20 January 2022: Review Articles
Impact of Arterial Hypertension on the Eye: A Review of the Pathogenesis, Diagnostic Methods, and Treatment of Hypertensive Retinopathy
Jacek Dziedziak1EF, Anna Zaleska-Żmijewska2DEF, Jacek Paweł Szaflik2DEF, Agnieszka Cudnoch-Jędrzejewska1ADEF*DOI: 10.12659/MSM.935135
Med Sci Monit 2022; 28:e935135
Abstract
ABSTRACT: The number of patients with arterial hypertension is continually increasing. Hypertension can cause organ complications, called hypertension-mediated organ damage (HMOD). One example is hypertensive retinopathy, in which high blood pressure (BP) damages both the retinal microcirculation and the retinal nerve fiber layer (RNFL). This can result in progressive and painless vision deterioration in some groups of patients. Unlike anywhere else in the human body, the microvasculature of the retina can be observed in vivo, and the progression of changes can be closely monitored. The harmful effect of increased BP on the eye is not only limited to hypertensive retinopathy, but can also lead to an exacerbation of diabetic retinopathy (DR) and to an increase in intraocular pressure (IOP), and it can also trigger the formation of thromboembolic lesions. This review presents an update on the pathogenesis of hypertensive retinopathy and the use of adaptive optics (AO) combined with optical coherence tomography (OCT) to evaluate the retinal microvasculature. The latest progress and directions of research in the field of hypertensive retinopathy are also discussed.
Keywords: Neovascularization, Pathologic, Hypertensive retinopathy, Hypertension, Retinal Neovascularization, Angiotensin-Converting Enzyme Inhibitors, Antihypertensive Agents, Humans, Tomography, Optical Coherence
Background
Hypertension can be diagnosed when systolic blood pressure (SBP) exceeds 140 mmHg and/or when diastolic blood pressure (DBP) is over 90 mmHg [1]. These criteria apply to the adult population. In children and adolescents, blood pressure (BP) norms are expressed on percentile charts, and average SBP and DBP should not exceed the 95th percentile [2]. Hypertension is a severe epidemiological problem. Currently, the number of people with hypertension exceeds 1.4 billion [3] and hypertension is responsible for more than 10 million deaths per year [4]. The number of patients is estimated to exceed 1.5 billion in 2025 [5]. Despite increasingly effective pharmacotherapy and a greater awareness of hypertension and its complications, this condition is still particularly dangerous [6]. For many years, it can proceed asymptomatically or it can display non-specific symptoms and is often diagnosed incidentally [7]. There are several organ complications, collectively referred to as hypertension-mediated organ damage (HMOD) in the course of vascular lesions that accompany hypertension, such as vascular endothelial damage, remodeling of small and large arteries, and vascular rarefaction [8]. This name (HMOD) has replaced the previously used term, target organ damage (TOD). HMOD includes ischemic and hemorrhagic stroke; coronary heart disease (CHD) with myocardial infarction (MI), proteinuria, and renal failure; and retinopathy and atherosclerotic changes, including the development of stenoses and aneurysms [9]. If hypertension remains untreated, or if it is diagnosed late, or if it is not controlled sufficiently, then the risk of developing HMOD increases [10].
Hypertensive retinopathy is an example of HMOD, which is caused by several vascular changes in the retinal microcirculation [11]. Signs of hypertensive retinopathy can be detected in 6–15% of the non-diabetic population aged 40 years and older [6], but this number is thought to be significantly underestimated [12]. It was first described by Marcus Gunn in the late 19th century, and the first classification of changes visible on the fundus of the eye was created by Keith, Wagener, and Barker (KWB) in the late 1930s [13]. This classification distinguishes the 4 stages of severity of the lesions: grade I – generalized retinal arteriolar narrowing; grade II – arteriovenous (AV) crossing and more severe generalized and focal areas of arteriolar narrowing; grade III – grade I and II with the additional presence of exudates and hemorrhages; and grade IV – papilledema, also called malignant hypertensive retinopathy [13]. To this day, this classification is widely used, despite its numerous limitations, including poor clinical and prognostic value (the most frequent are early findings of non-specific retinal alterations such as diffuse or focal arteriolar narrowing) and considerable interobserver and intraobserver variability [14]. There were several attempts made to modify the KWB classification. One of them was the classification proposed by Mitchell and Wong. This classification combined grades I and II of KWB classification into mild-grade hypertensive retinopathy, and distinguished moderate and malignant stages, which corresponded to grade III and IV of the KWB classification, respectively. Unfortunately, it did not meet expectations and only simplified clinical practice [15,16]. Lesions characteristic for advanced retinopathy have been proven to have a predictive value for HMOD [17]. It is increasingly suggested that less advanced changes are also important [18]. The fact that retinal vessels are terminal vessels and are highly susceptible to changes in BP may reflect the vasculature condition of the entire organism [19]. In addition, the development of hypertensive retinopathy can lead to a gradual, painless deterioration of vision and it can cause blindness as it progresses [20].
The aim of this review is to summarize current views on the pathophysiology, diagnostics, therapy, and predictive value of hypertensive retinopathy and to introduce novel and promising research directions in this field. Therefore, this review presents an update on the pathogenesis of hypertensive retinopathy and the use of AO combined with OCT to evaluate the retinal microvasculature.
Retinal Vasculature
Retinal vasculature is mainly derived from the central retinal artery (CRA), the first intraorbital branch of the ophthalmic artery. The CRA penetrates the optic nerve, and then enters the eye through the central part of the optic disc, where it is divided into 2 main branches. These branches divide further into arterioles which supply the peripheral parts of the retina. Each arteriole provides the vascularity of the inner nerve layer of 1 quadrant of the retina. The CRA is the terminal vessel, and when it is occluded, sudden blindness occurs [21,22]. Larger vessels lie in the inner part of the retina, near the inner limiting membrane. Arterioles form 2 layers of capillaries: the superficial vascular plexus (SVP) located in the nerve fiber and the ganglion cell layer; and the deep capillary plexus (DCP) which lies deeper in the inner nuclear layer. The development of optical coherence tomography angiography (OCTA) helped to better understand the complexity of retinal vasculature, whose vessels are only 100–300 μm in diameter [23]. In addition, the intermediate capillary plexus (ICP), which lies between the SVP and the DCP, and the radial peripapillary capillary plexus (RPCP), which supplies the nerve fiber layer (NFL) bundles, have been identified [22,24,25]. The retinal pigment epithelium (RPE) and the outer retina are nourished by diffusion from the choriocapillaris and separated from them by the blood-retinal barrier (BRB) [26]. The presence of the BRB prevents the leakage of macromolecules and other potentially harmful agents into the retina [27].
Pathogenesis of Retinopathy
VASOSPASM:
Despite the considerable attention given to hypertension and its complications, the pathogenesis of hypertensive retinopathy, especially the onset of the lesions, is not yet fully understood. It is believed that the first changes that occur in response to elevated BP in the vessels of the retina are vasospasm and an increase in vasomotor tone, which manifests as generalized vessel narrowing [6]. This is a consequence of the local regulation of blood flow and an attempt to control the volume of blood in the vascular bed by myogenic and metabolic mechanisms [28]. Since the ARIC (Atherosclerosis Risk in Communities) study, the arteriovenous ratio (AVR) is the most widely used method for assessing generalized retinal vasoconstriction [29]. It is based on the diameters of the central retinal artery equivalent (CRAE) and the central retinal venular equivalent (CRVE), also known as the central retinal vein equivalent [30]. A study of 223 subjects, of whom 86 were normotensive and 137 had early hypertension (up to 1 year since first appearing), showed that people with hypertension had lower CRAE and AVR, while no significant difference in CRVE was observed. Furthermore, patients with hypertension and retinopathy showed more significant arterial stiffness indices, expressed by the pulse wave velocity (PWV) and aortic augmentation index (AIx) [31]. Also, a lower AVR predicts an increase in the 3-year risk of CHD events in females [32]. Another study proved that a decrease in CRAE and an increase in CRVE correlates with higher mean arterial blood pressure (MABP) [33]. This indicates a reduction in the vascular lumen in hypertension. Inconsistent data about whether the diameter of the veins in hypertension increases or does not change might suggest that venular widening is associated with retinal ischemia and hypoperfusion, which appear later in hypertensive retinopathy.
EXACERBATION OF HYPERTENSION:
Continuously increasing blood pressure leads to arteriosclerosis. This results in an increase in blood flow resistance and a decrease in perfusion and, consequently, to retinal ischemia [34]. In addition, arteriosclerotic lesions cause several modifications in vessel architecture, such as intimal thickening, media-wall hyperplasia, and hyaline degeneration [34]. In fundoscopy, these changes manifest as a focal and diffuse narrowing of the arterial wall (resembling silver or copper wiring), accompanied by arteriovenous nicking, which is the result of increased pressure imposed on the venules by altered arterioles (Figure 1). Nicking is associated with both current and past BP, suggesting that it is a persistent and long-term marker of hypertension [18]. Observations carried out by Cheung et al have shown that increased arteriolar and venular tortuosity in the retina is also associated with chronic hypertension [35]. When hypertension is poorly controlled or reaches extremely high values, the damage progresses further. Over time this can lead to breakage of the BRB, the presence of hemorrhages, hard (lipid) exudates, and cotton wool spots, which are evidence of ischemia of the NFL [36]. Also, people with hypertension have been diagnosed with a lower amount of perifoveal arterioles and venules, and this may indicate gradual vascular occlusion and necrosis [37]. In advanced hypertension, an increase in intracranial pressure (ICP) can occur. When increased pressure of the cerebrospinal fluid is imposed on the optic nerve head and its vessels, it results in ischemia and edema of the optic disc (papilledema) [16,38,39].
DIFFERENCES IN VASCULAR CALIBER:
The presented pathogenesis appears to reflect a cascade of changes that occur in the course of hypertensive retinopathy in an orderly way. However, recent research suggests that the whole process might be much more complex. Lesions visible at the fundus of the eye may occur in a different order; for example, the presence of hemorrhages is not always preceded by generalized vasoconstriction [40]. Also, arterial stenosis is observed among people without hypertension [41] as a result of changes in the retinal vascular caliber (arteriolar narrowing and venular widening). A large meta-analysis on a group of 10 229 subjects indicates that these changes in retinal vessel diameter are associated with a higher risk of hypertension, which develops for up to 10 years after the onset of the changes. This observation supports the theory that changes in retinal microcirculation can precede arterial hypertension [42,43]. A decrease in arteriolar caliber also correlates with older age and low birth weight, as well as with a higher hematocrit, higher white cell count, and higher platelet count [30,44]. A meta-analysis by Chew et al proved that there is a close link between a decrease in both retinal arteriolar diameter and CRA diameter and hypertension, and that each 10-mmHg increase in MABP is associated with a 3 μm decrease in mean retinal arteriolar diameter [45]. This reduction occurs because high BP causes a gradual entropic remodeling of the arterioles, resulting in a decrease in the outer and inner diameter of the vessel [46].
PATHOMECHANISMS OF HYPERTENSIVE RETINOPATHY:
There are several mechanisms involved in the pathogenesis of hypertensive retinopathy. One of them is an elevated level of oxidative stress, expressed by an increase in the plasma markers gamma-glutamyltransferase and ferritin [47,48]. Other vital pathomechanisms are low-grade inflammation (increased high-sensitivity C-reactive protein, which is a marker of systemic low-grade inflammation, and is correlated with the prevalence and severity of hypertensive retinopathy) and increased platelet activation (the presence of hypertensive retinopathy correlated positively with urinary 11-dehydro-TXB2, a biochemical marker of platelet activation) [49–51]. Hypertension damages the vascular endothelium, which is located at the border between blood and the interstitial tissues. Its primary role is to control vasoregulation, hemostasis, and inflammation [52,53]. Nitric oxide (NO), which has an essential role in vasodilatation, is produced by the endothelial cells. The observation of the retinal flow after intravenous administration of NG-monomethyl-L-arginine (nitric oxide synthase inhibitor) in groups of normotensive and hypertensive patients showed significant changes. In the hypertensive group, the blood flow did not change, while in the group of normotensive patients the blood flow significantly decreased. This indicates a diminished role of NO-dependent vasodilatation in the regulation of blood flow among patients with hypertension and impaired endothelial function [54]. This was further confirmed in the hypertensive group by the elevated level of the von Willebrand factor, a substance which is stored in the endothelial cells, and by an increased concentration of angiotensin-converting enzyme (ACE, CD143), which is connected to their membranes [53].
THE ROLE OF THE RENIN-ANGIOTENSIN-ALDOSTERONE SYSTEM:
The renin-angiotensin-aldosterone system (RAAS) plays a vital role in the regulation of systemic and local BP. Its primary function is to control the concentration of sodium and potassium ions in body fluids, which is crucial in regulating the volume of circulating blood. The role of RAAS has been widely studied in the course of diseases such as diabetic retinopathy (DR) and glaucoma [55]. However, there is no major study focusing on RAAS and its relationship with hypertensive retinopathy. This fact is of great importance because its excessive activity can damage the vascular bed through persistent vasoconstriction. However, a local increase in the concentration of RAAS components can cause proliferation and inflammation and stimulate neovascularization [56]. In human model studies, the presence of local RAAS in the eye was confirmed, and many known components of this system were identified in the eye, such as its main effector, angiotensin II (Ang II). Ang II, by acting on the angiotensin II receptor type 1 (AT1-R) causes vasoconstriction and stimulates cell proliferation and fibrosis [56,57]. The Mas-receptors (Mas-R), which are also located in the eye, when stimulated by the product of the breakdown of Ang II to angiotensin 1–7 (Ang 1–7), has the opposite effects to AT1-R (anti-inflammatory, inhibition of cell proliferation, antifibrosis, and vasodilatation) [58]. Ang 1–7 administration resulted in a decrease in IOP in the animal model. IOP was also lowered when patients with hypertension were treated with an angiotensin-converting enzyme inhibitor (Captopril) and an AT1-R antagonist (Losartan) [59,60]. This observation is important because high IOP can lead to retinal perfusion disorders due to increased pressure imposed on the retinal vessels. Another AT1-R blocker, candesartan cilexetil, showed a positive impact on the regulation of retinal blood flow by improving endothelial-dependent NO vasodilatation among patients with hypertension [54]. An important step to better understand the impact of RAAS in hypertensive retinopathy may be a recent research study on the Ang II-elevated double-transgenic rat model [61]. The study indicated that a high level of Ang II correlates with the degeneration of retinal ganglion cells and alterations in the outer layer of the BRB. The study also showed that retinal RAAS is regulated by systemic alterations of the systemic RAAS, which is mainly expressed by changes in renin production by the RPE [61]. Furthermore, an increase in the systemic Ang II level correlates with a decrease in the retinal RAAS activity, and β-adrenergic influence increases the retinal RAAS activity, but the role of this process is yet to be explored [62]. The main factors involved in the pathogenesis of hypertensive retinopathy are presented in Figure 2.
POTENTIAL ROLE OF NEOVASCULARIZATION IN HYPERTENSIVE RETINOPATHY:
Neovascularization can have an essential role in the pathogenesis of hypertensive retinopathy. Angiogenesis is mostly controlled by a tight balance between pro-angiogenic and anti-angiogenic factors. Pathological mechanisms, such as ischemia, hypoxia, and inflammation, which cause endothelial damage, can promote neovascularization [63]. It should be emphasized that ischemia and hypoxia of the retina are important components of hypertensive retinopathy [64]. A review from 2012 highlights the potential role of the vascular endothelial growth factor (VEGF) family in the pathogenesis of hypertensive retinopathy. One of the best-known members of this family, VEGF-A, is crucial in neovascularization and is a major vascular permeability factor, whose increase correlates with essential hypertension [65]. VEGF is also an early marker of vascular endothelial damage [66]. Changes in its concentration can be significant in the early diagnosis of patients with microcirculation complications, such as hypertensive retinopathy. This is further confirmed by an increase in plasma VEGF levels in hypertensive patients with retinopathy [67]. VEGF can be released in response to hypoxia-mediated damage of the nerve fiber layer, and by activation of AT1-R [68]. A case report from 2016 first described a patient with proliferative retinopathy in the course of severe uncontrolled hypertension [69].
Diagnostics of Hypertensive Retinopathy
OPTICAL COHERENCE TOMOGRAPHY:
Recently, there has been significant progress in the area of visualizing the interior of the eye. Not only can large retinal vessels be observed, but it is also possible to go deeper to the level of the capillaries. The introduction of adaptive optics (AO) retinal imaging and optical coherence tomography (OCT) provided scientists with improved data on retinal vascular changes [75]. This is especially important for observing retinal microcirculation to predict cardiovascular risk [76]. Thanks to these new imaging techniques, the progression of the lesions can be closely monitored, as it makes it possible to perform vascular observation in vivo [77]. Spectral domain-OCT (SD-OCT) enabled faster scanning, denser sampling, and three-dimensional imaging [78]. A study using SD-OCT analysis on a group of 119 patients, 56 of whom had hypertension, showed that in the chronic stage (grade I and II of KWB) it is an accurate, reproducible, and convenient method for assessing CRA diameter, CRV diameter, and AVR [79]. In addition, SD-OCT showed a better correlation of these measurements with worsening visual acuity than the KWB classification due to the presence of subretinal fluid (SRF) in severely hypertensive patients (SBP >180 mmHg or DBP >110 mmHg). In their research, Ahn et al also proposed a new classification of hypertensive retinopathy based on OCT findings and consisting of 3 grades: mild-to-moderate, malignant without SRF, and malignant with SRF [80]. SD-OCT is also a useful tool for monitoring the decrease of retinal nerve fiber layer (RNFL) and the central macular thickness in grade IV hypertensive retinopathy [81]. The results of the Singapore Malay Eye Study highlight a link between a decrease in arteriolar and venular caliber and the thickening of RNFL, even after excluding patients with glaucoma [82]. Data from another study suggest that SD-OCT assessment should be added to standard hypertensive retinopathy evaluation in patients with systemic hypertension for the early diagnosis of HMOD [83].
ANGIO-OCT:
OCTA provides a quick and non-invasive assessment of retinal microcirculation. Retinal and choroidal microvascular changes are evaluated based on parameters such as the vessel density (VD), the area of the foveal avascular zone (FAZ), and the radial peripapillary capillary (RPC) network [84]. The assessment of retinal capillaries has been effective in DR, and its use in hypertensive retinopathy can also be effective. It enables the detection of even subtle differences in the architecture of the capillaries, which might be the earliest markers of ischemia and hypoxia, before arteriolar and venular modifications take place [85]. Moreover, recent studies with OCTA analysis indicate that vessel density in SVP decreases in the group of hypertensive patients with inadequate BP control [86]. Also, the reduction in retinal and choroidal VD is strictly related to the coronary artery and branch stenosis, making OCTA an efficient and non-invasive method for measuring and detecting early-stage CHD, which can reduce the occurrence of MI [87]. It is also reasonable to perform SD-OCT and OCTA analysis simultaneously. Unlike SD-OCT, OCTA cannot measure retinal vascular diameter, but provides an evaluation of the hemodynamics and vascular density of retinal blood flow [64]. Takayama et al expressed the need to adopt a new classification system based on the usage of OCTA and the measurement of choroidal vasculature in the fovea proximity, which is also reduced in arterial hypertension [88]. They demonstrated that changes in the SVP, the FAZ area, the capillary density, and the inner retinal thickness occurred in the group of hypertensive patients without hypertensive retinopathy. As mentioned earlier, subtle modifications in the vasculature can develop before the onset of hypertension. This implies that increased OCTA follow-up frequency may be useful for the supervision of retinal vascular and thickness changes in the group of patients at risk of developing hypertension [89].
ADAPTIVE OPTICS:
AO retinal imaging is another important tool in the precise visualization of retinal vasculature. With a resolution of less than 2 μm, it improved the quality of retinal images and helped in the assessment of cellular and subcellular details such as the condition of the endothelial cells and subtle fluid leakage [90]. The use of this technique enables direct measurement of the retinal vessel wall (VW) and the lumen diameter (LD). A semi-automated method used to measure vessel parameters had low intra-observer variability [91]. The wall-to-lumen ratio (WLR) is another effective vascular parameter that can be measured by coupling AO with scanning laser ophthalmoscopy (Figure 3) [92]. The increase in the WLR that occurs in hypertension is caused by a decrease in LD and the thickening of the arteriolar wall (Figure 4). This is used to detect subtle retinal microvascular modifications observed in the early remodeling process [93]. Its importance extends further, as an increase in the WLR correlates not only with age and with higher body mass index (BMI), but also with early-stage HMOD [94,95]. More extensive use of AO retinal imaging will be essential in the search for novel mechanisms of hypertension-related vascular alterations. It has already improved our understanding of the significance of capillary level changes in perfusion [90].
Therapeutic Approaches
Currently, the approach to the treatment of hypertensive retinopathy focuses mainly on the control of systemic BP within the limits of the norm, which is primarily achieved by oral pharmacotherapy. A decrease in BP obtained by the use of ACE-inhibitors can reduce the risk of progression of hypertensive retinopathy changes [96]. Also, such therapy results in an improvement of arteriolar architecture and rarefaction, and an increase in arteriolar density [97, 98]. In contrast to more severe retinopathy, an early manifestation is more likely to be reversible. For this reason, early detection of retinopathy lesions might prove helpful not only in the effective prevention of its further development but also in searching for new therapeutical opportunities [99]. The major obstacle in therapy based mainly on oral drug administration is poor compliance. A 2017 meta-analysis on a group of 12 603 hypertensive patients found non-adherence to pharmacotherapy in 45.1% of patients [100].
The latest reports also suggest that severe lesions associated with malignant hypertensive retinopathy can regress or even completely disappear. Strachan and McKnight described the case of a 34-year-old woman diagnosed with hypertension and stage IV hypertensive retinopathy on the KWB scale. Changes visible at the fundus of the eye retreated after 10 months of irbesartan (AT1-R antagonist), atenolol (β1-adrenergic antagonist), and amlodipine (a calcium channel blocker) therapy [101]. Another case report focused on a 23-year-old patient with deterioration in vision and malignant hypertensive retinopathy, which appeared 1 month after ceasing antihypertensive drugs. Six weeks after returning to pharmacotherapy, the signs of malignant hypertensive retinopathy disappeared, and visual acuity improved [102]. In addition, the presence of pheochromocytoma, a tumor that is characterized by frequent spikes in BP, can lead to rapid development of malignant hypertensive retinopathy. Treatment based on adrenalectomy and normalization of BP values was successful, and improvement of optic disc edema and hard and soft exudates was observed [103].
Unfortunately, there is a lack of research focusing primarily on the therapy of retinal lesions appearing in the course of hypertension. Most information on new directions of treatment come from case reports and studies with small sample sizes. One promising approach was based on the correlation between systemic hypertension and increased IOP. There were attempts to use topical administration of IOP-lowering drugs in systemic hypertension to improve blood flow and arteriolar blood saturation might. However, the application of timolol (β-adrenergic receptor antagonist) and dorzolamide (carbonic anhydrase inhibitor) had no effect or a minimal effect on retinal blood flow [104]. Interestingly, a recent report suggests that a diet rich in L-methylfolate and vitamin D might be beneficial for patients with hypertensive retinopathy. However, this approach needs to be confirmed in a larger group of patients [105].
Targeting neovascularization, which may play an essential role in the pathophysiology of hypertensive retinopathy, appears to be encouraging. Bevacizumab, an anti-VEGF antibody, is widely used in ophthalmology for the treatment of diseases associated with increased vascular permeability, such as exudative age-related macular degeneration (AMD) and DR [106]. Vascular leakage also appears in the course of malignant hypertensive retinopathy. When hypertensive retinopathy enters its exudative stage, BRB damage and increased vascular permeability occurs [36]. Intraocular injections of anti-VEGF antibody were used successfully in 2 patients with hypertension and malignant hypertensive retinopathy with the presence of macular and optic disc edema. One month after the injection, visual acuity improved, and the lesions characteristic of the exudative stage disappeared [107]. This is not the only report of the possible effectiveness of anti-VEGF antibody treatment in malignant retinopathy. Three other cases, 2 with the use of Bevacizumab and 1 with the use of Ranibizumab, which is also an anti-VEGF antibody, confirmed their efficacy [108–111]. The decision to apply a Ranibizumab injection was based on the lack of improvement in the retinal image in the course of malignant hypertensive retinopathy, despite extensive BP control. Therefore, targeting VEGF and neovascularization as a potential pathomechanism of malignant hypertensive retinopathy appears to be reasonable.
Predictive Value of Hypertensive Retinopathy
CARDIOVASCULAR RISK:
Changes in the retina that indicate a significant progression of retinopathy have been proven to be strongly associated with the occurrence of cardiovascular diseases. Advanced lesions can predict congestive heart failure and cardiovascular mortality, independent of BP and other risk factors [112]. Wang et al analyzed the results of 2 extensive cohort studies, the Beaver Dam Eye Study and the Blue Mountains Eye Study, which both assessed the diameter of the retinal vessels. These studies made it possible to establish a link between retinal vascular diameter and risk of CHD and stroke in middle-aged persons [113]. Another meta-analysis of over 21 000 patients indicates that both narrower retinal arterioles and wider retinal venules are correlated with a higher risk of CHD in women, but not in men. The above findings might support the thesis that coronary microvascular dysfunction plays an important role in the pathogenesis of CHD [114,115].
Hypertension, especially when insufficiently controlled or untreated, leads to the development of left ventricular hypertrophy (LVH). Previous studies have shown a close association between advanced hypertensive retinopathy and LVH, and that hypertensive retinopathy doubles the risk of LVH [116]. The results of recent research suggest that retinal arteriole narrowing may be as accurate as BP measurement in predicting LVH [117,118]. The coincidence of changes in retinal vessel diameter and HMOD, expressed as LVH and ventricular remodeling, has also been studied. Based on the images of digital retinography performed in hypertensive patients, AVR was shown to be correlated with the presence of HMOD in the form of LVH [119].
STROKE:
The evaluation of retinal arterioles is a model for assessing cerebral microcirculation, thanks to their great proximity, anatomical resemblance, and mutual vascularity (internal carotid artery supplies both the anterior brain and the retina) [120]. Hypertensive retinopathy has been proven to predict the long-term risk of stroke, even among patients with good hypertension control [12]. This suggests that the precise control of retinal microvasculature is of additional value in predicting vascular risk [121]. There is also evidence based on cohort studies that retinal microvascular changes are associated with dementia, cognitive impairment, and brain imaging abnormalities. The correlations are strongest for more severe retinal microvascular abnormalities [122].
CHRONIC KIDNEY DISEASE:
The development of chronic kidney disease (CKD) can occur due to severe hypertension. A study on a group of 224 non-diabetic CKD patients with hypertension showed that over 70% had hypertensive retinopathy. Furthermore, retinopathy advancement was independently associated with CKD severity, which was later confirmed in other studies [123, 124]. Recent research indicates that there is a lower density in both SVP and DCP among patients with simultaneous CKD and hypertension [125]. It has also been reported that the low retinal vascular density found in OCTA is associated with acute kidney injury (AKI) and can predict its onset [126].
On the contrary, Vaes et al showed that there is a lack of association between retinal vessel diameter and lung function parameters among patients diagnosed with chronic obstructive pulmonary disease (COPD) [127].
New Directions of Research
PATHOGENESIS:
Currently, there is an increasing number of studies in the field of hypertensive retinopathy. The majority of them are focused on better understanding the pathogenesis of this disease. One study indicated that endothelins, a group of vasoconstrictive agents produced by the endothelial cells, can cause BRB damage. It is mainly caused by the activation of endothelin receptors A and B (ETRA and ETRB) by endothelin 1 (ET-1). Stimulation of these receptors promotes vascular leakage and VEGF stimulation and is upregulated in the animal model of hypertension and diabetes. In addition, the antagonists of these receptors have been shown to reduce vascular damage and neovascularization, and have demonstrated a protective effect on the BRB. Also, the plasma ET-1 level is elevated in hypertensive retinopathy [128,129]. It is crucial to further explore the role of neovascularization in hypertensive retinopathy, as this might help achieve an additional therapeutical effect. Targeting the agents promoting neovascularization, such as platelet-derived growth factor (PDGF), pigment epithelium-derived factor (PEDF), hepatocyte growth factor (HGF), angiopoietins, and fibroblast growing factor (FGF), may be promising [130]. Research on the rat model of hypertensive complication of pregnancy, preeclampsia, confirmed the relationship between high BP, RAAS, and an increased level of pro-angiogenic factors, such as VEGF and PEDF. In preeclampsia, there is a massive endothelial dysfunction, which also causes slight retinal vessel narrowing [131]. Proteomic analysis of the vitreous humor might help to define the involvement of these factors in the pathogenesis of hypertensive retinopathy, thanks to its proximity to the damaged retina. One of the difficulties of this method might be the small sample size [132].
GENETIC RESEARCH:
Genetic testing can also provide new data. There have been attempts to locate the genes responsible for the diameter of the retinal vessels. So far, 8 single-nucleotide polymorphisms (SNPs) for CRVE and 2 for CRAE have been identified. Further research on their involvement in microvascular diseases might explain a predisposition to microcirculation disorders [133]. In addition, polymorphisms of ALK-1 and endoglin have been shown to be risk factors of cardiovascular events. Both ALK-1 and endoglin are transforming growth factor β1 (TGF-β1) receptors, which have a place in the regulation of BP and vascular homeostasis. In addition, it has been proven that TGF-β1 is involved in the thinning of the basal lamina of capillaries, a scaffolding for epithelium cells [134].
BIOMARKERS:
Serum uric acid (SUA) concentration is an independent risk factor for hypertension. As a large study on the Chinese population showed, it is also associated with hypertensive retinopathy. Elevated levels of SUA might lead to endothelial cell damage. In addition, the additional occurrence of high BP might have a negative cumulative effect on microcirculation and can be a mediator of vascular damage. It is possible that by reducing the SUA level, the risk of hypertensive retinopathy might be diminished [135]. Another substance, marinobufagenin, which is a biomarker of salt sensitivity that increases with salt intake, was found to be associated with reduced retinal microvascular artery dilatation in young normotensive adults. In addition, increased levels of marinobufagenin have been shown to promote endothelial damage and prevent BP reduction during the night, which is a physiological process [136].
ROLE OF INFLAMMATION:
There is significant emphasis on inflammation and its relationship with retinal microcirculation damage. The role of LMP10, a catalytic subunit of proteasome induced by interferon-γ with trypsin-like activity, has been emphasized. Ang II can cause upregulation of this subunit, and research on the mouse model and humans has shown its increased level and activity in the retina in hypertension. Moreover, knockout of LMP10 has attenuated the increase in retinal vascular permeability, remodeling, and inflammation. The inhibition of this subunit might be a potential therapeutic agent [137]. Another subunit of proteasome, β5i, has been shown to have a significant role in developing hypertensive retinopathy in the mouse model of hypertension. The β5i subunit decreases the stability of AT1R associated protein (ATRAP), which generally inhibits AT1R mediated retinopathy. Deletion of β5i alleviated the retinal thickness induced by Ang II, inflammation, oxidative stress, and remodeling. Overexpression tends to aggravate these effects [138]. A different study on the mouse model with hypertension has shown that G-protein coupled receptor 174 (GPR 174) plays a vital role in the regulation of the immune and inflammatory response. Knockout of the gene for this protein caused a decrease in vascular permeability and had a protective effect on the retina [139]. A recent study proposed glycoprotein acetyls (GlycA) as a new marker of chronic and cumulative inflammation. High GlycA levels were reported to be correlated with a wider venular caliber and narrower arteriolar caliber [140]. The association between GlycA and hypertensive retinopathy is yet to be examined.
ANIMAL MODELS:
Establishing a suitable experimental animal model for hypertensive retinopathy might contribute to significant progress in this field. Recently, a hypertensive BPH/2J mouse model was introduced that might be favorable for future diagnostics and research on retinal damage. It presents a similar hypertensive surge as that observed in humans [141]. A recent study on the well-known animal model of hypertension, spontaneously hypertensive rat (SHR), has shown that inhibition of an isoform of reactive oxygen-producing enzymes, NADPH oxidase (Nox), might prevent hypertension-related loss of vision. Inhibition of Nox 1 and Nox 4 had a protective effect on the BRB by the reduction of inflammation and the oxidative stress level. Inhibition of Nox 5 reduced retinal neovascularization and vascular permeability [142].
Conclusions
This review has shown that hypertensive retinopathy has a complex pathogenesis. Several damage processes might be involved with the probable essential role of neovascularization. Damage to the microvasculature may not be apparent on routine retinal examination. However, the combined use AO with OCT has begun to show how and when neovascularization may be a therapeutic target in hypertensive retinopathy.
Figures
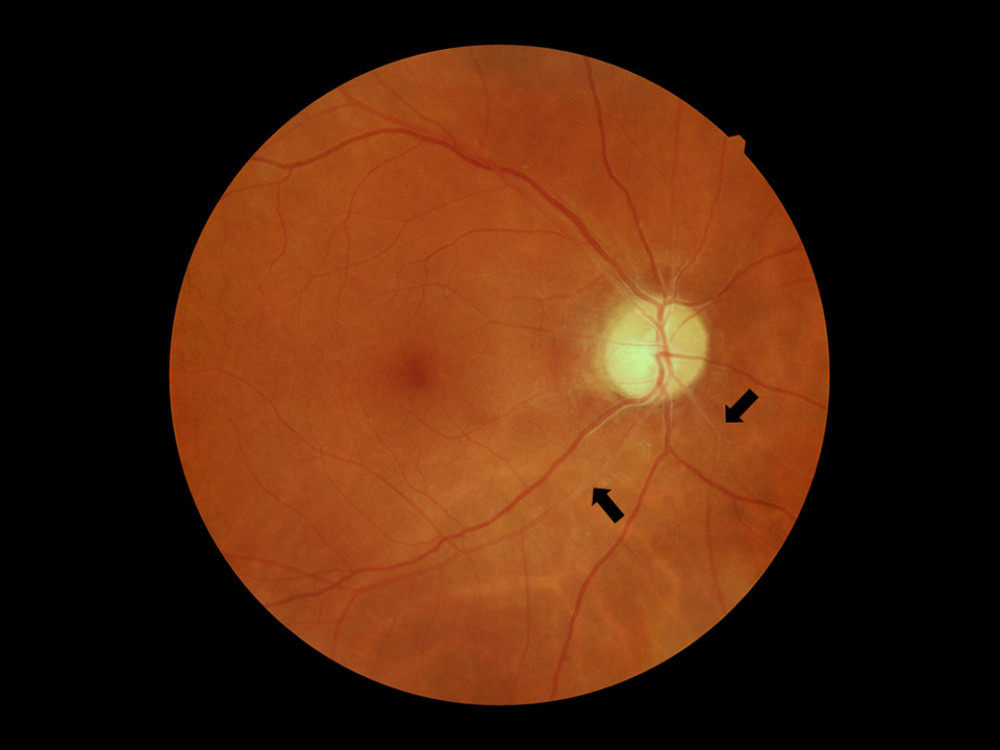 Figure 1. Patient with hypertensive retinopathy. Silver wiring sign (black arrows). Figure was prepared in Microsoft PowerPoint 2019 (Microsoft Corporation).
Figure 1. Patient with hypertensive retinopathy. Silver wiring sign (black arrows). Figure was prepared in Microsoft PowerPoint 2019 (Microsoft Corporation). 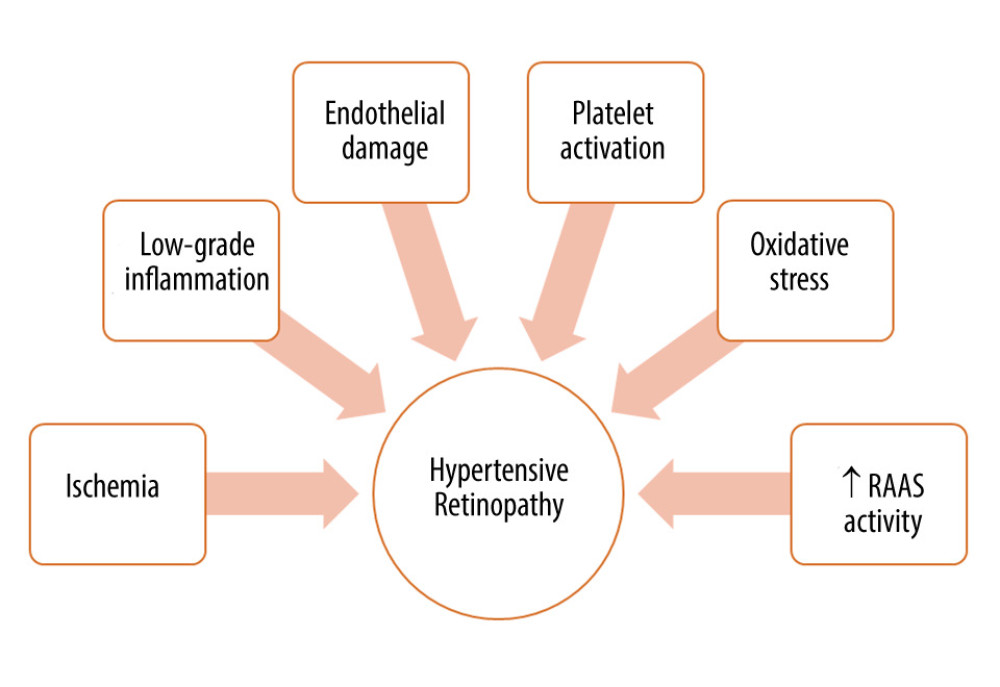 Figure 2. Factors involved in the pathogenesis of hypertensive retinopathy. RAAS – renin-angiotensin-aldosterone system. Figure was prepared in Microsoft PowerPoint 2019 (Microsoft Corporation).
Figure 2. Factors involved in the pathogenesis of hypertensive retinopathy. RAAS – renin-angiotensin-aldosterone system. Figure was prepared in Microsoft PowerPoint 2019 (Microsoft Corporation). 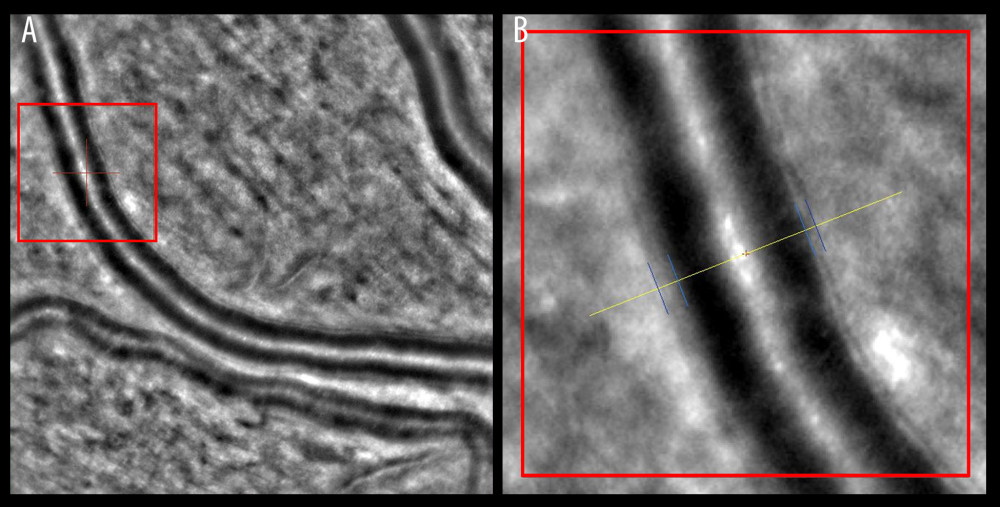 Figure 3. Wall-to-lumen ratio of patient without hypertension (0.240). (A) Image from rtx1 Adaptive Optics Retinal Camera. (B) Area in the red square enlarged. Figure was prepared in Microsoft PowerPoint 2019 (Microsoft Corporation).
Figure 3. Wall-to-lumen ratio of patient without hypertension (0.240). (A) Image from rtx1 Adaptive Optics Retinal Camera. (B) Area in the red square enlarged. Figure was prepared in Microsoft PowerPoint 2019 (Microsoft Corporation). 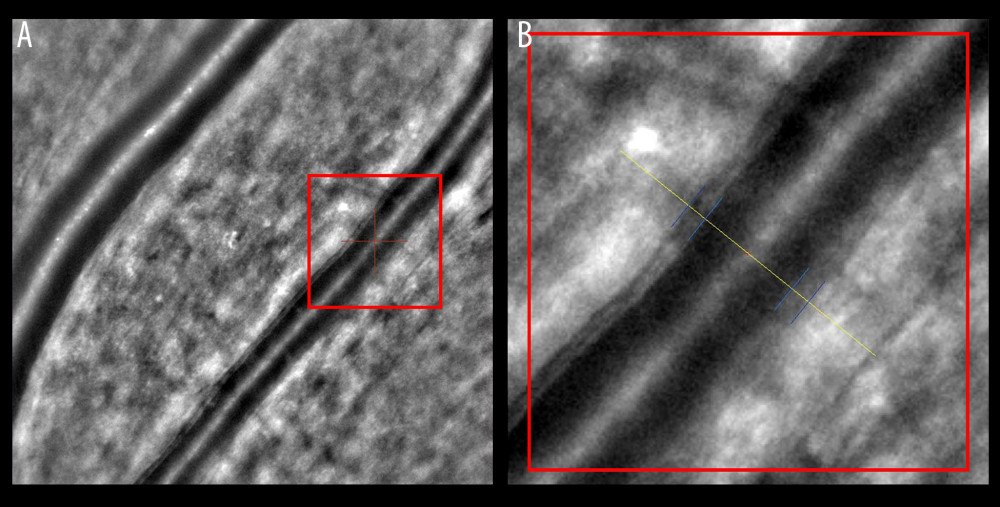 Figure 4. Increased wall-to-lumen ratio of patient with hypertension (0.370). (A) Image from rtx1 Adaptive Optics Retinal Camera. (B) Area in the red square enlarged. Figure was prepared in Microsoft PowerPoint 2019 (Microsoft Corporation).
Figure 4. Increased wall-to-lumen ratio of patient with hypertension (0.370). (A) Image from rtx1 Adaptive Optics Retinal Camera. (B) Area in the red square enlarged. Figure was prepared in Microsoft PowerPoint 2019 (Microsoft Corporation). References
1. Unger T, Borghi C, Charchar F, 2020 International Society of Hypertension Global Hypertension Practice Guidelines: Hypertension, 2020; 75(6); 1334-57
2. Dong Y, Song Y, Zou Z, Updates to pediatric hypertension guidelines: Influence on classification of high blood pressure in children and adolescents: J Hypertens, 2019; 37(2); 297-306
3. Mills KT, Bundy JD, Kelly TN, Global disparities of hypertension prevalence and control: A systematic analysis of population-based studies from 90 countries: Circulation, 2016; 134(6); 441-50
4. Whelton PK, Carey RM, Aronow WS, 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: Executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines: Hypertension, 2018; 71(6); 1269-324
5. Tabrizi JS, Sadeghi-Bazargani H, Farahbakhsh M, Prevalence and associated factors of prehypertension and hypertension in Iranian population: The Lifestyle Promotion Project (LPP): PLoS One, 2016; 11(10); e0165264
6. Bhargava M, Ikram MK, Wong TY, How does hypertension affect your eyes?: J Hum Hypertens, 2012; 26(2); 71-83
7. Varounis C, Katsi V, Nihoyannopoulos P, Cardiovascular hypertensive crisis: Recent evidence and review of the literature: Front Cardiovasc Med, 2017; 3; 51
8. Schmieder RE, End organ damage in hypertension: Dtsch Arztebl Int, 2010; 107(49); 866-73
9. Williams B, Mancia G, Spiering W, 2018 ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension: J Hypertens, 2018; 36(10); 1953-2041
10. Naser N, Dzubur A, Durak A, Blood pressure control in hypertensive patients, cardiovascular risk profile and the prevalence of masked uncontrolled hypertension (MUCH): Med Arch, 2016; 70(4); 274-79
11. Muiesan ML, Grassi G, Assessment of retinal vascular changes in hypertension: New perspectives: J Hypertens, 2006; 24(5); 813-14
12. Ong Y-T, Wong TY, Klein R, Hypertensive retinopathy and risk of stroke: Hypertension (Dallas, Tex: 1979), 2013; 62(4); 706-11
13. Wolffsohn JS, Hurcomb PG, Hypertension and the eye: Curr Hypertens Rep, 2002; 4(6); 471-76
14. Masaidi M, Cuspidi C, Giudici V, Is retinal arteriolar-venular ratio associated with cardiac and extracardiac organ damage in essential hypertension?: J Hypertens, 2009; 27(6); 1277-83
15. Aissopou EK, Papathanassiou M, Nasothimiou EG, The Keith-Wagener-Barker and Mitchell-Wong grading systems for hypertensive retinopathy: Association with target organ damage in individuals below 55 years: J Hypertens, 2015; 33(11); 2303-9
16. Wong TY, Mitchell P, Hypertensive retinopathy: N Engl J Med, 2004; 351(22); 2310-17
17. Porta M, Grosso A, Veglio F, Hypertensive retinopathy: There’s more than meets the eye: J Hypertens, 2005; 23(4); 683-96
18. Cheung CY, Ikram MK, Sabanayagam C, Wong TY, Retinal microvasculature as a model to study the manifestations of hypertension: Hypertension, 2012; 60(5); 1094-103
19. Ikram MK, Ong YT, Cheung CY, Wong TY, Retinal vascular caliber measurements: Clinical significance, current knowledge and future perspectives: Ophthalmologica, 2013; 229(3); 125-36
20. Tsukikawa M, Stacey AW, A Review of hypertensive retinopathy and chorioretinopathy: Clin Optom (Auckl), 2020; 12; 67-73
21. Ansari MW, Nadeem A, The blood supply to the eyeball: Atlas of Ocular Anatomy, 2016; 29-38
22. Riva CE, Pournaras CJ, Ocular circulation: Adler’s Physiology of the Eye, 2011; 243-45
23. Campbell JP, Zhang M, Hwang TS, Detailed vascular anatomy of the human retina by projection-resolved optical coherence tomography angiography: Sci Rep, 2017; 7; 42201
24. Henkind P, Radial peripapillary capillaries of the retina. I. Anatomy: human and comparative: Br J Ophthalmol, 1967; 51(2); 115-23
25. Provis JM, Development of the primate retinal vasculature: Prog Retin Eye Res, 2001; 20(6); 799-821
26. Sun Y, Smith LEH, Retinal vasculature in development and diseases: Annu Rev Vis Sci, 2018; 4; 101-22
27. Cunha-Vaz J, Bernardes R, Lobo C, Blood-retinal barrier: Eur J Ophthalmol, 2011; 21(Suppl 6); S3-9
28. Pournaras CJ, Rungger-Brändle E, Riva CE, Regulation of retinal blood flow in health and disease: Prog Retin Eye Res, 2008; 27(3); 284-330
29. Hubbard LD, Brothers RJ, King WN, Methods for evaluation of retinal microvascular abnormalities associated with hypertension/sclerosis in the Atherosclerosis Risk in Communities Study: Ophthalmology, 1999; 106(12); 2269-80
30. Sun C, Wang JJ, Mackey DA, Wong TY, Retinal vascular caliber: Systemic, environmental, and genetic associations: Surv Ophthalmol, 2009; 54(1); 74-95
31. Triantafyllou A, Anyfanti P, Gavriilaki E, Association between retinal vessel caliber and arterial stiffness in a population comprised of normotensive to early-stage hypertensive individuals: Am J Hypertens, 2014; 27(12); 1472-78
32. Wong TY, Klein R, Sharrett AR, Retinal arteriolar narrowing and risk of coronary heart disease in men and women. The Atherosclerosis Risk in Communities Study: JAMA, 2002; 287(9); 1153-59
33. Kawasaki R, Cheung N, Wang JJ, Retinal vessel diameters and risk of hypertension: The Multiethnic Study of Atherosclerosis: J Hypertens, 2009; 27(12); 2386-93
34. Katsi V, Marketou M, Vlachopoulos C, Impact of arterial hypertension on the eye: Curr Hypertens Rep, 2012; 14(6); 581-90
35. Cheung CY, Tay WT, Mitchell P, Quantitative and qualitative retinal microvascular characteristics and blood pressure: J Hypertens, 2011; 29(7); 1380-91
36. Liew G, Wang JJ, Rochtchina E, Complete blood count and retinal vessel calibers: PLoS One, 2014; 9(7); e102230
37. Ibrahim YW, Bots ML, Mulder PG, Number of perifoveal vessels in aging, hypertension, and atherosclerosis: The Rotterdam Study: Invest Ophthalmol Vis Sci, 1998; 39(6); 1049-53
38. Wong TY, Mitchell P, The eye in hypertension: Lancet, 2007; 369(9559); 425-35
39. Chatterjee S, Chattopadhyay S, Hope-Ross M, Lip PL, Hypertension and the eye: Changing perspectives: J Hum Hypertens, 2002; 16(10); 667-75
40. Pache M, Kube T, Wolf S, Kutschbach P, Do angiographic data support a detailed classification of hypertensive fundus changes?: J Hum Hypertens, 2002; 16(6); 405-10
41. Wang S, Xu L, Jonas JB, Major eye diseases and risk factors associated with systemic hypertension in an adult Chinese population: The Beijing Eye Study: Ophthalmology, 2009; 116(12); 2373-80
42. Ding J, Wai KL, McGeechan K, Retinal vascular caliber and the development of hypertension: A meta-analysis of individual participant data: J Hypertens, 2014; 32(2); 207-15
43. Ikram MK, Witteman JC, Vingerling JR, Retinal vessel diameters and risk of hypertension: The Rotterdam Study: Hypertension, 2006; 47(2); 189-94
44. Sasongko MB, Wong TY, Wang JJ, Retinal arteriolar changes: intermediate pathways linking early life exposures to cardiovascular disease?: Microcirculation, 2010; 17(1); 21-31
45. Chew SK, Xie J, Wang JJ, Retinal arteriolar diameter and the prevalence and incidence of hypertension: A systematic review and meta-analysis of their association: Curr Hypertens Rep, 2012; 14(2); 144-51
46. Hillard JG, Gast TJ, Chui TY, Retinal arterioles in hypo-, normo-, and hypertensive subjects measured using adaptive optics: Transl Vis Sci Technol, 2016; 5(4); 16
47. Karaca M, Coban E, Felek R, Unal M, The association of oxidative stress with hypertensive retinopathy: Clin Exp Hypertens, 2013; 35(1); 16-19
48. Coban E, Alkan E, Altuntas S, Akar Y, Serum ferritin levels correlate with hypertensive retinopathy: Med Sci Monit, 2010; 16(2); CR92-95
49. Coban E, Nizam I, Topal C, Akar Y, The association of low-grade systemic inflammation with hypertensive retinopathy: Clin Exp Hypertens, 2010; 32(8); 528-31
50. Newman A, Andrew N, Casson R, Review of the association between retinal microvascular characteristics and eye disease: Clin Exp Ophthalmol, 2018; 46(5); 531-52
51. Minuz P, Patrignani P, Gaino S, Determinants of platelet activation in human essential hypertension: Hypertension, 2004; 43(1); 64-70
52. Cohen JD, Overview of physiology, vascular biology, and mechanisms of hypertension: J Manag Care Pharm, 2007; 13(5 Suppl); S6-8
53. Goncharov NV, Nadeev AD, Jenkins RO, Avdonin PV, Markers and biomarkers of endothelium: When something is rotten in the state: Oxid Med Cell Longev, 2017; 2017; 9759735
54. Delles C, Michelson G, Harazny J, Impaired endothelial function of the retinal vasculature in hypertensive patients: Stroke, 2004; 35(6); 1289-93
55. Holappa M, Vapaatalo H, Vaajanen A, Many faces of renin-angiotensin system – focus on eye: Open Ophthalmol J, 2017; 11; 122-42
56. Vaajanen A, Luhtala S, Oksala O, Vapaatalo H, Does the renin-angiotensin system also regulate intra-ocular pressure?: Ann Med, 2008; 40(6); 418-27
57. Choudhary R, Kapoor MS, Singh A, Bodakhe SH, Therapeutic targets of renin-angiotensin system in ocular disorders: J Curr Ophthalmol, 2016; 29(1); 7-16
58. Vaajanen A, Kalesnykas G, Vapaatalo H, Uusitalo H, The expression of Mas-receptor of the renin-angiotensin system in the human eye: Graefes Arch Clin Exp Ophthalmol, 2015; 253(7); 1053-59
59. Vaajanen A, Vapaatalo H, Kautiainen H, Oksala O, Angiotensin (1–7) reduces intraocular pressure in the normotensive rabbit eye: Invest Ophthalmol Vis Sci, 2008; 49(6); 2557-62
60. Holappa M, Valjakka J, Vaajanen A, Angiotensin(1–7) and ACE2, “The Hot Spots” of renin-angiotensin system, detected in the human aqueous humor: Open Ophthalmol J, 2015; 9; 28-32
61. Reichhart N, Haase N, Crespo-Garcia S, Hypertensive retinopathy in a transgenic angiotensin-based model: Clin Sci (Lond), 2016; 130(13); 1075-88
62. Martins JR, Reichhart N, Kociok N, Systemic β adrenergic stimulation/sympathetic nerve system stimulation influences intraocular RAS through cAMP in the RPE: Exp Eye Res, 2019; 189; 107828
63. Campochiaro PA, Aiello LP, Rosenfeld PJ, Anti-vascular endothelial growth factor agents in the treatment of retinal disease: From bench to bedside: Ophthalmology, 2016; 123(10s); S78-88
64. Konstantinidis L, Guex-Crosier Y, Hypertension and the eye: Curr Opin Ophthalmol, 2016; 27(6); 514-21
65. Blann AD, Belgore FM, Constans J, Plasma vascular endothelial growth factor and its receptor Flt-1 in patients with hyperlipidemia and atherosclerosis and the effects of fluvastatin or fenofibrate: Am J Cardiol, 2001; 87(10); 1160-63
66. Felmeden DC, Blann AD, Lip GY, Angiogenesis: Basic pathophysiology and implications for disease: Eur Heart J, 2003; 24(7); 586-603
67. Tsai WC, Li YH, Huang YY, Plasma vascular endothelial growth factor as a marker for early vascular damage in hypertension: Clin Sci (Lond), 2005; 109(1); 39-43
68. Ferroni P, Della-Morte D, Palmirotta R, Angiogenesis and hypertension: The dual role of anti-hypertensive and anti-angiogenic therapies: Curr Vasc Pharmacol, 2012; 10(4); 479-93
69. Stryjewski TP, Papakostas TD, Vavvas D, Proliferative hypertensive retinopathy: JAMA Ophthalmol, 2016; 134(3); 345-46
70. van den Born BJ, Hulsman CA, Hoekstra JB, Value of routine funduscopy in patients with hypertension: Systematic review: BMJ, 2005; 331(7508); 73
71. MacGillivray TJ, Trucco E, Cameron JR, Retinal imaging as a source of biomarkers for diagnosis, characterization and prognosis of chronic illness or long-term conditions: Br J Radiol, 2014; 87(1040); 20130832
72. Maestri MM, Fuchs SC, Ferlin E, Detection of arteriolar narrowing in fundoscopic examination: Evidence of a low performance of direct ophthalmoscopy in comparison with a microdensitometric method: Am J Hypertens, 2007; 20(5); 501-5
73. Pakter HM, Ferlin E, Fuchs SC, Measuring arteriolar-to-venous ratio in retinal photography of patients with hypertension: Development and application of a new semi-automated method: Am J Hypertens, 2005; 18(3); 417-21
74. Wang JJ, Rochtchina E, Liew G, The long-term relation among retinal arteriolar narrowing, blood pressure, and incident severe hypertension: Am J Epidemiol, 2008; 168(1); 80-88
75. Koch E, Rosenbaum D, Brolly A, Morphometric analysis of small arteries in the human retina using adaptive optics imaging: Relationship with blood pressure and focal vascular changes: Journal of hypertension, 2014; 32(4); 890-98
76. Ponto KA, Werner DJ, Wiedemer L, Retinal vessel metrics: Normative data and their use in systemic hypertension: Results from the Gutenberg Health Study: J Hypertens, 2017; 35(8); 1635-45
77. Liew G, Wang JJ, Mitchell P, Wong TY, Retinal vascular imaging: A new tool in microvascular disease research: Circ Cardiovasc Imaging, 2008; 1(2); 156-61
78. Schuman JS, Spectral domain optical coherence tomography for glaucoma (an AOS thesis): Trans Am Ophthalmol Soc, 2008; 106; 426-58
79. Feng X, Wang H, Kong Y, Diagnosis of chronic stage of hypertensive retinopathy based on spectral domain optical coherence tomography: J Clin Hypertens (Greenwich), 2020; 22(7); 1247-52
80. Ahn SJ, Woo SJ, Park KH, Retinal and choroidal changes with severe hypertension and their association with visual outcome: Invest Ophthalmol Vis Sci, 2014; 55(12); 7775-85
81. Lee HM, Lee WH, Kim KN, Changes in thickness of central macula and retinal nerve fibre layer in severe hypertensive retinopathy: A 1-year longitudinal study: Acta Ophthalmol, 2018; 96(3); e386-92
82. Zheng Y, Cheung N, Aung T, Relationship of retinal vascular caliber with retinal nerve fiber layer thickness: The singapore malay eye study: Invest Ophthalmol Vis Sci, 2009; 50(9); 4091-96
83. Simsek EE, Kanar HS, Kanar BG, Can ocular OCT findings be as a predictor for end-organ damage in systemic hypertension?: Clin Exp Hypertens, 2020; 42(8); 733-37
84. Kashani AH, Chen C-L, Gahm JK, Optical coherence tomography angiography: A comprehensive review of current methods and clinical applications: Prog Retin Eye Res, 2017; 60; 66-100
85. Chua J, Chin CWL, Hong J, Impact of hypertension on retinal capillary microvasculature using optical coherence tomographic angiography: J Hypertens, 2019; 37(3); 572-80
86. Hua D, Xu Y, Zhang X, Retinal microvascular changes in hypertensive patients with different levels of blood pressure control and without hypertensive retinopathy: Curr Eye Res, 2021; 46(1); 107-14
87. Wang J, Jiang J, Zhang Y, Retinal and choroidal vascular changes in coronary heart disease: An optical coherence tomography angiography study: Biomed Opt Express, 2019; 10(4); 1532-44
88. Takayama K, Kaneko H, Ito Y, Novel classification of early-stage systemic hypertensive changes in human retina based on OCTA measurement of choriocapillaris: Sci Rep, 2018; 8(1); 15163
89. Hua D, Xu Y, Zeng X, Use of optical coherence tomography angiography for assessment of microvascular changes in the macula and optic nerve head in hypertensive patients without hypertensive retinopathy: Microvasc Res, 2020; 129; 103969
90. Burns SA, Elsner AE, Sapoznik KA, Adaptive optics imaging of the human retina: Prog Retin Eye Res, 2019; 68; 1-30
91. Mehta RA, Akkali MC, Jayadev C, Morphometric analysis of retinal arterioles in control and hypertensive population using adaptive optics imaging: Indian J Ophthalmol, 2019; 67(10); 1673-77
92. Streese L, Brawand LY, Gugleta K, New frontiers in noninvasive analysis of retinal wall-to-lumen ratio by retinal vessel wall analysis: Transl Vis Sci Technol, 2020; 9(6); 7
93. Meixner E, Michelson G, Measurement of retinal wall-to-lumen ratio by adaptive optics retinal camera: A clinical research: Graefes Arch Clin Exp Ophthalmol, 2015; 253(11); 1985-95
94. Park JB, Schiffrin EL, Small artery remodeling is the most prevalent (earliest?) form of target organ damage in mild essential hypertension: J Hypertens, 2001; 19(5); 921-30
95. Baleanu D, Ritt M, Harazny J, Wall-to-lumen ratio of retinal arterioles and arteriole-to-venule ratio of retinal vessels in patients with cerebrovascular damage: Invest Ophthalmol Vis Sci, 2009; 50(9); 4351-59
96. Klig JE, Ophthalmologic complications of systemic disease: Emerg Med Clin North Am, 2008; 26(1); 217-31
97. Hughes AD, Stanton AV, Jabbar AS, Effect of antihypertensive treatment on retinal microvascular changes in hypertension: J Hypertens, 2008; 26(8); 1703-7
98. Jumar A, Harazny JM, Ott C, Improvement in retinal capillary rarefaction after valsartan treatment in hypertensive patients: J Clin Hypertens (Greenwich), 2016; 18(11); 1112-18
99. Levy BI, Ambrosio G, Pries AR, Struijker-Boudier HA, Microcirculation in hypertension: A new target for treatment?: Circulation, 2001; 104(6); 735-40
100. Abegaz TM, Shehab A, Gebreyohannes EA, Nonadherence to antihypertensive drugs: A systematic review and meta-analysis: Medicine, 2017; 96(4); e5641
101. Strachan MW, McKnight JA, Images in clinical medicine. Improvement in hypertensive retinopathy after treatment of hypertension: N Engl J Med, 2005; 352(18); e17
102. Wong W, Gopal L, Yip CC, Hypertensive retinopathy and choroidopathy: CMAJ, 2020; 192(14); E371
103. Banerjee A, Nayak B, Verma G, Parija S, Resolution of grade IV hypertensive retinopathy in an adult with pheochromocytoma: Post-tumor resection: BMJ Case Rep, 2020; 13(2); e231245
104. Traustason S, Hardarson SH, Gottfredsdottir MS, Dorzolamide-timolol combination and retinal vessel oxygen saturation in patients with glaucoma or ocular hypertension: Br J Ophthalmol, 2009; 93(8); 1064-67
105. Wang J, Brown C, Shi C, Improving diabetic and hypertensive retinopathy with a medical food containing L-methylfolate: A preliminary report: Eye Vis (Lond), 2019; 6; 21
106. Jose V, Radhakrishna S, Pipalava P, Singh I, Bevacizumab for eye diseases – legal, regulatory, and ethical overview: Indian J Pharmacol, 2019; 51(6); 377-83
107. Kim EY, Lew HM, Song JH: J Ocul Pharmacol Ther, 2012; 28(3); 318-22
108. Salman AG, Intravitreal bevacizumab in persistent retinopathy secondary to malignant hypertension: Saudi J Ophthalmol, 2013; 27(1); 25-29
109. Al-Halafi AM, Tremendous result of bevacizumab in malignant hypertensive retinopathy: Oman J Ophthalmol, 2015; 8(1); 61-63
110. Padhy S, Kumar V, Dramatic response to intravitreal Bevacizumab in hypertensive retinopathy: Indian J Ophthalmol, 2018; 66(10); 1494-95
111. Georgiadis O, Kabanarou SA, Batsos G, Bilateral hypertensive retinopathy complicated with retinal neovascularization: Panretinal photocoagulation or intravitreal anti-VEGF treatment?: Case Rep Ophthalmol, 2014; 5(2); 231-38
112. Wong TY, McIntosh R, Hypertensive retinopathy signs as risk indicators of cardiovascular morbidity and mortality: Br Med Bull, 2005; 73–74; 57-70
113. Wang JJ, Liew G, Klein R, Retinal vessel diameter and cardiovascular mortality: Pooled data analysis from two older populations: Eur Heart J, 2007; 28(16); 1984-92
114. Liew G, Wang JJ, Retinal vascular signs: A Window to the heart?: Rev Esp Cardiol, 2011; 64(6); 515-21
115. McGeechan K, Liew G, Macaskill P, Meta-analysis: Retinal vessel caliber and risk for coronary heart disease: Ann Intern Med, 2009; 151(6); 404-13
116. Cuspidi C, Meani S, Salerno M, Retinal microvascular changes and target organ damage in untreated essential hypertensives: J Hypertens, 2004; 22(11); 2095-102
117. Cuspidi C, Negri F, Giudici V, Sala C, Retinal changes and cardiac remodelling in systemic hypertension: Ther Adv Cardiovasc Dis, 2009; 3(3); 205-14
118. Ali F, Tacey M, Lykopandis N, Microvascular narrowing and BP monitoring: A single centre observational study: PLoS One, 2019; 14(3); e0210625
119. Meazza R, Scardino C, Grosso Di Palma L, Target organ damage in hypertensive patients: correlation between retinal arteriovenular ratio and left ventricular geometric patterns: J Hum Hypertens, 2014; 28(4); 274-78
120. Rim TH, Teo AWJ, Yang HHS, Retinal vascular signs and cerebrovascular diseases: J Neuroophthalmol, 2020; 40(1); 44-59
121. Mitchell P, Wang JJ, Wong TY, Retinal microvascular signs and risk of stroke and stroke mortality: Neurology, 2005; 65(7); 1005-9
122. Heringa SM, Bouvy WH, van den Berg E, Associations between retinal microvascular changes and dementia, cognitive functioning, and brain imaging abnormalities: A systematic review: J Cereb Blood Flow Metab, 2013; 33(7); 983-95
123. Chillo P, Ismail A, Sanyiwa A, Hypertensive retinopathy and associated factors among nondiabetic chronic kidney disease patients seen at a tertiary hospital in Tanzania: A cross-sectional study: Int J Nephrol Renovasc Dis, 2019; 12; 79-86
124. Farrah TE, Dhillon B, Keane PA, The eye, the kidney, and cardiovascular disease: Old concepts, better tools, and new horizons: Kidney Int, 2020; 98(2); 323-42
125. Vadalà M, Castellucci M, Guarrasi G, Retinal and choroidal vasculature changes associated with chronic kidney disease: Graefes Arch Clin Exp Ophthalmol, 2019; 257(8); 1687-98
126. Alan G, Guenancia C, Arnould L, Retinal vascular density as a novel biomarker of acute renal injury after acute coronary syndrome: Sci Rep, 2019; 9(1); 8060
127. Vaes AW, Spruit MA, Van Keer K, Structural analysis of retinal blood vessels in patients with COPD during a pulmonary rehabilitation program: Sci Rep, 2020; 10(1); 31
128. Alrashdi SF, Deliyanti D, Wilkinson-Berka JL, Intravitreal administration of endothelin type A receptor or endothelin type B receptor antagonists attenuates hypertensive and diabetic retinopathy in rats: Exp Eye Res, 2018; 176; 1-9
129. Zhang Y, Zhao L, Li H, Wang Y, Risk factors for hypertensive retinopathy in a Chinese population with hypertension: The Beijing Eye study: Exp Ther Med, 2019; 17(1); 453-58
130. Cabral T, Mello LGM, Lima LH, Retinal and choroidal angiogenesis: A review of new targets: Int J Retina Vitreous, 2017; 3; 31
131. Ramírez-Montero C, Lima-Gómez V, Anguiano-Robledo L, Preeclampsia as predisposing factor for hypertensive retinopathy: Participation by the RAAS and angiogenic factors: Exp Eye Res, 2020; 193; 107981
132. Grus FH, Joachim SC, Pfeiffer N, Proteomics in ocular fluids: Proteomics Clin Appl, 2007; 1(8); 876-88
133. Jensen RA, Sim X, Smith AV, Novel genetic loci associated with retinal microvascular diameter: Circ Cardiovasc Genet, 2016; 9(1); 45-54
134. Garzon-Martinez M, Perretta-Tejedor N, Garcia-Ortiz L, Association of Alk1 and endoglin polymorphisms with cardiovascular damage: Sci Rep, 2020; 10(1); 9383
135. Chen X, Meng Y, Li J, Serum uric acid concentration is associated with hypertensive retinopathy in hypertensive chinese adults: BMC Ophthalmol, 2017; 17(1); 83
136. Strauss-Kruger M, Smith W, Wei W, Microvascular function in non-dippers: Potential involvement of the salt sensitivity biomarker, marinobufagenin-The African-PREDICT study: J Clin Hypertens (Greenwich), 2020; 22(1); 86-94
137. Wang S, Li J, Bai J, The immunoproteasome subunit LMP10 mediates angiotensin II-induced retinopathy in mice: Redox Biol, 2018; 16; 129-38
138. Wang S, Li J, Wang T, Ablation of immunoproteasome β5i subunit suppresses hypertensive retinopathy by blocking ATRAP degradation in mice: Mol Ther, 2020; 28(1); 279-92
139. Yue J, Zhao X, GPR174 suppression attenuates retinopathy in angiotensin II (Ang II)-treated mice by reducing inflammation via PI3K/AKT signaling: Biomed Pharmacother, 2020; 122; 109701
140. Liu M, Lycett K, Moreno-Betancur M, Inflammation mediates the relationship between obesity and retinal vascular calibre in 11–12 year-olds children and mid-life adults: Sci Rep, 2020; 10(1); 5006
141. Herat LY, Magno AL, Kiuchi MG, The Schlager mouse as a model of altered retinal phenotype: Neural Regen Res, 2020; 15(3); 512-18
142. Deliyanti D, Alrashdi Saeed F, Touyz Rhian M, Nox (NADPH Oxidase) 1, Nox4, and Nox5 promote vascular permeability and neovascularization in retinopathy: Hypertension, 2020; 75(4); 1091-101
Figures
 Figure 1. Patient with hypertensive retinopathy. Silver wiring sign (black arrows). Figure was prepared in Microsoft PowerPoint 2019 (Microsoft Corporation).
Figure 1. Patient with hypertensive retinopathy. Silver wiring sign (black arrows). Figure was prepared in Microsoft PowerPoint 2019 (Microsoft Corporation). Figure 2. Factors involved in the pathogenesis of hypertensive retinopathy. RAAS – renin-angiotensin-aldosterone system. Figure was prepared in Microsoft PowerPoint 2019 (Microsoft Corporation).
Figure 2. Factors involved in the pathogenesis of hypertensive retinopathy. RAAS – renin-angiotensin-aldosterone system. Figure was prepared in Microsoft PowerPoint 2019 (Microsoft Corporation). Figure 3. Wall-to-lumen ratio of patient without hypertension (0.240). (A) Image from rtx1 Adaptive Optics Retinal Camera. (B) Area in the red square enlarged. Figure was prepared in Microsoft PowerPoint 2019 (Microsoft Corporation).
Figure 3. Wall-to-lumen ratio of patient without hypertension (0.240). (A) Image from rtx1 Adaptive Optics Retinal Camera. (B) Area in the red square enlarged. Figure was prepared in Microsoft PowerPoint 2019 (Microsoft Corporation). Figure 4. Increased wall-to-lumen ratio of patient with hypertension (0.370). (A) Image from rtx1 Adaptive Optics Retinal Camera. (B) Area in the red square enlarged. Figure was prepared in Microsoft PowerPoint 2019 (Microsoft Corporation).
Figure 4. Increased wall-to-lumen ratio of patient with hypertension (0.370). (A) Image from rtx1 Adaptive Optics Retinal Camera. (B) Area in the red square enlarged. Figure was prepared in Microsoft PowerPoint 2019 (Microsoft Corporation). In Press
07 Mar 2024 : Clinical Research
Knowledge of and Attitudes Toward Clinical Trials: A Questionnaire-Based Study of 179 Male Third- and Fourt...Med Sci Monit In Press; DOI: 10.12659/MSM.943468
08 Mar 2024 : Animal Research
Modification of Experimental Model of Necrotizing Enterocolitis (NEC) in Rat Pups by Single Exposure to Hyp...Med Sci Monit In Press; DOI: 10.12659/MSM.943443
18 Apr 2024 : Clinical Research
Comparative Analysis of Open and Closed Sphincterotomy for the Treatment of Chronic Anal Fissure: Safety an...Med Sci Monit In Press; DOI: 10.12659/MSM.944127
08 Mar 2024 : Laboratory Research
Evaluation of Retentive Strength of 50 Endodontically-Treated Single-Rooted Mandibular Second Premolars Res...Med Sci Monit In Press; DOI: 10.12659/MSM.944110
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








