G, Xia A, Enterovirus 71 infection in children with hand, foot, and mouth disease in Shanghai, China: Epidemiology, clinical feature and diagnosis: Virol J, 2015; 12; 83
4. Zhang W, Huang B, She C, An epidemic analysis of hand, foot, and mouth disease in Zunyi, China between 2012 and 2014: Saudi Med J, 2015; 36; 593-98
5. Wong SS, Yip CC, Lau SK, Yuen KY, Human enterovirus 71 and hand, foot and mouth disease: Epidemiol Infect, 2010; 138; 1071-89
6. Chan KP, Goh KT, Chong CY, Epidemichand, foot and mouth disease caused by human enterovirus 71, Singapore: Emerg Infect Dis, 2003; 9; 78-85
7. Osterback R, Vuorinen T, Linna M, Coxsackievirus A6 and hand, foot, and mouth disease, Finland: EmergInfect Dis, 2009; 15; 1485-88
8. Zeng M, El Khatib NF, Tu S, Seroepidemiology of Enterovirus 71 infection prior to the 2011 season in children in Shanghai: J Clin Virol, 2012; 53; 285-89
9. Nguyen NT, Pham HV, Hoang CQ, Epidemiological and clinical characteristics of children who died from hand, foot and mouthdisease in Vietnam, 2011: BMC Infect Dis, 2014; 14; 341
10. Wu Y, Yeo A, Phoon MC, The largest outbreak of hand; foot and mouth disease in Singapore in 2008: The role of enterovirus 71 and coxsackievirus A strains: Int J Infect Dis, 2010; 14; e1076-81
11. Akiyoshi K, Suga T, Mori A, Enteroviruses in patients experiencing multiple episodes of hand, foot, and mouth disease in the same season in Kobe, Japan, 2011: Jpn J Infect Dis, 2012; 65; 459-61
12. Kim SJ, Kim JH, Kang JH, Risk factors for neurologic complications of hand, foot and mouth disease in the Republic of Korea, 2009: J Korean Med Sci, 2013; 28; 120-27
13. Yi L, Lu J, Kung HF, The virology and developments toward control of human enterovirus 71: Crit Rev Microbiol, 2011; 37; 313-27
14. Lin CW, Liu CC, Lu TC, Immunogenicity studies of bivalentin activated virions of EV71/CVA16 formulated with submicron emulsion systems: Biomed Res Int, 2014; 2014 670506
15. Chong P, Hsieh SY, Liu CC, Production of EV71 vaccine candidates: Hum Vaccin Immunother, 2012; 8; 1775-83
16. Lin YL, Yu CI, Hu YC, Enterovirustype 71 neutralizing antibodies in the serum of macaquemon keys immunized with EV71 virus-likeparticles: Vaccine, 2012; 30; 1305-12
17. Stewart M, Bhatia Y, Athmaran TN, Validation of a novel approach for the rapid production of immunogenic virus-like particles for blue tongue virus: Vaccine, 2010; 28; 3047-54
18. Cao L, Mao F, Pang Z, Protective effect of enterovirus71 (EV71) virus like particle vaccine against lethal EV71 infection in a neonatal mouse model: Mol Med Rep, 2015; 12; 2473-80
19. Zhang Y, Wang L, Liao Y, Similar protective immunity induced by an inactivated enterovirus 71 (EV71) vaccine in neonatal rhesus macaques and children: Vaccine, 2015; 33; 6290-97
20. Cai Y, Ku Z, Liu Q, A combination vaccine comprising of inactivated enterovirus 71 and coxsackievirus A16 elicits balanced protective immunity against both viruses: Vaccine, 2014; 32; 2406-12
21. Ku Z, Ye X, Huang X, Neutralizing antibodies induced by recombinant virus-like particles of enterovirus 71 genotype C4 inhibit infection at pre- and post-attachment steps: PLoS One, 2013; 8; e57601
22. Liu Q, Huang W, Nie J, A novel high-throughput vaccinia virus neutralization assay and pre-existing immunity in populations from different geographic regions in China: PLoS One, 2012; 7; e33392
23. Qin YY, Lu J, Li JLEstablishment of enterovirus 71-infected ICR neonatal mice model: J Jiangsu Univ (Med Ed), 2017; 27; 385-340 [in Chinese]
24. Chen Z, Lu S, Xu M, Role of miR-24, furin, and transforming growth factor-beta1 signal pathway in fibrosis after cardiac infarction: Med Sci Monit, 2017; 23; 65-70
25. Wang W, Duo J, Liu J, A mouse muscle-adapted enterovirus 71 strain with increased virulence in mice: Microbes Infect, 2011; 13; 862-70
26. Khong WX, Yan B, Yeo H, A non-mouse-adapted enterovirus 71 (EV71) strain exhibits neurotropism, causing neurological manifestations in a novel mouse model of EV71 infection: J Virol, 2012; 86; 2121-31
27. Wang YF, Chou CT, Lei HY, A mouse-adapted enterovirus 71 strain causes neurological disease in mice after oral infection: J Virol, 2004; 78; 7916-24
28. Meng T, Kolpe AB, Kiener TK, Display of VP1 on the surface of baculo virus and its immunogenicity against heterologous human enterovirus 71 strains in mice: PLoS One, 2011; 6; e21757
29. Foo DG, Alonso S, Chow VT, Passive protection against lethal enterovirus 71 infection in newborn mice by neutralizing antibodies elicited by a synthetic peptide: Microbes Infect, 2007; 9; 1299-306
30. Kim YI, Song JH, Kwon BE, Pros and cons of VP1-specific maternal IgG for the protection of Enterovirus 71 infection: Vaccine, 2015; 33; 6604-10
31. Chang LY, Hsiung CA, Lu CY, Status of cellular rather than humoral immunity is correlated with clinical outcome of enterovirus 71: Pediatr Res, 2006; 60; 466-71
32. Quan FS, Huang C, Compans RW, Virus-like particle vaccine induces protective immunity against homologous and heterologous strains of influenza virus: J Virol, 2007; 81; 3514-24
33. Emeny RT, Wheeler CM, Jansen KU, Priming of human papillomaviru stype 11-specific humoral and cellular immune responses in college-aged women with a virus-like particle vaccine: J Virol, 2002; 76; 7832-42
34. Xiu JH, Zhu H, Xu YF, Necrotizing myositis causes restrictive hypoventilation in a mouse model for human enterovirus 71 infection: Virol J, 2013; 10; 215
Figures
 Figure 1. Peptide injections decrease EV71 particle levels in muscle, small intestines, and brain tissues. (A) ELISA immunogenicity analysis for the synthesized peptides (1–20). Lanes 1–20 represent the synthesized peptides. (B) cDNA bands for EV71 distribution in tissues. Lanes 1, 2, and 3 show the level of EV71 virus in skeletal muscle, small intestines, and brain tissues, respectively. (C) Quantification and statistical analysis of relative mRNA expression of the EV71 virus by a semi-quantitative RT-PCR assay. ** P<0.01 vs. the Newborn EV71 group. EV71 – enterovirus 71.
Figure 1. Peptide injections decrease EV71 particle levels in muscle, small intestines, and brain tissues. (A) ELISA immunogenicity analysis for the synthesized peptides (1–20). Lanes 1–20 represent the synthesized peptides. (B) cDNA bands for EV71 distribution in tissues. Lanes 1, 2, and 3 show the level of EV71 virus in skeletal muscle, small intestines, and brain tissues, respectively. (C) Quantification and statistical analysis of relative mRNA expression of the EV71 virus by a semi-quantitative RT-PCR assay. ** P<0.01 vs. the Newborn EV71 group. EV71 – enterovirus 71. Figure 2. Peptide injections ameliorate inflammation. Hematoxylin and eosin staining of skeletal muscle tissues in the (A) Peptide 2, (B) Peptide 4, (C) Peptide 8, (D) Newborn, (E) Newborn peptide, and (F) Newborn EV71 groups. EV71 – enterovirus 71.
Figure 2. Peptide injections ameliorate inflammation. Hematoxylin and eosin staining of skeletal muscle tissues in the (A) Peptide 2, (B) Peptide 4, (C) Peptide 8, (D) Newborn, (E) Newborn peptide, and (F) Newborn EV71 groups. EV71 – enterovirus 71. Figure 3. Peptide injections decrease the number of lesions in the small intestines of EV71-infected mice. Hematoxylin and eosin staining of small intestines tissues in the (A) Peptide 2, (B) Peptide 4, (C) Peptide 8, (D) Newborn, (E) Newborn peptide, and (F) Newborn EV71 groups. EV71 – enterovirus 71.
Figure 3. Peptide injections decrease the number of lesions in the small intestines of EV71-infected mice. Hematoxylin and eosin staining of small intestines tissues in the (A) Peptide 2, (B) Peptide 4, (C) Peptide 8, (D) Newborn, (E) Newborn peptide, and (F) Newborn EV71 groups. EV71 – enterovirus 71. Figure 4. Peptides protect brain tissues from EV71 infections. Hematoxylin and eosin staining of brain tissues in the (A) Peptide 2, (B) Peptide 4, (C) Peptide 8, (D) Newborn, (E) Newborn peptide, and (F) Newborn EV71 groups. EV71 – enterovirus 71.
Figure 4. Peptides protect brain tissues from EV71 infections. Hematoxylin and eosin staining of brain tissues in the (A) Peptide 2, (B) Peptide 4, (C) Peptide 8, (D) Newborn, (E) Newborn peptide, and (F) Newborn EV71 groups. EV71 – enterovirus 71. In Press
15 Mar 2024 : Review article
Approaches and Challenges in the Current Management of Cytomegalovirus in Transplant Recipients: Highlighti...Ann Transplant In Press; DOI: 10.12659/AOT.941185
18 Mar 2024 : Original article
Does Antibiotic Use Increase the Risk of Post-Transplantation Diabetes Mellitus? A Retrospective Study of R...Ann Transplant In Press; DOI: 10.12659/AOT.943282
20 Mar 2024 : Original article
Transplant Nephrectomy: A Comparative Study of Timing and Techniques in a Single InstitutionAnn Transplant In Press; DOI: 10.12659/AOT.942252
28 Mar 2024 : Original article
Association Between FEV₁ Decline Rate and Mortality in Long-Term Follow-Up of a 21-Patient Pilot Clinical T...Ann Transplant In Press; DOI: 10.12659/AOT.942823
Most Viewed Current Articles
05 Apr 2022 : Original article
Impact of Statins on Hepatocellular Carcinoma Recurrence After Living-Donor Liver TransplantationDOI :10.12659/AOT.935604
Ann Transplant 2022; 27:e935604
12 Jan 2022 : Original article
Risk Factors for Developing BK Virus-Associated Nephropathy: A Single-Center Retrospective Cohort Study of ...DOI :10.12659/AOT.934738
Ann Transplant 2022; 27:e934738
22 Nov 2022 : Original article
Long-Term Effects of Everolimus-Facilitated Tacrolimus Reduction in Living-Donor Liver Transplant Recipient...DOI :10.12659/AOT.937988
Ann Transplant 2022; 27:e937988
15 Mar 2022 : Case report
Combined Liver, Pancreas-Duodenum, and Kidney Transplantation for Patients with Hepatitis B Cirrhosis, Urem...DOI :10.12659/AOT.935860
Ann Transplant 2022; 27:e935860