11 August 2020: Lab/In Vitro Research
Regulates the Proliferation and Migration of H9c2 Cells
Hongshu Wang1BCDEF, Yong Liu2BC, Shen Han1B, Yunfeng Zi1B, Yayong Zhang1B, Ruize Kong3B, Zu Liu1B, Zhibin Cai1B, Chongbin Zhong4B, Wei Liu5B, Lifeng Li4B, Lihong Jiang3AG*DOI: 10.12659/MSM.925388
Med Sci Monit 2020; 26:e925388
Abstract
BACKGROUND: The protein NKX2–5 affects mammalian heart development. In mice, the disruption of Nkx2–5 has been associated with arrhythmias, abnormal myocardial contraction, abnormal cardiac morphogenesis, and death. However, the details of the mechanisms are unclear. This study was designed to investigate them.
MATERIAL AND METHODS: Rat cardiomyocytes from the H9c2 cell line were used in our study. First, we knocked down Nkx2–5 in the H9c2 cells and then validated consequent changes in cell proliferation and migration. We then used RNA sequencing to determine the changes in transcripts. Finally, we validated these results by quantitative reverse transcription-polymerase chain reaction.
RESULTS: We confirmed that Nkx2–5 regulates the proliferation and migration of H9c2 cells. In our experiments, Nkx2–5 regulated the expression of genes related to proliferation, migration, heart development, and disease. Based on bioinformatics analysis, knockdown of Nkx2–5 caused differential expression of genes involved in cardiac development, calcium ion-related biological activity, the transforming growth factor (TGF)-β signaling pathway, pathways related to heart diseases, the MAPK signaling pathway, and other biological processes and signaling pathways.
CONCLUSIONS: Nkx2–5 may regulate proliferation and migration of the H9c2 cells through the genes Tgfb-2, Bmp10, Id2, Wt1, Hey1, and Cacna1g; rno-miR-1-3p; the TGF‑β signaling pathway; the MAPK signaling pathway; as well as other genes and pathways.
Keywords: Heart Defects, Congenital, Cell Line, Gene Expression Regulation, Homeobox Protein Nkx-2.5, RNA, Messenger, Reverse Transcriptase Polymerase Chain Reaction, Transforming Growth Factor beta
Background
Congenital heart disease (CHD), which arises from defective cardiac structure, has a global incidence of approximately 1% [1]. Individuals with CHD are at risk for heart failure and arrhythmias [2]. Common types of CHD include atrial septal defect (ASD), ventricular septal defect (VSD), patent ductus arteriosus, and tetralogy of Fallot [3], with ASD and VSD being the most common types [4]. ASD is a continuous interruption of the cardiac atrial septum, which can lead to heart failure, arrhythmia, and pulmonary hypertension. Among possible pathogenic factors leading to CHD [5, 6], genetic factors, especially abnormalities of
Human
Our study relied in part on miRNAs, which are noncoding RNAs that are highly evolutionarily conserved [18]. miRNAs can bind to mRNA to inhibit the expression of a target gene [19,20].
In our study, we knocked down the
Material and Methods
:
The shRNA lentiviral vector GV493 was designed and made by Shanghai GeneChem. GV493 contained the following elements: hU6 (promoter), MCS (polyclonal restriction site), CBh (promoter of the enhanced green fluorescent protein [GFP] gcGFP gene), gcGFP gene, internal ribosomal entry site, and puromycin resistance gene. The inserted sequence used for knocking down
RAT CARDIOMYOCYTE CELLS AND INFECTION BY SHRNA LENTIVIRAL VECTOR:
The rat cardiomyocyte cells from the H9c2(2-1) cell line were cultured with high-glucose Dulbecco’s modified Eagle’s medium. H9c2 cells were infected by shRNA lentiviral vector with a multiplicity of infection equal to 10. Seventy-two hours after infection, puromycin at a concentration of 0.4 μg/mL was used to kill uninfected cells.
QUANTITATIVE REVERSE TRANSCRIPTION-POLYMERASE CHAIN REACTION:
After the concentration of RNA from cells of the different groups was measured, the GoScript™ Reverse Transcription Mix Oligo(dT) (Promega) was used to obtain cDNA. In total, 2000 ng of RNA was used in the 20-μL reaction system. The cDNA was diluted to 40 ng/μL with nucleic acid-free water for quantitative reverse transcription-polymerase chain reaction (qRT-PCR). Eastep®qPCRMaster MixKit (Promega) was used to complete the qRT-PCR for genes according to the manufacturer’s instructions. All-in-One™ miRNA qRT-PCR Detection Kit (GeneCopoeia) was used to complete the qRT-PCR for miRNAs according to the manufacturer’s instructions. Some qRT-PCR primers for genes were designed by Tsingke Biological Technology. Primer sequence are listed in Table 1. The qRT-PCR primers for miRNAs and some genes were purchased from GeneCopoeia. Due to the trade secrets involved, the sequence information cannot be provided. The protocols for qRT-PCR of mRNAs and miRNAs are listed individually in Tables 2 and 3. Beta-actin and U6 genes were used as internal standards. The 2−ΔΔCt method was used to calculate the relative expression levels of genes, according to the cycle threshold values of the target mRNAs, miRNAs, and internal standards, respectively.
WESTERN BLOT:
Equal amounts of protein obtained from cells in the control group and the
CELL PROLIFERATION TEST:
The CCK8 method was used to detect the cells’ ability to proliferate. Cells from each group were seeded into 96-well plates at the same concentration and tested every 24 h. First, we removed the old culture medium and added 100 μL of fresh culture medium and 10 μL of CCK8 solution to each well. We then continued the cell cultures for 2 h. Finally, we measured the absorbance at 450 nm with a microplate reader and constructed the CCK8 cell proliferation curve according to the numerical values.
RNA SEQUENCING:
RNA was extracted from cells using Trizol reagent (Invitrogen), and the quantity and purity of RNA were validated. A chain-specific library was constructed by removing ribosomal RNA, and this library was sequenced using Illumina Novaseq™ 6000.
The small RNA-sequencing (RNA-seq) library was completed by using the TruSeq Small RNA Sample Prep Kits (Illumina), and this library was sequenced using Illumina Hiseq2000/2500 with a single-end read length of 50 bp. R package “ballgown” was used to screen the genes with a
STATISTICAL ANALYSIS:
The numerical results are described as the mean±standard deviation. GraphPad Prism (version 8.3.0) was used for statistical analysis and making statistical charts according to data (mean±standard deviation). The differences between the 2 groups were analyzed using
Results
:
H9c2 cells were infected with lentivirus and amplified after puromycin selection, and qRT-PCR and western blot analysis were used to validate the effect of shRNA on Nkx2–5. The qRT-PCR and western blot results (Figure 1) indicated that the shRNA knocked down the expression of Nkx2–5.
:
The CCK8 test was used to validate the effect of Nkx2–5 on the proliferative capacity of H9c2 cells. The results indicated that the knockdown of Nkx2–5 decreased the proliferative capacity of the H9c2 cells (Figure 2).
:
The cell scratch test was used to validate the effect of Nkx2–5 on the migration of H9c2 cells. The results indicated that the knockdown of Nkx2–5 increased the migration ability of the H9c2 cells (Figure 3).
:
To investigate the mechanisms leading to changes in the proliferation and migration of H9c2 cells, we used RNA-seq on the transcripts of cells. P-value <0.05 was used to identify the differentially expressed genes, and the results indicated that the knockdown of Nkx2–5 changed the expression levels of several genes. Gene Ontology (GO) enrichment analysis (Figure 4) suggested that enriched genes involved the extracellular space, extracellular matrix, calcium-dependent phospholipid binding, regulation of calcium ion-dependent exocytosis, calcium ion-regulated exocytosis of neurotransmitter, cardiac epithelial to mesenchymal transition, development of the cardiac bundle of His, and cardiac muscle cell proliferation. Pathway enrichment analysis (Figure 5) suggested that those genes are enriched in the transforming growth factor (TGF)-β signaling pathway, and pathways related to hypertrophic cardiomyopathy, dilated cardiomyopathy, and arrhythmogenic right ventricular cardiomyopathy.
:
The CCK8 test suggested that the knockdown of Nkx2–5 in H9c2 cells decreased cell proliferation. To investigate the mechanism, we selected and analyzed genes associated with cell proliferation based on their FPKM values. In the Nkx2–5 knockdown group, the results indicated that the expression of genes related to cell proliferation was changed. Among these genes, Bche, Cd81, Col18a1, Crlf1, Ednra, Emp2, Hmga1, Ptk2b, Rxfp2, and Serpine2 were upregulated, and Cenpe, Id2, Il1rl1, LOC100359539, Ndrg1, Nkx2–5, Ripor2, Tgfb2, Tnn, and Wt1 were downregulated. The expression of the genes is shown Table 4.
:
The knockdown of Nkx2–5 was found to increase cell migration. To clarify the mechanisms, we selected and analyzed genes related to migration based on their FPKM values. In the Nkx2–5 knockdown group, the results indicated that the expression of genes related to cell migration was changed. Among these genes, Cemip, Tgfb2, and Tnn were downregulated, and Aqp1, Col18a1, Efna1, Emp2, Itga7, Lcp1, Ptk2b, and S1pr1 were upregulated. The expression of the genes is shown in Table 5.
:
To clarify the functional mechanisms of NKX2–5 in the heart, we selected and analyzed genes associated with cardiovascular morphogenesis, function, and disease based on their FPKM values. In the Nkx2–5 knockdown group, the results indicated that the expression of Cited1, Id2, Lrp2, Olfm1, Olfm2, Pou5f1, Serpina3c, Tgfb2, Wnt4, Wt1, and Xdh was downregulated, while the expression of Aqp1, Cacna1g, Chrd, Dcn, Ednra, Efna1, Eln, Emp2, Heyl, Myo7a, Nalcn, Ptk2b, Rap1gap, Ren, S1pr1, Tenm4, and Thbs2 was upregulated. The expression of the genes is shown Table 6.
:
To clarify the function of miRNAs in the heart, we used a P-value <.05, rat species, and an miRbase database to screen for differentially expressed miRNAs. The miRNAs selected through this process are depicted in the heat map shown in Figure 6. The miRNAs are listed in Table 7. The target genes of the miRNAs were analyzed after prediction, and GO analysis (Figure 7) indicated that they are related to transcriptional regulation, redox, signal transduction, apoptosis, cell differentiation, cell proliferation, protein phosphorylation, proteolysis, intracellular signal transduction, protein ubiquitin, gene expression, protein binding, metal ion binding, ATP binding, homologous protein binding, homologous domain protein dimerization body activity, DNA binding, RNA binding, zinc ion binding, and calcium ion binding. Pathway enrichment analysis (Figure 8) suggested that the target genes are enriched in the MAPK signaling pathway and other signaling pathways.
VALIDATION OF RNA-SEQ:
We used qRT-PCR to validate the RNA-seq results. The results from the Nkx2–5 knockdown group (Figures 9, 10) showed that the expression of Tgfb-2 (0.62±0.03), Wnt4 (0.56±0.19), Xdh (0.70±0.12), Lrp2 (0.69±0.12), Cited1 (0.64±0.28), Syt1 (0.78±0.16), Emp2 (0.15±0.06), Pou5f (0.75±0.11), Itga7 (0.89±0.04), rno-miR-1-3p (0.14±0.04), rno-let-7a-5p (0.73±0.13), rno-miR-148b-3p (0.32±0.03), rno-miR-361-3p (0.09±0.01), and rno-miR-25-3p(0.45±0.03) was downregulated, and the expression of Id2 (1.58±0.16), Cacna1g (1.64±0.41), Wt1 (5.22±1.57), Hey1 (3.01±1.62), Olfml (2.65±0.31), Slrp1 (2.30±0.33), Nectin3 (1.80±0.27), Tenm4 (2.51±0.82), Bmp10 (35.62±3.18), rno-miR-1-5p (1.31±0.09), rno-miR-149-5p (1.37±0.22), and rno-miR-455-3p (1.72±0.28) was upregulated.
Discussion
In this study, we found that
Cardiac development includes the proliferation, migration, and differentiation of heart precursor cells [21,22]. Cardiac development starts on both sides of the front of the mesoderm, with cells from the heart-forming regions migrating and forming the heart tube [21,22]. The heart tube subsequently twists and cyclizes into a 3-dimensional heart structure [21,22]. With the differentiation of cardiac precursor cells, the heart gradually achieves contraction and relaxation related to the pumping function [23]. Therefore, the proliferation, migration, and differentiation of the heart precursor cells are necessary for cardiac morphogenesis and function. In a previous study, the knockout of
TGF-β2 has functions in many biological activities [25]. Mice with dysfunction of TGF-β2 have been shown to have developmental defects of multiple organs leading to death at birth [26]. In addition, TGF-β2-deficient mice were found to develop outflow tract malformations, permanent arterial trunks, membrane peripheral VSD, aortic valve hypertrophy, tricuspid valve deformity, and complete atrioventricular septal defect [27]. These findings suggest that the disruption of TGF-β2 results in the incomplete twisting of the heart tube and abnormal development of the atrioventricular septum [27]. In our study, we found that
Human
miRNA1 functions in the heart [44,45]. Previously, the knockout of miRNA1 resulted in a lack of the characteristic striped appearance in the mouse myocardium, and knockdown of miRNA1 resulted in VSD and cardiac dysfunction [46]. In our study, the knockdown of
Conclusions
Figures
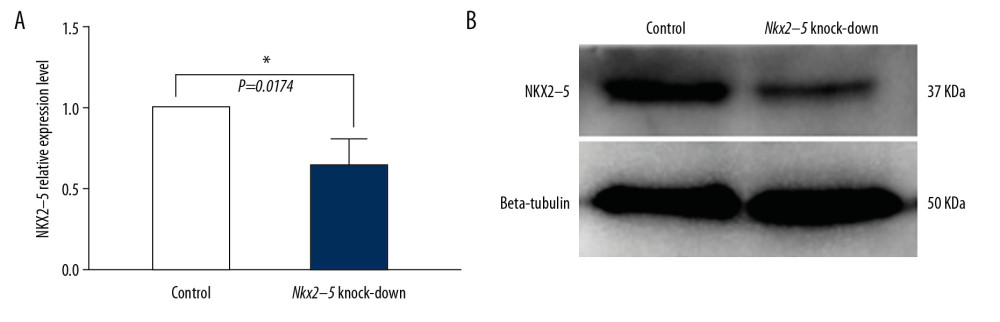 Figure 1. Verification of the knockdown effect of shRNA lentivirus on Nkx2–5 in H9c2 cells by quantitative reverse transcription-polymerase chain reaction (qRT-PCR) and western blot analysis, respectively. (A) qRT-PCR detection of the expression of Nkx2–5 in the control group and the Nkx2–5 knockdown group. (B) Western blot detection of the expression of Nkx2–5 in the control group and the Nkx2–5 knockdown group.
Figure 1. Verification of the knockdown effect of shRNA lentivirus on Nkx2–5 in H9c2 cells by quantitative reverse transcription-polymerase chain reaction (qRT-PCR) and western blot analysis, respectively. (A) qRT-PCR detection of the expression of Nkx2–5 in the control group and the Nkx2–5 knockdown group. (B) Western blot detection of the expression of Nkx2–5 in the control group and the Nkx2–5 knockdown group. 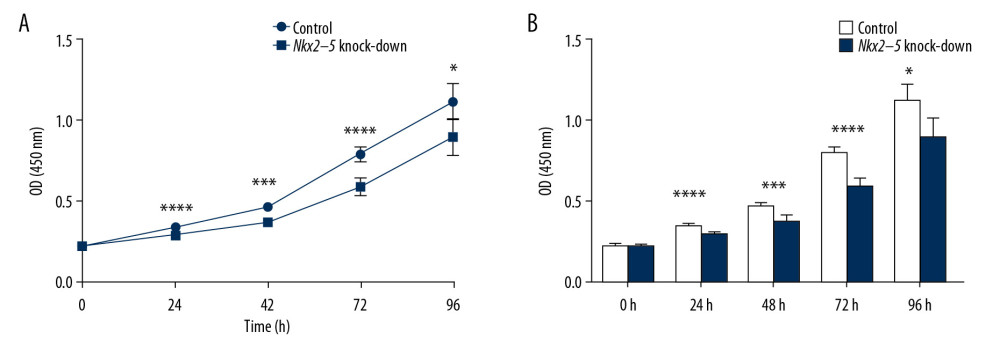 Figure 2. Knockdown of Nkx2–5 inhibited the proliferative capacity of H9c2 cells. The CCK8 method was used to detect the effect of Nkx2–5 on the proliferative capacity of H9c2 cells. (A) Cell growth was detected with the CCK8 method for 4 consecutive days. (B) Statistics of the CCK8 curve are shown.
Figure 2. Knockdown of Nkx2–5 inhibited the proliferative capacity of H9c2 cells. The CCK8 method was used to detect the effect of Nkx2–5 on the proliferative capacity of H9c2 cells. (A) Cell growth was detected with the CCK8 method for 4 consecutive days. (B) Statistics of the CCK8 curve are shown. 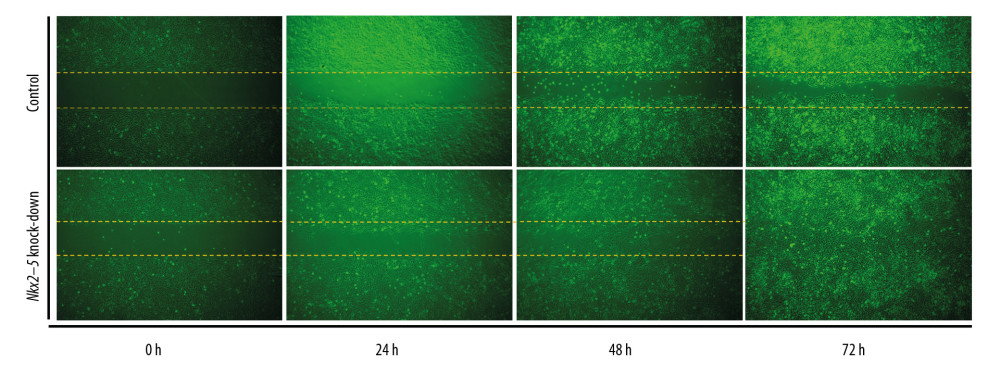 Figure 3. Knockdown of Nkx2–5 increase the migration ability of H9c2 cells. The cell scratch test was used to detect the effect of Nkx2–5 on the migration ability of H9c2 cells (0–72 h). Cells were seeded in the culture-insert, which was removed after 24 h, and cells were then continuously observed for 72 h.
Figure 3. Knockdown of Nkx2–5 increase the migration ability of H9c2 cells. The cell scratch test was used to detect the effect of Nkx2–5 on the migration ability of H9c2 cells (0–72 h). Cells were seeded in the culture-insert, which was removed after 24 h, and cells were then continuously observed for 72 h. 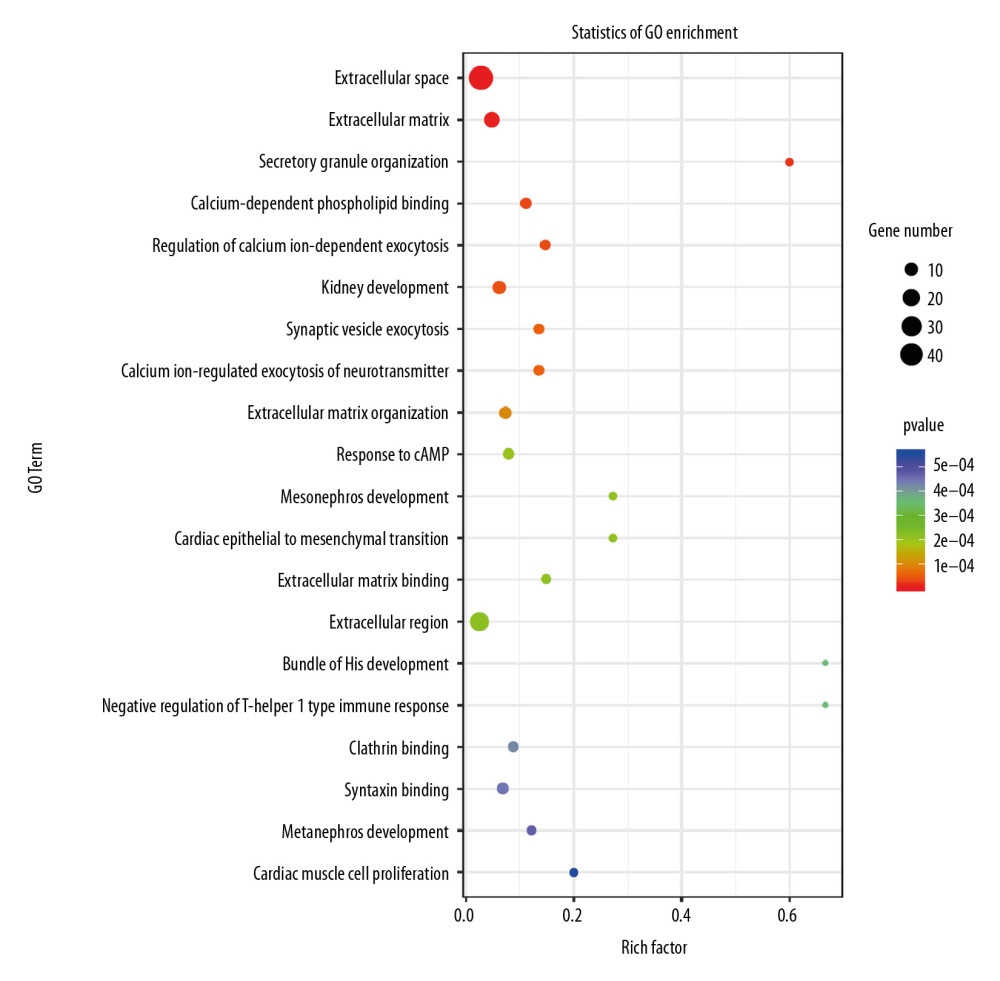 Figure 4. Gene Ontology (GO) analysis of the differential expression of genes caused by knockdown of Nkx2–5 indicated that the genes are enriched in many biological processes, including cardiac epithelial to mesenchymal transition, development of the cardiac bundle of His, and cardiac muscle cell proliferation.
Figure 4. Gene Ontology (GO) analysis of the differential expression of genes caused by knockdown of Nkx2–5 indicated that the genes are enriched in many biological processes, including cardiac epithelial to mesenchymal transition, development of the cardiac bundle of His, and cardiac muscle cell proliferation. 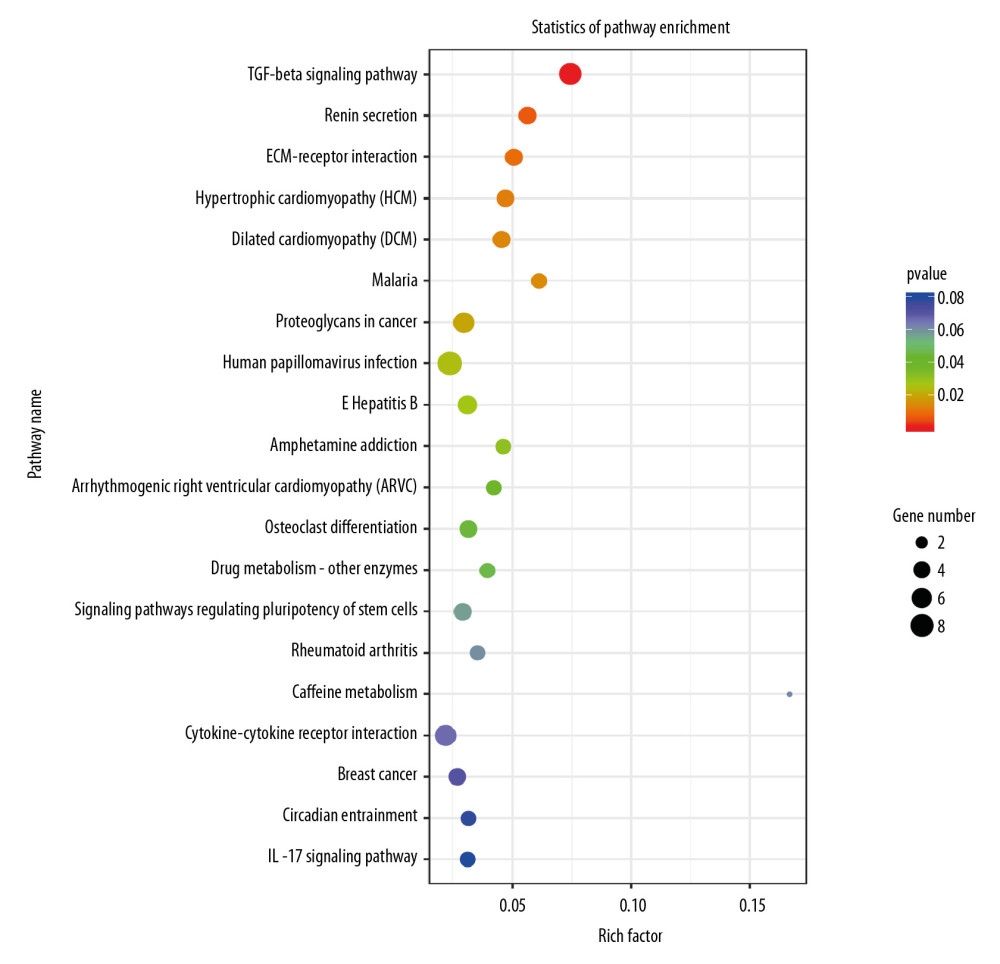 Figure 5. Pathway analysis of the differential expression of genes caused by knockdown of Nkx2–5 indicated that the genes are enriched in the transforming growth factor (TGF)-β signaling pathway, and pathways related to hypertrophic cardiomyopathy, dilated cardiomyopathy, and arrhythmogenic right ventricular cardiomyopathy.
Figure 5. Pathway analysis of the differential expression of genes caused by knockdown of Nkx2–5 indicated that the genes are enriched in the transforming growth factor (TGF)-β signaling pathway, and pathways related to hypertrophic cardiomyopathy, dilated cardiomyopathy, and arrhythmogenic right ventricular cardiomyopathy. 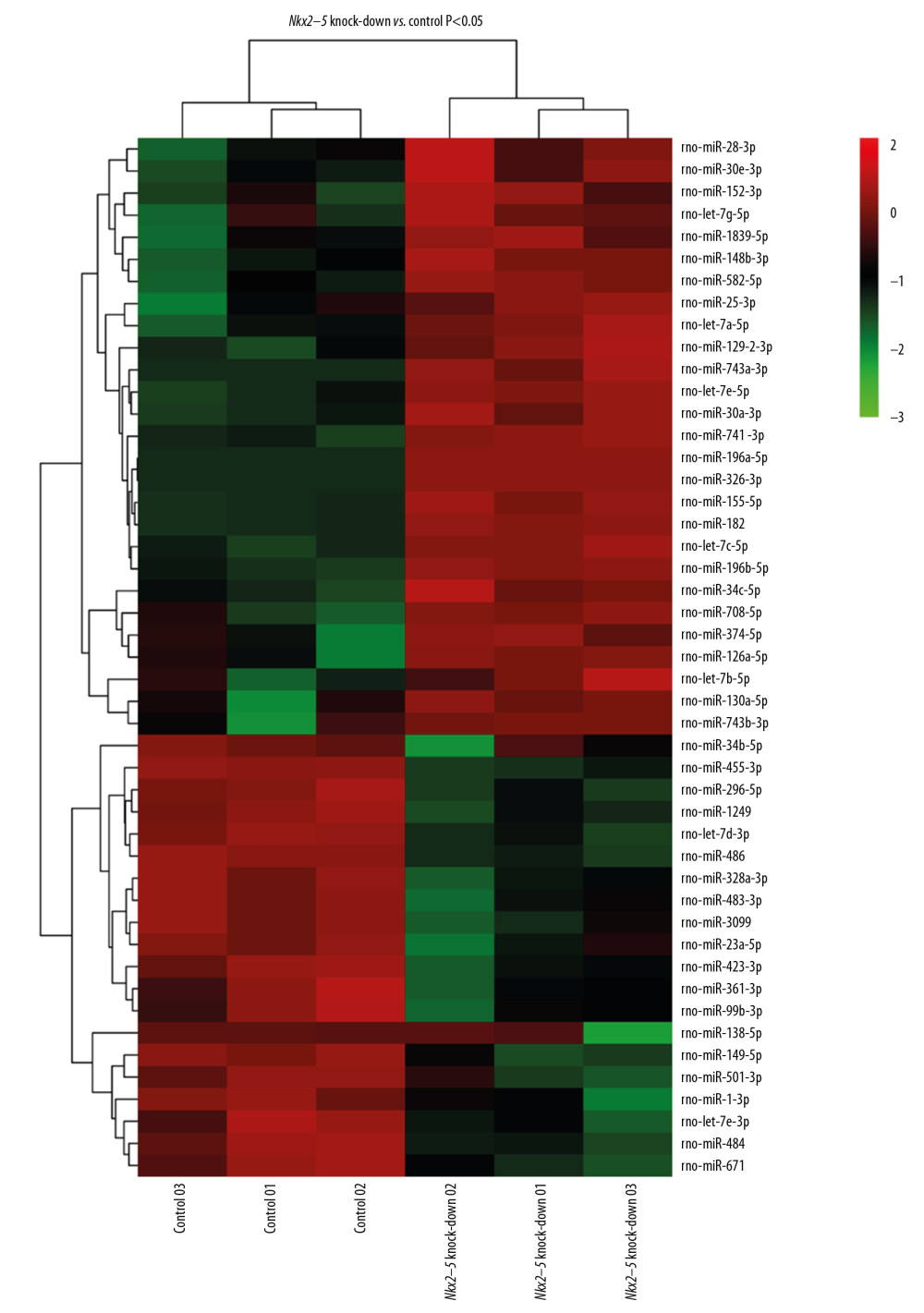 Figure 6. Heat map of the differentially expressed miRNAs following knockdown of Nkx2–5.
Figure 6. Heat map of the differentially expressed miRNAs following knockdown of Nkx2–5. 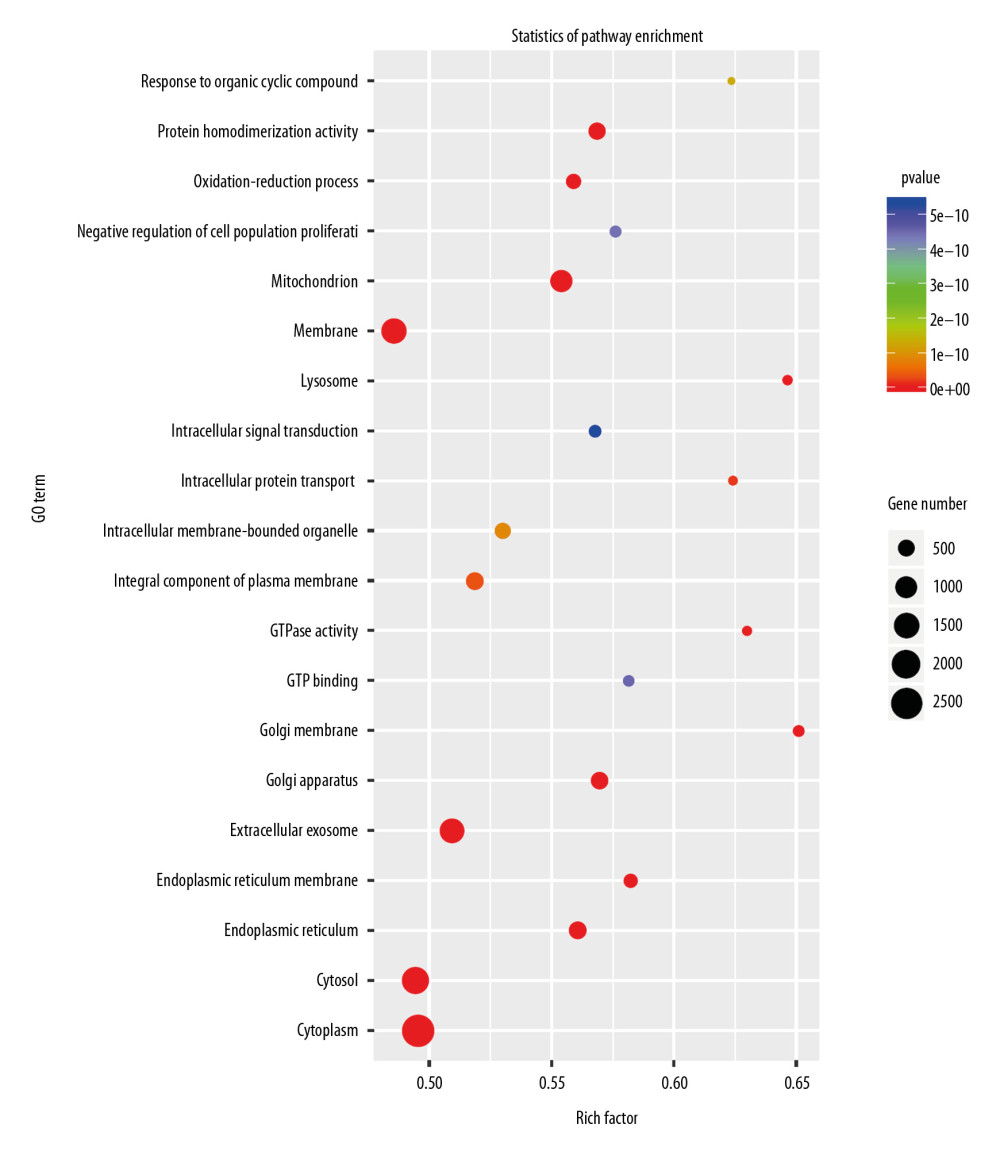 Figure 7. Gene Ontology (GO) enrichment analysis of the target genes of the differentially expressed miRNAs caused by knockdown of Nkx2–5 indicated that those genes function in many biological processes.
Figure 7. Gene Ontology (GO) enrichment analysis of the target genes of the differentially expressed miRNAs caused by knockdown of Nkx2–5 indicated that those genes function in many biological processes. 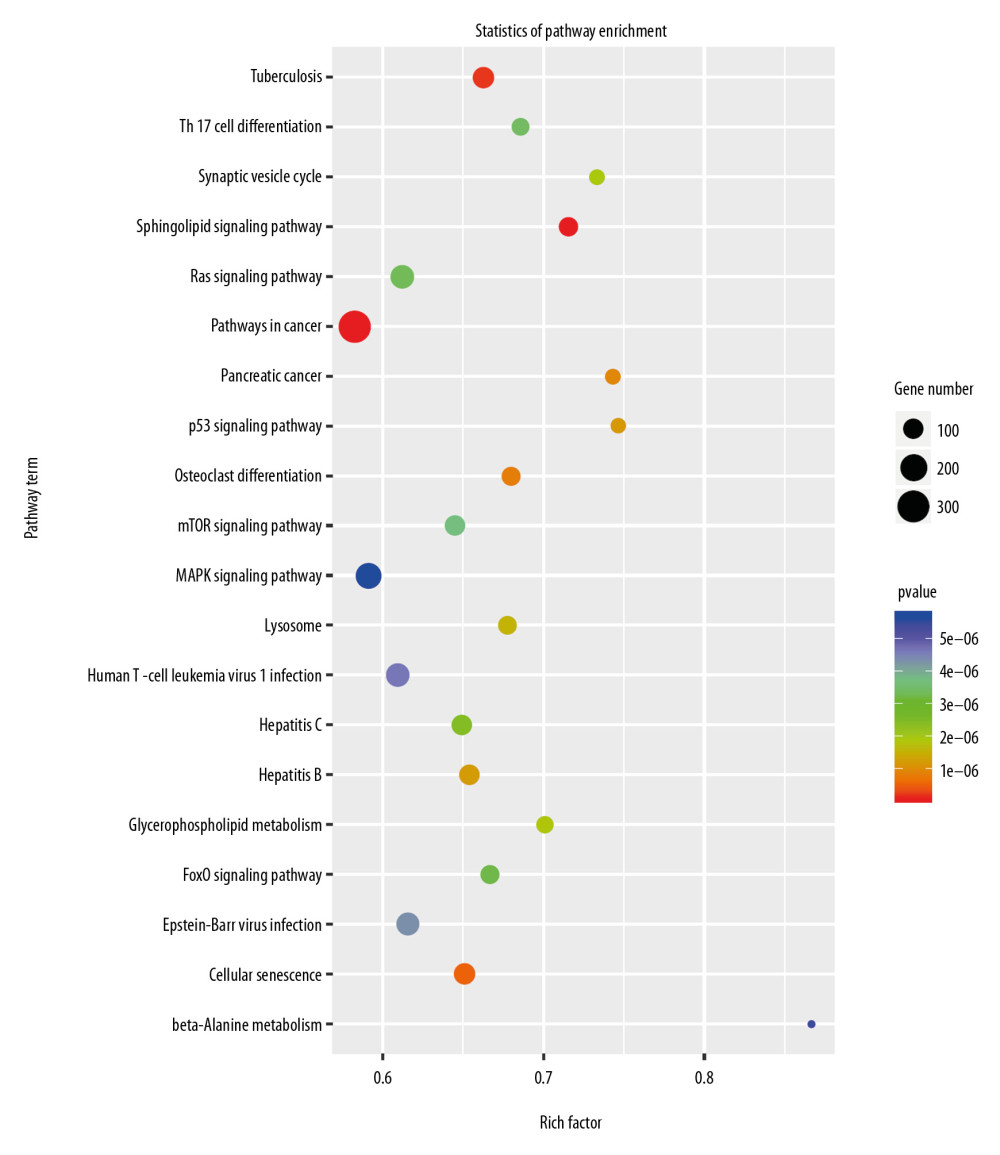 Figure 8. Pathway enrichment analysis of the target genes of miRNAs that were differentially expressed owing to knockdown of Nkx2–5 indicated that the target genes are enriched in the MAPK signaling pathway and other signaling pathways.
Figure 8. Pathway enrichment analysis of the target genes of miRNAs that were differentially expressed owing to knockdown of Nkx2–5 indicated that the target genes are enriched in the MAPK signaling pathway and other signaling pathways. 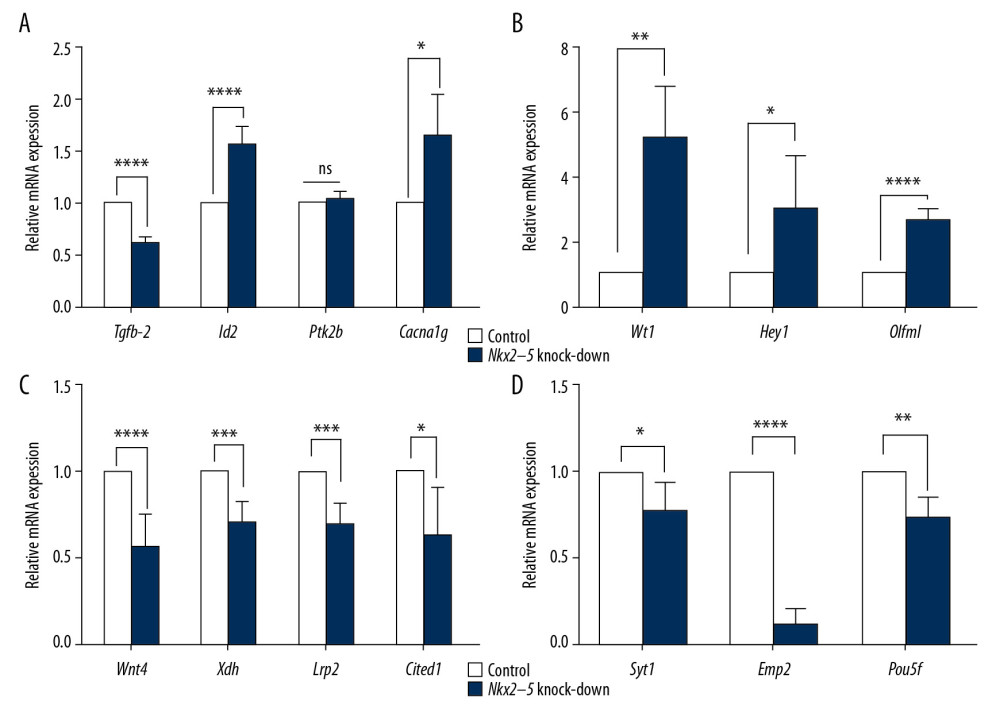 Figure 9. (A–D) Quantitative reverse transcription-polymerase chain reaction (qRT-PCR) analysis of the relative expression levels of Tgfb-2, Id2, Ptk2b, Cacna1g, Wt1, Heyl, Olfml, Wnt4, Xdh, Lrp2, Cited1, Syt1, Emp2, and Pou5f in the control group and in the Nkx2–5 knockdown group. The expression level of the genes in the control group was calculated as 1. The bar represents the fold change of the genes in the Nkx2–5 knockdown group compared with the control group. * P<.05; ** P<.01; *** P<.001; **** P<.0001.
Figure 9. (A–D) Quantitative reverse transcription-polymerase chain reaction (qRT-PCR) analysis of the relative expression levels of Tgfb-2, Id2, Ptk2b, Cacna1g, Wt1, Heyl, Olfml, Wnt4, Xdh, Lrp2, Cited1, Syt1, Emp2, and Pou5f in the control group and in the Nkx2–5 knockdown group. The expression level of the genes in the control group was calculated as 1. The bar represents the fold change of the genes in the Nkx2–5 knockdown group compared with the control group. * P<.05; ** P<.01; *** P<.001; **** P<.0001. 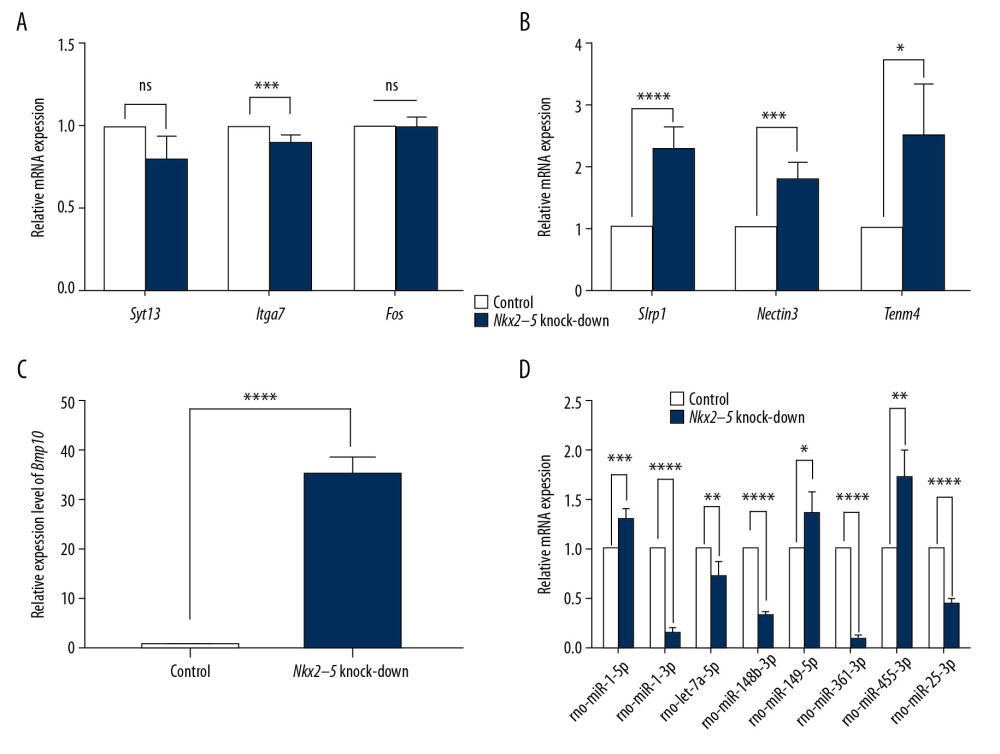 Figure 10. (A–D) Quantitative reverse transcription-polymerase chain reaction (qRT-PCR) analysis of the relative expression levels of Syt13, Itga7, Fos, Slrp1, Nectin3, Tenm4, Bmp10, rno-miR-1-5p, rno-miR-1-3p, rno-let-7a-5p, rno-miR-148b-3p, rno-miR-149-5p, rno-miR-361-3p, rno-miR-455-3p, and rno-miR-25-3p in the control group and in the Nkx2–5 knockdown group. The expression level of the genes in the control group was calculated as 1. The bar represents the fold change of the genes in the Nkx2–5 knockdown group compared with the control group. * P<.05; ** P<.01; *** P<.001; **** P<.0001.
Figure 10. (A–D) Quantitative reverse transcription-polymerase chain reaction (qRT-PCR) analysis of the relative expression levels of Syt13, Itga7, Fos, Slrp1, Nectin3, Tenm4, Bmp10, rno-miR-1-5p, rno-miR-1-3p, rno-let-7a-5p, rno-miR-148b-3p, rno-miR-149-5p, rno-miR-361-3p, rno-miR-455-3p, and rno-miR-25-3p in the control group and in the Nkx2–5 knockdown group. The expression level of the genes in the control group was calculated as 1. The bar represents the fold change of the genes in the Nkx2–5 knockdown group compared with the control group. * P<.05; ** P<.01; *** P<.001; **** P<.0001. Tables
Table 1. Sequence information of all quantitative reverse transcription-polymerase chain reaction (qRT-PCR) primers. Table 2. The protocol of quantitative reverse transcription-polymerase chain reaction (qRT-PCR) of mRNAs.
Table 2. The protocol of quantitative reverse transcription-polymerase chain reaction (qRT-PCR) of mRNAs. Table 3. The protocol of quantitative reverse transcription-polymerase chain reaction (qRT-PCR) of miRNAs.
Table 3. The protocol of quantitative reverse transcription-polymerase chain reaction (qRT-PCR) of miRNAs.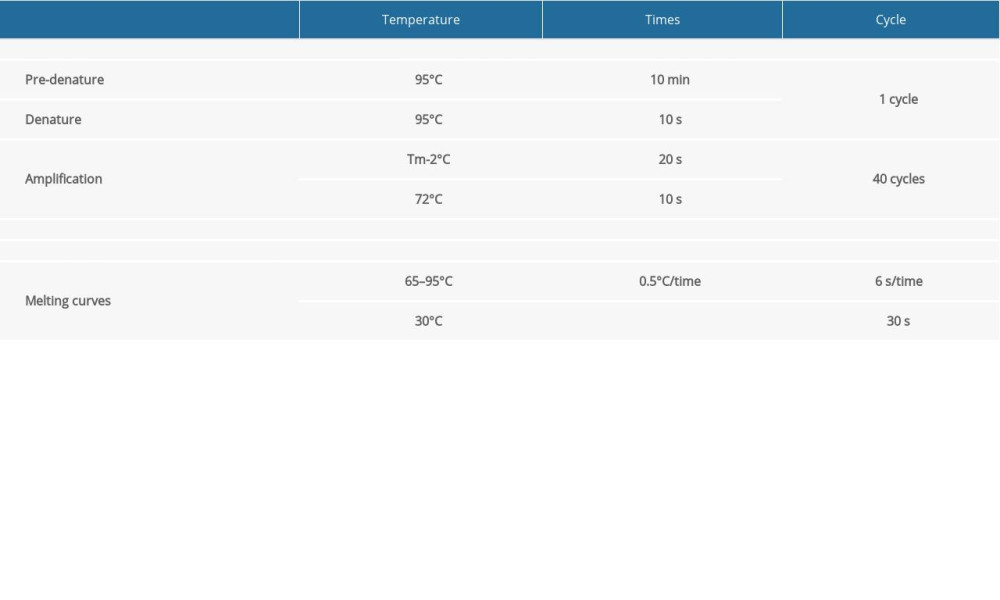 Table 4. The expression level of the differentially expressed genes related to cell proliferation according to FPKM value.
Table 4. The expression level of the differentially expressed genes related to cell proliferation according to FPKM value.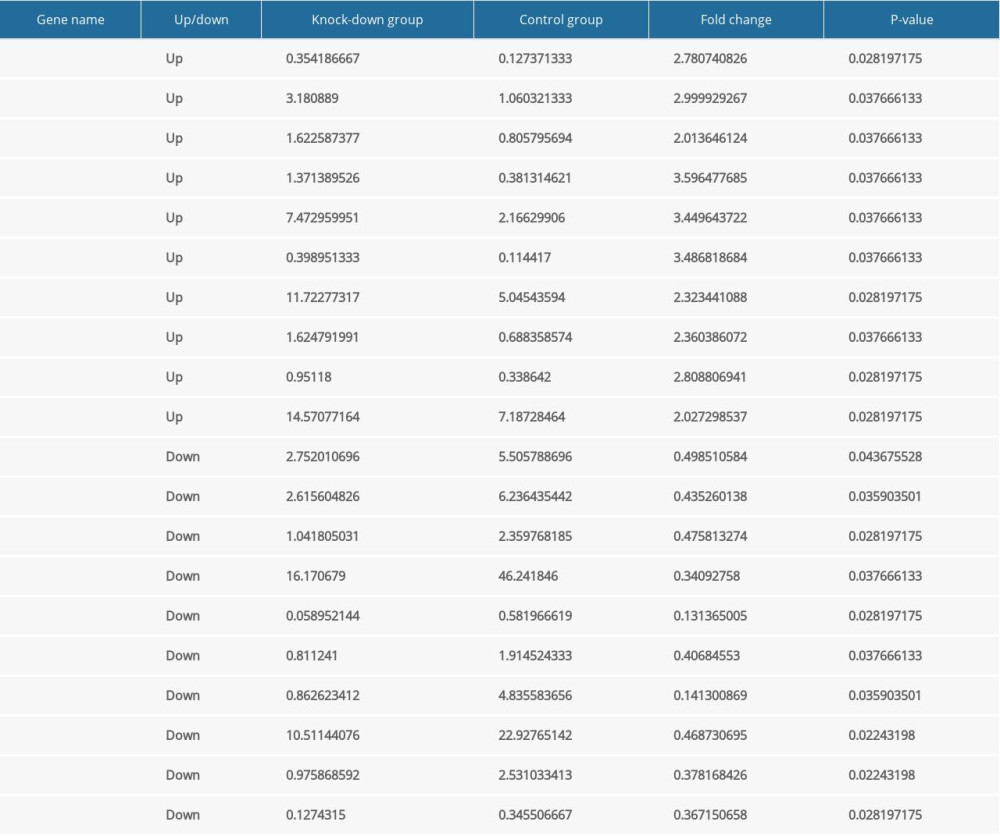 Table 5. The expression level of the differentially expressed genes related to cell migration according to FPKM value.
Table 5. The expression level of the differentially expressed genes related to cell migration according to FPKM value.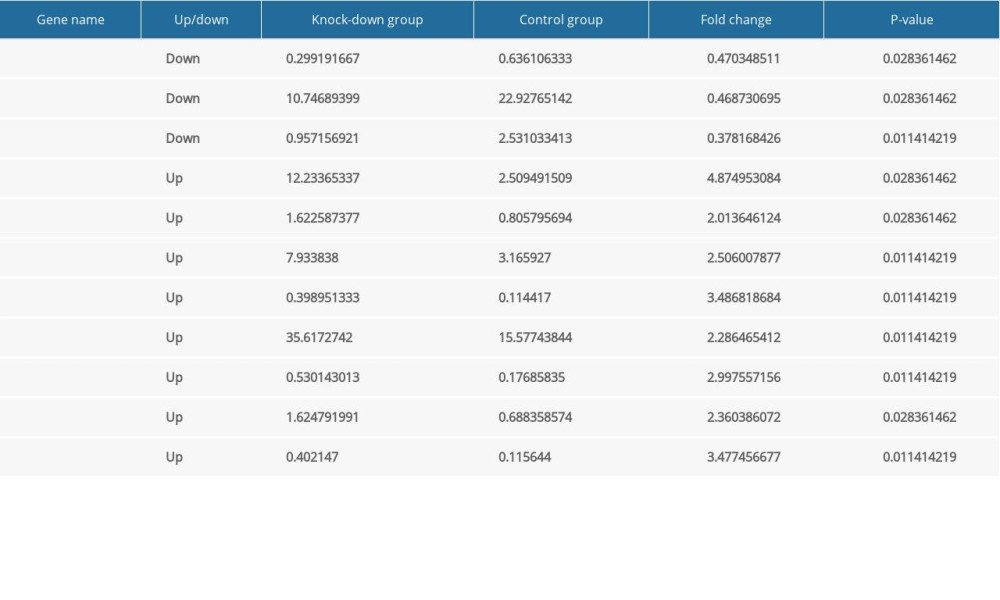 Table 6. The expression level of the differentially expressed genes related to cardiovascular development, function and disease according to FPKM value.
Table 6. The expression level of the differentially expressed genes related to cardiovascular development, function and disease according to FPKM value.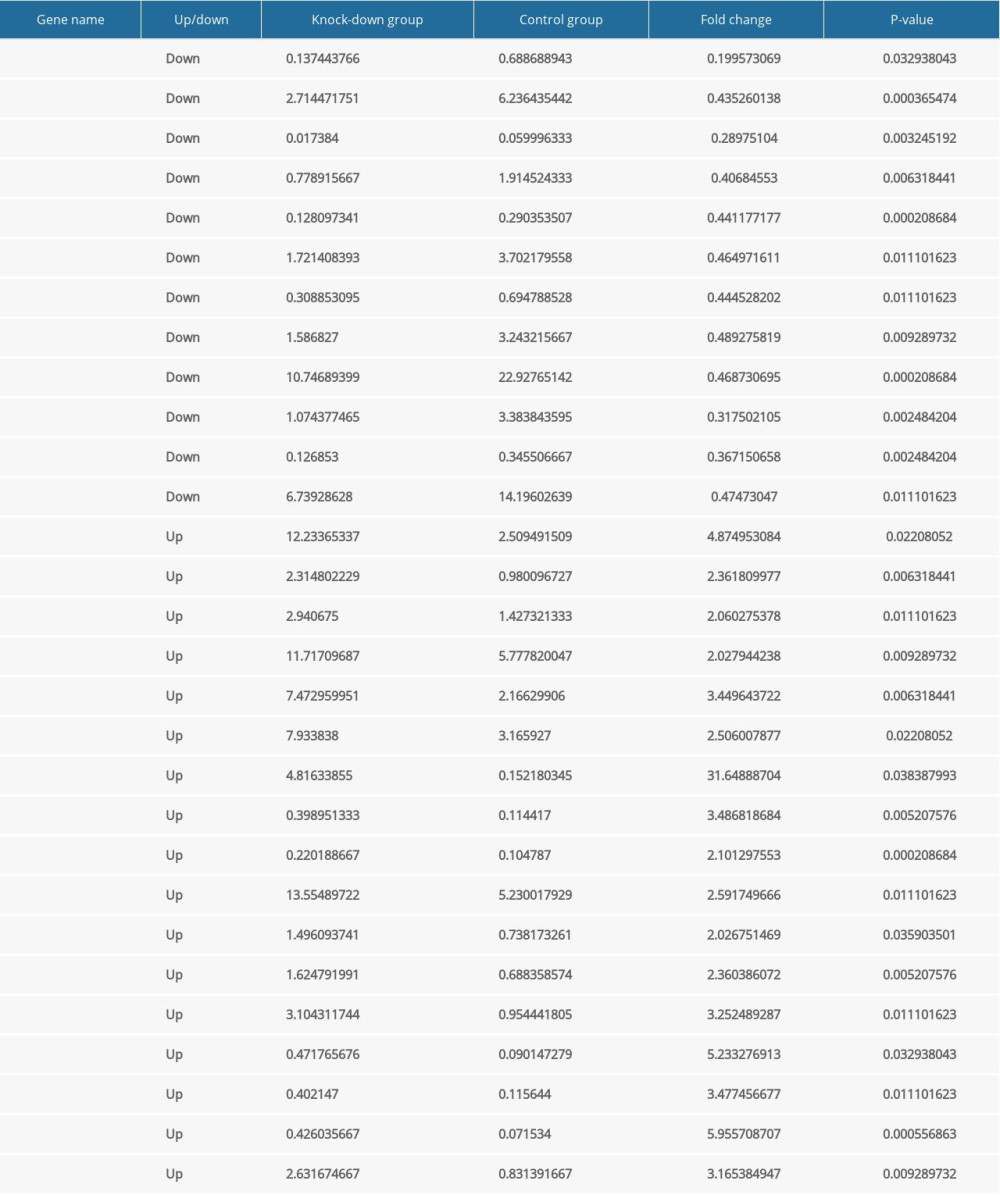 Table 7. The expression level of the differentially expressed miRNAs.
Table 7. The expression level of the differentially expressed miRNAs.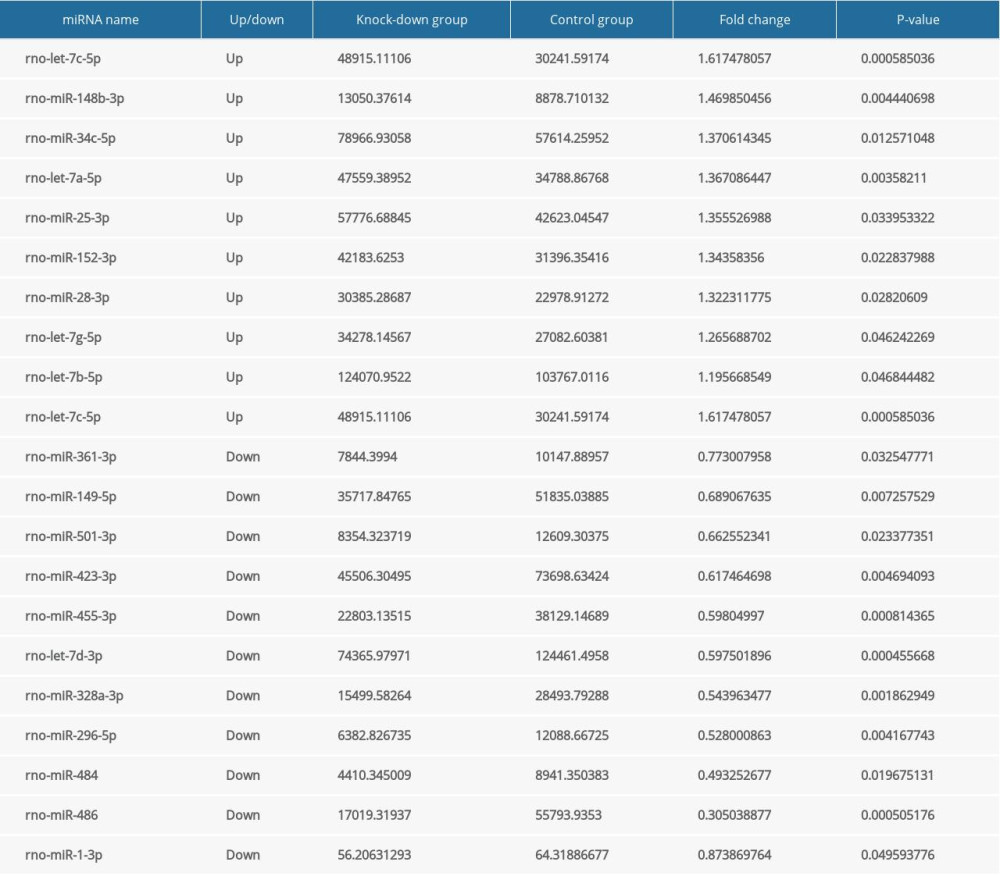
References
1. Liu Y, Chen S, Zühlke L, Global birth prevalence of congenital heart defects 1970–2017: updated systematic review and meta-analysis of 260 studies: Int J Epidemiol, 2019; 48(2); 455-63
2. Lal S, Kotchetkova I, Cao J, Heart failure admissions and poor subsequent outcomes in adults with congenital heart disease: Eur J Heart Fail, 2018; 20(4); 812-15
3. Zhao L, Chen L, Yang T, Birth prevalence of congenital heart disease in China, 1980–2019: A systematic review and meta-analysis of 617 studies: Eur J Epidemiol, 2020; 35; 631-42
4. Chung IM, Rajakumar G, Genetics of congenital heart defects: The NKX2–5 gene, a key player: Genes (Basel), 2016; 7(2); 6
5. Persson M, Razaz N, Edstedt Bonamy AK, Maternal overweight and obesity and risk of congenital heart defects: J Am Coll Cardiol, 2019; 73(1); 44-53
6. Moreau JLM, Kesteven S, Martin E, Gene-environment interaction impacts on heart development and embryo survival: Development, 2019; 146(4); dev172957
7. Pang S, Shan J, Qiao Y, Genetic and functional analysis of the NKX2–5 gene promoter in patients with ventricular septal defects: Pediatr Cardiol, 2012; 33(8); 1355-61
8. Shiojima I, Komuro I, Mizuno T: Circ Res, 1996; 79(5); 920-29
9. Billeter M, Homeodomain-type DNA recognition: Prog Biophys Mol Biol, 1996; 66(3); 211-25
10. Kasahara H, Lee B, Schott JJ, Loss of function and inhibitory effects of human CSX/NKX2. 5 homeoprotein mutations associated with congenital heart disease: J Clin Invest, 2000; 106(2); 299-308
11. Watada H, Mirmira RG, Kalamaras J, German MS, Intramolecular control of transcriptional activity by the NK2-specific domain in NK-2 homeodomain proteins: Proc Natl Acad Sci USA, 2000; 97(17); 9443-48
12. Kasahara H, Izumo S: Mol Cell Biol, 1999; 19(1); 526-36
13. Turbay D, Wechsler S, Blanchard K, Izumo S: Mol Med, 1996; 2(1); 86-96
14. Tanaka M, Chen Z, Bartunkova S: Development, 1999; 126(6); 1269-80
15. Harvey RP, Nk-2 homeobox genes and heart development: Dev Biol, 1996; 178(2); 203-16
16. Terada R, Warren S, Lu JT, Ablation of Nkx2-5 at mid-embryonic stage results in premature lethality and cardiac malformation: Cardiovasc Res, 2011; 91(2); 289-99
17. Choquet C, Nguyen THM, Sicard P, Deletion of Nkx2-5 in trabecular myocardium reveals the developmental origins of pathological heterogeneity associated with ventricular non-compaction cardiomyopathy: PLoS Genet, 2018; 14(7); e1007502
18. Gao L, Jiang F, MicroRNA (miRNA) profiling: Methods Mol Biol, 2016; 1381; 151-61
19. Murchison EP, Hannon GJ, miRNAs on the move: miRNA biogenesis and the RNAi machinery: Curr Opin Cell Biol, 2004; 16(3); 223-29
20. Denli AM, Tops BBJ, Plasterk RHA, Processing of primary microRNAs by the microprocessor complex: Nature, 2004; 432(7014); 231
21. Buckingham M, Meilhac S, Zaffran S, Building the mammalian heart from two sources of myocardial cells: Nat Rev Genet, 2005; 6(11); 826-35
22. Srivastava D, Making or breaking the heart: From lineage determination to morphogenesis: Cell, 2006; 126(6); 1037-48
23. Epstein JA, Cardiac development and implications for heart disease: N Engl J Med, 2010; 363(17); 1638-47
24. Lyons I, Parsons LM, Hartley L, Myogenic and morphogenetic defects in the heart tubes of murine embryos lacking the homeo box gene Nkx2-5: Genes Dev, 1995; 9(13); 1654-66
25. Massague J, Gomis RR, The logic of TGFbeta signaling: FEBS Lett, 2006; 580(12); 2811-20
26. Sanford LP, Ormsby I, Gittenberger-de Groot AC, TGFbeta2 knockout mice have multiple developmental defects that are non-overlapping with other TGFbeta knockout phenotypes: Development, 1997; 124(13); 2659-70
27. Bartram U, Molin DG, Wisse LJ, Double-outlet right ventricle and overriding tricuspid valve reflect disturbances of looping, myocardialization, endocardial cushion differentiation, and apoptosis in Tgf-β2-knockout mice: Circulation, 2001; 103(22); 2745-52
28. Jen Y, Manova K, Benezra R, Expression patterns of Id1, Id2, and Id3 are highly related but distinct from that of Id4 during mouse embryogenesis: Dev Dyn, 1996; 207(3); 235-52
29. Jongbloed MRM, Vicente-Steijn R, Douglas YL, Expression of Id2 in the second heart field and cardiac defects in Id2 knock-out mice: Dev Dyn, 2011; 240(11); 2561-77
30. Martinsen BJ, Frasier AJ, Baker CV, Lohr JL, Cardiac neural crest ablation alters Id2 gene expression in the developing heart: Dev Biol, 2004; 272(1); 176-90
31. Benezra R, Davis RL, Lockshon D, The protein Id: A negative regulator of helix-loop-helix DNA binding proteins: Cell, 1990; 61(1); 49-59
32. Jen Y, Manova K, Benezra R, Each member of the Id gene family exhibits a unique expression pattern in mouse gastrulation and neurogenesis: Dev Dyn, 1997; 208(1); 92-106
33. Lim JY, Kim WH, Kim J, Park SI, Induction of Id2 expression by cardiac transcription factors GATA4 and Nkx2.5: J Cell Biochem, 2008; 103(1); 182-94
34. Martínez-Estrada OM, Lettice LA, Essafi A, Wt1 is required for cardiovascular progenitor cell formation through transcriptional control of Snail and E-cadherin: Nat Genet, 2009; 42(1); 89-93
35. Rudat C, Kispert A, Wt1 and epicardial fate mapping: Circ Res, 2012; 111(2); 165-69
36. von Gise A, Zhou B, Honor LB, WT1 regulates epicardial epithelial to mesenchymal transition through beta-catenin and retinoic acid signaling pathways: Dev Biol, 2011; 356(2); 421-31
37. Fischer A, Gessler M, Hey genes in cardiovascular development: Trends Cardiovasc Med, 2003; 13(6); 221-26
38. Fischer A, Schumacher N, Maier M, The Notch target genes Hey1 and Hey2 are required for embryonic vascular development: Genes Dev, 2004; 18(8); 901-11
39. Fischer A, Steidl C, Wagner TU, Combined loss of Hey1 and HeyL causes congenital heart defects because of impaired epithelial to mesenchymal transition: Circ Res, 2007; 100(6); 856-63
40. Stowell SA, Kitajewski J, Hey, there’s a hole in my heart: Circ Res, 2007; 100(6); 764-65
41. Mangoni ME, Nargeot J, Properties of the hyperpolarization-activated current (I(f)) in isolated mouse sino-atrial cells: Cardiovasc Res, 2001; 52(1); 51-64
42. Mizuta E, Shirai M, Arakawa K: Biomed Res, 2010; 31(5); 301-5
43. Le Quang K, Naud P, Qi XY, Role of T-type calcium channel subunits in post-myocardial infarction remodelling probed with genetically engineered mice: Cardiovasc Res, 2011; 91(3); 420-28
44. Rao PK, Toyama Y, Chiang HR, Loss of cardiac microRNA-mediated regulation leads to dilated cardiomyopathy and heart failure: Circ Res, 2009; 105(6); 585-94
45. Mishima Y, Stahlhut C, Giraldez AJ, miR-1-2 gets to the heart of the matter: Cell, 2007; 129(2); 247-49
46. Zhao Y, Ransom JF, Li A, Dysregulation of cardiogenesis, cardiac conduction, and cell cycle in mice lacking miRNA-1-2: Cell, 2007; 129(2); 303-17
Figures
 Figure 1. Verification of the knockdown effect of shRNA lentivirus on Nkx2–5 in H9c2 cells by quantitative reverse transcription-polymerase chain reaction (qRT-PCR) and western blot analysis, respectively. (A) qRT-PCR detection of the expression of Nkx2–5 in the control group and the Nkx2–5 knockdown group. (B) Western blot detection of the expression of Nkx2–5 in the control group and the Nkx2–5 knockdown group.
Figure 1. Verification of the knockdown effect of shRNA lentivirus on Nkx2–5 in H9c2 cells by quantitative reverse transcription-polymerase chain reaction (qRT-PCR) and western blot analysis, respectively. (A) qRT-PCR detection of the expression of Nkx2–5 in the control group and the Nkx2–5 knockdown group. (B) Western blot detection of the expression of Nkx2–5 in the control group and the Nkx2–5 knockdown group. Figure 2. Knockdown of Nkx2–5 inhibited the proliferative capacity of H9c2 cells. The CCK8 method was used to detect the effect of Nkx2–5 on the proliferative capacity of H9c2 cells. (A) Cell growth was detected with the CCK8 method for 4 consecutive days. (B) Statistics of the CCK8 curve are shown.
Figure 2. Knockdown of Nkx2–5 inhibited the proliferative capacity of H9c2 cells. The CCK8 method was used to detect the effect of Nkx2–5 on the proliferative capacity of H9c2 cells. (A) Cell growth was detected with the CCK8 method for 4 consecutive days. (B) Statistics of the CCK8 curve are shown. Figure 3. Knockdown of Nkx2–5 increase the migration ability of H9c2 cells. The cell scratch test was used to detect the effect of Nkx2–5 on the migration ability of H9c2 cells (0–72 h). Cells were seeded in the culture-insert, which was removed after 24 h, and cells were then continuously observed for 72 h.
Figure 3. Knockdown of Nkx2–5 increase the migration ability of H9c2 cells. The cell scratch test was used to detect the effect of Nkx2–5 on the migration ability of H9c2 cells (0–72 h). Cells were seeded in the culture-insert, which was removed after 24 h, and cells were then continuously observed for 72 h. Figure 4. Gene Ontology (GO) analysis of the differential expression of genes caused by knockdown of Nkx2–5 indicated that the genes are enriched in many biological processes, including cardiac epithelial to mesenchymal transition, development of the cardiac bundle of His, and cardiac muscle cell proliferation.
Figure 4. Gene Ontology (GO) analysis of the differential expression of genes caused by knockdown of Nkx2–5 indicated that the genes are enriched in many biological processes, including cardiac epithelial to mesenchymal transition, development of the cardiac bundle of His, and cardiac muscle cell proliferation. Figure 5. Pathway analysis of the differential expression of genes caused by knockdown of Nkx2–5 indicated that the genes are enriched in the transforming growth factor (TGF)-β signaling pathway, and pathways related to hypertrophic cardiomyopathy, dilated cardiomyopathy, and arrhythmogenic right ventricular cardiomyopathy.
Figure 5. Pathway analysis of the differential expression of genes caused by knockdown of Nkx2–5 indicated that the genes are enriched in the transforming growth factor (TGF)-β signaling pathway, and pathways related to hypertrophic cardiomyopathy, dilated cardiomyopathy, and arrhythmogenic right ventricular cardiomyopathy. Figure 6. Heat map of the differentially expressed miRNAs following knockdown of Nkx2–5.
Figure 6. Heat map of the differentially expressed miRNAs following knockdown of Nkx2–5. Figure 7. Gene Ontology (GO) enrichment analysis of the target genes of the differentially expressed miRNAs caused by knockdown of Nkx2–5 indicated that those genes function in many biological processes.
Figure 7. Gene Ontology (GO) enrichment analysis of the target genes of the differentially expressed miRNAs caused by knockdown of Nkx2–5 indicated that those genes function in many biological processes. Figure 8. Pathway enrichment analysis of the target genes of miRNAs that were differentially expressed owing to knockdown of Nkx2–5 indicated that the target genes are enriched in the MAPK signaling pathway and other signaling pathways.
Figure 8. Pathway enrichment analysis of the target genes of miRNAs that were differentially expressed owing to knockdown of Nkx2–5 indicated that the target genes are enriched in the MAPK signaling pathway and other signaling pathways. Figure 9. (A–D) Quantitative reverse transcription-polymerase chain reaction (qRT-PCR) analysis of the relative expression levels of Tgfb-2, Id2, Ptk2b, Cacna1g, Wt1, Heyl, Olfml, Wnt4, Xdh, Lrp2, Cited1, Syt1, Emp2, and Pou5f in the control group and in the Nkx2–5 knockdown group. The expression level of the genes in the control group was calculated as 1. The bar represents the fold change of the genes in the Nkx2–5 knockdown group compared with the control group. * P<.05; ** P<.01; *** P<.001; **** P<.0001.
Figure 9. (A–D) Quantitative reverse transcription-polymerase chain reaction (qRT-PCR) analysis of the relative expression levels of Tgfb-2, Id2, Ptk2b, Cacna1g, Wt1, Heyl, Olfml, Wnt4, Xdh, Lrp2, Cited1, Syt1, Emp2, and Pou5f in the control group and in the Nkx2–5 knockdown group. The expression level of the genes in the control group was calculated as 1. The bar represents the fold change of the genes in the Nkx2–5 knockdown group compared with the control group. * P<.05; ** P<.01; *** P<.001; **** P<.0001. Figure 10. (A–D) Quantitative reverse transcription-polymerase chain reaction (qRT-PCR) analysis of the relative expression levels of Syt13, Itga7, Fos, Slrp1, Nectin3, Tenm4, Bmp10, rno-miR-1-5p, rno-miR-1-3p, rno-let-7a-5p, rno-miR-148b-3p, rno-miR-149-5p, rno-miR-361-3p, rno-miR-455-3p, and rno-miR-25-3p in the control group and in the Nkx2–5 knockdown group. The expression level of the genes in the control group was calculated as 1. The bar represents the fold change of the genes in the Nkx2–5 knockdown group compared with the control group. * P<.05; ** P<.01; *** P<.001; **** P<.0001.
Figure 10. (A–D) Quantitative reverse transcription-polymerase chain reaction (qRT-PCR) analysis of the relative expression levels of Syt13, Itga7, Fos, Slrp1, Nectin3, Tenm4, Bmp10, rno-miR-1-5p, rno-miR-1-3p, rno-let-7a-5p, rno-miR-148b-3p, rno-miR-149-5p, rno-miR-361-3p, rno-miR-455-3p, and rno-miR-25-3p in the control group and in the Nkx2–5 knockdown group. The expression level of the genes in the control group was calculated as 1. The bar represents the fold change of the genes in the Nkx2–5 knockdown group compared with the control group. * P<.05; ** P<.01; *** P<.001; **** P<.0001. Tables
 Table 1. Sequence information of all quantitative reverse transcription-polymerase chain reaction (qRT-PCR) primers.
Table 1. Sequence information of all quantitative reverse transcription-polymerase chain reaction (qRT-PCR) primers. Table 2. The protocol of quantitative reverse transcription-polymerase chain reaction (qRT-PCR) of mRNAs.
Table 2. The protocol of quantitative reverse transcription-polymerase chain reaction (qRT-PCR) of mRNAs. Table 3. The protocol of quantitative reverse transcription-polymerase chain reaction (qRT-PCR) of miRNAs.
Table 3. The protocol of quantitative reverse transcription-polymerase chain reaction (qRT-PCR) of miRNAs. Table 4. The expression level of the differentially expressed genes related to cell proliferation according to FPKM value.
Table 4. The expression level of the differentially expressed genes related to cell proliferation according to FPKM value. Table 5. The expression level of the differentially expressed genes related to cell migration according to FPKM value.
Table 5. The expression level of the differentially expressed genes related to cell migration according to FPKM value. Table 6. The expression level of the differentially expressed genes related to cardiovascular development, function and disease according to FPKM value.
Table 6. The expression level of the differentially expressed genes related to cardiovascular development, function and disease according to FPKM value. Table 7. The expression level of the differentially expressed miRNAs.
Table 7. The expression level of the differentially expressed miRNAs. Table 1. Sequence information of all quantitative reverse transcription-polymerase chain reaction (qRT-PCR) primers.
Table 1. Sequence information of all quantitative reverse transcription-polymerase chain reaction (qRT-PCR) primers. Table 2. The protocol of quantitative reverse transcription-polymerase chain reaction (qRT-PCR) of mRNAs.
Table 2. The protocol of quantitative reverse transcription-polymerase chain reaction (qRT-PCR) of mRNAs. Table 3. The protocol of quantitative reverse transcription-polymerase chain reaction (qRT-PCR) of miRNAs.
Table 3. The protocol of quantitative reverse transcription-polymerase chain reaction (qRT-PCR) of miRNAs. Table 4. The expression level of the differentially expressed genes related to cell proliferation according to FPKM value.
Table 4. The expression level of the differentially expressed genes related to cell proliferation according to FPKM value. Table 5. The expression level of the differentially expressed genes related to cell migration according to FPKM value.
Table 5. The expression level of the differentially expressed genes related to cell migration according to FPKM value. Table 6. The expression level of the differentially expressed genes related to cardiovascular development, function and disease according to FPKM value.
Table 6. The expression level of the differentially expressed genes related to cardiovascular development, function and disease according to FPKM value. Table 7. The expression level of the differentially expressed miRNAs.
Table 7. The expression level of the differentially expressed miRNAs. In Press
15 Apr 2024 : Laboratory Research
The Role of Copper-Induced M2 Macrophage Polarization in Protecting Cartilage Matrix in OsteoarthritisMed Sci Monit In Press; DOI: 10.12659/MSM.943738
07 Mar 2024 : Clinical Research
Knowledge of and Attitudes Toward Clinical Trials: A Questionnaire-Based Study of 179 Male Third- and Fourt...Med Sci Monit In Press; DOI: 10.12659/MSM.943468
08 Mar 2024 : Animal Research
Modification of Experimental Model of Necrotizing Enterocolitis (NEC) in Rat Pups by Single Exposure to Hyp...Med Sci Monit In Press; DOI: 10.12659/MSM.943443
18 Apr 2024 : Clinical Research
Comparative Analysis of Open and Closed Sphincterotomy for the Treatment of Chronic Anal Fissure: Safety an...Med Sci Monit In Press; DOI: 10.12659/MSM.944127
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








