04 September 2020: Original Paper
Transplantation of a Novel Recombinant Adeno-Associated Virus (pAAV-HE1B19K-TE1A) Demonstrates Higher Anti-Tumor Effects in Tumor Cells
Zhuo-Qing Li1BCDEF, Hong Shen2BCDE, Kun-Xian Shu3BCDF, Jian-Jun Li1BCDF, Yin Tang4BCD, Jing-Jing Su4BCF, Jun Yan1BCD, Jie Yang3BCF, Ze-Qing Wang1BCF, Yan Qiu4BC, Yong Yang1CF, Yang Liu4CF, Yong Zhou5BDEG*DOI: 10.12659/AOT.925013
Ann Transplant 2020; 25:e925013
Abstract
BACKGROUND: Oncolytic viruses (OVs) can specifically infect and kill tumor cells. Adeno-associated virus (AAV) is a widely-studied OV. This study aimed to construct a tumor-targeted recombinant AAV using genetic engineering technology.
MATERIAL AND METHODS: The transgene plasmid pAAV-HE1B19K-TE1A was constructed with 4 genes (hTERT, E1A, HKII, and E1B19K) and co-transfected with pAAV-RC and pHelper to tumor cells (HepG2, A549, BGC-803) and normal cells (HUVEC). rAAV was verified with fluorescence microscopy. Quantitative PCR (qPCR) assay was used to test the titer of rAAV in each cell line. Apoptosis was analyzed using qPCR and Western blot assay. MTT was used to detect the effect of rAAV on cell viability.
RESULTS: The pAAV-HE1B19K-TE1A transgene plasmid was successfully structured. pAAV-HE1B19K-TE1A was highly expressed in all tumor cells. The titers of pAAV-HE1B19K-TE1A in HepG2, A549, and BGC-803 were 7.4×10⁷, 1.4×10⁸, and 1.1×10⁸ gc/μl, respectively. pAAV-HE1B19K-TE1A significantly decreased cell viability of tumor cells compared to that in HUVEC (p<0.05). pAAV-HE1B19K-TE1A remarkably triggered cleaved caspase 3 (C-caspase 3) activity in tumor cells compared to that in untransfected tumor cells (p<0.05). pAAV-HE1B19K-TE1A significantly induced release of cytochrome C (Cyto C) in tumor cells compared to that in untransfected tumor cells (p<0.05). pAAV-HE1B19K-TE1A demonstrated no toxicity to vital tissues of animals.
CONCLUSIONS: Tumor-targeted rAAV was successfully produced using the Helper-free system with recombinant plasmid, demonstrating high efficacy in decreasing viability of tumor cells without adverse effects on normal cells.
Keywords: Bioengineering, Dependovirus, Tumor Cells, Cultured, Hep G2 Cells, Human Umbilical Vein Endothelial Cells, Transfection
Background
Cancer is the second leading cause of death in the world after cardiovascular diseases. Oncolytic viruses (OVs) are replication-defective viruses that can specifically recognize and infect tumor cells, eventually causing cell swelling and destroying tumor cells [1]. Adeno-associated virus (AAV) is one of the most widely applied OVs. AAV, as an un-enveloped single-stranded DNA virus, belongs to the
Human telomere is a special structure composed of non-coding repeat sequence (TTAGGG) and binding proteins at the end of the chromosome [9,10]. These regions are gradually shortened in cell division, resulting in the loss of basic genetic information and eventually causing cell death [11,12]. Telomerase is a RNA-dependent reverse-transcriptional DNA polymerase that is responsible for telomere elongation [13]. Telomerase is mainly composed of human telomere reverse transcriptase (hTERT), telomere RNA (TR), and telomerase-associated proteins [14,15]. Telomerase can use its own RNA as a template to transcribe and synthesize telomere DNA, which is also added to the end of the chromosome, maintaining the integrity and stability of chromosomes [16]. The hTERT gene is only highly expressed in most tumors and immortalized cell lines, while expression of the hTERT gene is turned off in mature and differentiated normal cells [16]. Therefore, the telomerase gene is specifically transcribed and expressed in most tumor cells [16]. It has been reported that the activation of telomerase can enable tumor cells to gain the ability of unlimited proliferation, which is a key step in tumor occurrence and development [17]. hTERT is the main regulatory subunit of telomerase activity, the high expression of which is caused by complete activation of its promoter in tumor cells. Therefore, hTERT promoter is considered to be tumor-specific [18].
Hexokinase (HK) is the first enzyme in glycolysis, and is also the rate-limiting enzyme in tumor tissues [19]. With the increase of its expression and activity in tumor tissues, the tumor tissues can still obtain enough energy under the condition of hypoxia [19,20]. Many intermediate products of glycolysis can be used by tumor cells to synthesize proteins, nucleic acids, and lipids, thus providing necessary materials for the growth and proliferation of tumor cells [21]. HK II is an extensively studied glycolytic enzyme that plays a dual role in tumors: one is to upregulate the level of glycolysis, and the other is to bind to volt-dependent anion channel (VDAC) in the outer membrane of mitochondria to inhibit apoptosis [22,23]. Therefore, HK II promoter demonstrates tumor specificity.
In this study, we synthesized a tumor-targeted recombined adeno-associated virus (rAAV)-carrying targeting gene using the AAV Helper-free system and evaluated its anti-tumor effects.
Material and Methods
MATERIALS:
Plasmid pAAV-IRES-ZsGreen was purchased from Addgene (Cambridge, MA, USA). DNA and RNA extraction kits, DNA fragment gel extraction kit, restriction enzymes, and
AAV TRANSGENE PLASMID CONSTRUCTION:
The core promoter of HKII (294 bp) and E1B19K (528 bp) and the core promoter of hTERT (370 bp) and E1A (984 bp) were reported on NCBI. The elements were synthesized in tandem and cloned into
HELPER-FREE PRODUCTION OF RAAV:
HepG2 and HUVEC cells were cultured in DMEM supplemented with 10% FBS and 10% penicillin-streptomycin. A549 was cultured with F12K medium containing 10% FBS and 10% penicillin-streptomycin. BGC-803 was cultured in RPMI-1640 medium supplemented with 10% FBS and 10% penicillin-streptomycin. All of the above cells were seeded in 60-mm dishes and cultured at 37°C and in a 5% CO2 atmosphere to a confluence of 60%–70%. At 2 h before transfection, all cells were cultured in medium containing FBS without antibody. Then, these cells were transiently co-transfected with rAAV plasmids pAAV-RC, pHelper, and pAAV-HE1B19K-TE1A at an equimolar ratio using Lip2000 as a DNA carrier agent. After 72 h, ZsGreen expression was visualized using direct fluorescence microscopy and all cells were harvested and lysed using the freeze-thaw method to obtain recombined pAAV-HE1B19K-TE1A (rAAV). Then, rAAV was used to infect cells again until the third viral generation. The isolation and purification procedure was conducted as described by Crosson et al. [24].
RAAV QUANTITATIVE ANALYSIS:
Titers of rAAV were determined by quantitative real-time PCR (qRT-PCR) using SYBR Green PCR master mix. In brief, the purified rAAV was treated with protease K at 50°C for 1 h, then 95°C for 29 min for inactivation. The number of genome copies of rAAV was detected using primers targeting the
GENE EXPRESSION DETERMINED BY PCR ASSAY:
qPCR was carried out to assess the expression of E1B19K, caspase-3 and cytochrome C (Cyto C). Total RNAs were isolated and complementary DNA (cDNA) was synthesized using the reverse transcription method. The conditions for PCR assay were: initial denaturation at 95°C for 5 min, 30 cycles of 95°C for 30 s, 60°C for 30 s, and 72°C for 1 min, followed by final extension at 72°C for 10 min. The transcript level of the housekeeping gene GAPDH was detected to normalize expression of target genes. The expression levels of these 3 genes were analyzed using 2−ΔΔCT method [25]. The gene-specific primers for the reference gene and target genes, including E1B19K, caspase 3, cytochrome C, HKII, and GAPDH, are illustrated in Table 1. The primers were designed using the Primer 5 primer designing tool and synthesized by Western Biotech (Chongqing, China),
PROTEIN EXPRESSION DETERMINED BY WESTERN BLOT ASSAY:
Western blot analysis for cleaved caspase-3 (C-caspase 3) and cytochrome C were performed to analyze apoptosis levels of all cell lines. Total proteins were extracted from lysed cells and the concentration was determined using the BCA Protein Assay Kit (Cat. No. P0010, Beyotime Biotech, Shanghai, China) according to the protocol of the manufacturer. Samples were separated on 10% SDS-PAGE gel and electro-transferred onto PVDF membranes before blocking with 5% non-fat dry milk for 1 h at room temperature. Then, the PVDF membrane was incubated with rabbit anti-cleaved caspase 3 antibody (Cat. No. AC033, Beyotime Biotech, Shanghai, China), mouse anti-cytochrome C (Cat. No. sc-13156, Santa Cruz Biotech, Santa Cruz, CA, USA), and mouse anti-β-actin antibody (Cat. No. sc-8432, Santa Cruz Biotech) at room temperature for 30 min. After washing with PBS 3 times, PVDF membranes were incubated with secondary antibodies at room temperature for 2 h. After washing with BPS 3 times, the specific protein bands were visualized using an ECL kit. β-actin was used as the internal control to normalize the C-caspase 3 and cytochrome C expression.
CELL VIABILITY EVALUATION WITH MTT ASSAY:
MTT assay was carried out to detect cell survival and growth (cell viability). Cells were seeded onto a 96-well plate and we adjusted the density to 2×104 cells per well. After infecting with rAAV for 24 h and 72 h, each cell line was incubated with MTT solution (at final concentration of 5 mg/ml) for 4 h, and then the absorbance of cells in plates was detected using an ELISA reader at a wavelength of 595 nm.
ACUTE TOXICITY TEST:
Acute toxicity testing was conducted to assess the general toxicity of rAAV. Total of 10 ml rAAV (107 infectious units) was injected into C57b1/6 male mice through the tail vein. Fourteen days after injection, the heart, liver, spleen, lung, and kidney tissues were collected to assess the safety of rAAV using hematoxylin-eosin (HE) staining.
STATISTICAL ANALYSIS:
Data were analyzed with SPSS software 20.0 (SPSS, Inc., Chicago, IL, USA). Data were expressed as mean±standard deviation (SD) and were analyzed with Tukey’s post hoc test validated by ANOVA to compare differences among groups. At least 6 replicates were carried out for every experiment or test. A
Results
PAAV-HE1B19K-TE1A TRANSGENE PLASMID WAS SUCCESSFULLY STRUCTURED:
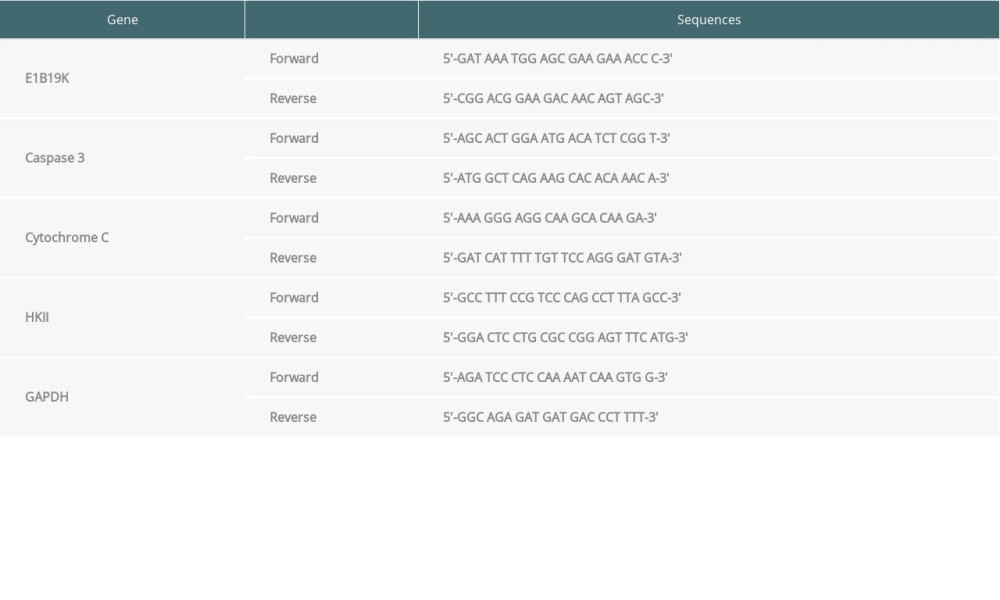
The pAAV-IRES-ZsGreen and pUC57-HE1B19K-TE1A plasmids were digested with restriction endonuclease Mlu1 and BamH1 (Figure 1A). Through digestion, the fragment HE1B19K-TE1A and open-circular pAAV-IRES-ZsGreen plasmid were obtained (Figure 1A). The large fragment of digested pAAV-IRES-Green and small fragment of digested pUC57-HE1B19K-TE1A (HE1B19K-TE1A) were purified and then ligated to construct pAAV-HE1B19K-TE1A (Figure 1B). The positive clones were verified with restriction digestion (Figure 1C) and PCR assay (Figure 1D). The results showed that both restriction (Figure 1C) and PCR (Figure 1D) images demonstrated an obvious fragment (294 bp) in the positive clone, which was the HKII gene.
PAAV-HE1B19K-TE1A WAS HIGHLY EXPRESSED IN TUMOR CELLS:
At 72 h after transfection, the ZsGreen signal was obviously visible in A549 (Figure 2A), HepG2 (Figure 2B), and BGC-803 (Figure 2C), but without signals in HUVEC (Figure 2D). These results suggest that hTERT promoter is only highly expressed in tumor cells. After transfecting with pAAV-HE1B19K-TE1A, expression of E1B19K in tumor cells was significantly higher compared to that in the pAAV-IRES-Zs Green group (Figure 3, p<0.05). However, there were no obvious effects of pAAV-HE1B19K-TE1A transfection on E1B19K expression in normal HUVECs (Figure 3). These results suggest that both hTERT and HKII promoters are highly activated in tumor cells; therefore, E1B19K and E1A protein may play important roles in rAAV packaging.
TITERS OF PAAV-HE1B19K-TE1A:
Due to the appropriate amplification plot of ITR for pAAV-IRES-ZsGreen (Figure 4A) and pAAV-HE1B19K-TE1A (Figure 4B) plasmid, the titers of pAAV-HE1B19K-TE1A were detected using qPCR with ITR primers. After collecting pAAV-HE1B19K-TE1A from lysed cells, HepG2, A549, and BGC-803 cells demonstrated 7.4×107, 1.4×108, and 1.1×108 gc/μl virus genome copies, respectively (Figure 4C).
PAAV-HE1B19K-TE1A REDUCED TUMOR CELL VIABILITY:
The inhibitory effects of pAAV-HE1B19K-TE1A on viability of tumor cells were determined with MTT assay. The pAAV-HE1B19K-TE1A infection demonstrated an obvious inhibitory effect on the cell viability of tumor cells (HepG2, A549, and BGC-803 cells) at 24 h (Figure 5A) and 48 h (Figure 5B) after transfection, in a time-dependent manner. However, no obvious inhibitory effect of pAAV-HE1B19K-TE1A on cell viability of the normal HUVEC cells was discovered (Figure 5).
PAAV-HE1B19K-TE1A TRIGGERED CASPASE 3 ACTIVITY IN TUMOR CELLS:
To clarify the mechanism by which pAAV-HE1B19K-TE1A causes a decrease of cell viability in tumor cells, caspase 3 activity was determined using Western blot assay (Figure 6A) to examine cleaved caspase 3 (C-caspase 3) levels. The findings indicated that pAAV-HE1B19K-TE1A transfection remarkably promoted the C-caspase 3 expression compared to that in un-transfected tumor cells (or pAAV-IRES-Zs Green group) in HepG2 cells (Figure 6B, p<0.05), A549 cells (Figure 6C, p<0.05), and BGC-803 cells (Figure 6D, p<0.05). However, there were no significant effects of pAAV-HE1B19K-TE1A transfection on C-caspase 3 expression in HUVEC cells (Figure 6E, p>0.05).
The qPCR assay also illustrated that pAAV-HE1B19K-TE1A transfection remarkably enhanced C-caspase 3 gene expression in HepG2 (Figure 7A, p<0.05), A549 (Figure 7B, p<0.05), and BGC-803 (Figure 7C, p<0.05) compared to that in blank tumor cells. However, pAAV-HE1B19K-TE1A transfection triggered no obvious changes of C-caspase 3 gene expression in HUVEC cells (Figure 7D, p>0.05).
PAAV-HE1B19K-TE1A INDUCED RELEASE OF CYTO C IN TUMOR CELLS:
We assessed cytochrome C (Cyto C) expression in tumor cells using Western blot (Figure 6A) and qPCR assays. The Western blot assay results showed that pAAV-HE1B19K-TE1A transfection obviously induced Cyto C release in HepG2 cells (Figure 6B, p<0.05), A549 cells (Figure 6C, p<0.05), and BGC-803 cells (Figure 6D, p<0.05), but did not trigger Cyto C release in normal HUVEC cells (Figure 6E, p>0.05). qPCR assay also showed that pAAV-HE1B19K-TE1A transfection triggered the release of Cyto C in HepG2 (Figure 7A, p<0.001), A549 (Figure 7B, p<0.001), and BGC-803 (Figure 7C, p<0.001), but not in HUVEC cells (Figure 7D, p<0.001).
PAAV-HE1B19K-TE1A DEMONSTRATED NO TOXICITY TO TISSUES OF ANIMALS:
HE staining showed that pAAV-HE1B19K-TE1A caused no obvious pathological changes in the heart, liver, spleen, lung, and kidney of the treated mice, equal to the status of the normal control mice (Figure 8). This result suggests that treatment with pAAV-HE1B19K-TE1A caused no acute toxicity to the heart, liver, spleen, lung, and kidney. Therefore, pAAV-HE1B19K-TE1A appears to be safe for tail vein injection.
Discussion
In contrast to the non-replicating virus vector used in traditional gene therapy, OVs are have high reproduction and are assembled by special plasmids and without propagation in normal cells. In theory, they have higher anti-tumor efficiency and fewer adverse effects. OVs can propagate in tumor cells and occupy the materials, energy, and places of the host cells, eventually destroying the cells and releasing offspring virus to infect neighboring tumor cells [1]. Moreover, OVs can express toxic proteins, induce inflammatory cytokines such as TNF, and trigger the immune response [26]. Gene therapy using conditional replicated AAV has become an important focus of tumor gene therapy [27]. There are 2 main approaches to constructing targeted rAAV. One approach is to remove genes that are necessary for the virus to replicate in normal cells but are not needed in tumor cells, and the other is that gene expression is regulated by a tumor-specific promoter. rAAV vector, which is the best-known gene vector system, overcomes the shortcomings that other gene expression vectors cannot overcome [28,29]. rAAV vector has a wide range of transfection, with high transfection efficiency, to drive gene expression stably
In this study, recombinant AAV was successfully produced using the Helper-free system in tumor cells and obtained replication ability with E1A and E1B19K genes. Tumor-specific promoters, hTERT and HKII, were inserted upstream of E1A and E1B19K gene frames, respectively, to regulate the expression of these 2 genes. This process causes the rAAV to only replicate in tumor cells. The construction of this vector gives the gene therapy vectors dual targeted effects on tumor cells. These results showed that all of the tumor cells (HepG2, A549, and BGC-803) demonstrated higher ZsGreen signals, but HUVEC did not. These results suggest that hTERT promoter is only highly expressed in tumor cells. However, due to the different cell lines with different growth characteristics, the ZsGreen signals were different in various tumor cells lines. Moreover, HKII and hTERT promoters could effectively mediate the tumor-specific expression of E1B19K and E1A genes, but not in normal cells. In addition,
Conclusions
The result of this study indicated that the tumor-targeted rAAV was successfully produced using the Helper-free system with the recombinant plasmid, demonstrating high ability to decrease the cell viability of tumor cells without adverse effects on normal cells. In the future, it may be possible that the plasmid pAAV-HE1B19K-TE1A can be inserted with special genes targeted to the tumor markers in rAAV to enhance the ability of specific infection.
Figures
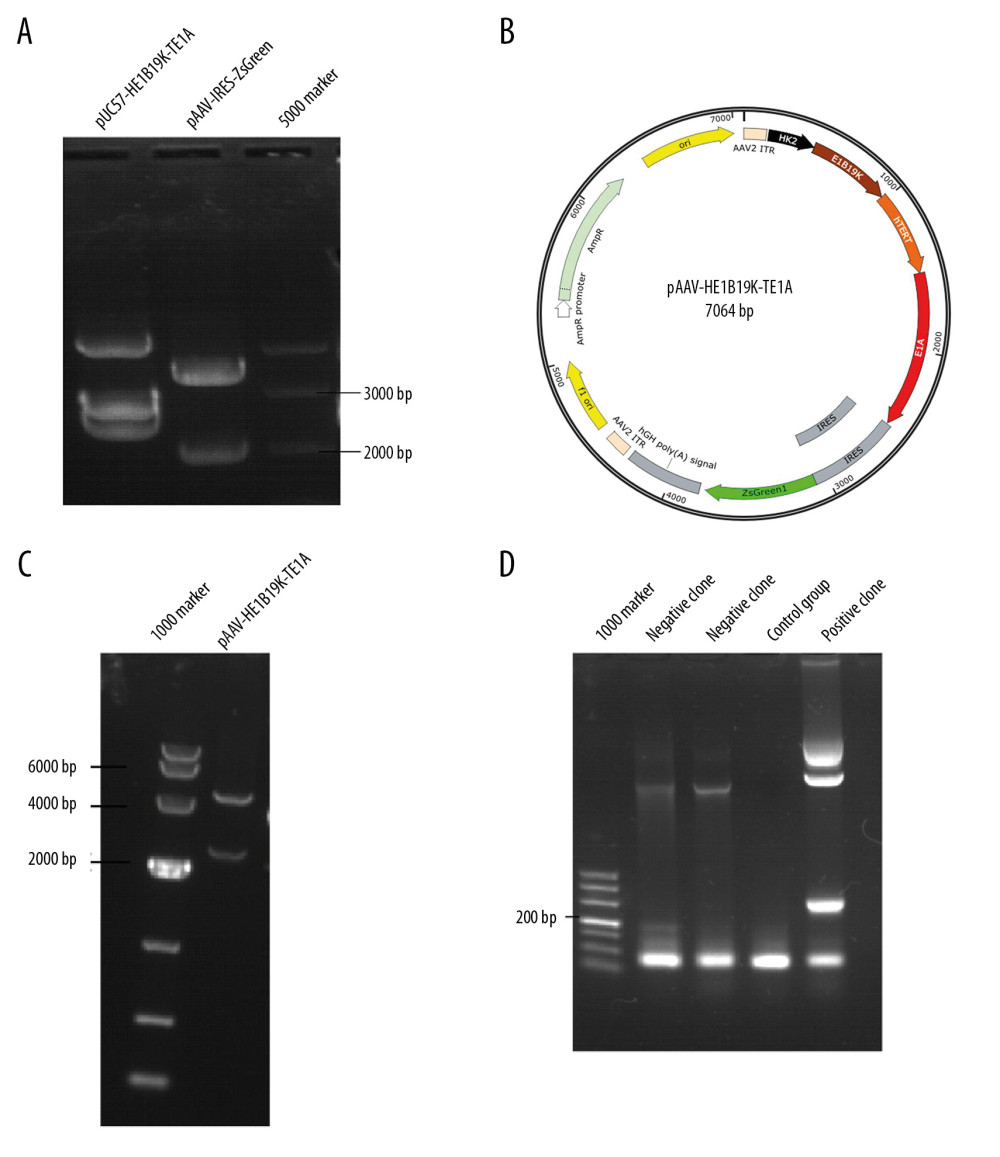 Figure 1. Synthesis of the new transgene plasmid pAAV-HE1B19K-TE1A. (A) Restriction digestion of pUC57-HE1B19K-TE1A and pAAV-IRES-ZsGreen with Mlu1 and BamH1. (B) The structure of the pAAV-HE1B19K-TE1A. (C) Positive clones were verified by restriction digestion. (D) Positive clones were verified by PCR assay.
Figure 1. Synthesis of the new transgene plasmid pAAV-HE1B19K-TE1A. (A) Restriction digestion of pUC57-HE1B19K-TE1A and pAAV-IRES-ZsGreen with Mlu1 and BamH1. (B) The structure of the pAAV-HE1B19K-TE1A. (C) Positive clones were verified by restriction digestion. (D) Positive clones were verified by PCR assay. 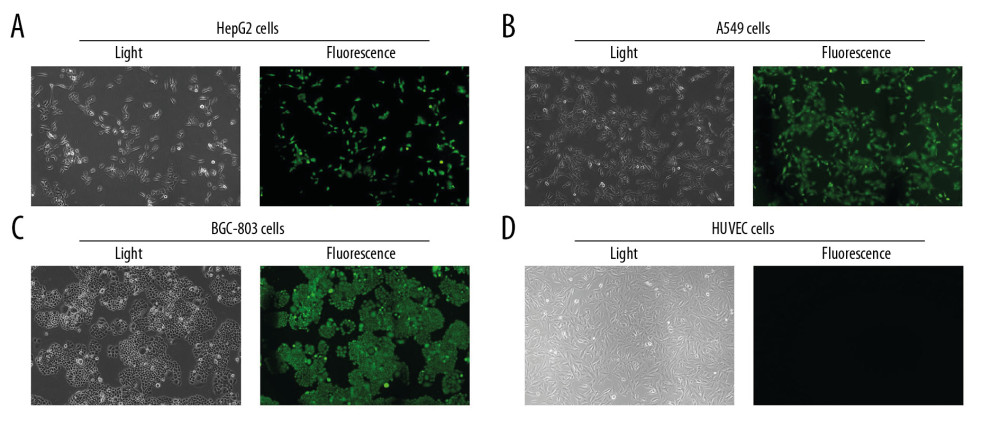 Figure 2. Determination for pAAV-HE1B19K-TE1A transfection in tumor cells. ZsGreen expression was observed in HepG2 (A), A549 (B), and BGC-803 (C), but not in HUVEC cells (D).
Figure 2. Determination for pAAV-HE1B19K-TE1A transfection in tumor cells. ZsGreen expression was observed in HepG2 (A), A549 (B), and BGC-803 (C), but not in HUVEC cells (D).  Figure 3. E1B19K expression in cells detected using qPCR assay. (A – HepG2 cells, B – A549 cells, C – BGC-803 cells, D – HUVEC cells. 1 – cells without transfection, 2 – cells transfected with pAAV-IRES-ZsGreen, pHelper, and pAAV-RC, 3 – cells transfected with pAAV-HE1B19K-TE1A, pHelper, and pAAV-RC). *** p<0.0001 vs. pAAV-IRES-ZsGreen group in HepG2, A549, or BGC-803 cells.
Figure 3. E1B19K expression in cells detected using qPCR assay. (A – HepG2 cells, B – A549 cells, C – BGC-803 cells, D – HUVEC cells. 1 – cells without transfection, 2 – cells transfected with pAAV-IRES-ZsGreen, pHelper, and pAAV-RC, 3 – cells transfected with pAAV-HE1B19K-TE1A, pHelper, and pAAV-RC). *** p<0.0001 vs. pAAV-IRES-ZsGreen group in HepG2, A549, or BGC-803 cells. 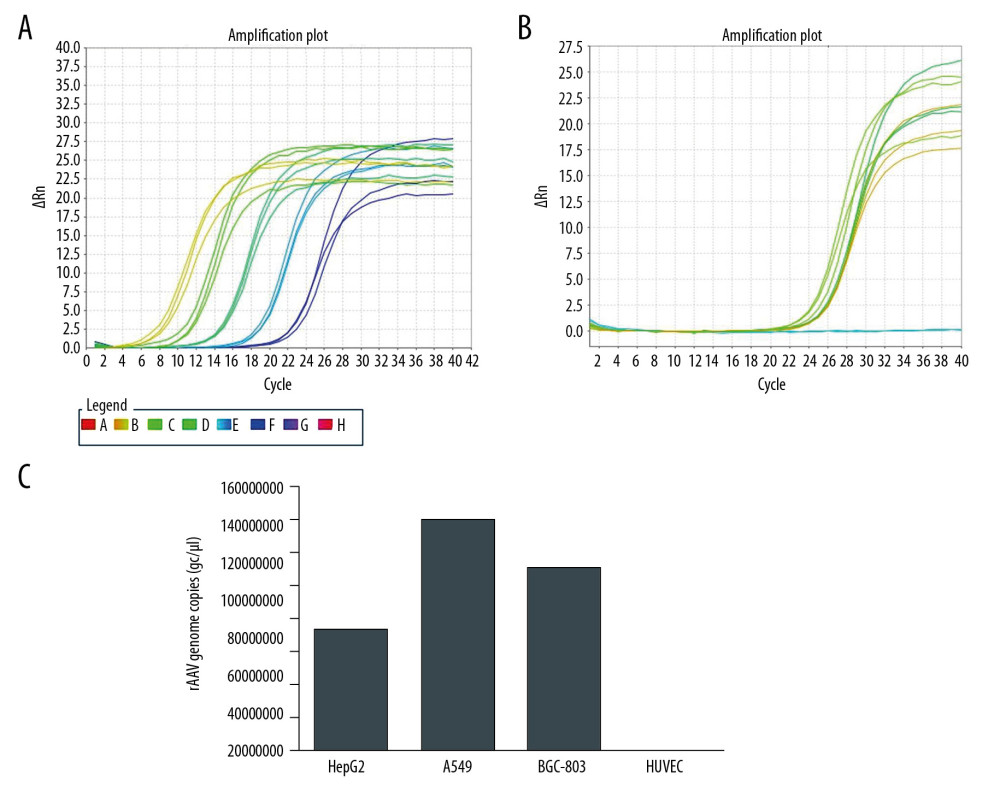 Figure 4. Titers of pAAV-HE1B19K-TE1A were detected using qPCR with ITR primers. (A) Standard amplification plot of ITR for plasmid pAAV-IRES-ZsGreen. (B) Amplification plot of ITR for pAAV-HE1B19K-TE1A. (C) pAAV-HE1B19K-TE1A genome copies in each cell line.
Figure 4. Titers of pAAV-HE1B19K-TE1A were detected using qPCR with ITR primers. (A) Standard amplification plot of ITR for plasmid pAAV-IRES-ZsGreen. (B) Amplification plot of ITR for pAAV-HE1B19K-TE1A. (C) pAAV-HE1B19K-TE1A genome copies in each cell line. 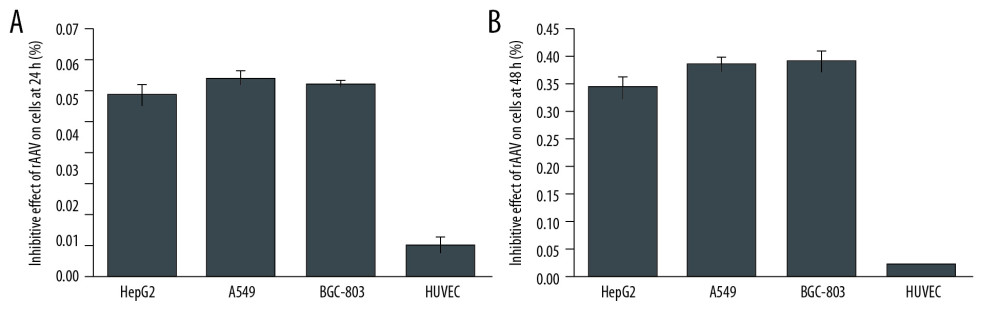 Figure 5. MTT assay for measuring the inhibitory effect of pAAV-HE1B19K-TE1A on tumor cells. (A) Inhibitive effects at 24 h after transfection. (B) Inhibitive effects at 48 h after transfection.
Figure 5. MTT assay for measuring the inhibitory effect of pAAV-HE1B19K-TE1A on tumor cells. (A) Inhibitive effects at 24 h after transfection. (B) Inhibitive effects at 48 h after transfection. 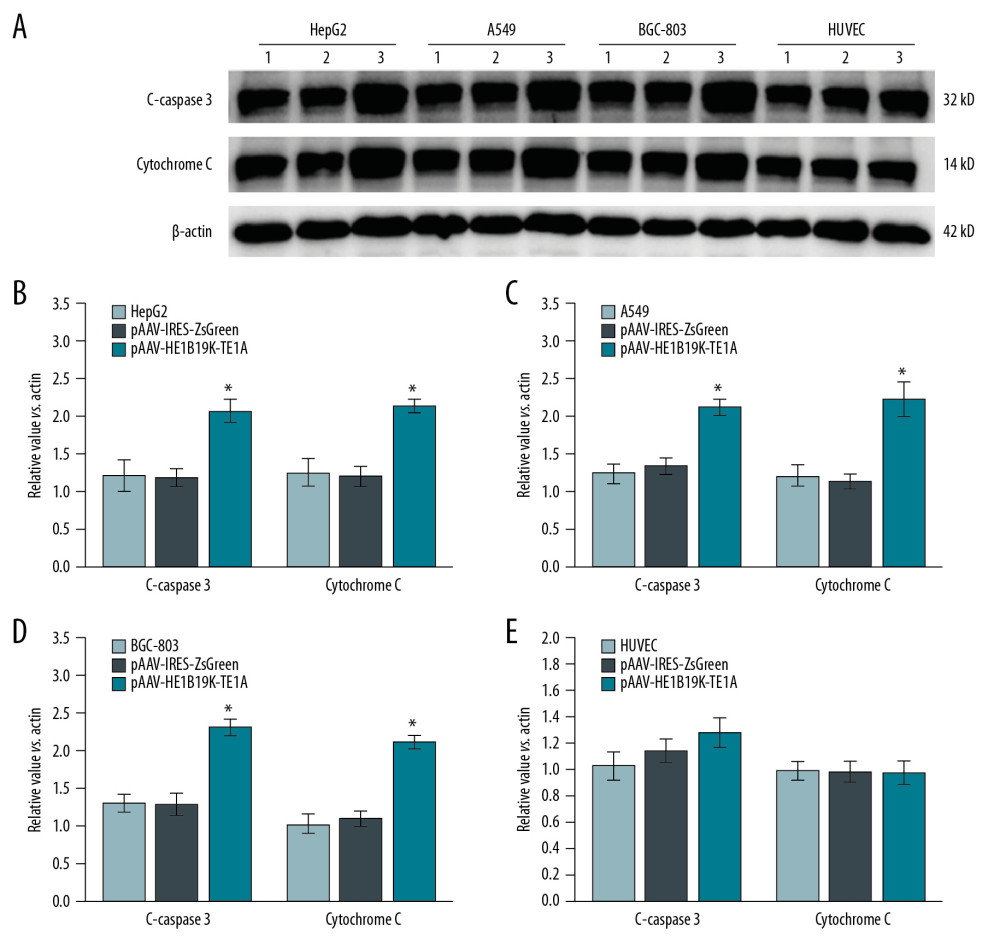 Figure 6. Production of C-caspase 3 and cytochrome C proteins in tumor cells and normal cells. (A) Western blot images. Relative C-caspase 3 and cytochrome C expression in HepG2 (B), A549 (C), BGC-803 (D), and HUVEC (E) was statistically analyzed. 1 – Tumor cells (HepG2, A549, BGC-803, or HUVEC cells) without transfection, 2 – Tumor cells (HepG2, A549, BGC-803, or HUVEC cells) transfected with pAAV-IRES-ZsGreen, pHelper, and pAAV-RC, 3 – Tumor cells (HepG2, A549, BGC-803, or HUVEC cells) transfected with pAAV-HE1B19K-TE1A, pHelper, and pAAV-RC). * p<0.05 vs. pAAV-IRES-ZsGreen group in HepG2, A549, or BGC-803 cells.
Figure 6. Production of C-caspase 3 and cytochrome C proteins in tumor cells and normal cells. (A) Western blot images. Relative C-caspase 3 and cytochrome C expression in HepG2 (B), A549 (C), BGC-803 (D), and HUVEC (E) was statistically analyzed. 1 – Tumor cells (HepG2, A549, BGC-803, or HUVEC cells) without transfection, 2 – Tumor cells (HepG2, A549, BGC-803, or HUVEC cells) transfected with pAAV-IRES-ZsGreen, pHelper, and pAAV-RC, 3 – Tumor cells (HepG2, A549, BGC-803, or HUVEC cells) transfected with pAAV-HE1B19K-TE1A, pHelper, and pAAV-RC). * p<0.05 vs. pAAV-IRES-ZsGreen group in HepG2, A549, or BGC-803 cells. 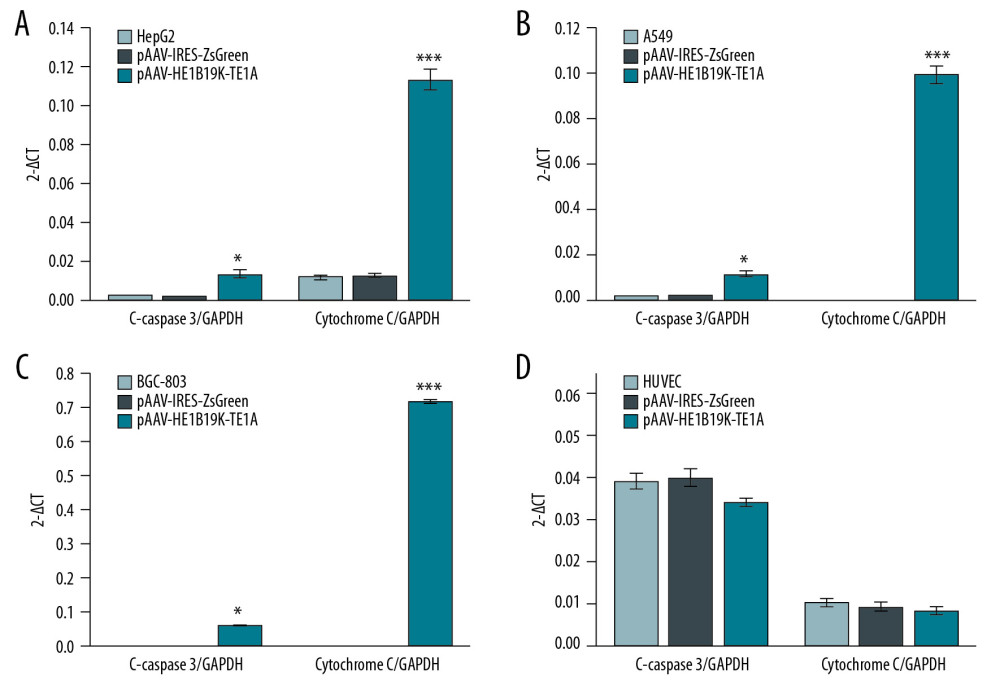 Figure 7. Effects of pAAV-HE1B19K-TE1A transfection on C-caspase 3 and cytochrome C expression in tumor cells and HUVEC cells using PCR assay. (A) Relative C-caspase 3 gene expression (C-caspase 3/GAPDH) and relative cytochrome C gene expression (cytochrome C/GAPDH) in HepG2 cells. (B) Relative C-caspase 3/GAPDH and relative cytochrome C/GAPDH in A549 cells. (C) Relative C-caspase 3/GAPDH and relative cytochrome C/GAPDH in BGC-803 cells. (D) Relative C-caspase 3/GAPDH and relative cytochrome C/GAPDH in HUVEC cells. * p<0.05 and *** p<0.0001 vs. pAAV-IRES-ZsGreen group in HepG2, A549, or BGC-803 cells.
Figure 7. Effects of pAAV-HE1B19K-TE1A transfection on C-caspase 3 and cytochrome C expression in tumor cells and HUVEC cells using PCR assay. (A) Relative C-caspase 3 gene expression (C-caspase 3/GAPDH) and relative cytochrome C gene expression (cytochrome C/GAPDH) in HepG2 cells. (B) Relative C-caspase 3/GAPDH and relative cytochrome C/GAPDH in A549 cells. (C) Relative C-caspase 3/GAPDH and relative cytochrome C/GAPDH in BGC-803 cells. (D) Relative C-caspase 3/GAPDH and relative cytochrome C/GAPDH in HUVEC cells. * p<0.05 and *** p<0.0001 vs. pAAV-IRES-ZsGreen group in HepG2, A549, or BGC-803 cells. 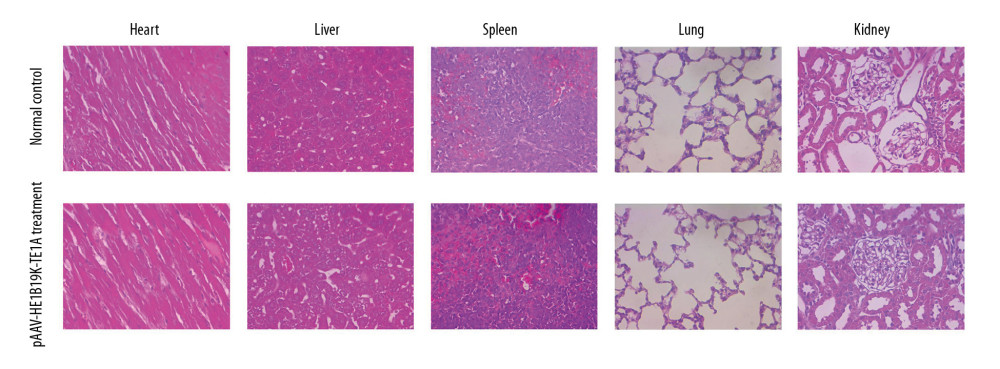 Figure 8. HE staining for analyzing pathological changes of vital organs of mice after pAAV-HE1B19K-TE1A injection and in the vital organs of normal mice. For the pAAV-HE1B19K-TE1A-injected mice, at 2 weeks after injection, the tissues (heart, liver, spleen, lung, and kidney) were collected for toxicity evaluation. Magnification, 400×.
Figure 8. HE staining for analyzing pathological changes of vital organs of mice after pAAV-HE1B19K-TE1A injection and in the vital organs of normal mice. For the pAAV-HE1B19K-TE1A-injected mice, at 2 weeks after injection, the tissues (heart, liver, spleen, lung, and kidney) were collected for toxicity evaluation. Magnification, 400×. References
1. Bommareddy PK, Shettigar M, Kaufman HL, Integrating oncolytic viruses in combination cancer immunotherapy: Nat Rev Immunol, 2018; 18; 498-513
2. Sirika P, Wei Z, Fang C, Adeno-associated virus (AAV) serotypes have distinctive interactions with domains of the cellular AAV Receptor: J Virol, 2017; 91; e00391-417
3. Qin S, Miguel S-E, Guangping G, Production of recombinant adeno-associated viruses (rAAVs) by transient transfection: Cold Spring Harb Protoc, 2020; 2020 095596
4. Li C, Samulski RJ, Engineering adeno-associated virus vectors for gene therapy: Nat Rev Genet, 2020; 21; 255-72
5. Samulski RJ, Muzyczka N, AAV-mediated gene therapy for research and therapeutic purposes: Annu Rev Virol, 2014; 1; 427-51
6. Trapani I, Adeno-associated viral vectors as a tool for large gene delivery to the retina: Genes (Basel), 2019; 10; 287
7. Xiao X, Li J, Samulski RJ, Production of high-titer recombinant adeno-associated virus vectors in the absence of helper adenovirus: J Virol, 1998; 72; 2224-32
8. Matsushita T, Okada T, Inaba T, The adenovirus E1A and E1B19K genes provide a helper function for transfection-based adeno-associated virus vector production: J Gen Virol, 2004; 85; 2209-14
9. Greider CW, Telomere length regulation: Annu Rev Biochem, 1996; 65; 337-65
10. Lee M, Teber ET, Holmes O, Telomere sequence content can be used to determine ALT activity in tumours: Nucleic Acids Res, 2018; 46; 4903-18
11. Komata T, Kanzawa T, Kondo Y, Telomerase as a therapeutic target for malignant gliomas: Oncogene, 2002; 21; 656-63
12. Farahzadi R, Fathi E, Mesbah-Namin SA, Zinc sulfate contributes to promote telomere length extension via increasing telomerase gene expression, telomerase activity and change in the TERT gene promoter CpG island methylation status of human adipose-derived mesenchymal stem cells: PLoS One, 2017; 12; e0188052
13. Counter CM, Gupta J, Harley CB, Telomerase activity in normal leukocytes and in hematologic malignancies: Blood, 1995; 85; 2315-20
14. Nakamura TM, Cech TR, Reversing time: Origin of telomerase: Cell, 1998; 92; 587-90
15. Aalbers AM, Kajigaya S, Van den Heuvel-Eibrink MM, Human telomere disease due to disruption of the CCAAT box of the TERC promoter: Blood, 2012; 119; 3060-63
16. Kim NW, Piatyszek MA, Prowse KR, Specific association of human telomerase activity with immortal cells and cancer: Science, 1994; 266; 2011-15
17. Takakura M, Kyo S, Kanaya T, Cloning of human telomerase catalytic subunit (hTERT) gene promoter and identification of proximal core promoter sequences essential for transcriptional activation in immortalized and cancer cells: Cancer Res, 1999; 59; 551-57
18. Ramlee MK, Wang J, Toh WX, Transcription regulation of the human telomerase reverse transcriptase (hTERT) gene: Genes (Basel), 2016; 7; 50
19. Illsinger S, Goken C, Brockmann M, Effect of tacrolimus on energy metabolism in human umbilical endothelial cells: Ann Transplant, 2011; 16; 68-75
20. Lee HG, Kim H, Son T, Regulation of HK2 expression through alterations in CpG methylation of the HK2 promoter during progression of hepatocellular carcinoma: Oncotarget, 2016; 7; 41798-810
21. Anderson M, Marayati R, Moffitt R, Hexokinase 2 promotes tumor growth and metastasis by regulating lactate production in pancreatic cancer: Oncotarget, 2017; 8; 56081-94
22. Shoshan-Barmatz V, Mizrachi D, VDAC1: From structure to cancer therapy: Front Oncol, 2012; 2; 164
23. Mathupala SP, Rempel A, Pedersen PL, Glucose catabolism in cancer cells: identification and characterization of a marked activation response of the type II hexokinase gene to hypoxic conditions: J Biol Chem, 2001; 276; 43407-12
24. Crosson SM, Dib P, Smith JK, Helper-free production of laboratory grade AAV and purification by iodixanol density gradient centrifugation. molecular therapy: Methods Clin Devel, 2018; 10; 1-7
25. Livak KJ, Schmittgen TD, Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method: Methods (San Diego, CA), 2001; 25; 402-8
26. Crompton AM, Kirn DH, From ONYX-015 to armed vaccinia viruses: the education and evolution of oncolytic virus development: Curr Cancer Drug Targets, 2007; 7; 133-39
27. Wang Z, Cheng F, Engelhardt JF, Development of a novel recombinant adeno-associated virus production system using human bocavirus 1 helper genes: Mol Ther, 2018; 11; 40-51
28. Monahan PE, Samulski RJ, AAV vectors: Is clinical success on the horizon?: Gene Ther, 2000; 7; 24-30
29. Matsushita T, Elliger S, Elliger C, Adeno-associated virus vectors can be efficiently produced without helper virus: Gene Ther, 1998; 5; 938-45
30. Linden RM, Berns KI, Site-specific integration by adeno-associated virus: A basis for a potential gene therapy vector: Gene Ther, 1997; 4; 4-5
Figures
 Figure 1. Synthesis of the new transgene plasmid pAAV-HE1B19K-TE1A. (A) Restriction digestion of pUC57-HE1B19K-TE1A and pAAV-IRES-ZsGreen with Mlu1 and BamH1. (B) The structure of the pAAV-HE1B19K-TE1A. (C) Positive clones were verified by restriction digestion. (D) Positive clones were verified by PCR assay.
Figure 1. Synthesis of the new transgene plasmid pAAV-HE1B19K-TE1A. (A) Restriction digestion of pUC57-HE1B19K-TE1A and pAAV-IRES-ZsGreen with Mlu1 and BamH1. (B) The structure of the pAAV-HE1B19K-TE1A. (C) Positive clones were verified by restriction digestion. (D) Positive clones were verified by PCR assay. Figure 2. Determination for pAAV-HE1B19K-TE1A transfection in tumor cells. ZsGreen expression was observed in HepG2 (A), A549 (B), and BGC-803 (C), but not in HUVEC cells (D).
Figure 2. Determination for pAAV-HE1B19K-TE1A transfection in tumor cells. ZsGreen expression was observed in HepG2 (A), A549 (B), and BGC-803 (C), but not in HUVEC cells (D). Figure 3. E1B19K expression in cells detected using qPCR assay. (A – HepG2 cells, B – A549 cells, C – BGC-803 cells, D – HUVEC cells. 1 – cells without transfection, 2 – cells transfected with pAAV-IRES-ZsGreen, pHelper, and pAAV-RC, 3 – cells transfected with pAAV-HE1B19K-TE1A, pHelper, and pAAV-RC). *** p<0.0001 vs. pAAV-IRES-ZsGreen group in HepG2, A549, or BGC-803 cells.
Figure 3. E1B19K expression in cells detected using qPCR assay. (A – HepG2 cells, B – A549 cells, C – BGC-803 cells, D – HUVEC cells. 1 – cells without transfection, 2 – cells transfected with pAAV-IRES-ZsGreen, pHelper, and pAAV-RC, 3 – cells transfected with pAAV-HE1B19K-TE1A, pHelper, and pAAV-RC). *** p<0.0001 vs. pAAV-IRES-ZsGreen group in HepG2, A549, or BGC-803 cells. Figure 4. Titers of pAAV-HE1B19K-TE1A were detected using qPCR with ITR primers. (A) Standard amplification plot of ITR for plasmid pAAV-IRES-ZsGreen. (B) Amplification plot of ITR for pAAV-HE1B19K-TE1A. (C) pAAV-HE1B19K-TE1A genome copies in each cell line.
Figure 4. Titers of pAAV-HE1B19K-TE1A were detected using qPCR with ITR primers. (A) Standard amplification plot of ITR for plasmid pAAV-IRES-ZsGreen. (B) Amplification plot of ITR for pAAV-HE1B19K-TE1A. (C) pAAV-HE1B19K-TE1A genome copies in each cell line. Figure 5. MTT assay for measuring the inhibitory effect of pAAV-HE1B19K-TE1A on tumor cells. (A) Inhibitive effects at 24 h after transfection. (B) Inhibitive effects at 48 h after transfection.
Figure 5. MTT assay for measuring the inhibitory effect of pAAV-HE1B19K-TE1A on tumor cells. (A) Inhibitive effects at 24 h after transfection. (B) Inhibitive effects at 48 h after transfection. Figure 6. Production of C-caspase 3 and cytochrome C proteins in tumor cells and normal cells. (A) Western blot images. Relative C-caspase 3 and cytochrome C expression in HepG2 (B), A549 (C), BGC-803 (D), and HUVEC (E) was statistically analyzed. 1 – Tumor cells (HepG2, A549, BGC-803, or HUVEC cells) without transfection, 2 – Tumor cells (HepG2, A549, BGC-803, or HUVEC cells) transfected with pAAV-IRES-ZsGreen, pHelper, and pAAV-RC, 3 – Tumor cells (HepG2, A549, BGC-803, or HUVEC cells) transfected with pAAV-HE1B19K-TE1A, pHelper, and pAAV-RC). * p<0.05 vs. pAAV-IRES-ZsGreen group in HepG2, A549, or BGC-803 cells.
Figure 6. Production of C-caspase 3 and cytochrome C proteins in tumor cells and normal cells. (A) Western blot images. Relative C-caspase 3 and cytochrome C expression in HepG2 (B), A549 (C), BGC-803 (D), and HUVEC (E) was statistically analyzed. 1 – Tumor cells (HepG2, A549, BGC-803, or HUVEC cells) without transfection, 2 – Tumor cells (HepG2, A549, BGC-803, or HUVEC cells) transfected with pAAV-IRES-ZsGreen, pHelper, and pAAV-RC, 3 – Tumor cells (HepG2, A549, BGC-803, or HUVEC cells) transfected with pAAV-HE1B19K-TE1A, pHelper, and pAAV-RC). * p<0.05 vs. pAAV-IRES-ZsGreen group in HepG2, A549, or BGC-803 cells. Figure 7. Effects of pAAV-HE1B19K-TE1A transfection on C-caspase 3 and cytochrome C expression in tumor cells and HUVEC cells using PCR assay. (A) Relative C-caspase 3 gene expression (C-caspase 3/GAPDH) and relative cytochrome C gene expression (cytochrome C/GAPDH) in HepG2 cells. (B) Relative C-caspase 3/GAPDH and relative cytochrome C/GAPDH in A549 cells. (C) Relative C-caspase 3/GAPDH and relative cytochrome C/GAPDH in BGC-803 cells. (D) Relative C-caspase 3/GAPDH and relative cytochrome C/GAPDH in HUVEC cells. * p<0.05 and *** p<0.0001 vs. pAAV-IRES-ZsGreen group in HepG2, A549, or BGC-803 cells.
Figure 7. Effects of pAAV-HE1B19K-TE1A transfection on C-caspase 3 and cytochrome C expression in tumor cells and HUVEC cells using PCR assay. (A) Relative C-caspase 3 gene expression (C-caspase 3/GAPDH) and relative cytochrome C gene expression (cytochrome C/GAPDH) in HepG2 cells. (B) Relative C-caspase 3/GAPDH and relative cytochrome C/GAPDH in A549 cells. (C) Relative C-caspase 3/GAPDH and relative cytochrome C/GAPDH in BGC-803 cells. (D) Relative C-caspase 3/GAPDH and relative cytochrome C/GAPDH in HUVEC cells. * p<0.05 and *** p<0.0001 vs. pAAV-IRES-ZsGreen group in HepG2, A549, or BGC-803 cells. Figure 8. HE staining for analyzing pathological changes of vital organs of mice after pAAV-HE1B19K-TE1A injection and in the vital organs of normal mice. For the pAAV-HE1B19K-TE1A-injected mice, at 2 weeks after injection, the tissues (heart, liver, spleen, lung, and kidney) were collected for toxicity evaluation. Magnification, 400×.
Figure 8. HE staining for analyzing pathological changes of vital organs of mice after pAAV-HE1B19K-TE1A injection and in the vital organs of normal mice. For the pAAV-HE1B19K-TE1A-injected mice, at 2 weeks after injection, the tissues (heart, liver, spleen, lung, and kidney) were collected for toxicity evaluation. Magnification, 400×. In Press
15 Mar 2024 : Review article
Approaches and Challenges in the Current Management of Cytomegalovirus in Transplant Recipients: Highlighti...Ann Transplant In Press; DOI: 10.12659/AOT.941185
18 Mar 2024 : Original article
Does Antibiotic Use Increase the Risk of Post-Transplantation Diabetes Mellitus? A Retrospective Study of R...Ann Transplant In Press; DOI: 10.12659/AOT.943282
20 Mar 2024 : Original article
Transplant Nephrectomy: A Comparative Study of Timing and Techniques in a Single InstitutionAnn Transplant In Press; DOI: 10.12659/AOT.942252
28 Mar 2024 : Original article
Association Between FEV₁ Decline Rate and Mortality in Long-Term Follow-Up of a 21-Patient Pilot Clinical T...Ann Transplant In Press; DOI: 10.12659/AOT.942823
Most Viewed Current Articles
05 Apr 2022 : Original article
Impact of Statins on Hepatocellular Carcinoma Recurrence After Living-Donor Liver TransplantationDOI :10.12659/AOT.935604
Ann Transplant 2022; 27:e935604
12 Jan 2022 : Original article
Risk Factors for Developing BK Virus-Associated Nephropathy: A Single-Center Retrospective Cohort Study of ...DOI :10.12659/AOT.934738
Ann Transplant 2022; 27:e934738
22 Nov 2022 : Original article
Long-Term Effects of Everolimus-Facilitated Tacrolimus Reduction in Living-Donor Liver Transplant Recipient...DOI :10.12659/AOT.937988
Ann Transplant 2022; 27:e937988
15 Mar 2022 : Case report
Combined Liver, Pancreas-Duodenum, and Kidney Transplantation for Patients with Hepatitis B Cirrhosis, Urem...DOI :10.12659/AOT.935860
Ann Transplant 2022; 27:e935860