28 May 2021: Original Paper
Risk Factors Affecting Outcomes in Pediatric Liver Transplantation: A Real-World Single-Center Experience
Suk Kyun Hong1ABCDEF, Nam-Joon Yi1ABCDEF*, Kwangpyo Hong1BE, Eui Soo Han1BE, Jeong-Moo Lee1BE, YoungRok Choi1BE, Kwang-Woong Lee1BE, Kyung-Suk Suh1BEDOI: 10.12659/AOT.929145
Ann Transplant 2021; 26:e929145
Abstract
BACKGROUND: Despite liver transplantation (LT) being the standard treatment for pediatric end-stage liver disease, complications often persist and can adversely affect the post-transplant outcomes. This study aimed to identify the risk factors affecting the outcomes in pediatric LT patients.
MATERIAL AND METHODS: Data from pediatric patients who underwent primary LT from March 1988 to December 2018 were retrospectively analyzed. Chronic liver disease was defined as an explanted liver showing fibrosis regardless of grade, cirrhosis, or any other underlying disease that may cause progressive liver injury leading to fibrosis or cirrhosis.
RESULTS: A total of 255 pediatric patients underwent LT during the study period. Their 1-, 5-, and 10-year overall survival rates were 90.5%, 88.4%, and 87.8%, respectively. According to multivariate analysis, while liver disease without underlying chronic liver disease (P=0.024) and a pediatric end-stage liver disease (PELD) score ≥30 (P=0.036) were the only factors associated with worse survival, body weight <6 kg (P=0.050), whole-liver DDLT compared to LDLT (P=0.001), fulminant liver failure (P=0.008), and postoperative hepatic artery complications (P<0.001) were associated with worse graft survival. Liver disease without underlying chronic liver disease was the only factor independently associated with hepatic artery complications (P=0.003).
CONCLUSIONS: Greater caution is recommended in pediatric patients with liver disease unaccompanied by underlying chronic liver disease, high PELD score, or low body weight to improve survival after LT. Hepatic artery complication was the only surgical complication affecting the graft survival outcome, especially in patients having liver disease without underlying chronic liver disease.
Keywords: End stage liver disease, Graft Survival, Hepatic Artery, Liver Transplantation, Child, Child, Preschool, Infant, Living Donors, Risk Factors, Severity of Illness Index
Background
Since the first liver transplant (LT), which was performed by Starzl in 1963, the outcomes of LT have gradually improved due to development in surgical techniques, perioperative management, and immunosuppressive agents. LT is now considered the treatment of choice for acute liver failure and chronic end-stage liver disease in both adults and children [1].
In 1988, our center performed the first LT in Korea, for a 14-year-old girl suffering from Wilson disease; she survived for 30 years after LT [2]. Living donor liver transplantation (LDLT) was first performed in Korea in 1999, in a 1-year-old boy with biliary atresia who is still alive. Since the first LT in 1988, more than 250 pediatric LTs have been performed. Our pediatric LT program has developed into 3 stages [3]. The LT program, which includes adult and pediatric LTs, was set up in the early stage (1988–2007). The improvement in survival after LDLT and the prospective collection of donor data started in the middle stage (2008–2011). In the recent stage (2012–2018), recipient data were prospectively collected and a specialized LT program for children was created; furthermore, there was an increase in the number of split LTs. Under this program, we previously reported that the rate of hepatic artery complications was higher in pediatric recipients with metabolic liver disease than in those with biliary atresia [3]. However, the study had several limitations, including confinement to metabolic liver disease and biliary atresia as disease entities.
This study aimed to evaluate the outcomes of pediatric LT in Korea and identify the risk factors affecting the outcomes, including hepatic artery complications.
Material and Methods
STATISTICAL ANALYSIS:
Results were expressed as mean and standard deviation (SD) for continuous data and as numbers with percentages for categorical data. Overall patient survival and graft survival rates were calculated using the Kaplan-Meier method and compared between groups (male vs female, age <1 vs ≥1 year, body weight <6 vs ≥6 kg, early vs middle vs recent period of LT, LDLT vs whole-liver DDLT vs split DDLT, BA vs others, fulminant vs others, chronic liver disease vs others, pediatric end-stage liver disease [PELD] score <30 vs ≥30, Child-Pugh score A vs B or C, hepatic artery complication yes vs no, portal vein complication yes vs no, hepatic vein complication yes vs no, bile duct complication yes vs no, acute cellular rejection yes vs no, post-transplant lymphoproliferative disorder yes vs no, donor sex male vs female, donor age <18 vs ≥18 years, and relationship to recipient mother vs other than mother) using a log-rank test. Factors independently associated with recipient and graft survival (
Results
BASELINE CHARACTERISTICS:
The demographic and clinical characteristics of patients and their donors are summarized in Table 1. Of the 255 patients, 116 were male and 139 were female. The mean age was 59.8 months and the mean body weight was 19.2 kg. There was a significant majority of LDLTs (64.3%); biliary atresia (55.7%) was the most common underlying liver disease, followed by metabolic liver disease (13.3%). The proportion of liver disease with underlying chronic liver disease was 77.6%. The mean Child-Pugh and PELD scores were 8.2 and 13.0, respectively. Of the 255 donors, 139 were male and 116 were female. The mean donor age was 28.9 years and the mean body weight was 60.8 kg. Among the 164 patients who underwent LDLT, the mother (n=79) was the most common donor, followed by the father (n=64).
SURVIVAL OUTCOMES AND RISK FACTORS:
The 1-, 5-, and 10-year overall survival rates were 90.5%, 88.4%, and 87.8%, respectively (Figure 1A), while the 1-. 5-, and 10-year graft survival rates were 89.7%, 88.5%, and 87.2%, respectively (Figure 2A). Graft failure was recorded when the patient underwent retransplantation or when the graft was no longer functioning at the time of death. The most common cause of graft failure was primary nonfunction, followed by hepatic artery complications and acute cellular rejection (Table 2). Univariate analysis showed that factors that were significantly associated with patient survival were body weight (<6 vs ≥6 kg), type of LT (LDLT vs whole-liver DDLT vs split DDLT), underlying liver disease (fulminant vs others, with vs without chronic liver disease), PELD score (<30 vs ≥30) and postoperative hepatic artery complications (Table 3). Multivariate analysis showed that liver disease without underlying chronic liver disease (hazard ratio [HR]: 2.69, confidence interval [CI]: 1.14–6.38, P=0.024) (Figure 1B) and PELD score (HR: 2.81, CI: 1.07–7.37, P=0.036) (Figure 1C) were the only factors that were independently associated with overall survival.
Factors affecting graft survival were body weight <6 kg (P=0.035), type of LT (LDLT vs whole-liver DDLT vs split DDLT) (P=0.043), liver disease other than biliary atresia (P=0.015), fulminant liver failure (P=0.001), liver disease without underlying chronic liver disease (P=0.004), and postoperative hepatic artery complications (P < 0.001) according to the univariate analysis (Table 4). Multivariate analysis showed that body weight <6 kg (HR: 2.91, CI: 1.00–8.45, P=0.050) (Figure 2B), whole-liver DDLT compared to LDLT (HR: 3.93, CI: 1.71–9.03, P=0.001) (Figure 2C), fulminant liver failure (HR: 3.13, CI 1.35–7.27, P=0.008) (Figure 2D), and postoperative hepatic artery complications (HR: 6.1, CI: 2.42–15.36, P<0.001) (Figure 2E) were the only factors that were independently associated with graft survival.
RISK FACTORS AND OUTCOMES OF HEPATIC ARTERY COMPLICATIONS:
Hepatic artery complications are surgical complications that are critical (ie, they directly result in patient or graft death); therefore, another univariate analysis was performed to identify the risk factors of postoperative hepatic artery complications.
According to the univariate analysis, liver disease other than biliary atresia (P=0.036), fulminant liver failure (P=0.013) and liver disease without underlying chronic liver disease (P=0.004) were significantly associated with postoperative hepatic artery complications (Table 5). Multivariate analysis identified that liver disease without underlying chronic liver disease was the only factor independently associated with hepatic artery complications (HR: 5.22, CI: 1.73–15.76, P=0.003).
Among 14 patients who experienced postoperative hepatic artery complications (HAT: n=11, HAS: n=3), 4 patients (28.6%) eventually died. Six patients underwent retransplantation; 4 of them underwent thrombectomy and hepatic artery reconstruction before retransplantation. Furthermore, 6 patients underwent hepatic artery reconstruction without retransplantation. Consequently, 2 patients eventually died due to graft failure, while the others survived. One patient who underwent thrombolysis survived without retransplantation while another patient who only underwent arteriography without further intervention died. The details of patients who experienced hepatic artery complications are summarized in Table 6.
Discussion
The overall patient and graft survival rates observed in this study were excellent and consistent with previous studies conducted worldwide on LDLT and DDLT [9–11]. The risk factors for overall and graft survival rates from the present study also matched the results of previous studies [9–11].
Children with higher PELD scores were more likely to be hospitalized on parenteral nutrition and under intensive care unit management at the time of LT, which reflects a worse pre-LT condition. The PELD score has been used as a predictor of mortality in children with chronic liver diseases listed for LT as well as in children with acute liver failure [12]. Previous studies showed that patients with PELD scores >20 do not show significantly poorer survival compared to that of those with a PELD score <20; another study showed that the cutoff PELD score was 33 as there was a significant survival difference at that point [3,12]. Similarly, the present study showed no significant survival difference when the PELD cutoff value was 15 or 20, whereas a significant difference in survival was seen when the PELD cutoff value was 30.
Despite the significance of both fulminant liver failure and liver disease without underlying chronic liver disease as risk factors for overall survival in the univariate analysis, fulminant liver failure was not a significant risk factor in the multivariate analysis. Liver disease not accompanied by chronic liver diseases such as fulminant liver failure, hepatoblastoma, and immature teratoma, and other metabolic liver diseases (glycogen storage disease, tyrosinemia type 1, primary hyperoxaluria, urea cycle defect, and factor H deficiency) were more predictive of the survival outcome than fulminant liver failure alone. On the contrary, isolated fulminant liver failure and not liver disease without chronic liver disease was a significant independent risk factor for graft survival. This can be explained by the fact that 5 patients without chronic liver disease died of causes other than graft failure, and only one of these patients had fulminant liver failure. In other words, a patient with liver disease other than fulminant liver failure without underlying chronic liver disease is likely to die of causes other than graft failure.
Whole-liver DDLT compared to LDLT was another significant independent risk factor for graft survival (
A bodyweight <6 kg and postoperative hepatic artery complications were also associated with increased graft loss in the multivariate analysis. Young age and/or low body weight are well-known risk factors for high mortality and graft loss after pediatric LT [14–18]. Although assessment of body weight itself may often be misleading because of the effects of organomegaly and ascites, body weight is still a useful variable because it is easy to measure. Various surgical innovations are required to improve the outcomes of pediatric LT, especially in patients with body weight <6 kg and those with a liver disease without underlying chronic liver disease [4,19–24]. Therefore, increased care is required as the number of small children undergoing LT and split LT has recently increased in Korea [25].
Hepatic artery complications, including HAT and HAS, are strong risk factors for graft loss. Hepatic artery complications are well known as one of the most severe complications leading to increased morbidity, graft loss, and, eventually, patient death [26–31]. The incidence of HAT has been reported to be 3–18% after pediatric LT [25,32–34]. In our study, among 14 cases, 2 underwent non-surgical treatment, 10 underwent surgery, and 6 eventually underwent retransplantation. Owing to the organ shortage in Korea, as in other Asian countries, patients with hepatic artery complications cannot get a deceased organ at the appropriate time despite having a 1A priority status on the waiting list. Among the 8 patients who could not get retransplanted, 5 survived via an aggressive treatment strategy.
Dividing the disease category by biliary atresia vs others, fulminant liver failure vs others, and with vs without chronic liver disease may lead to some overlapping. Biliary atresia, which rapidly progresses to biliary cirrhosis and results in complications related to portal hypertension, would be included in the other than fulminant liver failure group while also being categorized in the liver disease with underlying chronic liver disease group. Considering the confounding effect and the results of multivariate analysis, liver disease without underlying chronic liver disease, rather than biliary atresia or fulminant liver failure itself, is more strongly associated with hepatic artery complications.
According to our previous study that compared the outcomes of pediatric patients with biliary atresia and metabolic liver disease undergoing LT, the rate of hepatic artery complications was higher in patients with metabolic liver disease than in those with biliary atresia [3]. In accordance with the present study, the results indicate that the rate of hepatic artery complications may increase in children without chronic liver disease not characterized by portal hypertension. Several studies reported that smaller or younger children have a higher incidence of hepatic artery complications [26,33–35]. Furthermore, children with chronic liver disease were significantly younger (80.9 vs 53.7 months;
It is well known that biliary complications occur in up to 50% of patients after HAT [39,40]. The present study also shows a significantly higher frequency of biliary complications in children with hepatic artery complications compared to those without hepatic artery complications (10.0% vs 35.7%;
Subgroup analysis was performed to identify any specific disease associated with poor prognosis in patients with and without chronic liver disease. Disease entities with <5 patients were grouped to strengthen the statistical power. There was no statistically significant in-group disease entity associated with poor overall survival both in patients with and without chronic liver disease (
There are some limitations to our study. This was a retrospective study that depended on the completeness of the medical records. Since this study was performed in a single center which mostly performs LDLT, the results may not be directly generalizable to other centers that frequently perform DDLT. To draw a definitive and general conclusion, a multicenter study including all patients who are considered for LT would be needed. The Korean Organ Transplantation Registry is an ongoing prospective multicenter registration database that has been in operation since 2014. Further studies using this database after adequate long-term follow-up are needed to validate the results.
Conclusions
Greater caution is recommended in pediatric patients with a high PELD score and/or without chronic liver disease undergoing LT to improve patient survival. To improve graft survival, greater caution is recommended for patients with low body weight and/or patients with liver disease not accompanied by underlying chronic liver disease. Hepatic artery complications were the only surgical complications affecting graft survival. Therefore, further technical innovations and careful management are required to deal with hepatic artery reconstruction, especially in patients without chronic liver disease.
Figures
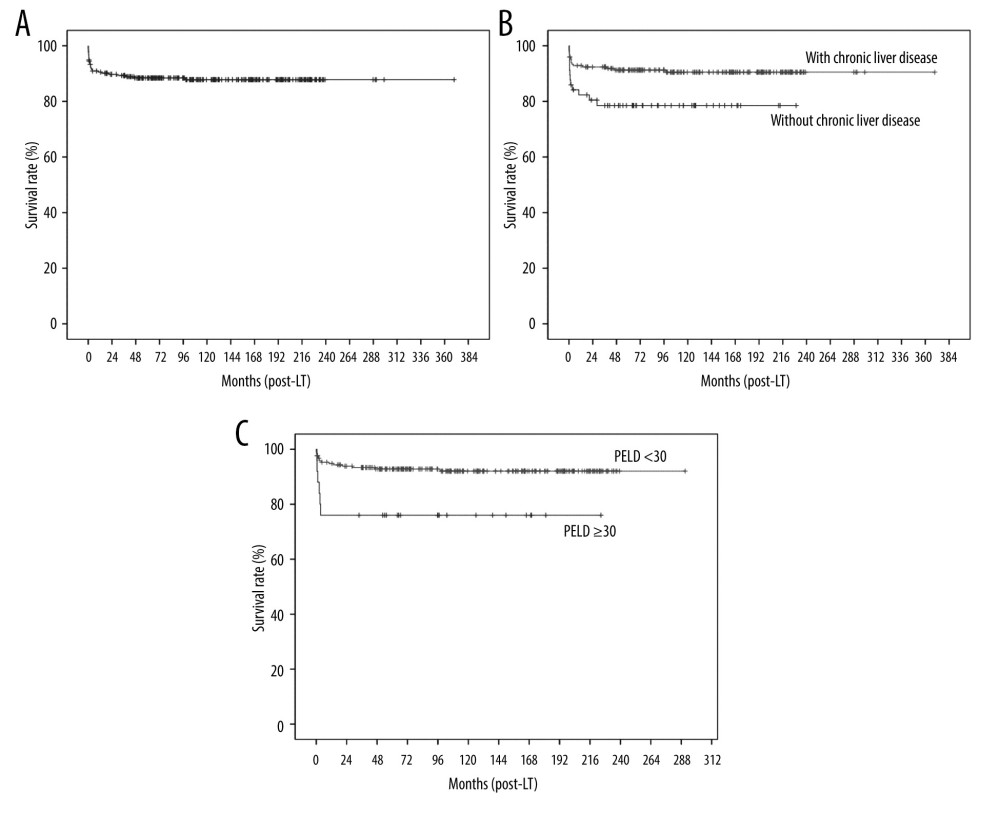 Figure 1. Kaplan-Meier analysis of overall survival: (A) all patients, (B) according to liver disease (with vs without chronic liver disease), and (C) according to pediatric end-stage liver disease (PELD) score.
Figure 1. Kaplan-Meier analysis of overall survival: (A) all patients, (B) according to liver disease (with vs without chronic liver disease), and (C) according to pediatric end-stage liver disease (PELD) score. 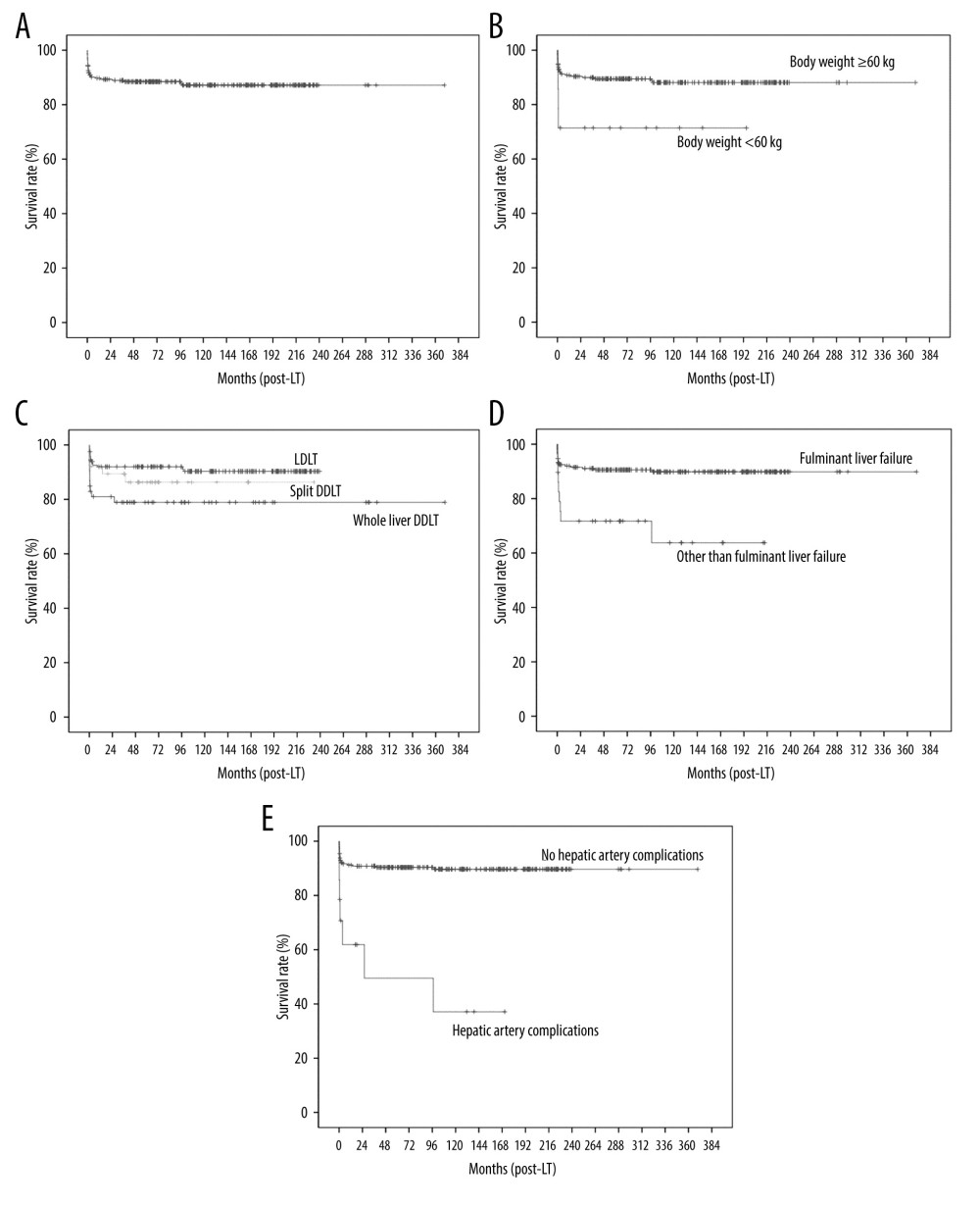 Figure 2. Kaplan-Meier analysis of graft survival: (A) all patients, (B) according to body weight, (C) according to type of LT (LDLT vs split DDLT vs whole-liver DDLT), (D) according to liver disease (fulminant liver failure vs other than fulminant liver failure), and (E) according to hepatic artery complications.
Figure 2. Kaplan-Meier analysis of graft survival: (A) all patients, (B) according to body weight, (C) according to type of LT (LDLT vs split DDLT vs whole-liver DDLT), (D) according to liver disease (fulminant liver failure vs other than fulminant liver failure), and (E) according to hepatic artery complications. Tables
Table 1. Demographic and clinical characteristics of pediatric patients and their donors.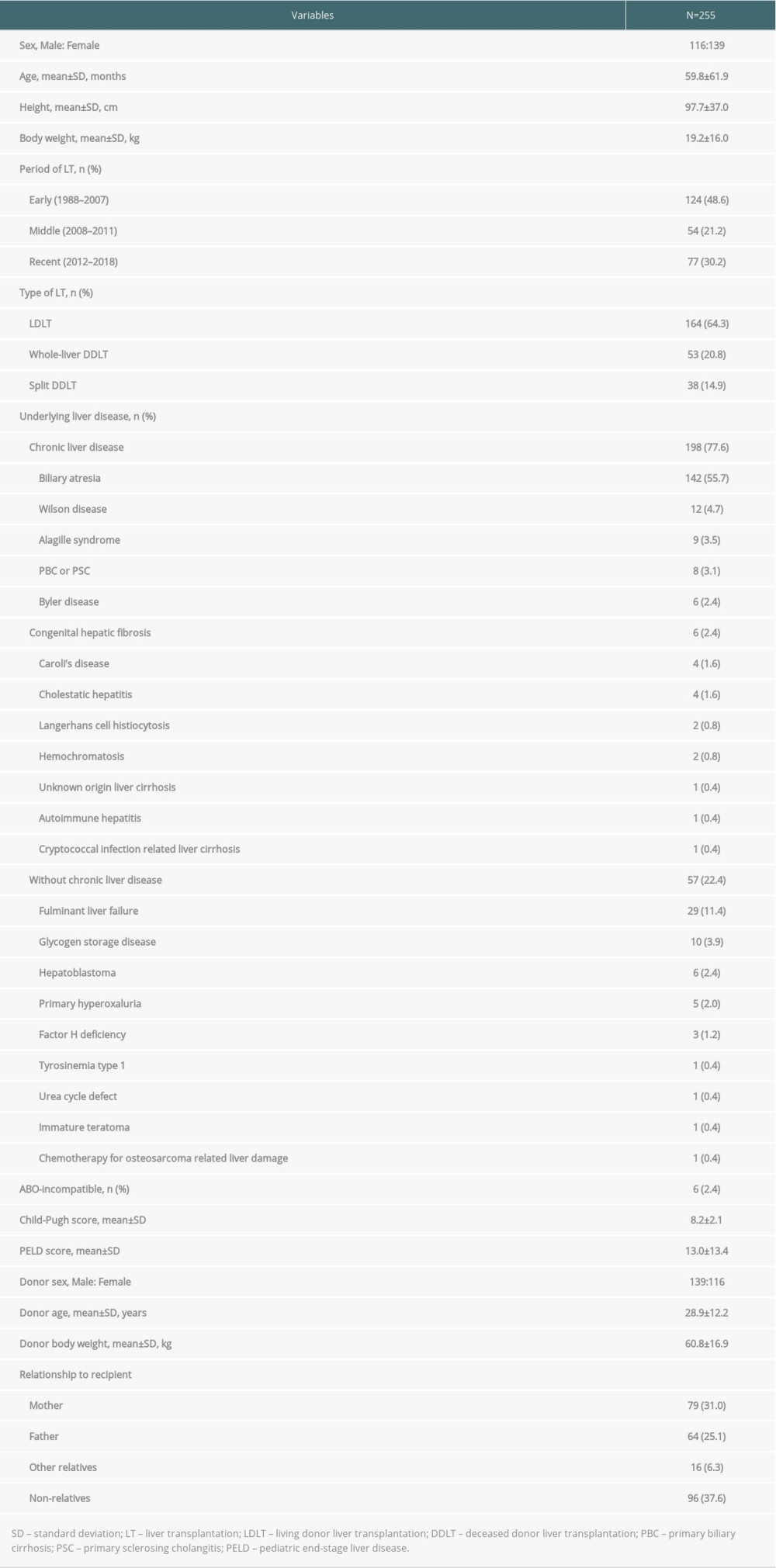 Table 2. Cause of graft failure and death.
Table 2. Cause of graft failure and death.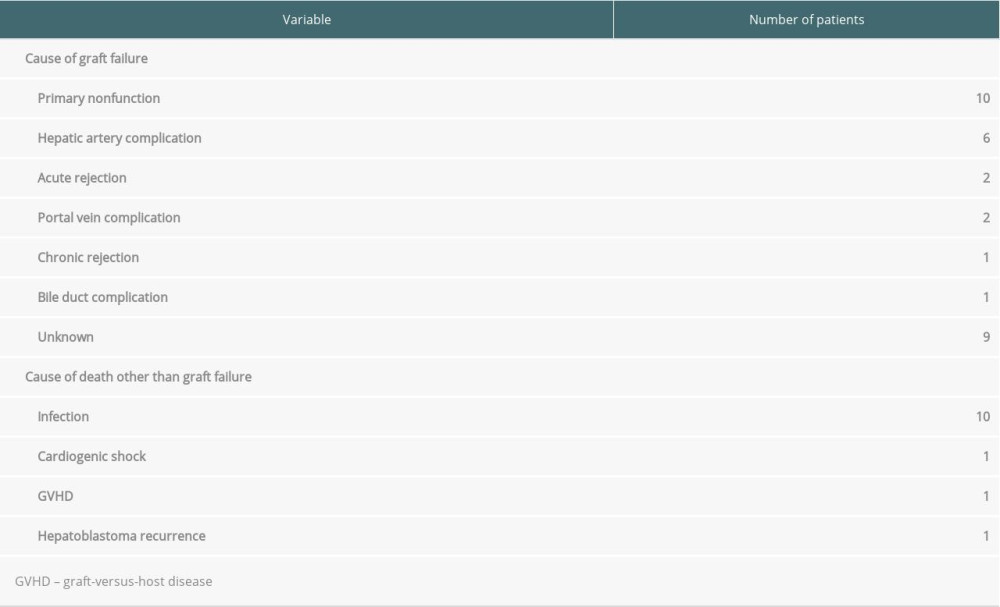 Table 3. Univariate and multivariate analysis of factors associated with patient survival.
Table 3. Univariate and multivariate analysis of factors associated with patient survival.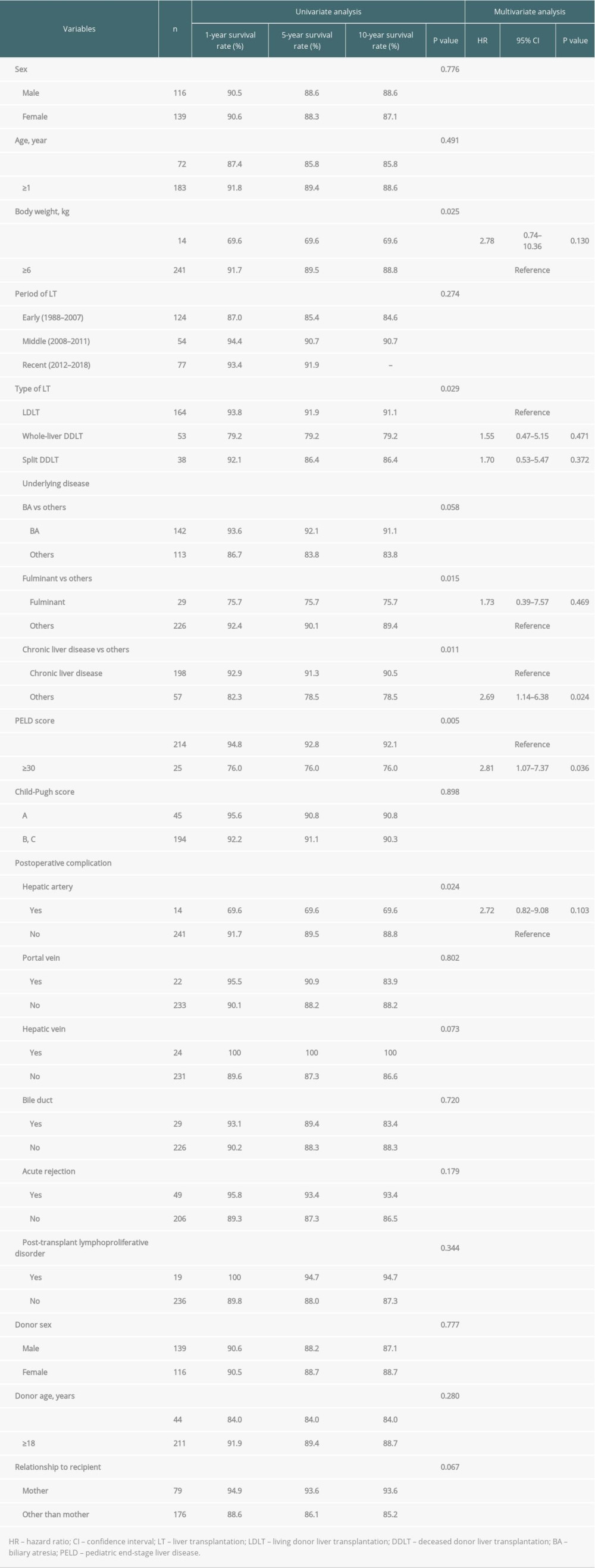 Table 4. Univariate and multivariate analysis of factors associated with graft survival.
Table 4. Univariate and multivariate analysis of factors associated with graft survival.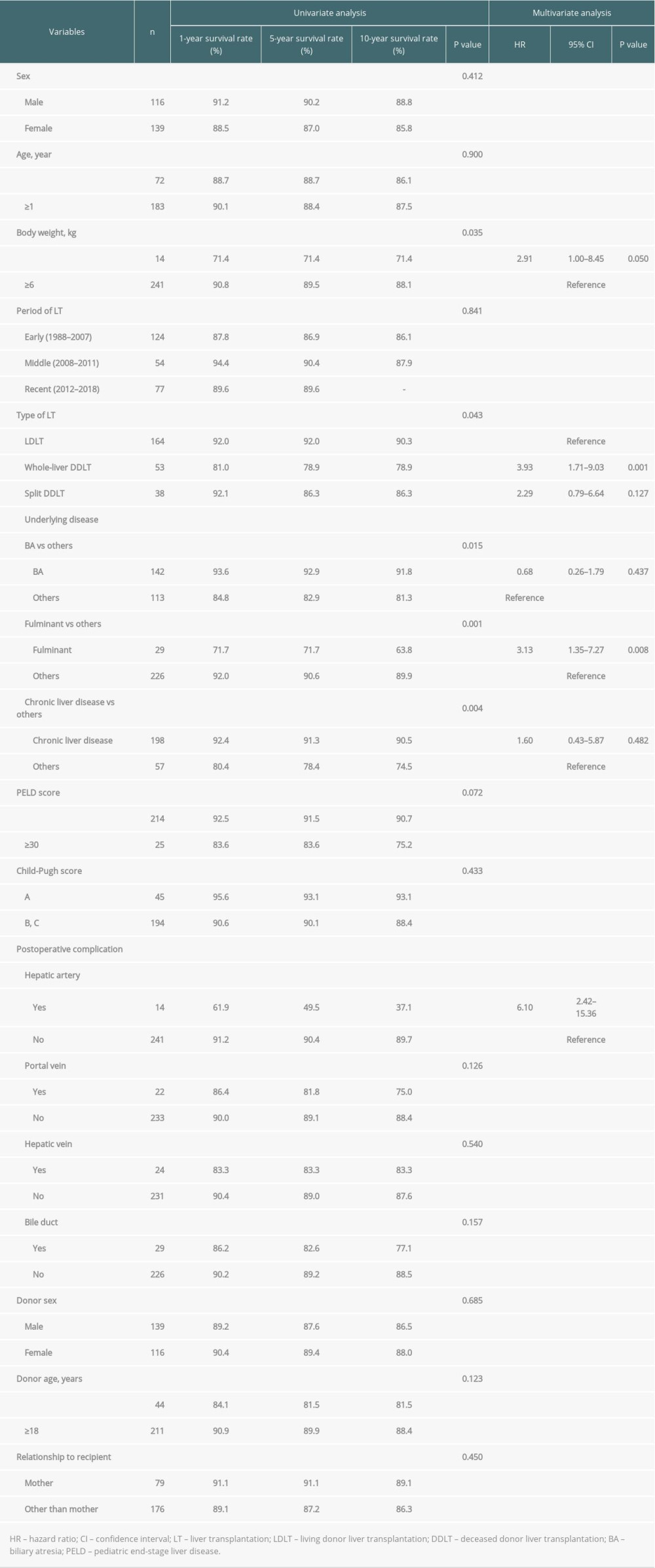 Table 5. Univariate and multivariate analysis of factors associated with postoperative hepatic artery complication.
Table 5. Univariate and multivariate analysis of factors associated with postoperative hepatic artery complication.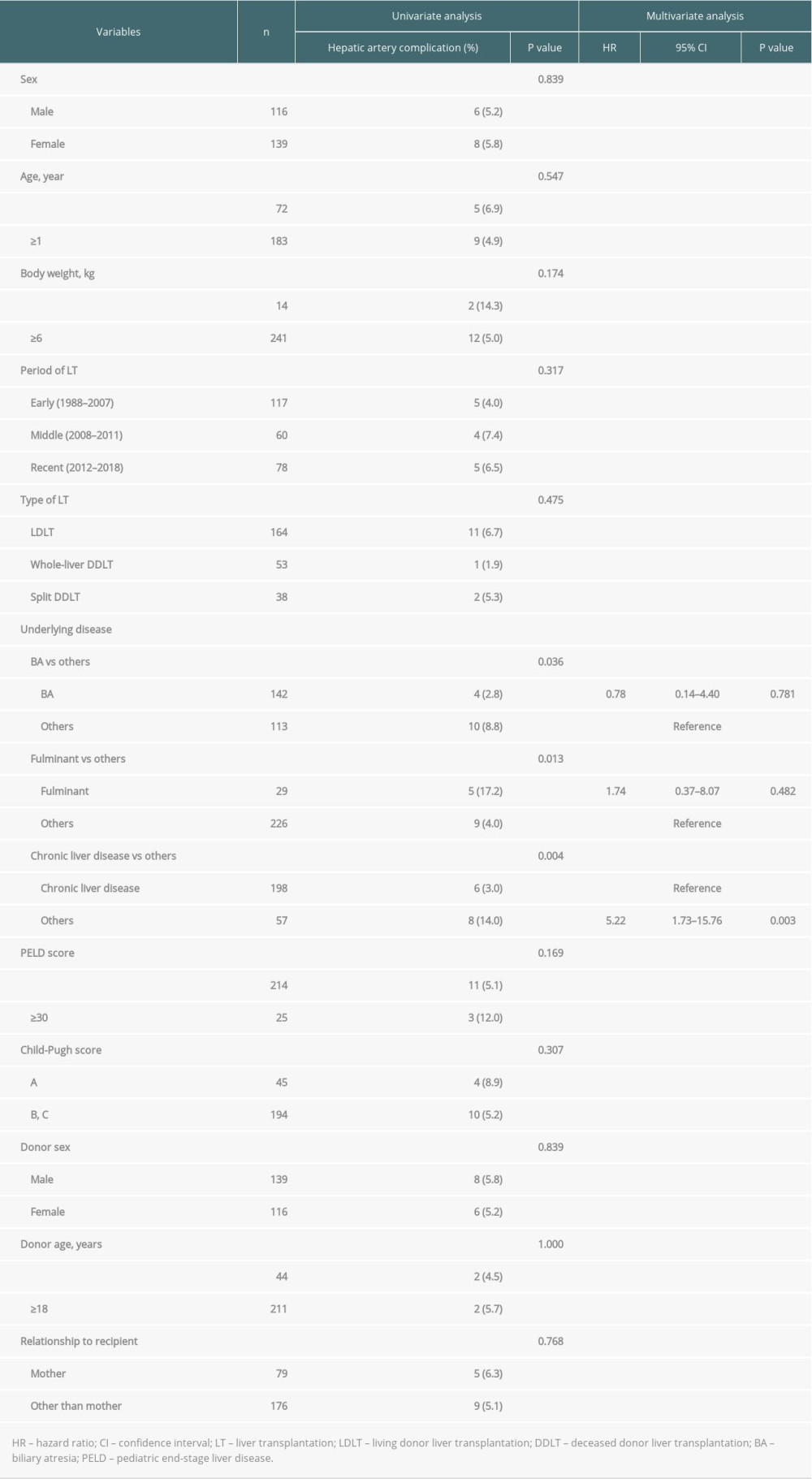 Table 6. Details of patients who had hepatic artery complication after LT.
Table 6. Details of patients who had hepatic artery complication after LT.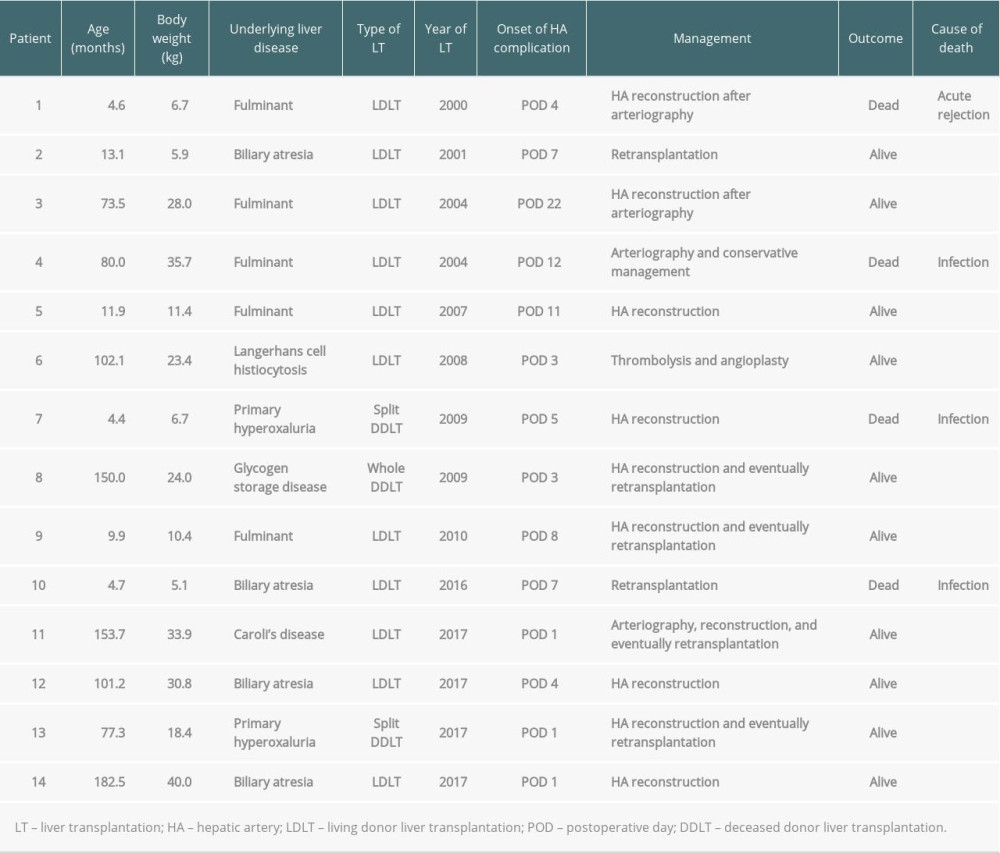
References
1. Rawal N, Yazigi N, Pediatric liver transplantation: Pediatr Clin North Am, 2017; 64; 677-84
2. Kim ST, Park YH, Lee KU, An experience of liver transplantation in Korea: J Korean Soc Transplant, 1988; 2; 27-36
3. Hong SK, Yi NJ, Chang H, The rate of hepatic artery complications is higher in pediatric liver transplant recipients with metabolic liver diseases than with biliary atresia: J Pediatr Surg, 2018; 53; 1516-22
4. Yi NJ, Lee JM, Kim H, Simple ellipsoid reconstruction technique for a hypoplastic portal vein during pediatric liver transplantation: Liver Transpl, 2016; 22; 854-58
5. Clavien PA, Barkun J, de Oliveira ML, The Clavien-Dindo classification of surgical complications: Five-year experience: Ann Surg, 2009; 250; 187-96
6. Park YS, Kim KW, Lee SJ, Hepatic arterial stenosis assessed with doppler US after liver transplantation: Frequent false-positive diagnoses with tardus parvus waveform and value of adding optimal peak systolic velocity cutoff: Radiology, 2011; 260; 884-91
7. Pulitano C, Joseph D, Sandroussi C, Hepatic artery stenosis after liver transplantation: Is endovascular treatment always necessary?: Liver Transpl, 2015; 21; 162-68
8. Demetris AJ, Bellamy C, Hübscher SG, 2016 comprehensive update of the Banff working group on liver allograft pathology: Introduction of antibody-mediated rejection: Am J Transplant, 2016; 16; 2816-35
9. Soltys KA, Mazariegos GV, Squires RH, Late graft loss or death in pediatric liver transplantation: An analysis of the SPLIT database: Am J Transplant, 2007; 7; 2165-71
10. Farmer DG, Venick RS, McDiarmid SV, Predictors of outcomes after pediatric liver transplantation: An analysis of more than 800 cases performed at a single institution: J Am Coll Surg, 2007; 204; 904-14
11. Kasahara M, Umeshita K, Inomata Y, Long-term outcomes of pediatric living donor liver transplantation in Japan: An analysis of more than 2200 cases listed in the registry of the japanese liver transplantation society: Am J Transplant, 2013; 13; 1830-39
12. Sanchez MC, D’Agostino DE, Pediatric end-stage liver disease score in acute liver failure to assess poor prognosis: J Pediatr Gastroenterol Nutr, 2012; 54; 193-96
13. Yankol Y, Fernandez LA, Kanmaz T, Results of pediatric living donor compared to deceased donor liver transplantation in the PELD/MELD era: Experience from two centers on two different continents: Pediatr Transplant, 2016; 20; 72-82
14. Yang SC, Huang CJ, Chen CL, Living donor liver transplantation with body-weight more or less than 10 kilograms: World J Gastroenterol, 2015; 21; 7248-53
15. Arnon R, Annunziato R, Miloh T, Liver transplantation in children weighing 5 kg or less: Analysis of the UNOS database: Pediatr Transplant, 2011; 15; 650-58
16. Noujaim HM, Mayer DA, Buckles JA, Techniques for and outcome of liver transplantation in neonates and infants weighing up to 5 kilograms: J Pediatr Surg, 2002; 37; 159-6
17. Lucianetti A, Guizzetti M, Bertani A, Liver transplantation in children weighting less than 6 kg: The Bergamo experience: Transplant Proc, 2005; 37; 1143-45
18. Neto JS, Carone E, Pugliese V, Living donor liver transplantation for children in Brazil weighing less than 10 kilograms: Liver Transpl, 2007; 13; 1153-58
19. Hwang S, Kim DY, Ahn CS, Computational simulation-based vessel interposition reconstruction technique for portal vein hypoplasia in pediatric liver transplantation: Transplant Proc, 2013; 45; 255-58
20. Rela M, Technique of hepatic arterial anastomosis in living donor pediatric auxiliary partial orthotopic liver transplantation: Liver Transpl, 2013; 19; 1046-48
21. Yamaoka Y, Tanaka K, Ozawa K, Liver transplantation from living-related donors: Clin Transpl, 1993; 179-83
22. Shirouzu Y, Kasahara M, Morioka D, Vascular reconstruction and complications in living donor liver transplantation in infants weighing less than 6 kilograms: The Kyoto experience: Liver Transpl, 2006; 12; 1224-32
23. Mitchell A, John PR, Mayer DA, Improved technique of portal vein reconstruction in pediatric liver transplant recipients with portal vein hypoplasia: Transplantation, 2002; 73; 1244-47
24. Kanazawa H, Sakamoto S, Fukuda A, Living-donor liver transplantation with hyperreduced left lateral segment grafts: A single-center experience: Transplantation, 2013; 95; 750-54
25. Kim JM, Kim KM, Yi NJ, Pediatric liver transplantation outcomes in Korea: J Korean Med Sci, 2013; 28; 42-47
26. Ma N, Song Z, Dong C, Risk factors of hepatic artery thrombosis in pediatric deceased donor liver transplantation: Pediatr Surg Int, 2019; 35; 853-59
27. Seda-Neto J, Antunes da Fonseca E, Pugliese R, Twenty years of experience in pediatric living donor liver transplantation: Focus on hepatic artery reconstruction, complications, and outcomes: Transplantation, 2016; 100; 1066-72
28. Herrero A, Souche R, Joly E, Early hepatic artery thrombosis after liver transplantation: What is the impact of the arterial reconstruction type?: World J Surg, 2017; 41; 2101-10
29. Alobaidi R, Anton N, Cave D, Predicting early outcomes of liver transplantation in young children: the EARLY study: World J Hepatol, 2018; 10; 62-72
30. Feng MX, Zhang JX, Wan P, Hepatic artery reconstruction in pediatric liver transplantation: Experience from a single group: Hepatobiliary Pancreat Dis Int, 2020; 19; 307-10
31. Nacoti M, Ruggeri GM, Colombo G, Thrombosis prophylaxis in pediatric liver transplantation: A systematic review: World J Hepatol, 2018; 10; 752-60
32. Chen CL, Concejero A, Wang CC, Living donor liver transplantation for biliary atresia: A single-center experience with first 100 cases: Am J Transplant, 2006; 6; 2672-79
33. Jain A, Costa G, Marsh W, Thrombotic and nonthrombotic hepatic artery complications in adults and children following primary liver transplantation with long-term follow-up in 1000 consecutive patients: Transpl Int, 2006; 19; 27-37
34. Uchida Y, Sakamoto S, Egawa H, The impact of meticulous management for hepatic artery thrombosis on long-term outcome after pediatric living donor liver transplantation: Clin Transplant, 2009; 23; 392-99
35. Alexopoulos SP, Nekrasov V, Cao S, Effects of recipient size and allograft type on pediatric liver transplantation for biliary atresia: Liver Transpl, 2017; 23; 221-33
36. Uflacker R, Pariente DM, Angiographic findings in biliary atresia: Cardiovasc Intervent Radiol, 2004; 27; 486-90
37. Ho CW, Shioda K, Shirasaki K, The pathogenesis of biliary atresia: A morphological study of the hepatobiliary system and the hepatic artery: J Pediatr Gastroenterol Nutr, 1993; 16; 53-60
38. dos Santos JL, da Silveira TR, da Silva VD, Medial thickening of hepatic artery branches in biliary atresia. A morphometric study: J Pediatr Surg, 2005; 40; 637-42
39. Pinna AD, Smith CV, Furukawa H, Urgent revascularization of liver allografts after early hepatic artery thrombosis: Transplantation, 1996; 62; 1584-87
40. Stringer MD, Marshall MM, Muiesan P, Survival and outcome after hepatic artery thrombosis complicating pediatric liver transplantation: J Pediatr Surg, 2001; 36; 888-91
41. Kamath BM, Yin W, Miller H, Outcomes of liver transplantation for patients with Alagille syndrome: The studies of pediatric liver transplantation experience: Liver Transpl, 2012; 18; 940-48
42. Kamath BM, Schwarz KB, Hadzić N, Alagille syndrome and liver transplantation: J Pediatr Gastroenterol Nutr, 2010; 50; 11-15
Figures
 Figure 1. Kaplan-Meier analysis of overall survival: (A) all patients, (B) according to liver disease (with vs without chronic liver disease), and (C) according to pediatric end-stage liver disease (PELD) score.
Figure 1. Kaplan-Meier analysis of overall survival: (A) all patients, (B) according to liver disease (with vs without chronic liver disease), and (C) according to pediatric end-stage liver disease (PELD) score. Figure 2. Kaplan-Meier analysis of graft survival: (A) all patients, (B) according to body weight, (C) according to type of LT (LDLT vs split DDLT vs whole-liver DDLT), (D) according to liver disease (fulminant liver failure vs other than fulminant liver failure), and (E) according to hepatic artery complications.
Figure 2. Kaplan-Meier analysis of graft survival: (A) all patients, (B) according to body weight, (C) according to type of LT (LDLT vs split DDLT vs whole-liver DDLT), (D) according to liver disease (fulminant liver failure vs other than fulminant liver failure), and (E) according to hepatic artery complications. Tables
 Table 1. Demographic and clinical characteristics of pediatric patients and their donors.
Table 1. Demographic and clinical characteristics of pediatric patients and their donors. Table 2. Cause of graft failure and death.
Table 2. Cause of graft failure and death. Table 3. Univariate and multivariate analysis of factors associated with patient survival.
Table 3. Univariate and multivariate analysis of factors associated with patient survival. Table 4. Univariate and multivariate analysis of factors associated with graft survival.
Table 4. Univariate and multivariate analysis of factors associated with graft survival. Table 5. Univariate and multivariate analysis of factors associated with postoperative hepatic artery complication.
Table 5. Univariate and multivariate analysis of factors associated with postoperative hepatic artery complication. Table 6. Details of patients who had hepatic artery complication after LT.
Table 6. Details of patients who had hepatic artery complication after LT. Table 1. Demographic and clinical characteristics of pediatric patients and their donors.
Table 1. Demographic and clinical characteristics of pediatric patients and their donors. Table 2. Cause of graft failure and death.
Table 2. Cause of graft failure and death. Table 3. Univariate and multivariate analysis of factors associated with patient survival.
Table 3. Univariate and multivariate analysis of factors associated with patient survival. Table 4. Univariate and multivariate analysis of factors associated with graft survival.
Table 4. Univariate and multivariate analysis of factors associated with graft survival. Table 5. Univariate and multivariate analysis of factors associated with postoperative hepatic artery complication.
Table 5. Univariate and multivariate analysis of factors associated with postoperative hepatic artery complication. Table 6. Details of patients who had hepatic artery complication after LT.
Table 6. Details of patients who had hepatic artery complication after LT. In Press
15 Mar 2024 : Review article
Approaches and Challenges in the Current Management of Cytomegalovirus in Transplant Recipients: Highlighti...Ann Transplant In Press; DOI: 10.12659/AOT.941185
18 Mar 2024 : Original article
Does Antibiotic Use Increase the Risk of Post-Transplantation Diabetes Mellitus? A Retrospective Study of R...Ann Transplant In Press; DOI: 10.12659/AOT.943282
20 Mar 2024 : Original article
Transplant Nephrectomy: A Comparative Study of Timing and Techniques in a Single InstitutionAnn Transplant In Press; DOI: 10.12659/AOT.942252
28 Mar 2024 : Original article
Association Between FEV₁ Decline Rate and Mortality in Long-Term Follow-Up of a 21-Patient Pilot Clinical T...Ann Transplant In Press; DOI: 10.12659/AOT.942823
Most Viewed Current Articles
05 Apr 2022 : Original article
Impact of Statins on Hepatocellular Carcinoma Recurrence After Living-Donor Liver TransplantationDOI :10.12659/AOT.935604
Ann Transplant 2022; 27:e935604
12 Jan 2022 : Original article
Risk Factors for Developing BK Virus-Associated Nephropathy: A Single-Center Retrospective Cohort Study of ...DOI :10.12659/AOT.934738
Ann Transplant 2022; 27:e934738
22 Nov 2022 : Original article
Long-Term Effects of Everolimus-Facilitated Tacrolimus Reduction in Living-Donor Liver Transplant Recipient...DOI :10.12659/AOT.937988
Ann Transplant 2022; 27:e937988
15 Mar 2022 : Case report
Combined Liver, Pancreas-Duodenum, and Kidney Transplantation for Patients with Hepatitis B Cirrhosis, Urem...DOI :10.12659/AOT.935860
Ann Transplant 2022; 27:e935860








