24 August 2022: Clinical Research
Risk Factors and Pathogen Spectrum in Continuous Ambulatory Peritoneal Dialysis-Associated Peritonitis: A Single Center Retrospective Study
Supei Yin1ABCDEFG, Ming Tang1ABCDE, Zhengsheng Rao1ABCD, Ximing Chen1ABDE, Mengjuan Zhang1ABCDEFG, Ling Liu1ABCDE, Keqin Zhang1ABCDEFG*DOI: 10.12659/MSM.937112
Med Sci Monit 2022; 28:e937112
Abstract
BACKGROUND: To investigate the incidence, risk factors, pathogen distribution, and drug resistance patterns in continuous ambulatory peritoneal dialysis-associated peritonitis (CAPDP).
MATERIAL AND METHODS: Clinical data for 248 patients who underwent continuous ambulatory peritoneal dialysis (CAPD) treatment in a single center in China from March 2018 to January 2021 were retrospectively collected. The patients were divided into the CAPDP group (n=40) and the non-CAPDP group (n=208) according to whether peritonitis occurred. The incidence rate, risk factors, bacterial distribution, and drug sensitivity of CAPDP were analyzed.
RESULTS: The incidence of CAPDP was 16.13%, and 87.5% of patients with CAPDP continued CAPDP treatment after anti-infection treatment. Patients with and without CAPDP were clearly distinguished, on the basis of their clinical characteristics, by using principal component analysis (PCA) methods. Logistic regression analysis found that body mass index (BMI; P=0.0095), albumin (P=0.016), albumin/globulin ratio (P=0.018), C-reactive protein (P=0.0001), and rapid transport (P=0.034) were independent risk factors for CAPDP. The main pathogens causing the CAPDP were Staphylococcus epidermidis (50.00%), Staphylococcus capitis (13.33%), and Escherichia coli (10.00%). Among the pathogenic bacteria, the main drugs to which gram-negative cocci were sensitive were imipenem, meropenem, piperacillin/tazobactam, cefoperazone/sulbactam, ceftazidime, and tigecycline. The main drugs to which gram-positive cocci were sensitive were vancomycin, teicoplanin, and linezolid. The drug resistance rate of pathogenic bacteria to penicillin G, ampicillin, compound trimethoprim, cefepime, ceftriaxone, and amoxicillin-clavulanic acid drugs was 36.26-100%.
CONCLUSIONS: BMI, albumin, albumin/globulin ratio, C-reactive protein, and rapid transport are independent risk factors for CAPDP. Gram-positive bacteria are the main pathogens of CAPDP and are sensitive to vancomycin, teicoplanin, and linezolid.
Keywords: Peritoneal Dialysis, peritonitis, Risk Factors, Anti-Bacterial Agents, Bacteria, C-Reactive Protein, Drug Resistance, Bacterial, globulins, Humans, linezolid, Microbial Sensitivity Tests, Peritoneal Dialysis, Continuous Ambulatory, Teicoplanin, Vancomycin
Background
Current research points out that continuous ambulatory peritoneal dialysis (CAPD) is one of the main alternative therapies for patients with end-stage renal disease, which has proven to be an important method for peritoneal dialysis (PD) [1–3]. CAPD has obvious advantages in reducing the occurrence of peritonitis, improving the adequacy of dialysis, protecting residual renal function, and improving the quality of life of patients [2,3].
Peritoneal dialysis-associated peritonitis is an important complication of PD that can cause nearly 20% of PD failures and 3.5–10.0% of patient deaths [4–7]. With the development of dialysis equipment, disinfection measures, and increased awareness of aseptic practice, the infection rate of continuous ambulatory peritoneal dialysis-associated peritonitis (CAPDP) patients has decreased significantly [4–7]. However, CAPDP is still an independent risk factor that affects the efficacy and mortality of CAPD patients [4–7]. CAPDP can lead to an increase in hospitalization rate, loss of peritoneal function, and decline of residual renal function, which seriously affects the quality of life of patients [4–7]. CAPDP is also the direct and main reason for patients to withdraw from PD treatment for a short time or permanently [4–7]. Studies have shown that for every 0.5 times/year increase in the incidence of CAPDP, the risk of death increases by 4% to 11% [4–9]. At the same time, in recent years, with the wide application of antibiotics, antibiotic resistance in pathogens and, consequently, difficulty in clinical treatment of CAPDP have increased significantly [8,9]. Systematic research on the risk factors for CAPDP, along with the characteristics of the pathogenic bacteria seen in CAPDP and their drug resistance, is of great significance for preventing the occurrence of CAPDP and guiding clinical drug use. Therefore, this retrospective study from a single center in China aimed to study the factors associated with the development of peritonitis and the causes of infection in patients treated with CAPD between 2018 and 2021.
Material and Methods
PATIENT SELECTION AND CLINICAL DATA:
A retrospective study design was conducted in the current investigation. Sample size was calculated using the website (http://powerandsamplesize.com/Calculators/) in line with the Strengthening the Reporting of Observational Studies in Epidemiology (STROPE) statement for observational studies. The parameter setting of the calculated sample size needed to test the odds ratio was the following: power (1 – β, 90%), type I error (α, 0.05), and odds ratio (OR, 1.3). The sample sizes necessary for the main factor were 285. Clinical data from 248 patients who underwent CAPD in our hospital from March 2018 to January 2021 were retrospectively collected for analysis. The patients were divided into the CAPDP group (n=40) and the non-CAPDP group (n=208) according to whether peritoneal dialysis-associated peritonitis (PDAP) occurred. The inclusion criteria were as follows: 1) patients who were followed up for CAPD; 2) patients who were older than 18 years old; 3) patients who met the inclusion criteria for chronic renal failure [10]; 4) patients who met the indications for PD; 5) patients who use CAPD for PD. Bearing in mind that the main purpose of exclusion is to avoid factors that may cause bias and error in the research results, the exclusion criteria included the following: 1) peritonitis caused by other diseases, such as gastrointestinal perforation; 2) patients who cannot cooperate with follow-up and use of CAPD; 3) People with hematological diseases prone to bleeding, such as leukemia, lymphoma, hemophilia, aplastic anemia; 4) patients who have mental system diseases and cannot complete CAPD. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. All patients voluntarily participated in this study and signed an informed consent agreement. This study was approved by the Committee of The Second Affiliated Hospital of Chongqing Medical University and was carried out in accordance with the Declaration of Helsinki.
CAPDP DIAGNOSTIC CRITERIA AND EFFICACY JUDGEMENT:
The diagnosis of CAPDP is based on the 2016 International Society for Peritoneal Dialysis standards, and CAPDP was diagnosed if it met 2 of the following 3 criteria: 1) presence of symptoms and signs of peritonitis, turbid dialysate, and/or abdominal pain/fever; 2) white blood cell count in the exudate >100×106/L and the proportion of neutrophils >50%; and 3) positive culture of PD fluid [11]. Criteria for treatment outcomes included: 1) cure: clinical symptoms are completely relieved, laboratory indicators return to normal, no need to remove the PD catheter, no antibiotic treatment, and no recurrence within 30 days; 2) recurrence: peritonitis that recurs within 4 weeks after the peritonitis is cured, with the same pathogenic bacteria, or peritonitis with a negative culture; 3) withdrawal: the peritonitis leads to removal of the PD catheter, transfer to hemodialysis, and cessation of treatment; 4) death: the patient dies of active peritonitis or dies within 2 weeks of the onset of peritonitis [11,12].
BACTERIAL CULTURE, IDENTIFICATION AND DRUG SENSITIVITY TESTING METHODS:
All the peritoneal fluid samples were collected when patients were admitted to the hospital and sent to the laboratory of the hospital for routine examination and bacterial culture. Bacterial culture was performed with a Bact/ALERT3D360 automatic culture instrument from the French Mérieux Company (Lyon, France). The identification of positive specimens and drug susceptibility tests were completed by the VITEK 2 COMPACT automatic microbial identification system of the French Mérieux Company (Lyon, France). The results were determined in accordance with the American National Clinical Laboratory Standards.
CLINICAL DATA COLLECTION:
The clinical data for the CAPDP and non-CAPDP groups were collected for analysis immediately after admission. The parameters that were compared and analyzed included sex, age, primary disease, education level, body mass index (BMI), catheterization time, complications, routine blood tests, liver and kidney function, blood glucose, blood lipids, electrolytes, transport type, pathogenic bacteria culture results, drug sensitivity test results, and prognosis.
STATISTICAL ANALYSIS:
SPSS 20.0 software was used for statistical analysis of the data. The Kolmogorov-Smirnov single sample test was used to calculate the normal distribution of continuous variables before performing further comparisons. Normally distributed measurement data were expressed as the mean±standard deviation, and comparisons between groups were performed by
Results
CLINICAL DATA AND OUTCOME ANALYSIS OF PATIENTS WITH CAPDP:
A total of 248 patients with CAPD were included in the current study, including 40 patients in the CAPDP group and 208 patients in the non-CAPDP group. The incidence of CAPDP was 16.13%. In the CAPDP group, 35 patients (87.5%) with CAPDP were able to continue on CAPD after treatment of the infection, and 5 (12.5%) patients withdrew from treatment due to poor infection control. There was no death or recurrence. As shown in Table 1, single factor analysis showed that there were no significant differences in age, sex, education level, diabetes, primary disease, alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, total bilirubin, direct bilirubin, uric acid, blood creatinine, urea, blood lipids, blood potassium, blood calcium, blood sodium, blood chlorine, total bile acid, unconjugated bilirubin, white blood cell count, red blood cell count, hemoglobin concentration, percentage of neutrophils, neutrophil count, lymphocyte percentage, or parathyroid hormone between the CAPDP group and the non-CAPDP group (P>0.05). There were significant differences in BMI (19.74±2.22 vs 21.79±2.96, P<0.001), albumin (33.81±4.19 vs 37.89±5.02, P=0.008), albumin/globulin ratio (1.42±0.29 vs 1.79±0.74, P=0.012), platelet count (132.49±53.67 vs 170.75±69.90, P=0.031), C-reactive protein (13.01±8.82 vs 5.60±4.95, P<0.001), and rapid transport (32.50% vs 12.98%, P=0.014) between the CAPDP group and the non-CAPDP group.
LOGISTIC REGRESSION ANALYSIS OF RISK FACTORS IN PATIENTS WITH CAPDP:
As shown in Table 2, both univariate and multivariate analysis found that BMI, albumin, albumin/globulin ratio, C-reactive protein, and rapid transport are independent risk factors for CAPDP. Multivariate logistic regression analysis found that BMI (β=−0.5919, OR=0.554, P=0.0095), albumin (β=−0.474, OR=0.985, P=0.016), albumin/globulin ratio (β=−3.253, OR=0.874, P=0.018), C-reactive protein (β=0.294, OR=1.342, P=0.0001), and rapid transport (β=−2.134, OR=0.876, P=0.034) were independent risk factors for CAPDP.
PCA AND RANDOM FOREST ANALYSIS OF RISK FACTORS IN PATIENTS WITH CAPDP:
Except for logistical regression analysis, the unsupervised learning method of PCA was employed to distinguish CAPDP from non-CAPDP patients. However, we could not find any effects that were discernable with such methods (Figure 1). Random forest, another frequently used classification algorithm, was employed in the current data analysis. The classification accuracy of the random forest method depends on user-defined parameters N and m, where N=100 and m=3 are selected for optimal CAPDP and non-CAPDP classification. The out-of-bag estimate of error rate 0.34 was used to measure the variance importance of patients who developed CAPDP. Mean decreased accuracy and Gini’s test are shown in Figure 2A and 2B. The most important source of variance for detecting patients who developed CAPDP was C-reactive protein. The analysis revealed that, at the optimal cut-off value of 1.5 for the random forest model, the sensitivity was 92.5% and the specificity was 98.3%, with an area under the curve (AUC) of 0.929 (95% confidence interval, 0.857–1) (Figure 2C).
ANALYSIS OF BACTERIAL DISTRIBUTION AND DRUG RESISTANCE RATE IN PATIENTS WITH CAPDP:
As shown in Table 3, specimens from 40 patients with CAPDP were submitted for examination, of which 30 specimens were positive for infection (the positive rate was 75.00%). In the present study, multiple infections and repeated infections were not found. Twenty-one positive specimens (70.00%) were infected with gram-positive bacteria, 8 positive specimens (26.67%) were infected with gram-negative bacteria, and 1 positive specimen (3.33%) was infected with a fungus (Aspergillus). Among the positive strains, Staphylococcus epidermidis (50.00%), Staphylococcus capitis (13.33%), and Escherichia coli (10.00%) were the main species. As shown in Table 4, in the drug sensitivity analysis of pathogenic bacteria, we found that the main drugs to which the gram-negative bacteria were sensitive were imipenem, meropenem, piperacillin/tazobactam, cefoperazone/sulbactam sodium, and ceftazidime, and the main drugs to which the gram-positive bacteria were sensitive were vancomycin, teicoplanin, linezolid, and tigecycline. In this study, patients with CAPDP showed strong resistance to penicillin G, ampicillin, compound trimethoprim, cefepime, ceftriaxone, amoxicillin-clavulanic acid, and other drugs (the resistance rate was 36.26–100%).
Discussion
In this study, we found that the incidence of CAPDP was 16.13%. Logistic regression analysis found that BMI, albumin, albumin/globulin ratio, C-reactive protein, and rapid transport were independent risk factors for CAPDP. Gram-positive bacteria, especially
Previous studies have shown that risk factors for peritonitis associated with PD include hypoalbuminemia, nasal
Pathogen detection is the key to guiding clinical PDAP treatment, and the proportion of culture-negative peritonitis, according to International Society for Peritoneal Dialysis (ISPD) guidelines, should not exceed 20% [29]. In the present study, the positive detection rate for pathogenic bacteria was 75.00%, which is slightly lower than the recommendations in the ISPD guidelines. In terms of the spectrum of pathogenic bacteria, this study found that gram-positive bacteria accounted for 70.00% of the CAPDP, gram-negative bacteria accounted for 26.67% of the CAPDP, and fungi accounted for 3.33% of the CAPDP. The most important pathogenic bacteria in this PD center are gram-positive bacteria, and
The present study has certain limitations. First, our study is a retrospective study, and the study design has inherent limitations and biases. Second, our study was a small-sample, single-center study, and the research conclusions therefore have limitations. Large-sample, multicenter studies are needed to confirm our results. Third, due to differences in the underlying disease, population, and living environment, the results of our study are subject to inherent errors and biases. Fourth, the observation time of our study was short, and long-term studies with large samples are needed to further explore the present findings. Fifth, our study has inherent limitations and risks of bias in research design.
Conclusions
The present study found that BMI, albumin, albumin/globulin ratio, C-reactive protein, and rapid transport are independent risk factors for CAPDP. Gram-positive bacteria are the main pathogens seen in CAPDP and are sensitive to vancomycin, teicoplanin, and linezolid.
Figures
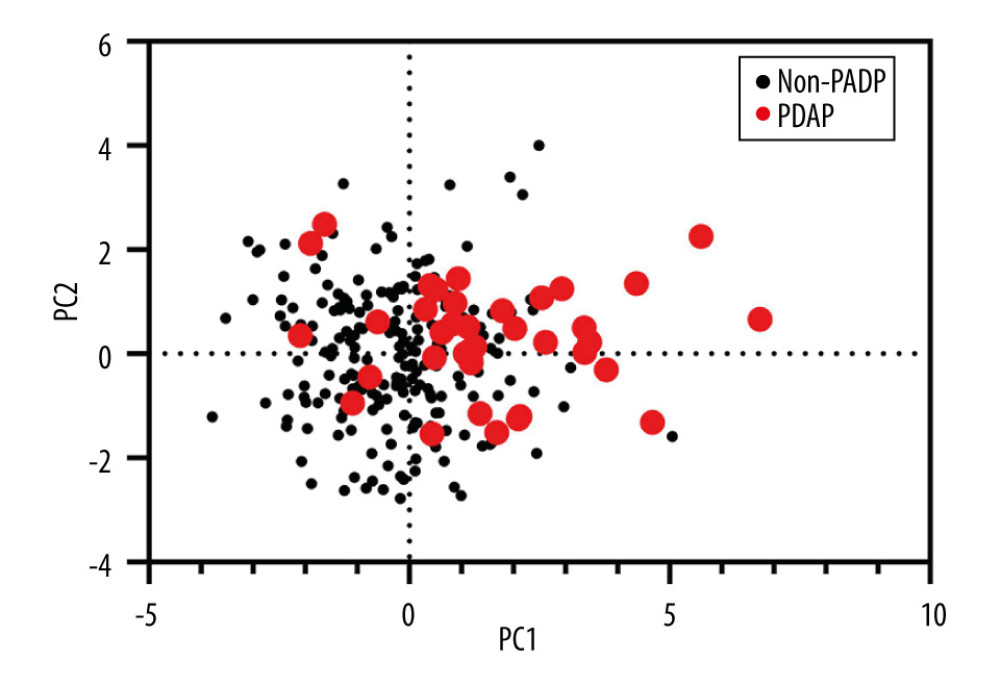 Figure 1. Principal component analysis (PCA) of clinical characteristics between patients with peritoneal dialysis-associated peritonitis (PDAP) and without PDAP.
Figure 1. Principal component analysis (PCA) of clinical characteristics between patients with peritoneal dialysis-associated peritonitis (PDAP) and without PDAP. 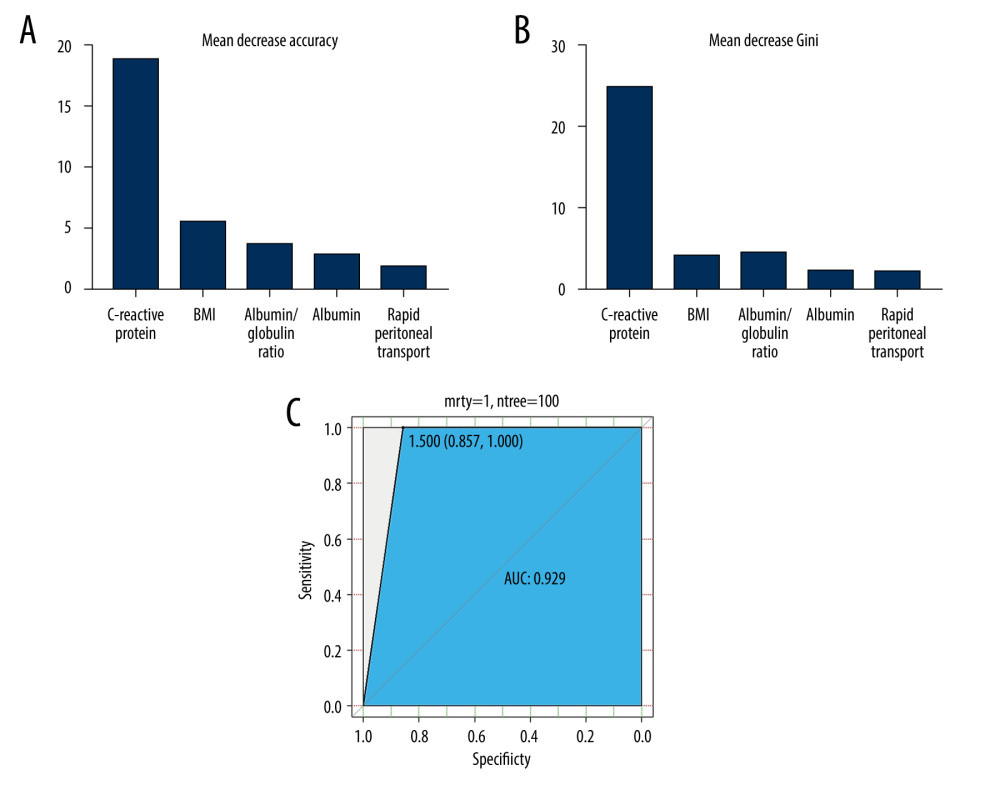 Figure 2. (A) Mean decrease in accuracy in predicting the occurrence of peritoneal dialysis-associated peritonitis (PDAP) when using the random forest model. (B) Mean decrease in the Gini coefficient in predicting the occurrence of PDAP when using the random forest model. (C) The results of the receiver operating characteristic curve (ROC) analysis, using random forest prediction value to detect the occurrence of PDAP.
Figure 2. (A) Mean decrease in accuracy in predicting the occurrence of peritoneal dialysis-associated peritonitis (PDAP) when using the random forest model. (B) Mean decrease in the Gini coefficient in predicting the occurrence of PDAP when using the random forest model. (C) The results of the receiver operating characteristic curve (ROC) analysis, using random forest prediction value to detect the occurrence of PDAP. Tables
Table 1. Comparative analysis of clinical data for patients with automated peritoneal dialysis-associated peritonitis. Table 2. Logistic regression analysis of risk factors in patients with continuous ambulatory peritoneal dialysis-associated peritonitis.
Table 2. Logistic regression analysis of risk factors in patients with continuous ambulatory peritoneal dialysis-associated peritonitis.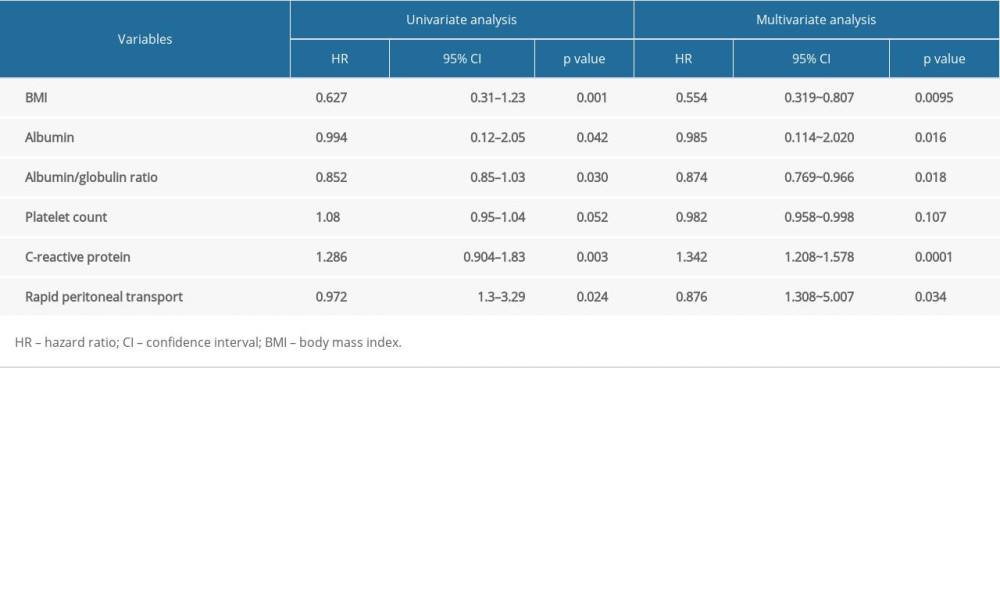 Table 3. Bacteria culture and distribution of peritoneal fluid.
Table 3. Bacteria culture and distribution of peritoneal fluid.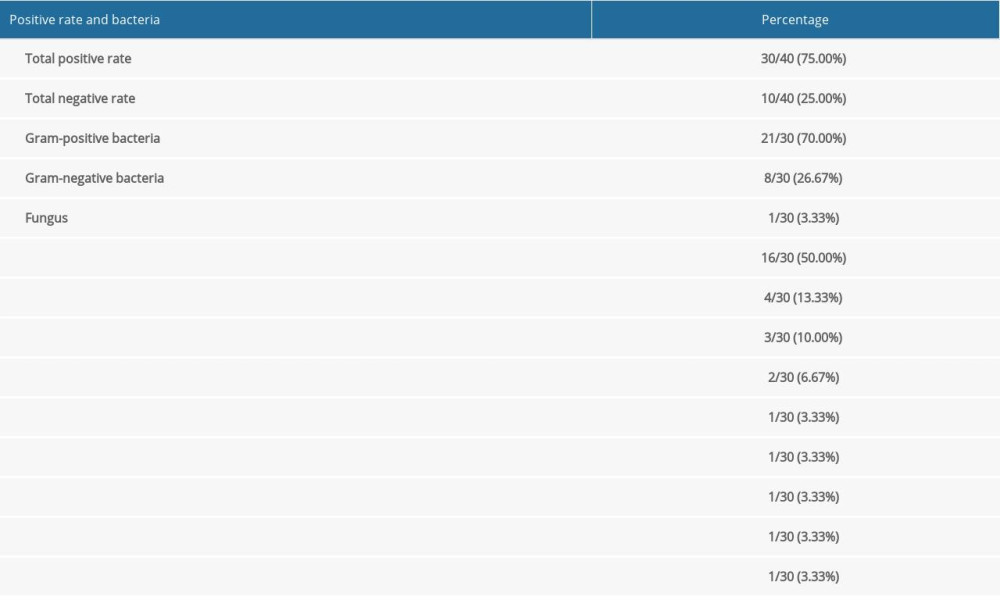 Table 4. Analysis of drug susceptibility and drug resistance rate of common bacteria with positive peritoneal fluid (%).
Table 4. Analysis of drug susceptibility and drug resistance rate of common bacteria with positive peritoneal fluid (%).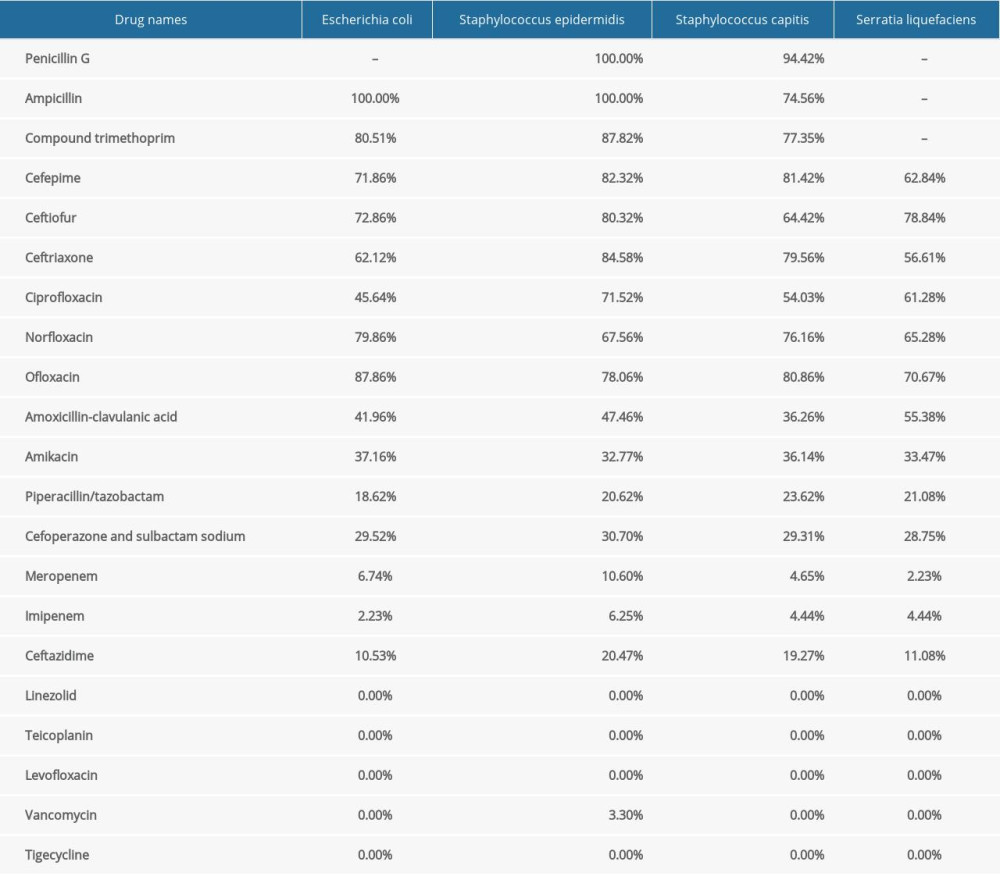
References
1. Li PK, Chow KM, Van de Luijtgaarden MW, Changes in the worldwide epidemiology of peritoneal dialysis: Nat Rev Nephrol, 2016; 13(2); 90-103
2. Woodrow G, Fan SL, Reid C, Renal association clinical practice guideline on peritoneal dialysis in adults and children: BMC Nephrol, 2017; 18(1); 333
3. Jeloka TK, Abraham G, Bhalla AK, Continuous ambulatory peritoneal dialysis peritonitis guidelines-consensus statement of Peritoneal Dialysis Society of India-2020: Indian J Nephrol; 31(5); 425-34 202
4. Mujais S, Microbiology and outcomes of peritonitis in North America: Kidney Int Suppl, 2006; 10(3); S55-62
5. Salzer WL, Peritoneal dialysis-related peritonitis: challenges and solutions: Int J Nephrol Renovasc Dis, 2018; 11(2); 173-86
6. Tian Y, Xie X, Xiang S, Risk factors and outcomes of high peritonitis rate in continuous ambulatory peritoneal dialysis patients: A retrospective study: Medicine (Baltimore), 2016; 95(49); e5569
7. Hsieh YP, Wang SC, Chang CC, The negative impact of early peritonitis on continuous ambulatory peritoneal dialysis patients: Perit Dial Int, 2014; 34(6); 627-35
8. Andy Tang SO, Carolisna YI, Sakura D, Demographic characteristics and outcomes of continuous ambulatory peritoneal dialysis related peritonitis in Miri General Hospital, Malaysia: Med J Malaysia, 2019; 74(4); 270-74
9. de Fijter CW, Jakulj L, Amiri F, Intraperitoneal meropenem for polymicrobial peritoneal dialysis-related peritonitis: Perit Dial Int, 2016; 36(5); 572-73
10. Meersch M, Schmidt C, Zarbock A, Patient with chronic renal failure undergoing surgery: Curr Opin Anaesthesiol, 2016; 29(3); 413-20
11. Li PK, Szeto CC, Piraino B, ISPD peritonitis recommendations: 2016 update on prevention and treatment: Perit Dial Int, 2016; 36(5); 481-508
12. Boudville N, Kemp A, Clayton P, Recent peritonitis associates with mortality among patients treated with peritoneal dialysis: J Am Soc Nephrol, 2012; 23(8); 1398-405
13. Michaela CB, Keith S, Kerssens J, Peritoneal dialysis-associated peritonitis rates and outcomes in a national cohort are not improving in the post-millennium (2000–2007): Perit Dial Int, 2011; 31(6); 639-50
14. Al Sahlawi M, Bargman JM, Perl J, Peritoneal dialysis-associated peritonitis: Suggestions for management and mistakes to avoid: Kidney Med, 2020; 2(4); 467-75
15. Yekinni IO, Viker T, Hunter R, Design and proof-of-concept evaluation of a touchless connector system for preventing peritoneal dialysis-associated peritonitis: BMJ Innov, 2022; 8(2); 98-104
16. Ghali JR, Bannister KM, Brown FG, Microbiology and outcomes of peritonitis in Australian peritoneal dialysis patients: Perit Dial Int, 2011; 31(6); 651-62
17. Liao CT, Zheng CM, Lin YC, Aberrant serum parathyroid hormone, calcium, and phosphorus as risk factors for peritonitis in peritoneal dialysis patients: Sci Rep, 2021; 11(1); 1171
18. Ong LM, Ch’ng CC, Wee HC, Risk of peritoneal dialysis-related peritonitis in a multi-racial Asian population: Perit Dial Int, 2017; 37(1); 35-43
19. Liakopoulos V, Nikitidou O, Kalathas T, Peritoneal dialysis-related infections recommendations: 2016 update: What is new? Int Urol Nephrol, 2017; 49(12); 2177-84
20. Kotsanas D, Polkinghorne KR, Korman TM, Risk factors for peritoneal dialysis-related peritonitis: Can we reduce the incidence and improve patient selection?: Nephrology (Carlton), 2007; 12(3); 239-45
21. Cheng LT, Chen W, Tang W, Does loss of residual renal function lead to malnutrition in peritoneal dialysis patients?: Clin Nephrol, 2006; 66(3); 192-201
22. Contreras-Velázquez JC, Soto V, Jaramillo-Rodríguez Y, Clinical outcomes and peritoneal histology in patients starting peritoneal dialysis are related to diabetic status and serum albumin levels: Kidney Int Suppl, 2008; 8(108); S34-41
23. Wang X, Han Q, Wang T, Serum albumin changes and mortality risk of peritoneal dialysis patients: Int Urol Nephrol, 2020; 52(3); 565-71
24. Laura T, Alan K, Nancy GB, Course of C-reactive protein during continuous peritoneal dialysis-associated peritonitis: Nephrology (Carlton), 2005; 10(5); 442-45
25. Hind CR, Thomson SP, Winearls CG, Serum C-reactive protein concentration in the management of infection in patients treated by continuous ambulatory peritoneal dialysis: J Clin Pathol, 1985; 38(4); 459-63
26. Yilmaz FM, Yilmaz G, Akay H, Evaluation of a card test for procalcitonin in continuous ambulatory peritoneal dialysis peritonitis: Ann Clin Biochem, 2007; 44(Pt 5); 482-84
27. van Esch S, Krediet RT, Struijk DG, Prognostic factors for peritonitis outcome: Contrib Nephrol, 2012; 178; 264-70
28. Coronel F, Cigarrán S, Herrero JA, Peritoneal protein losses in diabetic patients starting peritoneal dialysis: Is there a relationship with diabetic vascular lesions?: Adv Perit Dial, 2009; 25; 115-18
29. Beth P, Judith Bi, Edwina B, ISPD position statement on reducing the risks of peritoneal dialysis-related infections: Perit Dial Int, 2011; 31(6); 614-30
30. Trinh E, Hanley JA, Nadeau-Fredette AC, A comparison of technique survival in Canadian peritoneal dialysis and home hemodialysis patients: Nephrol Dial Transplant, 2019; 34(11); 1941-49
31. Alwakeel JS, Alsuwaida A, Akram A, Outcome and complications in peritoneal dialysis patients: A five-year single center experience: Saudi J Kidney Dis Transpl, 2011; 22(2); 245-51
32. Coronel F, Cigarrán S, Herrero JA, Morbidity and mortality in diabetic patients on peritoneal dialysis. Twenty-five years of experience at a single centre: Nefrologia, 2010; 30(6); 626-32
33. Kussmann M, Schuster L, Zeitlinger M: Eur J Clin Microbiol Infect Dis, 2018; 37(6); 1091-98
34. Ballinger AE, Palmer SC, Wiggins KJ, Treatment for peritoneal dialysis-associated peritonitis: Cochrane Database Syst Rev, 2014; 26(4); CD005284
35. Szeto CC, Li PK, Peritoneal dialysis-associated peritonitis: Clin J Am Soc Nephrol, 2019; 14(7); 1100-5
Figures
 Figure 1. Principal component analysis (PCA) of clinical characteristics between patients with peritoneal dialysis-associated peritonitis (PDAP) and without PDAP.
Figure 1. Principal component analysis (PCA) of clinical characteristics between patients with peritoneal dialysis-associated peritonitis (PDAP) and without PDAP. Figure 2. (A) Mean decrease in accuracy in predicting the occurrence of peritoneal dialysis-associated peritonitis (PDAP) when using the random forest model. (B) Mean decrease in the Gini coefficient in predicting the occurrence of PDAP when using the random forest model. (C) The results of the receiver operating characteristic curve (ROC) analysis, using random forest prediction value to detect the occurrence of PDAP.
Figure 2. (A) Mean decrease in accuracy in predicting the occurrence of peritoneal dialysis-associated peritonitis (PDAP) when using the random forest model. (B) Mean decrease in the Gini coefficient in predicting the occurrence of PDAP when using the random forest model. (C) The results of the receiver operating characteristic curve (ROC) analysis, using random forest prediction value to detect the occurrence of PDAP. Tables
 Table 1. Comparative analysis of clinical data for patients with automated peritoneal dialysis-associated peritonitis.
Table 1. Comparative analysis of clinical data for patients with automated peritoneal dialysis-associated peritonitis. Table 2. Logistic regression analysis of risk factors in patients with continuous ambulatory peritoneal dialysis-associated peritonitis.
Table 2. Logistic regression analysis of risk factors in patients with continuous ambulatory peritoneal dialysis-associated peritonitis. Table 3. Bacteria culture and distribution of peritoneal fluid.
Table 3. Bacteria culture and distribution of peritoneal fluid. Table 4. Analysis of drug susceptibility and drug resistance rate of common bacteria with positive peritoneal fluid (%).
Table 4. Analysis of drug susceptibility and drug resistance rate of common bacteria with positive peritoneal fluid (%). Table 1. Comparative analysis of clinical data for patients with automated peritoneal dialysis-associated peritonitis.
Table 1. Comparative analysis of clinical data for patients with automated peritoneal dialysis-associated peritonitis. Table 2. Logistic regression analysis of risk factors in patients with continuous ambulatory peritoneal dialysis-associated peritonitis.
Table 2. Logistic regression analysis of risk factors in patients with continuous ambulatory peritoneal dialysis-associated peritonitis. Table 3. Bacteria culture and distribution of peritoneal fluid.
Table 3. Bacteria culture and distribution of peritoneal fluid. Table 4. Analysis of drug susceptibility and drug resistance rate of common bacteria with positive peritoneal fluid (%).
Table 4. Analysis of drug susceptibility and drug resistance rate of common bacteria with positive peritoneal fluid (%). In Press
08 Mar 2024 : Clinical Research
Evaluation of Foot Structure in Preschool Children Based on Body MassMed Sci Monit In Press; DOI: 10.12659/MSM.943765
15 Apr 2024 : Laboratory Research
The Role of Copper-Induced M2 Macrophage Polarization in Protecting Cartilage Matrix in OsteoarthritisMed Sci Monit In Press; DOI: 10.12659/MSM.943738
07 Mar 2024 : Clinical Research
Knowledge of and Attitudes Toward Clinical Trials: A Questionnaire-Based Study of 179 Male Third- and Fourt...Med Sci Monit In Press; DOI: 10.12659/MSM.943468
08 Mar 2024 : Animal Research
Modification of Experimental Model of Necrotizing Enterocolitis (NEC) in Rat Pups by Single Exposure to Hyp...Med Sci Monit In Press; DOI: 10.12659/MSM.943443
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








