24 June 2020: Clinical Research
Germline BRCA2 Truncating Mutation in Familial Esophageal Squamous Cell Carcinoma: A Case Controlled Study in China
Zhong Liang1ABCDEF*, Weidong Hu2ABCD, Shuping Li3ABCDEF, Zhenhong Wei4BCDEF, Zijiang Zhu1CDDOI: 10.12659/MSM.923926
Med Sci Monit 2020; 26:e923926
Abstract
BACKGROUND: Germline mutations of BRCA2 have been reported in various malignancies. We investigated BRCA2 germline mutations in familial clusters with esophageal squamous cell carcinoma (ESCC).
MATERIAL AND METHODS: We screened the DNA of familial ESCC patients for BRCA2 germline mutations with whole gene sequencing. Multiple BRCA2 mutations including one novel splice variant, c.426-2A>G were identified. Other family members, sporadic ESCC patients, and controls were also assessed for the novel mutation.
RESULTS: The mutation c.426-2A>G was found in 2 affected ESCC sisters and 7 other family members. The splice variant mutation results in exon 5 skipping with a frame shift leading to a premature stop codon in exon 6 and truncation. Novel mutation tracking ruled out single nucleotide polymorphism (SNP) in 100 chromosomes of healthy individuals.
CONCLUSIONS: BRCA2 germline mutation in ESCC patients may play a role in genetic susceptibility to familial ESCC. Genetic analysis of BRCA2 in patients with familial ESCC could provide opportunities for targeted therapies.
Keywords: Carcinoma, Squamous Cell, Esophageal Neoplasms, Genes, BRCA2, Protein Isoforms, Asians, BRCA2 Protein, Case-Control Studies, esophageal squamous cell carcinoma, Exons, Genetic Predisposition to Disease, Germ Cells, Germ-Line Mutation, Mutation, Pedigree, Polymorphism, Single Nucleotide, Whole Genome Sequencing
Background
Esophageal cancer has a high mortality rate with overall 5-year survival of 15% to 25% which declines to 5% in advanced stages [1]. Esophageal cancer has 2 major histologic subtypes with different epidemiology, pathogenesis, and clinicopathological features. Esophageal squamous cell carcinoma (ESCC) is, worldwide, the more common subtype with a distinct geographic distribution that is predominantly prevalent in esophageal cancer belt stretched from northern and western China towards central Asia and northern Iran [2]. In contrast, adenocarcinoma is more frequently seen in western countries. While adenocarcinoma is thought to develop in the setting of gastroesophageal reflux disease and Barrett’s esophagus, major risk factors for the development of ESCC include tobacco smoking, alcohol intake, diet (lack of fresh fruits and vegetable, hot beverages) and exposure to certain chemical factors such as polycyclic aromatic hydrocarbons (PAH) [3,4].
There is growing evidence in the literature that supports an important role for genetic and epigenetic predisposition in the carcinogenesis of ESCC, in addition to the environmental factors, particularly in the areas with a high-risk population [5–10]. Yet, the exact molecular biology of ESCC carcinogenesis has not yet been clearly identified. Environmental insults such as smoking and excessive alcohol consumption can cause DNA alterations and in the absence of intact DNA repair mechanism; this will lead to disrupt of the normal cell cycle which promotes tumorigenesis [11]. The role of genetic aberrations and subsequent impairment of DNA repair and cell cycle control has been shown in upper aerodigestive SCC [12].
BRCA2 is a gene that exerts an important role in DNA repair. It is involved in homologous recombination repair of double-strand DNA breaks. It is located in chromosome 13 at 13q12.3 and encodes a protein with 3418 amino acids [13]. In addition to breast cancer, as was originally identified, germline mutations in the BRCA2 gene have been associated with increased risk of various other cancers such as ovarian cancer, melanoma, and cancers of the pancreas and liver [14–18]. In the current study, we evaluated family clusters with ESCC for possible germline mutations in BRCA2 and identified a novel BRCA2 variant in a family, one with multiple primary cancers, as well as several other mutations with unknown clinical significance and polymorphisms in BRCA2 gene.
Material and Methods
In this cohort, we retrospectively identified and enrolled 5 family clusters with familial ESCC (ESCC diagnosed in at least 2 first-degree relatives), and 50 patients with sporadic ESCC with no familial history of esophageal cancers as a control group. Informed consents were obtained in accordance with institutional ethics committee guidelines. The institutional ethics committee approved the protocols of the study and patients were enrolled in our hospital. The study has been reported in line with the STROCSS (Strengthening the Reporting of Cohort Studies in Surgery) criteria [19]. A blood sample of 3 mL was obtained from each individual. For all samples, DNA was extracted and purified using standard methods.
Five families with affected probands were analyzed for BRCA2 mutations in germline DNA by whole gene sequencing in accordance to previously published protocol [20]. Patient’s nucleotide sequence was compared with reference sequences: BRCA2 GeneBank U43746. Large genomic deletion detection of BRCA2 gene was performed using multiplex ligation-dependent probe amplification (MLPA) assay.
The exons 4–6 of BRCA2 were next scanned by a specific primer to investigate the novel variation detected by whole gene sequencing in family members. Polymerase chain reaction (PCR) was carried out in a volume of 20 μL with approximately 100 ng of genomic DNA, 1× PCR buffer, 2 mM MgCl2, 200 μmol mix, 10 pmol of both primer (forward: 5′-AAATACACGGTTTCCAGCAG-3′, reverse: -GAAACAAACTCCCACATACCAC-3′) and 1 unit Taq polymerase (GenetBio, South Korea). An initial denaturation of 94°C for 5 minutes was followed by 30 cycles (94°C for 30 seconds, 61°C for 30 seconds, 72°C for 30 seconds) and a final extension of 72°C for 5 minutes. The amplified products were visualized by electrophoresis in ethidium bromide stained 2% agarose gel; 10 μl of the PCR reaction product was subjected to digestion with 1 unit of AvaII (New England Biolabs) at 37°C for 6 hours. The results were analyzed in 2.5% agarose gel.
For RNA analysis, whole blood was collected in EDTA tubes. RNA quality and quantity were evaluated at an absorbance of 260 and 280 nm using an ultraviolet spectrophotometer. First strand cDNA was synthesized from 1 μg total RNA using oligo(dt)18 as amplification of cDNA was conducted with flanking exonic primers designed by oligo software. RT-PCR was performed by the specific primer (forward: TCTTCGCACAGTGAAAACTA, reverse: TTCAGATGCTTCTTCATTT) in a final volume of 20 μL that contained 2 μL of cDNA. Samples were denatured at 94°C for 30 seconds, 58°C for 30 seconds, and 72°C for 30 seconds and extension step at 72°C for 5 minutes. The fragment size was 304 bp. RT-PCR products were confirmed by direct sequencing (Macrogen).
Results
The whole gene mutation analysis of patients with familial ESCC is summarized in Tables 1–5 (for patients 1–5 respectively). Our analysis revealed one novel mutation which is located in the intronic sequence of BRCA2 in one patient with familial ESCC. Familial pedigree is shown in Figure 1. The sequence of the region of interest was submitted to splice site predictor program (NNSPLICE version 9) which confirmed c.426-2A>G mutation at the splice site, as heterozygote in Patient Number 4.
To characterize the impact of the novel mutation on splicing site, RNA was isolated from peripheral blood and cDNA was synthesized with specific primers spanning the exons that involved variation. cDNA analysis revealed an unexpectedly small product size in electrophoresis. Comparison of mutant RNA with normal RNA showed a smaller size in the amplified fragment. Direct sequencing of the amplified fragment revealed aberrant RNA splicing effect in exon 5, which predicted to result in skipping of exon 5 in the splicing process and premature truncation of protein during translation (Figure 2). Sequencing revealed that mutation at the splice site leads to exon 5 skipping and disrupted the coding reading frame in exon 6, which in turn created a frameshift with a premature stop codon in exon 6 and subsequent truncation of the in BRCA2 protein. We then investigated the same mutation in other family members of this proband. The proband was a female diagnosed with ESCC at age 53 years. Her symptoms included weight loss and dysphagia, and an upper endoscopy revealed her tumor at 35 cm and a biopsy proved ESCC. She underwent neoadjuvant chemoradiation and subsequent esophagectomy and pathology revealed a complete pathologic response with no nodal involvement. Two years after diagnosis of ESCC, she was diagnosed with invasive ductal carcinoma of the left breast and underwent left mastectomy with sentinel lymph node biopsy which showed no nodal involvement. Her tumor was estrogen receptor positive, progesterone receptor positive, and human epidermal growth factor receptor 2 (HER2) positive. Six months after her diagnosis of ESCC, her sister was also diagnosed with ESCC with symptoms of dysphagia, at the age of 48 years old. She also underwent neoadjuvant chemotherapy and esophagectomy; her tumor also showed a complete pathologic response with no nodal involvement. Mutation analysis revealed the same mutation in her as well. Screening of other family members revealed that other sisters were negative for this mutation while brothers carried the mutation (Figure 1). Members of the family have been under surveillance for ESCC and breast cancer, and to date have not been diagnosed with any cancers. We also screened the DNA of 50 ESCC patients with no family history of esophageal cancer, and the mutation was not identified in any other person.
Discussion
In this study, we report a novel BRCA2 germline mutation at the splicing site c.426-2A>G in familial ESCC and concomitant breast cancer in one of the patients. This mutation leads to a premature stop codon and affects the splicing process. RT-PCR analysis of the proband revealed exon skipping and was predicted to result in a truncated protein. Alteration in exon-intron junctions will impact mRNA splicing fidelity [21].
RAD51 is a protein that is essential for DNA recombination. RAD51-BRCA2 complex and the interaction between the 2 molecules is crucial for their role in DNA repair [22]. The binding site for RAD51 is an 8 conserved sequence motifs in BRCA2, called BRC repeats (located between residues 990 and 2100) [23]. The truncated BRCA2 protein lacks the crucial BRC domain for binding to RAD51 and the RAD51-BRCA2 complex will not be formed to participate in DNA repair process [24]. Lack of functional BRCA2, similar to RAD51, leads to chromosome breakage and genome instability during cell division [25].
Hu et al. reported loss of heterozygosity (LOH) in chromosome 13 of patients with ESCC [26] and later, for the first time, reported germline (Cys315Ser, Pro3300Ser, and Arg118His) and somatic mutations of BRCA2 in patients with familial as well as sporadic esophageal cancer. Nevertheless, they showed that BRCA2 was not a target for LOH [27]. They next focused on familial ESCC in a high-risk area in China and reported 12% germline mutations, including 3 novel ones, and concluded that germline mutation in BRCA2 was associated with genetic susceptibility to familial esophageal squamous cell carcinoma. They also showed segregation of C315S mutation in the siblings of the proband in their study [28]. On the other hand, Zhong et al. reported germline mutations in sporadic ESCC from an area with low risk of esophageal cancer in China [29]. In another study from a high-risk area in India, Kaushal et al. reported 2 germline mutations in 3 familial ESCC patients while none of the sporadic ESCC patients or healthy controls had any germline mutations [6]. Akbari et al. studied germline mutations in the coding regions of BRCA2 in a cohort of patients with Turkmen ethnic background in northern Iran. They reported 2 novel deletions which lead to a premature stop codon and translates to a truncated protein. They also detected k3326X mutation in 8 familial ESCC patients, as the most frequent mutation associated with genetic susceptibility to familial ESCC [5]. The product of this mutation was a truncated protein. A robust association between k3326X mutation and upper aerodigestive tract cancers was demonstrated by Delahaye-Sourdeix et al. in another report, studying multiple patient populations from different continents [30].
In the familial ESCC patients we studied, a similar pattern of genetic alterations was observed. His372Asn is a common polymorphism (rs144848) that was found in all the studied familial ESCC patients, and it has been shown to be associated with increased risk of non-Hodgkin lymphoma in a meta-analysis [31]. Phe599Ser is another single nucleotide variant which was observed in all the familial ESCC tested and has not been widely studied but has been considered to likely be pathogenic [32]. Patients with familial ESCC also shared multiple unclassified variant intervening sequences (IVS). Further studies are required to better understand the clinical significance of these unclassified variants.
Multiple other primary cancers have been reported in patients with esophageal cancer [33–35]. Gastric cancer, colon cancer, and head and neck cancer are the most common malignancies associated with esophageal cancer as a metachronous or synchronous tumor. It is hypothesized that a history of smoking and the concept of field cancerization plays an important role in the development of multiple primary cancer. Based on this theory, exposure of the epithelium (head and neck and gastrointestinal tract) to the carcinogens, such as tobacco and alcohol, promotes carcinogenesis in multiple sites [33]. Breast cancer as a second primary tumor in patients with ESCC, however, it has a lower prevalence in patients with multiple primary cancers. Our findings support the hypothesis that genetic susceptibility might play an important role in metachronous familial ESCC and breast cancer.
Conclusions
Our findings have potentially important clinical implications. Genetic analysis of BRCA2 in patients with familial ESCC should be considered and could identify family members at higher risk, both for esophageal cancer and future risk for breast cancer. Moreover, genetic alterations of BRCA2 have potential therapeutic significance with recent advancements in PARP inhibitors in BRCA2 related tumors [36]. Screening for BRCA2 mutations in familial esophageal cancer patients could provide opportunities for targeted therapies.
Figures
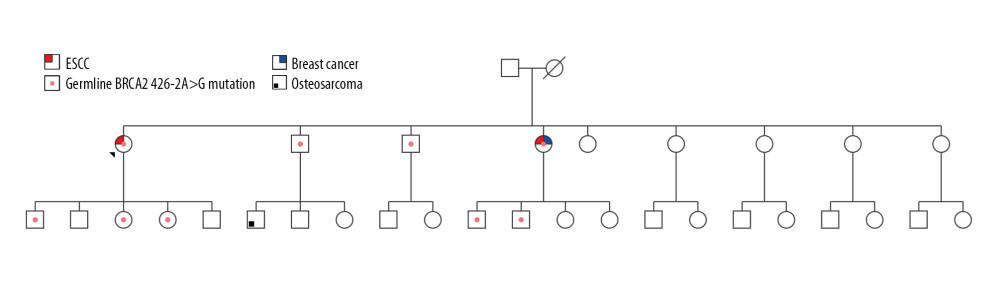 Figure 1. Pedigree of 2 patients with familial ESCC who shared the truncating mutation 426-2A>G. “ + ”shows family members who carry this mutation and “ − ”represents patients without this mutation.
Figure 1. Pedigree of 2 patients with familial ESCC who shared the truncating mutation 426-2A>G. “ + ”shows family members who carry this mutation and “ − ”represents patients without this mutation. 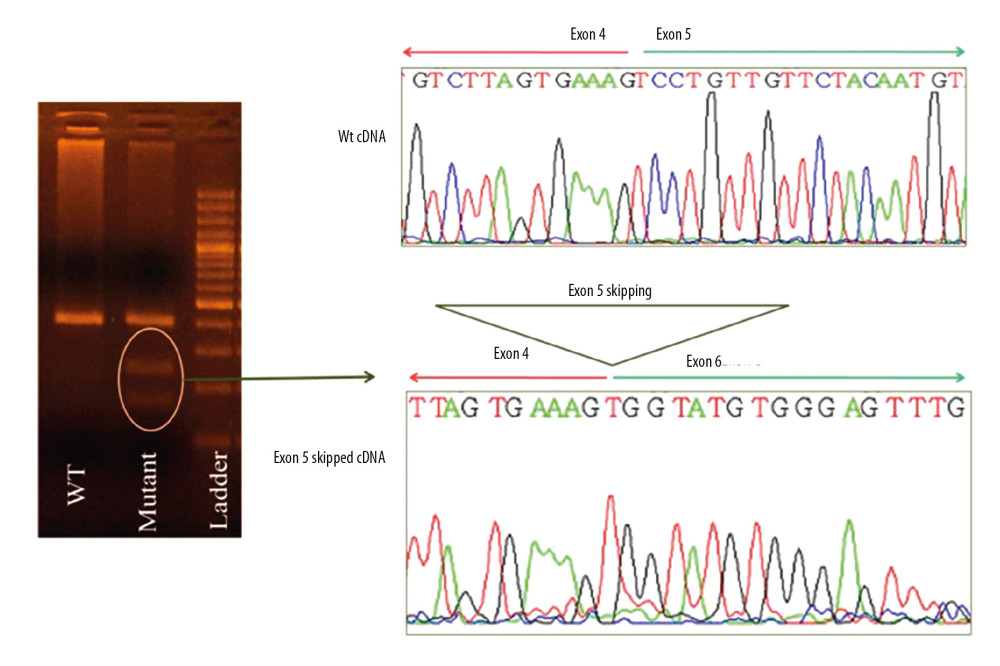 Figure 2. Analysis of cDNA of Patient Number 4 with 426-2A>G mutation. Sequencing revealed skipping of exon 5 and a premature stop codon at the exon 6.
Figure 2. Analysis of cDNA of Patient Number 4 with 426-2A>G mutation. Sequencing revealed skipping of exon 5 and a premature stop codon at the exon 6. Tables
Table 1. Summary of mutations in the BRCA2 gene in patient #1 with familial esophageal cancer.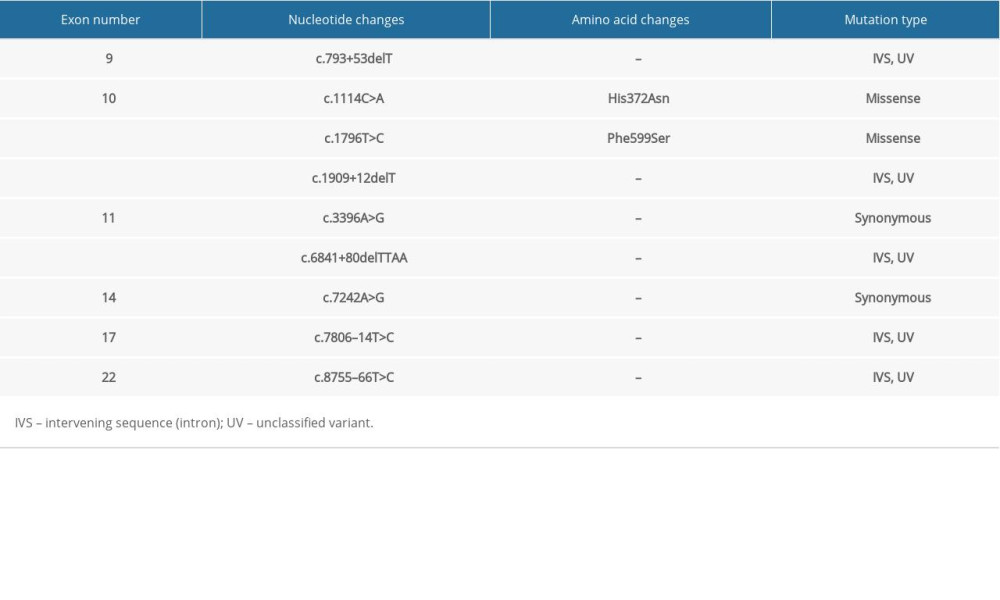 Table 2. Summary of mutations in the BRCA2 gene in patient #2 with familial esophageal cancer.
Table 2. Summary of mutations in the BRCA2 gene in patient #2 with familial esophageal cancer.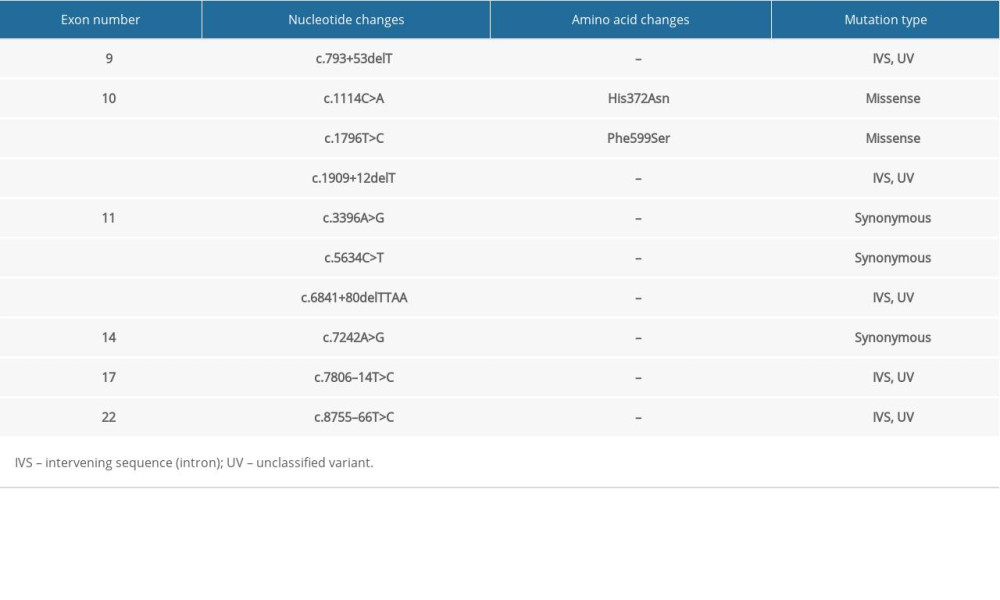 Table 3. Summary of mutations in the BRCA2 gene in patient #3 with familial esophageal cancer.
Table 3. Summary of mutations in the BRCA2 gene in patient #3 with familial esophageal cancer.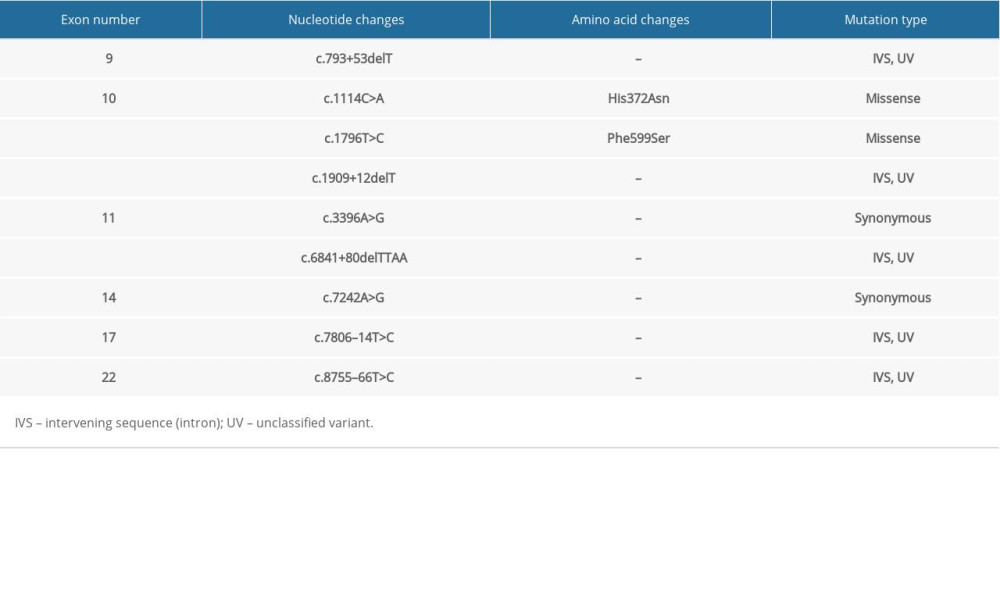 Table 4. Summary of mutations in the BRCA2 gene in patient #4 with familial esophageal cancer.
Table 4. Summary of mutations in the BRCA2 gene in patient #4 with familial esophageal cancer.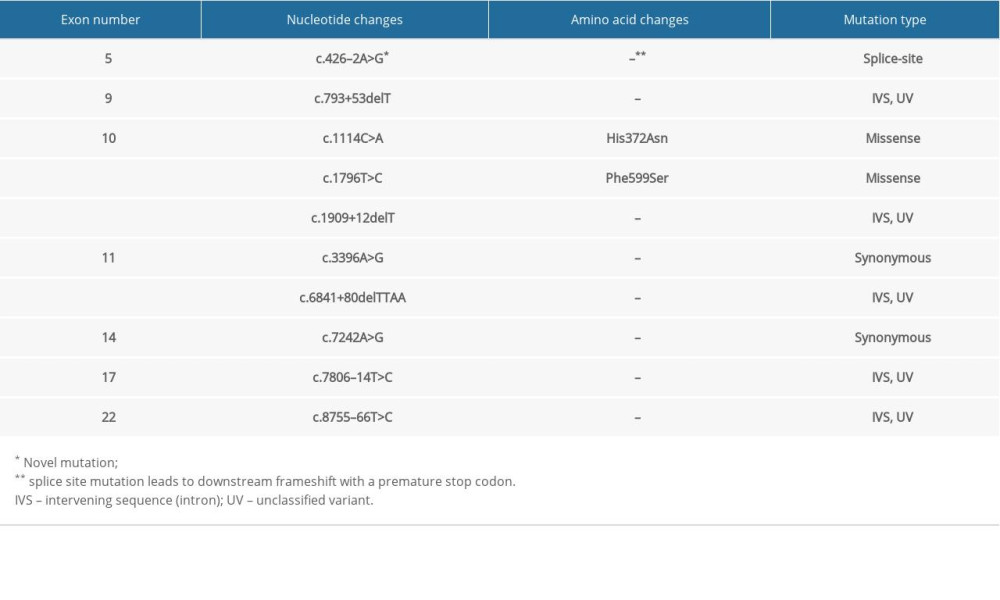 Table 5. Summary of mutations in the BRCA2 gene in patient #5 with familial esophageal cancer.
Table 5. Summary of mutations in the BRCA2 gene in patient #5 with familial esophageal cancer.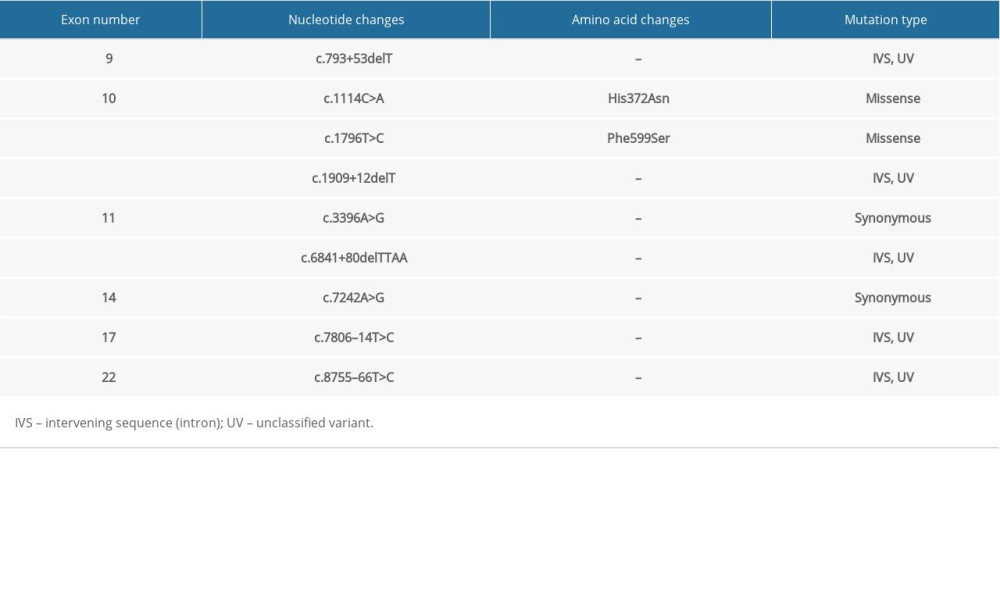
Reference
1. Pennathur A, Gibson MK, Jobe BA, Oesophageal carcinoma: Lancet, 2013; 381(9864); 400-12
2. Umar SB, Fleischer DE, Esophageal cancer: Epidemiology, pathogenesis and prevention: Nat Clin Pract Gastroenterol Hepatol, 2008; 5(9); 517-26
3. Islami F, Pourshams A, Nasrollahzadeh D, Tea drinking habits and oesophageal cancer in a high risk area in northern Iran: Population based case-control study: BMJ, 2009; 338; b929
4. Naghibzadeh Tahami A, Khanjani N, Yazdi Feyzabadi V, Opium as a risk factor for upper gastrointestinal cancers: A population-based case-control study in Iran: Arch Iran Med, 2014(1); 2-6
5. Akbari MR, Malekzadeh R, Nasrollahzadeh D, Germline BRCA2 mutations and the risk of esophageal squamous cell carcinoma: Oncogene, 2008; 27(9); 1290-96
6. Kaushal M, Chattopadhyay I, Phukan R, Contribution of germ line BRCA2 sequence alterations to risk of familial esophageal cancer in a high risk area of India: Dis Esophagus, 2010; 23(1); 71-75
7. Hofmann W, Horn D, Huttner C, The BRCA2 variant 8204G>A is a splicing mutation and results in an in-frame deletion of the gene: J Med Genet, 2003; 40(3); e23
8. Tran GD, Sun XD, Abnet CC, Prospective study of risk factors for esophageal and gastric cancers in the Linxian general population trial cohort in China: Int J Cancer, 2005; 113(3); 456-63
9. Khalilipour N, Baranova A, Jebelli A, Familial esophageal squamous cell carcinoma with damaging rare/germline mutations in KCNJ12/KCNJ18 and GPRIN2 genes: Cancer Genet, 2018; 221; 46-52
10. Abbaszadegan MR, Raziee HR, Ghafarzadegan K, Aberrant p16 methylation, a possible epigenetic risk factor in familial esophageal squamous cell carcinoma: Int J Gastrointest Cancer, 2005; 36(1); 47-54
11. Hoeijmakers JH, Genome maintenance mechanisms for preventing cancer: Nature, 2010; 411(6835); 366-74
12. Scully C, Field JK, Tanzawa H, Genetic aberrations in oral or head and neck squamous cell carcinoma (SCCHN): 1. Carcinogen metabolism, DNA repair and cell cycle control: Oral Oncol, 2000; 36(3); 256-63
13. Petrucelli N, Daly MB, Feldman GL, Hereditary breast and ovarian cancer due to mutations in BRCA1 and BRCA2: Genet Med, 2010; 12(5); 245-59
14. Wooster R, Bignell G, Lancaster J, Identification of the breast cancer susceptibility gene BRCA2: Nature, 1995; 378(6559); 92-189
15. Katagiri T, Nakamura Y, Miki Y, Mutations in the BRCA2 gene in hepatocellular carcinomas: Cancer Res, 1996; 56(20); 4575-77
16. Narod SA, Modifiers of risk of hereditary breast and ovarian cancer: Nat Rev Cancer, 2002; 2(2); 113-23
17. Salo-Mullen EE, O’Reilly EM, Kelsen DP, Identification of germline genetic mutations in patients with pancreatic cancer: Cancer, 2015; 121(24); 4382-88
18. Monnerat C, Chompret A, Kannengiesser C, BRCA1, BRCA2, TP53, and CDKN2A germline mutations in patients with breast cancer and cutaneous melanoma: Fam Cancer, 2007; 6(4); 453-61
19. Agha RA, Borrelli MR, Vella-Baldacchino M, The STROCSS statement: Strengthening the reporting of cohort studies in surgery: Int J Surg, 2017; 46; 198-202
20. Kwong A, Ng EK, Wong CL, Identification of BRCA1/2 founder mutations in Southern Chinese breast cancer patients using gene sequencing and high resolution DNA melting analysis: PLoS One, 2012; 7(9); e43994
21. Acedo A, Sanz DJ, Duran M, Comprehensive splicing functional analysis of DNA variants of the BRCA2 gene by hybrid minigenes: Breast Cancer Res, 2012; 14(3); R87
22. Pellegrini L, Yu DS, Lo T, Insights into DNA recombination from the structure of a RAD51-BRCA2 complex: Nature, 2002; 420(6913); 287-93
23. Wong AK, Pero R, Ormonde PA, RAD51 interacts with the evolutionarily conserved BRC motifs in the human breast cancer susceptibility gene BRCA2: J Biol Chem, 1997; 272(51); 31941-44
24. Evers B, Jonkers J, Mouse models of BRCA1 and BRCA2 deficiency: Past lessons, current understanding and future prospects: Oncogene, 2006; 25(43); 5885-97
25. Silva MC, Bryan KE, Morrical MD, Defects in recombination activity caused by somatic and germline mutations in the multimerization/BRCA2 binding region of human RAD51 protein: DNA Repair (Amst), 2017; 60; 64-76
26. Hu N, Roth MJ, Emmert-Buck MR, Allelic loss in esophageal squamous cell carcinoma patients with and without family history of upper gastrointestinal tract cancer: Clin Cancer Res, 1999; 5(11); 3476-82
27. Hu N, Li G, Li WJ, Infrequent mutation in the BRCA2 gene in esophageal squamous cell carcinoma: Clin Cancer Res, 2002; 8(4); 1121-26
28. Hu N, Wang C, Han XY, Evaluation of BRCA2 in the genetic susceptibility of familial esophageal cancer: Oncogene, 2004; 23(3); 852-58
29. Zhong L, Zhu ZZ, Shen Y, Frequent germline mutation in the BRCA2 gene in esophageal squamous cell carcinoma patients from a low-risk Chinese population: Asian Pac J Cancer Prev, 2011; 12(7); 1771-76
30. Delahaye-Sourdeix M, Anantharaman D, Timofeeva MN, A rare truncating BRCA2 variant and genetic susceptibility to upper aerodigestive tract cancer: J Natl Cancer Inst, 2015; 107(5) djv037
31. Li Q, Guan R, Qiao Y, Association between the BRCA2 rs144848 polymorphism and cancer susceptibility: A meta-analysis: Oncotarget, 2017; 8(24); 39818-32
32. NCBI: NIH ClinVar, 2017 https//:www.ncbi.nlm.nih.gov/clinvar/RCV000521047/#clinical-assertions
33. Baba Y, Yoshida N, Kinoshita K, Clinical and prognostic features of patients with esophageal cancer and multiple primary cancers: A retrospective single-institution study: Ann Surg, 2018; 267(3); 478-83
34. Otowa Y, Nakamura T, Takiguchi G, Safety and benefit of curative surgical resection for esophageal squamous cell cancer associated with multiple primary cancers: Eur J Surg Oncol, 2016; 42(3); 407-11
35. Kumagai Y, Kawano T, Nakajima Y, Multiple primary cancers associated with esophageal carcinoma: Surg Today, 2001; 31(10); 872-76
36. Ledermann J, Harter P, Gourley C, Olaparib maintenance therapy in patients with platinum-sensitive relapsed serous ovarian cancer: A preplanned retrospective analysis of outcomes by BRCA status in a randomised phase 2 trial: Lancet Oncol, 2014; 15(8); 852-61
Figures
 Figure 1. Pedigree of 2 patients with familial ESCC who shared the truncating mutation 426-2A>G. “ + ”shows family members who carry this mutation and “ − ”represents patients without this mutation.
Figure 1. Pedigree of 2 patients with familial ESCC who shared the truncating mutation 426-2A>G. “ + ”shows family members who carry this mutation and “ − ”represents patients without this mutation. Figure 2. Analysis of cDNA of Patient Number 4 with 426-2A>G mutation. Sequencing revealed skipping of exon 5 and a premature stop codon at the exon 6.
Figure 2. Analysis of cDNA of Patient Number 4 with 426-2A>G mutation. Sequencing revealed skipping of exon 5 and a premature stop codon at the exon 6. Tables
 Table 1. Summary of mutations in the BRCA2 gene in patient #1 with familial esophageal cancer.
Table 1. Summary of mutations in the BRCA2 gene in patient #1 with familial esophageal cancer. Table 2. Summary of mutations in the BRCA2 gene in patient #2 with familial esophageal cancer.
Table 2. Summary of mutations in the BRCA2 gene in patient #2 with familial esophageal cancer. Table 3. Summary of mutations in the BRCA2 gene in patient #3 with familial esophageal cancer.
Table 3. Summary of mutations in the BRCA2 gene in patient #3 with familial esophageal cancer. Table 4. Summary of mutations in the BRCA2 gene in patient #4 with familial esophageal cancer.
Table 4. Summary of mutations in the BRCA2 gene in patient #4 with familial esophageal cancer. Table 5. Summary of mutations in the BRCA2 gene in patient #5 with familial esophageal cancer.
Table 5. Summary of mutations in the BRCA2 gene in patient #5 with familial esophageal cancer. Table 1. Summary of mutations in the BRCA2 gene in patient #1 with familial esophageal cancer.
Table 1. Summary of mutations in the BRCA2 gene in patient #1 with familial esophageal cancer. Table 2. Summary of mutations in the BRCA2 gene in patient #2 with familial esophageal cancer.
Table 2. Summary of mutations in the BRCA2 gene in patient #2 with familial esophageal cancer. Table 3. Summary of mutations in the BRCA2 gene in patient #3 with familial esophageal cancer.
Table 3. Summary of mutations in the BRCA2 gene in patient #3 with familial esophageal cancer. Table 4. Summary of mutations in the BRCA2 gene in patient #4 with familial esophageal cancer.
Table 4. Summary of mutations in the BRCA2 gene in patient #4 with familial esophageal cancer. Table 5. Summary of mutations in the BRCA2 gene in patient #5 with familial esophageal cancer.
Table 5. Summary of mutations in the BRCA2 gene in patient #5 with familial esophageal cancer. In Press
06 Mar 2024 : Clinical Research
Comparison of Outcomes between Single-Level and Double-Level Corpectomy in Thoracolumbar Reconstruction: A ...Med Sci Monit In Press; DOI: 10.12659/MSM.943797
21 Mar 2024 : Meta-Analysis
Economic Evaluation of COVID-19 Screening Tests and Surveillance Strategies in Low-Income, Middle-Income, a...Med Sci Monit In Press; DOI: 10.12659/MSM.943863
10 Apr 2024 : Clinical Research
Predicting Acute Cardiovascular Complications in COVID-19: Insights from a Specialized Cardiac Referral Dep...Med Sci Monit In Press; DOI: 10.12659/MSM.942612
06 Mar 2024 : Clinical Research
Enhanced Surgical Outcomes of Popliteal Cyst Excision: A Retrospective Study Comparing Arthroscopic Debride...Med Sci Monit In Press; DOI: 10.12659/MSM.941102
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








