19 June 2020: Animal Study
Effects of Combined Bushen Zhichan Recipe and Levodopa in a Rodent Model of Parkinson Disease: Potential Mechanisms
Wenhao Li1BCDEF, Han Gao1BCDEF, Wentao Li1ABCDEFG*DOI: 10.12659/MSM.922345
Med Sci Monit 2020; 26:e922345
Abstract
BACKGROUND: Parkinson disease is characterized by the loss of neurons in the substantia nigra, and under pathological conditions, glutamate can produce excitotoxic effects on nerve cells. The astrocytic excitatory amino acid transporter (EAAT) 1 can be functionally upregulated and targeted to functional compartments, resulting in reduced excitotoxicity. levodopa is the gold standard for the treatment of Parkinson disease, but prolonged levodopa treatment often leads to the development of abnormal involuntary movements. Numerous studies suggest the potential beneficial effects of traditional Chinese medicine on Parkinson disease.
MATERIAL AND METHODS: We validated the efficacy of a Bushen Zhichan recipe combined with levodopa in a rodent Parkinson disease model and explored its possible mechanisms.
RESULTS: Rats in the combined levodopa and Bushen Zhichan recipe group performed significantly better than the control group in the open field and forelimb function experiments. The number of midbrain dopaminergic neurons in rats in the levodopa and Bushen Zhichan recipe group was greater compared to controls. The levodopa and Bushen Zhichan recipe group exhibited decreased glutamate receptors and increased γ-aminobutyric acid receptors in the striatum. At the same time, EAAT1 was increased and EAAT2 was synchronized with the number of glutamate receptors.
CONCLUSIONS: Our results indicate that levodopa combined with Bushen Zhichan recipe significantly improves behavior and protects dopaminergic neurons in a rodent Parkinson disease model, and suggest that the mechanism involves the decrease of excitatory amino acid toxicity and the increase in the expression of EAAT1.
Keywords: Dopamine Plasma Membrane Transport Proteins, Levodopa, Receptors, Ionotropic Glutamate, Behavior, Animal, Cistanche, Cornus, Corpus Striatum, Dioscorea, dopaminergic neurons, Drugs, Chinese Herbal, Excitatory Amino Acid Transporter 1, Excitatory Amino Acid Transporter 2, Fallopia multiflora, Forelimb, Medial Forebrain Bundle, Mesencephalon, open field test, Oxidopamine, Parkinsonian Disorders, Rehmannia
Background
Parkinson disease is the second most common neurodegenerative disease in high-income countries, with rates of 14 per 100 000 people in the total population and 160 per 100 000 in individuals aged 65 years or older [1]. It is characterized by the loss of dopaminergic neurons in the substantia nigra [2]. Glutamate-mediated excitotoxicity is initiated rapidly after ischemic brain injury and contributes to neuronal damage [3]. Glutamate receptors include ionotropic glutamate receptors (iGluRs) and metabotropic glutamate receptors (mGluRs). Ionotropic glutamate receptors (iGluRs) are ligand-gated cation channels and are subdivided into a-amino-3-hydroxy-5-methyl-4 isoxazole propionic acid (AMPA), N-methyl-D-aspartate (NMDA), and kainate receptors [4–7]. 80% of AMPA receptors in the synapse are composed of Glur1/Glur2, while extrasynaptic Glur1/Glur2 accounts for more than 95% of all AMPA receptors (AMPARs) [8]. Increased stimulation of AMPARs is implicated in the pathophysiological adaptations of medium-spiny neurons (MSN) induced by dopamine neuron depletion and chronic L-DOPA treatment [9]. NMDA exhibits high permeability to calcium ions and has been proposed that the NMDA receptor is a heterotetraploid composed of 2 NR1 subunits and 2 NR2 subunits, whereas NR1/NR2B heteromers also are expressed at extra-synaptic sites [10]. Excitatory amino acid transporters (EAATs) play a crucial role in clearing excessive glutamate in the synaptic cleft [11], and they include EAAT1 (GLAST GLAST1), EAAT2 (Glt-1), EAAT3 (EAAC1), EAAT4, and EAAT5. Glutamate transporter-1 (GLT-1, also called EAAT2) is responsible for transporting nearly 90% of the glutamate in astrocytes (Km=2 uM) [12], and glutamate/aspartate transporter (GLAST, also called EAAT1) has the greatest glutamate transport efficiency (Km=77 uM). Clinical evidence indicates that reactive astrocytes and activated microglial cells express EAAT1, suggesting neuroprotective potential following ischemia [13]. Moreover, the pharmacological upregulation of EAAT1 is associated with reduced infarct volume and improved neurological outcomes following middle cerebral artery occlusion in rats [14,15]. There is evidence that EAAT1 is functionally upregulated and targeted to functional compartments, resulting in reduced excitotoxicity [16].
levodopa is the gold standard for the treatment of Parkinson disease [17], but prolonged L-dopa treatment often leads to the development of abnormal involuntary movements [18]. Many studies show the potential effects of traditional Chinese medicine (TCM) on Parkinson disease [19–22]. The Bushen Zhichan recipe used in the current study is a clinical intervention developed by Director Wentao Li (Encephalopathy Department, Shanghai Municipal Hospital of Traditional Chinese Medicine). It was from the classic prescription-Liuwei Dihuang Pills. And it is composed of Rehmanniae Radix Praeparata (12 g), Dioscoreae Rhizoma (12 g), Cornifructus (12 g), Polygoni Multiflori Radix (3 g), and Cistanches Herba (9 g). Many of these drugs have been experimentally proven to have protective effects on nerves. Studies have reported that the main extracts of Polygonum multiflorum can inhibit MPP+induce PC12 cell apoptosis by inhibiting reactive oxygen species (ROS) production, regulating JNK activation, and improving mitochondrial function [23]. Rehmannia glutinosa extract sterol pretreatment can protect dopaminergic neurons from lipopolysaccharide (LPS)-induced neurotoxicity, and it also exhibits neuroprotective effects on MPP+-induced cerebral neuronal oxidative stress [24].
We used a rodent Parkinson disease model to verify the behavioral improvements and protective effects on dopaminergic neurons of this Bushen Zhichan recipe when combined with levodopa. We assessed levels of surface receptors, including iGluRs and EAAT1/EAAT2, and inhibitory amino acid receptors and transporters in the midbrain and striatum to explore possible mechanisms.
Material and Methods
PREPARATION OF DRUGS:
The Bushen Zhichan recipe was composed of Rehmanniae Radix Praeparata (Shudihuang 12 g), Dioscoreae Rhizoma (Shanyao 12 g), Corni Fructus (Shanzhuyu 12 g), Polygoni Multiflori Radix (Heshouwu 3 g), and Cistanches Herba (Roucongrong 9 g). All drugs were purchased and prepared by the Pharmacy of Shanghai Municipal Hospital of Traditional Chinese Medicine. The herbs were boiled in water according to the traditional method of herbal preparation. The filtrates were concentrated and dried in vacuum at 60°C. The concentrated extracts were then dried by lyophilization to obtain the Bushen Zhichan recipe extracts. The extracts were dissolved by 0.9% normal saline and stored at 4°C. Every 50 mL solution contains Chinese herbal medicine Rehmanniae Radix Praeparata 12 g, Dioscoreae Rhizoma 12 g, Corni Fructus 12 g, Polygoni Multiflori Radix 3 g, and Cistanches Herba 9 g.
ANIMALS TREATMENTS:
Male Sprague-Dawley rats (weighing 180–200 g) were purchased from the Shanghai SLAC Animal Laboratory (Shanghai, China), and housed 4 to 5 rats per cage. Rats received a standard chow diet in a controlled environment (temperature 23°C, relative humidity 50% 70%, 12-hour light/dark cycle, lights on from 7: 00 am to 7: 00 pm). After 5 days of adaptive feeding, rats were deeply anesthetized with 10% chloral hydrate (4 mL⁄kg, intraperitoneal) and fixed in a stereotaxic frame to ensure that bregma and lambda were in the same horizontal plane. Rats were then injected with 6-hydroxydopamine (16 ug 6-OHDA, 4 ug/uL of saline containing 0.1% ascorbic acid) via a Hamilton syringe into the 2 coordinates in medial forebrain bundle, at a rate of 0.1 uL/minute [anteroposterior=−4.2/−3.6; lateral=−1.2/−1.7; ventrodorsal=−7.8/−7.8]. Then 50 000 units of antibiotics were injected per day for the next 3 days. Twelve days later, the rats were tested for contralateral turns with a 0.05 mg/kg intraperitoneal injection of apomorphine for 40 minutes, and only those rats consistently making at least 210 contralateral turns were considered to be exhibiting Parkinson disease like behavior [25].
These Parkinson disease model rats were divided into 3 groups (Parkinson disease (PD) control group, levodopa group, and levodopa combined with Bushen Zhichan recipe group; n=4 for each group). The levodopa group was given 20 mg/kg/day levodopa (1 mg/mL of 0.5% CMC-NA, Roche, Shanghai) by gavage based on previous protocols clinical dosing [26]. The levodopa combined with Bushen Zhichan recipe group was separately given 20 mg/kg/day levodopa (2 mg/mL of 0.5% CMC-NA) and Bushen Zhichan recipe (10 mL/kg of 0.9% NS) by gavage successively. The amount of Bushen Zhichan recipe was determined according to clinical dosing and the body surface area of the rat. The PD model control group and an additional no treatment group (near anesthesia, no surgery) were given saline containing 0.5% CMC-NA. All groups were gavaged twice a day for 4 weeks.
OPEN FIELD TEST:
The open field box was 50 cm high and 50 cm long, the inner wall was black, and the bottom surface was divided into 25 squares of 2×2 cm. A digital camera was placed directly above it so that the field of view included the entire floor. Experimenters and computers were located in another room to reduce interference with animals, and the laboratory background noise was kept below 65 dB. We selected the following variables to assess: the time of the animal in the middle of the box, the distance travelled in the middle, the total distance, and the numbers of stools.
FORELIMB FUNCTION EXPERIMENT:
The forelimb function experiment was performed 30 minutes following each gavage in the last 3 days. The experimenter fixed the posterior and hind limbs of the rat’s body in one hand to elevate it above the surface, and the other hand placed the forelimbs on the surface. The rats were moved obliquely to one side (moving 90 cm within 5 seconds), and the number of steps with forelimbs during this movements was recorded. The numbers of steps of both forelimbs were measured alternately.
BRAIN EXTRACTING:
Following the behavioral tests, rats were anesthetized, and the brains were extracted. The right injured semi-brain was separated. And the midbrain was sectioned for paraffin embedding and western blotting. The striatum was quickly removed and stored at −80°C for western blotting.
IMMUNOFLUORESCENCE:
Paraffin-embedded midbrain tissue was subjected to immunofluorescence to detect the dopamine transporter (DAT) and mGluRs. The sections were treated in 0.02 M phosphate-buffered saline (PBS, pH 7.4) 3×15 minutes, treated with 3% H2O2-methanol 1×15 minutes, and treated in 0.02 M PBS (pH 7.4) for 15 minutes. Antigen retrieval with citric acid-sodium citrate (pH=6.0) was completed, followed by treatment with 0.4% Triton X-100 in Tris-buffered saline (TBS) for 1 hour at room temperature (RT). This was followed by TBST incubation with 5% goat serum for 30 min at RT. Samples were applied to slides with DAT (Abcam,ab184451), NR1 (CST, 5704s), NR2B (CST, 14544), GluR1 (CST, 13185), and GluR2 (Santa Cruz, Sc-518265) primary antibodies, sealed 4°C for 20 hours; washed 3 times for 5 minutes each with PBS, and secondary antibodies were then applied (Beyotime, A516, Cy3-labeled goat anti-rabbit IgG, or Beyotime, A0568, FITC-labeled goat anti-mouse IgG). All antibodies in immunofluorescence were diluted 1: 100. They were incubated in the dark at 37°C for 60 to 90 minutes, washed 3 times for 5 minutes each with PBS, treated with DAPI (Beyotime, C1005) at 37°C for 5 minutes, washed 3 times for 5 minutes each with PBS, then an antifade mounting medium was added prior to sealing. Images were collected with fluorescence microscopy, and the photos by analyzed with Image Pro Plus 6.0 (Media Cybernetics, MD, USA).
WESTERN BLOTTING: The striatal and midbrain tissues were stored at −80°C and subjected to lysis to extract proteins by subcellular fractionation [27] (Figure 1). Membrane-associated proteins such as mGluRs and EAATs used pellets for detection, and other proteins used total detection. Equal amounts of total protein mixed with the loading buffer were heated to 100°C for 10 minutes and then re-dissolved by SDS-PAGE using 10% acrylamide for electrophoresis. Proteins were transferred onto an NC membrane for 1 hour at 100 V. After blocking with 0.1% PBST containing 5% skim milk for 1 hour at room temperature, membranes were incubated with the primary antibodies in 0.1% PBST with 5% skim milk at 4°C overnight, then incubated for 1 hour at RT in the secondary antibody solution. All antibodies in western blotting were diluted 1: 1000. Chemiluminescence was detected with ECL solution (Tanon, no. 180–5001). Bands were scanned by the Image Lab 5.1 software image system (Bio-Rad, USA). The aforementioned methods were used to detect DAT (Abcam, ab184451), cytochrome C (Santa Cruz, Sc-13156), amino acid receptor GluR1 (CST, 13185), GluR2 (Santa Cruz, Sc-518265), NR1 (CST, 5704s), NR2B (CST, 14544), GABAA (Santa Cruz, SC-376252), glial fibrillary acidic protein GFAP (CST, 80788), amino acid transporter EAAT1 (Santa Cruz, Sc-515839), EAAT2 (Santa Cruz, Sc-365634), and GABAT-3 (Santa Cruz, SC-376001). The stained lipids were quantified by ImageJ (Schneider et al., 2012).
STATISTICAL ANALYSIS:
All data are expressed in terms of the mean value with its standard deviation (mean±SD). Differences in the data among each group were analyzed by one-way analysis of variance with Tukey’s test using SPSS Version 19.0.
Results
BEHAVIORAL TESTING:
Rats in the 4 groups were tested for open field behavior and forelimb function after 28 days of gavage. In the open field test, levodopa combined with Bushen Zhichan recipe significantly improved the stools times (0.8571±0.8997), total distance (2129±159.9), middle distance (433.1±41.99), and middle duration (52.08±15.72) value compared with PD controls (stools time, 3.429±2.149; total distance, 1687±165.4; middle distance, 663.2±86.84; and middle duration 92.62±15.48) (Figure 2). There was no significant difference between levodopa group and the PD model group. There was a trend of a therapeutic effect of the levodopa combined with TCM compared to levodopa. In the forelimb function experiment, the levodopa combined with TCM group (1.400±0.5477) and levodopa group values (2.600±0.8944) were superior to those of the PD model group (5.200±0.8367).
DOPAMINERGIC NEURON PROTECTION:
We detected dopaminergic neurons in midbrain with western blotting and immunofluorescence. Western blotting revealed that the normal group (1.039±0.2285) was significantly different from the model group (0.4057±0.1347) and levodopa group (0.5054±0.2114) (Figure 3). The levodopa combined with TCM group (0.6100±0.3120) was not significantly different from the normal controls, model, or levodopa groups. Due to the low number of rats in each group, more animals may be needed to identify a significant difference. In the immunofluorescence experiments, we located the midbrain-based coordinates from the Paxinos and Watson brain atlas. We observed a decrease in dopamine neurons in the levodopa combined with TCM group relative to the PD model and levodopa groups (Figure 4).
DETECTION OF EXCITATORY AMINO ACID RECEPTORS AND INHIBITORY AMINO ACID RECEPTORS:
Striatal tissues stored at −80°C were subjected to lysis to extract proteins. The results of western blotting indicated a decrease in excitatory amino acid toxicity in the levodopa combined with TCM group (Figure 5). GluN2B levels in the levodopa combined with Bushen Zhichan recipe group (0.1924±0.06684) were significantly lower than the model group (0.4783±0.1313), but there was no significant difference compared with the levodopa group (0.3114±0.07850). GluR2 of the levodopa combined with Bushen Zhichan recipe group (0.3381±0.1565) was also significantly lower than the model group (0.7163±0.1884) but was not significantly lower than the levodopa group (0.5880±0.07669). GABAA levels in the levodopa combined with Bushen Zhichan recipe group were significantly higher than the other 3 groups. The immunofluorescence data supported these differences in amino acid receptors and inhibitory amino acid receptors (Figure 4). We also evaluated cytochrome C (Figure 3), a marker for excitatory amino acid toxicity, which exhibited significant improvement in the levodopa combined with Bushen Zhichan recipe group (0.3659±0.08758) relative to the model group (0.8533±0.1366).
DETECTION OF EXCITATORY AMINO ACID TRANSPORTERS AND INHIBITORY AMINO ACID TRANSPORTERS:
The expression of EAAT2 was increased in the model and levodopa groups, while levels in levodopa combined with Bushen Zhichan recipe group (0.2258±0.08954) were significantly reduced compared with the levodopa group (0.5139±0.05705) (Figure 6). The expression levels of EAAT1 in the levodopa combined with Bushen Zhichan recipe group (0.8069±0.2528) were significantly higher than that in the normal group (0.3635±0.04814) and the model group (0.3321±0.1064). Expression levels of the inhibitory amino acid transporter GABAT-3 were similar between the groups.
Discussion
In this experiment, we verified behavioral improvements in the levodopa combined with Bushen Zhichan recipe group of Parkinson disease rats with open field tests and forelimb function experiments. Protective effects of levodopa combined with Bushen Zhichan recipe on midbrain dopamine neurons were confirmed. We then assessed excitatory and inhibitory amino acid receptors to evaluate attenuated excitatory amino acid toxicity. It has recently been proposed that NMDA blockade may be responsible for the lack of efficacy of NMDA receptor antagonists in human stroke and traumatic brain injury trials [28]. Expression levels of EAAT1 and EAAT2 and their ratios indicate an increase in the dopamine transporter efficiency of the levodopa combined with Bushen Zhichan recipe group. The change of EAAT1 may be the reason why the Bushen Zhichan recipe protected dopamine neurons. We hypothesized that there is a compound in Bushen Zhichan that can increase the glutamate uptake efficiency of astrocytes through EAAT1. There have been reports of different results compared to the present study, which may be related to differences in PD model, administration, and timing of treatment. For example, in the striatum of Parkinson disease rats, it has been reported that the expression of AMPA receptors may be elevated [29], decreased [30], or unchanged [31].
There are 2 commonly used preparation methods for animal models of Parkinson disease. One is to destroy dopaminergic neurons by injecting 6-OHDA into the nigrostriatal pathway [32], and the other is to inject MPTP through the abdominal cavity (1 -methyl-4-phenyl-1,2,3,6 tetrahydropyridine) to prepare PD models through the function of mitochondrial disruption [33,34]. In this experiment, we used the traditional localized injection of 6-OHDA to damage the dopaminergic neurons. The experimental process is more complicated, the time is longer, and the molding rate is acceptable. There is also a large difference in the dosage of 6-OHDA, mainly between 8 and 20 ug at each nucleus site [35,36]. Based on the results of our previous preliminary experiments and literature data, we selected a 20 ug dose for each site to ensure the degree of damage to the dopaminergic neurons and improve the modeling rate. In the choice of behavioral testing, we also tried the rotating rod experiment and the hanger experiment. However, because the rotating rod experiment could not be performed when the weight of the rat was over, and the hanger experiment had a large subjective effect, we chose to give up these 2 experiments.
The results of this experiment show the protective effect of levodopa combined with the Bushen Zhichan recipe group on dopaminergic neurons. In inhibiting the excitatory amino acid toxicity, the levodopa combined with the Bushen Zhichan recipe group showed a good effect compared to using levodopa alone. However, in terms of behavioral results and DAT expression, despite the improvement trend, the levodopa combined with Bushen Zhichan recipe group did not show a significant statistical improvement over the levodopa group. The main reason we consider this is that the time of medication is too late, the dopaminergic neurons have been much destroyed and the Bushen Zhichan recipe cannot play a neuroprotective effect earlier and longer. Therefore, we speculate that using early-stage Parkinson disease models and early administration can improve the experimental results.
TCM has been used for centuries to treat conditions such as trembling hands and shaking of the head and remains popular for the management of Parkinson disease in Asian countries such as China, Korea, and Japan [37]. The World Health Organization recognizes TCM in its influential global medical compendium. In 2017, the Chinese government canceled the clinical TCM toxicology experiments. This change in regulations reflects the difference between Chinese and modern medicine. Due to extensive historical use, Chinese medicine has already been informally tested in a clinical setting. Not only in the clinical aspect, but also in the basic research level, the research of Chinese medicine in Parkinson disease has also made significant progress. For example, Tianma Gouteng Yin can reduce alpha-synuclein protein levels and prevent loss of dopaminergic neurons in the brains of Drosophila [38].
TCM, especially prescriptions, is challenging to adapt to modern pharmacological research methods, but there have been many studies of the therapeutic effects of TCM on Parkinson disease related to its physical and chemical properties [39]. Chinese medicines have great potential in the treatment of neurological diseases. Future studies will involve additional experiments to analyze the components of the Bushen Zhichan recipe and determine its role and mechanisms in astrocytes
Conclusions
Our study results confirmed that the Bushen Zhichan recipe combined with levodopa treatment had beneficial effects on behavior and dopaminergic neuron protection in a rodent model of Parkinson disease, and suggest that the mechanism involves the decrease of excitatory amino acid toxicity and the increase in the expression of EAAT1.
Figures
 Figure 1. Methods for the subcellular fractionation of proteins.
Figure 1. Methods for the subcellular fractionation of proteins. 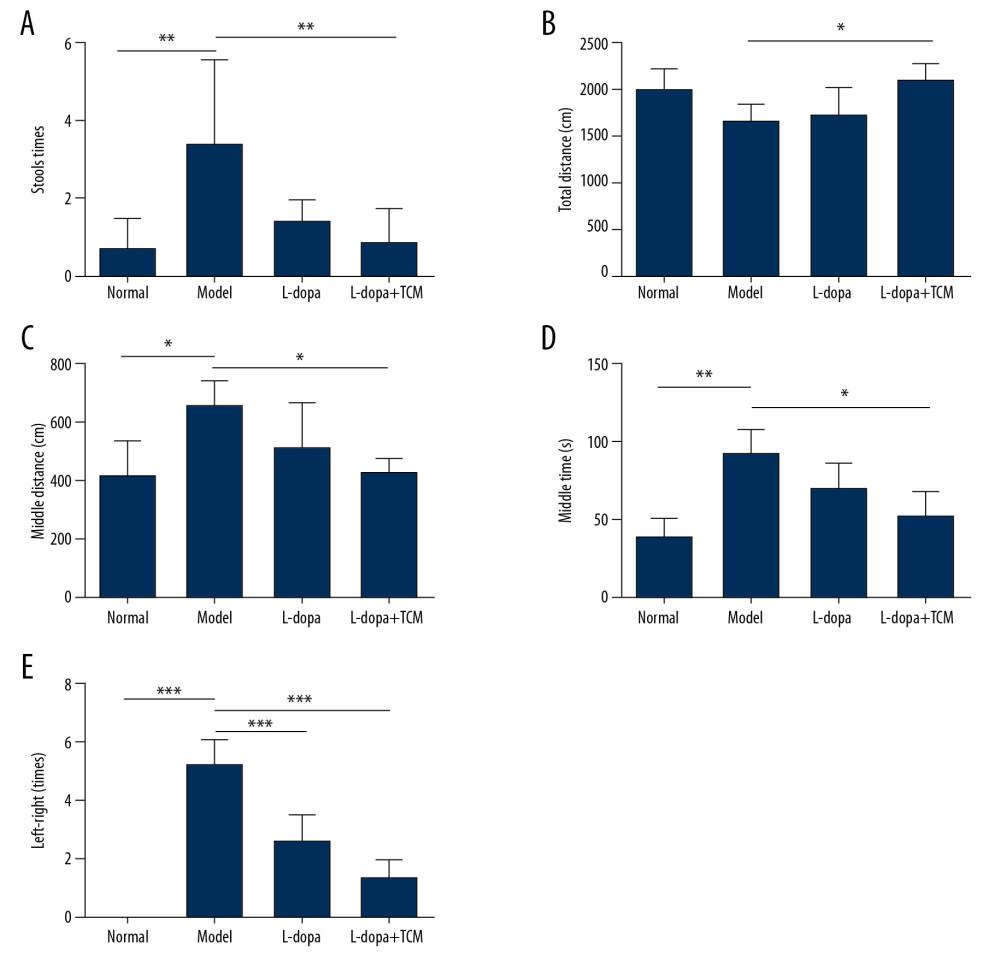 Figure 2. levodopa combined with Bushen Zhichan recipe significantly improved the behavioral testing results. Open field test (A–D) and forelimb function experiment (E) were performed after 28 days of gavage. levodopa combined with Bushen Zhichan recipe significantly improved the stools times (A, 0.8571±0.8997), total distance (B, 2129±159.9), middle distance (C, 433.1±41.99), middle duration (D, 52.08±15.72) value and forelimb function results (1.400±0.5477) compared with PD controls. * P<0.05; ** P<0.01; *** P<0.001.
Figure 2. levodopa combined with Bushen Zhichan recipe significantly improved the behavioral testing results. Open field test (A–D) and forelimb function experiment (E) were performed after 28 days of gavage. levodopa combined with Bushen Zhichan recipe significantly improved the stools times (A, 0.8571±0.8997), total distance (B, 2129±159.9), middle distance (C, 433.1±41.99), middle duration (D, 52.08±15.72) value and forelimb function results (1.400±0.5477) compared with PD controls. * P<0.05; ** P<0.01; *** P<0.001. 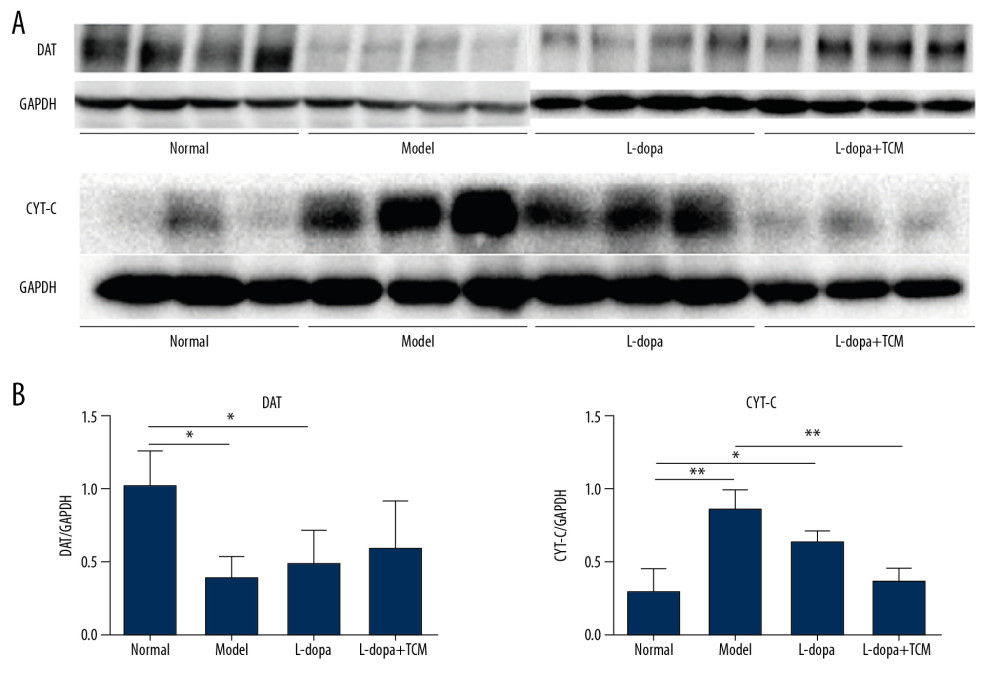 Figure 3. Midbrain tissue detection of DAT and cytochrome C by western blotting. (A) western blotting for DAT and cytochrome C, (B) analyzing for the expression of DAT and cytochrome C. The expressions of cytochrome C in levodopa combined with Bushen Zhichan recipe group (0.3659±0.08758) were significantly lower than the model group (0.8533±0.1366). * P<0.05; ** P<0.01; *** P<0.001.
Figure 3. Midbrain tissue detection of DAT and cytochrome C by western blotting. (A) western blotting for DAT and cytochrome C, (B) analyzing for the expression of DAT and cytochrome C. The expressions of cytochrome C in levodopa combined with Bushen Zhichan recipe group (0.3659±0.08758) were significantly lower than the model group (0.8533±0.1366). * P<0.05; ** P<0.01; *** P<0.001. 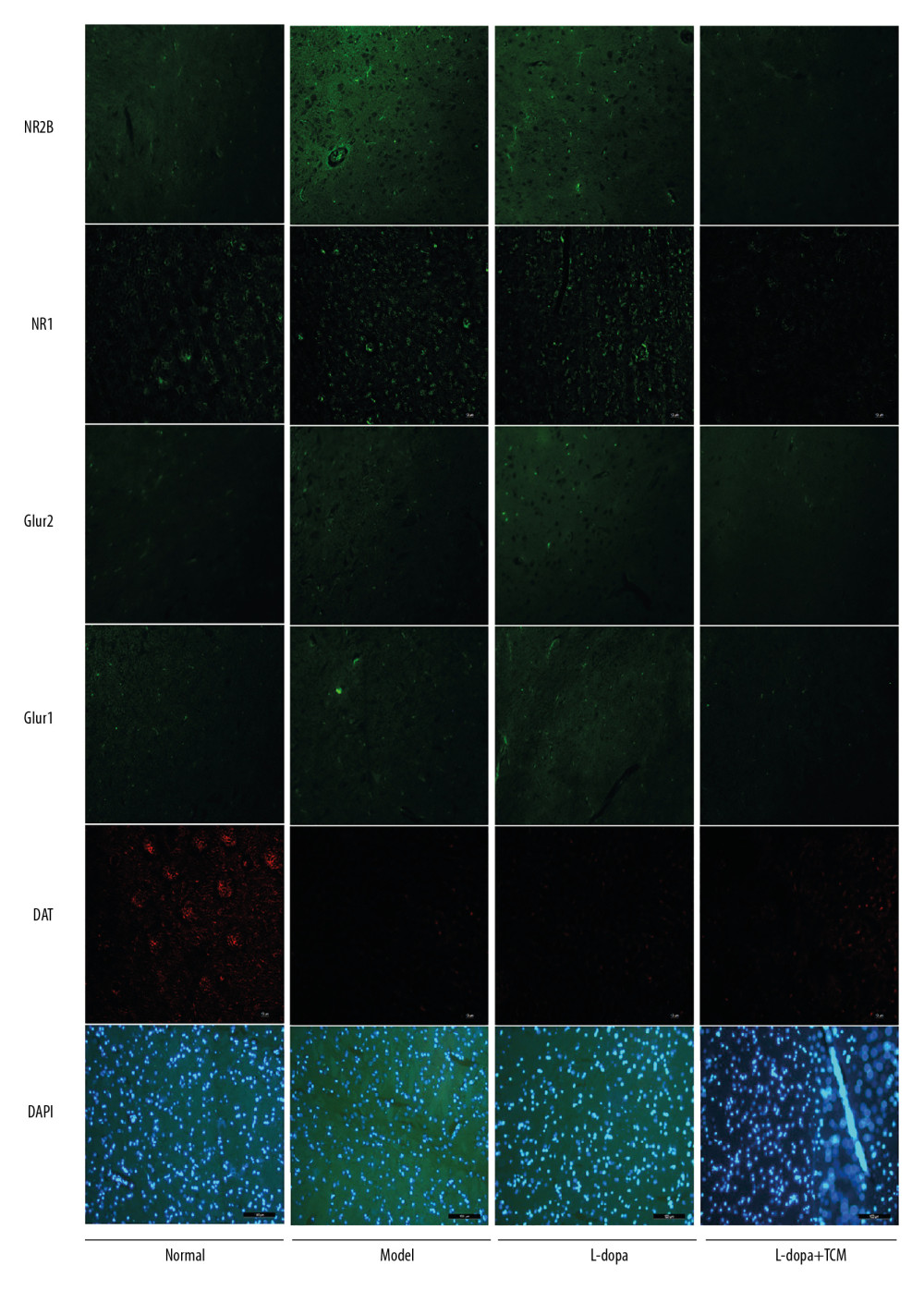 Figure 4. Immunofluorescence pictures showed that levodopa combined with Bushen Zhichan recipe protected the DAT cells and reduced the expressions of excitatory amino acid receptors. The scale of the pictures is 100 microns, and the magnification is 400 times.
Figure 4. Immunofluorescence pictures showed that levodopa combined with Bushen Zhichan recipe protected the DAT cells and reduced the expressions of excitatory amino acid receptors. The scale of the pictures is 100 microns, and the magnification is 400 times. 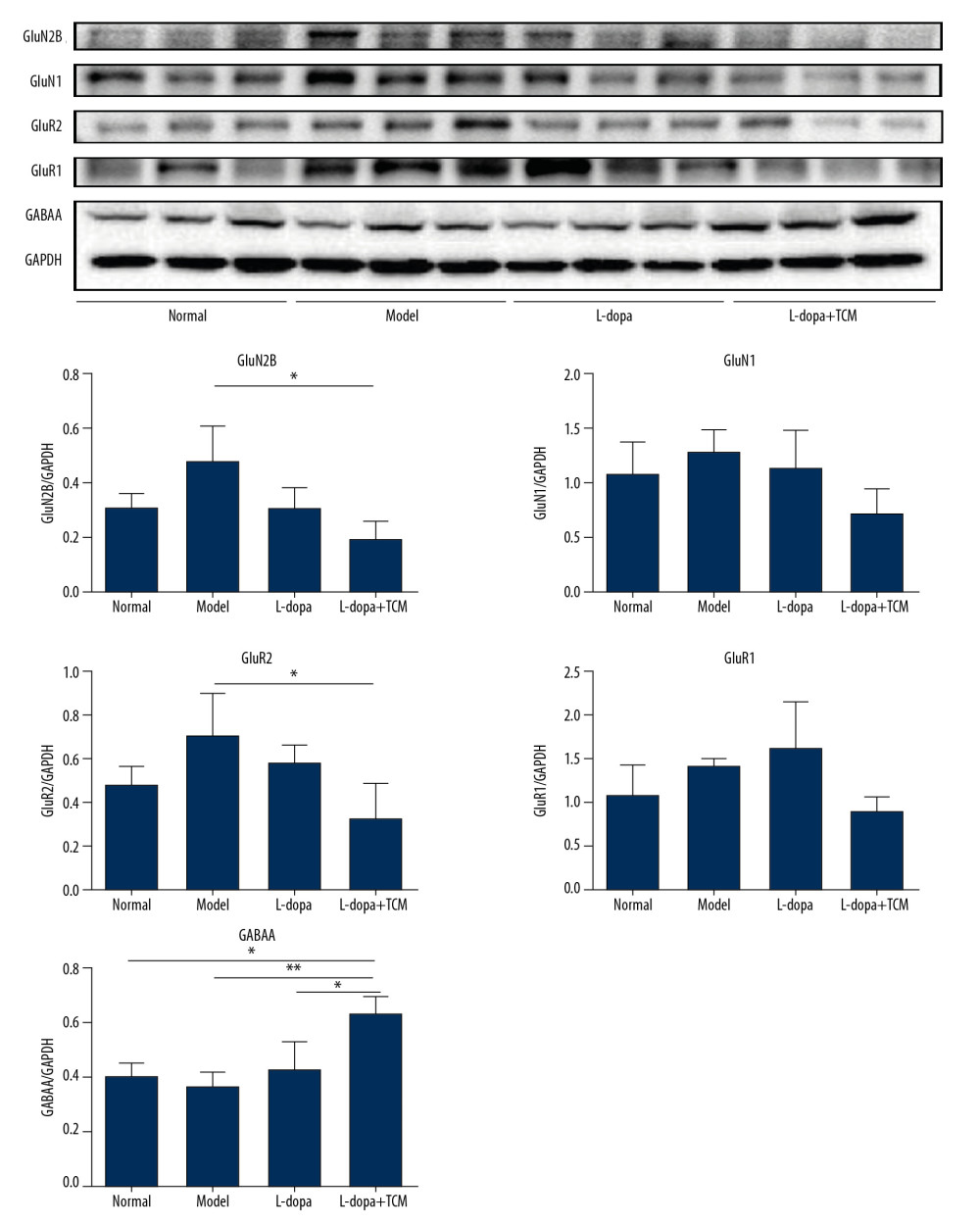 Figure 5. The striatal tissue detection of GABAA, GluR1, GluR2, GluN1, and GluN2B by western blotting. GABAA levels in the levodopa combined with Bushen Zhichan recipe group were significantly higher than the other 3 groups. Data are expressed as mean±standard deviation, * P<0.05; ** P<0.01; *** P<0.001.
Figure 5. The striatal tissue detection of GABAA, GluR1, GluR2, GluN1, and GluN2B by western blotting. GABAA levels in the levodopa combined with Bushen Zhichan recipe group were significantly higher than the other 3 groups. Data are expressed as mean±standard deviation, * P<0.05; ** P<0.01; *** P<0.001. 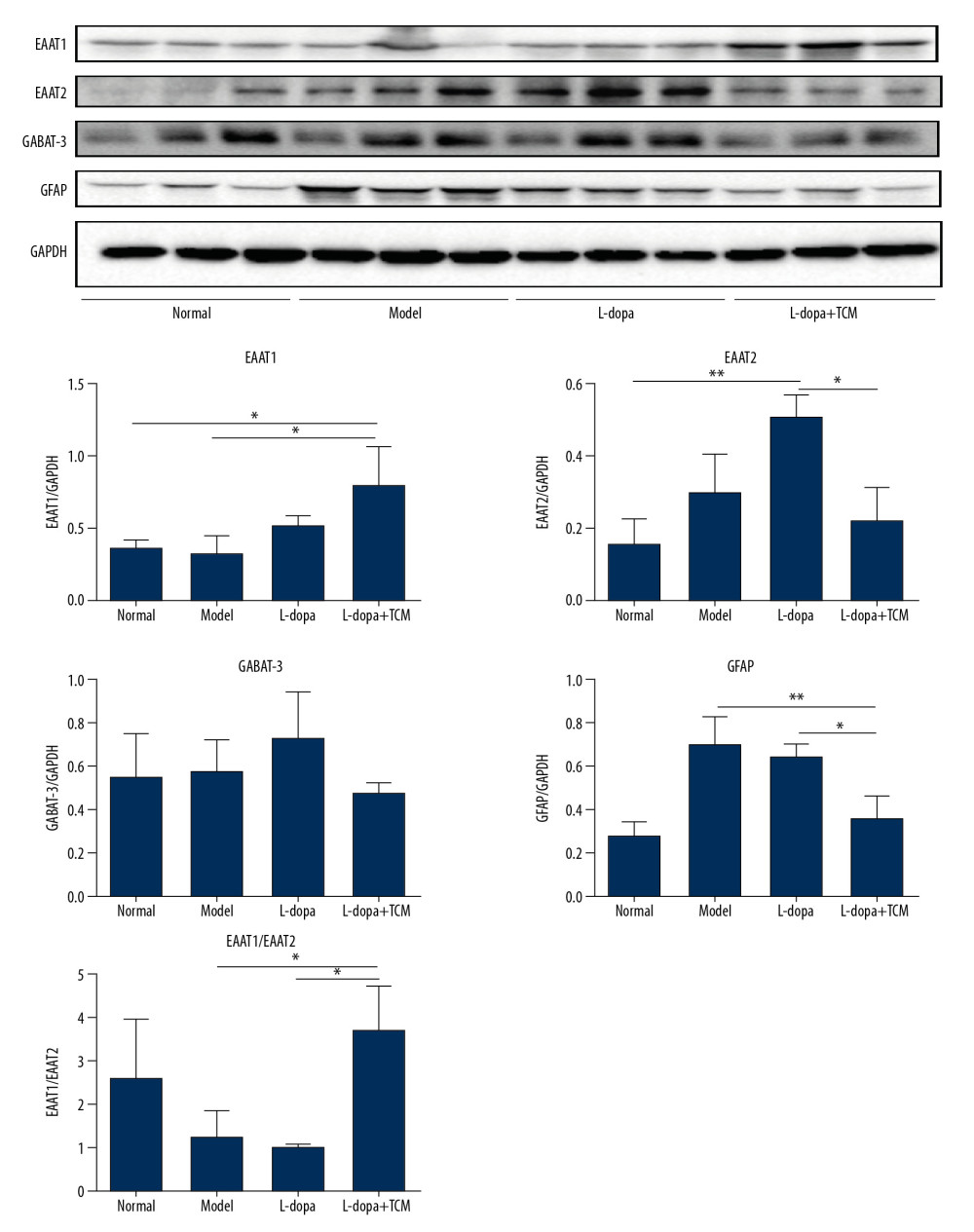 Figure 6. The striatal tissue detection of GFAP, GABAT-3, EAAT1, and EAAT2 by western blotting. The expression levels of EAAT1 in the levodopa combined with Bushen Zhichan recipe group (0.8069±0.2528) were significantly higher than that in the model group (0.3321±0.1064). Data are expressed as mean±standard deviation, * P<0.05; ** P<0.01; *** P<0.001.
Figure 6. The striatal tissue detection of GFAP, GABAT-3, EAAT1, and EAAT2 by western blotting. The expression levels of EAAT1 in the levodopa combined with Bushen Zhichan recipe group (0.8069±0.2528) were significantly higher than that in the model group (0.3321±0.1064). Data are expressed as mean±standard deviation, * P<0.05; ** P<0.01; *** P<0.001. References
1. Hirtz D, Thurman DJ, Gwinn-Hardy K, How common are the “common” neurologic disorders?: Neurology, 2007; 68(5); 326-37
2. Michel PP, Hirsch EC, Hunot S, Understanding dopaminergic cell death pathways in parkinson disease: Neuron, 2016; 90(4); 675-91
3. Dirnagl U, Iadecola C, Moskowitz MA, Pathobiology of ischaemic stroke: An integrated view: Trends Neurosci, 1999; 22; 391-97
4. Hollmann M, Heinemann S, Cloned glutamate receptors: Annu Rev Neurosci, 1994; 17; 31-108
5. Seeburg PH, The TINS/TiPS Lecture the molecular biology of mammalian glutamate receptor channels: Trends Neurosci, 1993; 16; 359-65
6. Monaghan DT, Bridges RJ, Cotman CW, The excitatory amino acid receptors: Their classes, pharmacology, and distinct properties in the function of the central nervous system: Annu Rev Pharmacol Toxicol, 1989; 29; 365-402
7. Ozawa S, Kamiya H, Tsuzuki K, Glutamate receptors in the mammalian central nervous system: Prog Neurobiol, 1998; 54; 581-618
8. Lu W, Bushong EA, Shih TP, The cell-autonomous role of excitatory synaptic transmission in the regulation of neuronal structure and function: Neuron, 2013; 78(3); 433-39
9. Sgambato-Faure V, Cenci MA, Glutamatergic mechanisms in the dyskinesias induced by pharmacological dopamine replacement and deep brain stimulation for the treatment of Parkinson disease: Prog Neurobiol, 2012; 96; 69-86
10. Chen BS, Roche KW, Regulation of NMDA receptors by phosphorylation: Neuropharmacology, 2007; 53; 362-68
11. Zhang Y, He X, Meng X, Regulation of glutamate transporter trafficking by Nedd4-2 in a Parkinson’s disease model: Cell Death Dis, 2017; 8(2); e2574
12. Kim K, Lee SG, Kegelman TP, Role of excitatory amino acid transporter-2 (EAAT2) and glutamate in neurodegeneration: Opportunities for developing novel therapeutics: J Cell Physiol, 2011; 226(10); 2484-93
13. Beschorner R, Simon P, Schauer N, Reactive astrocytes and activated microglial cells express EAAT1, but not EAAT2, reflecting a neuroprotective potential following ischaemia: Histopathology, 2007; 50(7); 897-910
14. Mori T, Tateishi N, Kagamiishi Y, Attenuation of a delayed increase in the extracellular glutamate level in the peri-infarct area following focal cerebral ischemia by a novel agent ONO-2506: Neurochem Int, 2004; 45(2–3); 381-87
15. Matsuura S, Ikegaya Y, Yamada MK: Glia, 2002; 37(2); 178-82
16. Šerý O, Sultana N, Kashem MA, GLAST but not least – distribution, function, genetics and epigenetics of l-glutamate transport in brain – focus on GLAST/EAAT1: Neurochem Res, 2015; 40(12); 2461-72
17. Koller WC, Hutton JT, Tolosa E, Capilldeo R, Immediate-release and controlled-release carbidopa/levodopa in PD: a 5-year randomized multicenter study. Carbidopa/levodopa Study Group: Neurology, 1999; 53(5); 1012-19
18. Jankovic J, Motor fluctuations and dyskinesias in Parkinson disease: Clinical manifestations: Mov Disord, 2005; 20(Suppl 11); S11-16
19. Okamoto H, Iyo M, Ueda K, Yokukan-san: A review of the evidence for use of this Kampo herbal formula in dementia and psychiatric conditions: Neuropsychiatr Dis Treat, 2014; 10; 1727-42
20. Iseki C, Furuta T, Suzuki M, Acupuncture alleviated the nonmotor symptoms of Parkinson’s disease including pain, depression, and autonomic symptoms: Case Rep Neurol Med, 2014; 2014 953109
21. Pan W, Kwak S, Liu Y, Traditional Chinese medicine improves activities of daily living in Parkinson’s disease: Parkinsons Dis, 2011; 2011 789506
22. Sun C, Wang D, Jiang W, Quantitative evaluation of Chinese herb medicine in the treatment of sialorrhea and frequent nighttime urination in patients with Parkinson’s disease: Evid Based Complement Alternat Med, 2017; 2017 3045260
23. Li X, Li Y, Chen J, Tetrahydroxystilbene glucoside attenuates MPP+-induced apoptosis in PC12 cells by inhibiting ROS generation and modulating JNK activation: Neurosci Lett, 2010; 483(1); 1-5
24. Tian YY, Jiang B, An LJ, Bao YM, Neuroprotective effect of catalpol against MPP(+)-induced oxidative stress in mesencephalic neurons: Eur J Pharmacol, 2007; 568(1–3); 142-48
25. Schwarting RK, Huston JP, The unilateral 6-hydroxydopamine lesion model in behavioral brain research. Analysis of functional deficits, recovery and treatments: Prog Neurobiol, 1996; 50(2–3); 275-331
26. Pellegrini C, Antonioli L, Colucci R, Effects of L-DOPA/benserazide co-treatment on colonic excitatory cholinergic motility and enteric inflammation following dopaminergic nigrostriatal neurodegeneration: Neuropharmacology, 2017; 123; 22-33
27. Gu Z, Liu W, Yan Z, β-Amyloid impairs AMPA receptor trafficking and function by reducing Ca2+/calmodulin-dependent protein kinase II synaptic distribution: J Biol Chem, 2009; 284(16); 10639-49
28. Ikonomidou C, Turski L, Why did NMDA receptor antagonists fail clinical trials for stroke and traumatic brain injury?: Lancet Neurol, 2002; 1(6); 383-86
29. Betarbet R, Porter RH, Greenamyre JT, GluR1 glutamate receptor subunit is regulated differentially in the primate basal ganglia following nigrostriatal dopamine denervation: J Neurochem, 2000; 74(3); 1166-74
30. Lai SK, Tse YC, Yang MS, Gene expression of glutamate receptors GluR1 and NR1 is differentially modulated in striatal neurons in rats after 6-hydroxydopamine lesion: Neurochem Int, 2003; 43(7); 639-53
31. Silverdale MA, Crossman AR, Brotchie JM, Striatal AMPA receptor binding is unaltered in the MPTP-lesioned macaque model of Parkinson’s disease and dyskinesia: Exp Neurol, 2002; 174(1); 21-28
32. Ungerstedt U, Arbuthnott GW, Quantitative recording of rotational behavior in rats after 6-hydroxy-dopamine lesions of the nigrostriatal dopamine system: Brain Res, 1970; 24; 485-93
33. Burns RS, Chiueh CC, Markey SP, A primate model of parkinsonism: selective destruction of dopaminergic neurons in the pars compacta of the substantia nigra by N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine: Proc Natl Acad Sci USA, 1983; 80(14); 4546-50
34. Arai N, Misugi K, Goshima Y, Misu Y, Evaluation of a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-treated C57 black mouse model for parkinsonism: Brain Res, 1990; 515(1–2); 57-63
35. Alam M, Heissler HE, Schwabe K, Krauss JK, Deep brain stimulation of the pedunculopontine tegmental nucleus modulates neuronal hyperactivity and enhanced beta oscillatory activity of the subthalamic nucleus in the rat 6-hydroxydopamine model: Exp Neurol, 2012; 233(1); 233-42
36. Yuan H, Sarre S, Ebinger G, Michotte Y, Histological, behavioural and neurochemical evaluation of medial forebrain bundle and striatal 6-OHDA lesions as rat models of Parkinson’s disease: J Neurosci Methods, 2005; 144(1); 35-45
37. Kum WF, Durairajan SS, Bian ZX, Treatment of idiopathic Parkinson’s disease with traditional Chinese herbal medicine: A randomized placebo-controlled pilot clinical study: Evid Based Complement Alternat Med, 2011; 2011 724353
38. Liu LF, Song JX, Lu JH, Tianma Gouteng Yin, a Traditional Chinese Medicine decoction, exerts neuroprotective effects in animal and cellular models of Parkinson’s disease: Sci Rep, 2015; 5; 16862
39. Tang C, Ye Y, Feng Y, Quinn RJ, TCM, brain function and drug space: Nat Prod Rep, 2016; 33(1); 6-25
Figures
 Figure 1. Methods for the subcellular fractionation of proteins.
Figure 1. Methods for the subcellular fractionation of proteins. Figure 2. levodopa combined with Bushen Zhichan recipe significantly improved the behavioral testing results. Open field test (A–D) and forelimb function experiment (E) were performed after 28 days of gavage. levodopa combined with Bushen Zhichan recipe significantly improved the stools times (A, 0.8571±0.8997), total distance (B, 2129±159.9), middle distance (C, 433.1±41.99), middle duration (D, 52.08±15.72) value and forelimb function results (1.400±0.5477) compared with PD controls. * P<0.05; ** P<0.01; *** P<0.001.
Figure 2. levodopa combined with Bushen Zhichan recipe significantly improved the behavioral testing results. Open field test (A–D) and forelimb function experiment (E) were performed after 28 days of gavage. levodopa combined with Bushen Zhichan recipe significantly improved the stools times (A, 0.8571±0.8997), total distance (B, 2129±159.9), middle distance (C, 433.1±41.99), middle duration (D, 52.08±15.72) value and forelimb function results (1.400±0.5477) compared with PD controls. * P<0.05; ** P<0.01; *** P<0.001. Figure 3. Midbrain tissue detection of DAT and cytochrome C by western blotting. (A) western blotting for DAT and cytochrome C, (B) analyzing for the expression of DAT and cytochrome C. The expressions of cytochrome C in levodopa combined with Bushen Zhichan recipe group (0.3659±0.08758) were significantly lower than the model group (0.8533±0.1366). * P<0.05; ** P<0.01; *** P<0.001.
Figure 3. Midbrain tissue detection of DAT and cytochrome C by western blotting. (A) western blotting for DAT and cytochrome C, (B) analyzing for the expression of DAT and cytochrome C. The expressions of cytochrome C in levodopa combined with Bushen Zhichan recipe group (0.3659±0.08758) were significantly lower than the model group (0.8533±0.1366). * P<0.05; ** P<0.01; *** P<0.001. Figure 4. Immunofluorescence pictures showed that levodopa combined with Bushen Zhichan recipe protected the DAT cells and reduced the expressions of excitatory amino acid receptors. The scale of the pictures is 100 microns, and the magnification is 400 times.
Figure 4. Immunofluorescence pictures showed that levodopa combined with Bushen Zhichan recipe protected the DAT cells and reduced the expressions of excitatory amino acid receptors. The scale of the pictures is 100 microns, and the magnification is 400 times. Figure 5. The striatal tissue detection of GABAA, GluR1, GluR2, GluN1, and GluN2B by western blotting. GABAA levels in the levodopa combined with Bushen Zhichan recipe group were significantly higher than the other 3 groups. Data are expressed as mean±standard deviation, * P<0.05; ** P<0.01; *** P<0.001.
Figure 5. The striatal tissue detection of GABAA, GluR1, GluR2, GluN1, and GluN2B by western blotting. GABAA levels in the levodopa combined with Bushen Zhichan recipe group were significantly higher than the other 3 groups. Data are expressed as mean±standard deviation, * P<0.05; ** P<0.01; *** P<0.001. Figure 6. The striatal tissue detection of GFAP, GABAT-3, EAAT1, and EAAT2 by western blotting. The expression levels of EAAT1 in the levodopa combined with Bushen Zhichan recipe group (0.8069±0.2528) were significantly higher than that in the model group (0.3321±0.1064). Data are expressed as mean±standard deviation, * P<0.05; ** P<0.01; *** P<0.001.
Figure 6. The striatal tissue detection of GFAP, GABAT-3, EAAT1, and EAAT2 by western blotting. The expression levels of EAAT1 in the levodopa combined with Bushen Zhichan recipe group (0.8069±0.2528) were significantly higher than that in the model group (0.3321±0.1064). Data are expressed as mean±standard deviation, * P<0.05; ** P<0.01; *** P<0.001. In Press
05 Mar 2024 : Clinical Research
Role of Critical Shoulder Angle in Degenerative Type Rotator Cuff Tears: A Turkish Cohort StudyMed Sci Monit In Press; DOI: 10.12659/MSM.943703
06 Mar 2024 : Clinical Research
Comparison of Outcomes between Single-Level and Double-Level Corpectomy in Thoracolumbar Reconstruction: A ...Med Sci Monit In Press; DOI: 10.12659/MSM.943797
21 Mar 2024 : Meta-Analysis
Economic Evaluation of COVID-19 Screening Tests and Surveillance Strategies in Low-Income, Middle-Income, a...Med Sci Monit In Press; DOI: 10.12659/MSM.943863
10 Apr 2024 : Clinical Research
Predicting Acute Cardiovascular Complications in COVID-19: Insights from a Specialized Cardiac Referral Dep...Med Sci Monit In Press; DOI: 10.12659/MSM.942612
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








