20 July 2020: Clinical Research
Predictive Value of Fibrinogen-to-Albumin Ratio for Post-Contrast Acute Kidney Injury in Patients Undergoing Elective Percutaneous Coronary Intervention
Can Wang1BCDE, Gaoye Li1F, Xiaomei Liang1C, Chunyu Qin1C, Qiuhu Luo1B, Rui Song1C, Wuxian Chen1AG*DOI: 10.12659/MSM.924498
Med Sci Monit 2020; 26:e924498
Abstract
BACKGROUND: Post-contrast acute kidney injury (PC-AKI) is a contributor to adverse outcomes after percutaneous coronary intervention (PCI). This study aimed to investigate whether fibrinogen-to-albumin ratio (FAR), a novel inflammation-based risk index, can predict the occurrence of PC-AKI in patients undergoing elective PCI.
MATERIAL AND METHODS: We retrospectively enrolled 291 patients who underwent elective PCI from June 2017 to June 2019. PC-AKI was defined as an increase in serum creatinine ≥0.3 mg/dL (≥26.5 μmol/L), or ≥1.5 times baseline within 48 to 72 hours after PCI. The area under the receiver-operating characteristic curve (AUC), continuous net reclassification improvement (NRI), and integrated discrimination improvement (IDI) were calculated to make comparison for PC-AKI prediction.
RESULTS: PC-AKI occurred in 43 patients (14.8%). FAR showed an AUC of 0.691 (95% confidence interval: 0.64–0.74; P<0.001) in predicting PC-AKI. In stepwise multivariable logistic regression, FAR was independently associated with the occurrence of PC-AKI along with hypertension, diabetes, hemoglobin, estimated glomerular filtration rate, and left ventricular ejection fraction. FAR significantly improved PC-AKI prediction over Mehran risk score in the continuous NRI and IDI, but not AUC.
CONCLUSIONS: FAR is independently associated with the occurrence of PC-AKI, and can significantly improve PC-AKI prediction over Mehran risk score in patients undergoing elective PCI.
Keywords: Acute Kidney Injury, Biological Markers, Coronary Artery Disease, percutaneous coronary intervention, Albumins, Contrast Media, Creatinine, Elective Surgical Procedures, Fibrinogen, Glomerular Filtration Rate, Logistic Models, Predictive Value of Tests, Risk Factors, Stroke Volume, Ventricular Function, Left
Background
Acute kidney injury (AKI) that follows intravascular administration of iodine-based contrast media is a common complication with an overall incidence of approximate 3% to 15% after percutaneous coronary intervention (PCI) [1,2]. The European Society of Urogenital Radiology (ESUR) [3] has recently updated their guidelines and recommends that the term post-contrast acute kidney injury (PC-AKI) should replace the older term of contrast-induced nephropathy to describe a decrease in renal function after the use of contrast media. Common risk factors of PC-AKI include primary renal insufficiency, diabetes, anemia, heart failure, and high contrast volume [4]. Although PC-AKI is generally transient and reversible, it remains a serious clinical problem due to its high incidence and association with adverse outcomes. Patients with PC-AKI generally confer prolonged hospitalization, high economic burden, high risk of the requirement for dialysis, and increased mortality [1,2,5,6].
Although the pathological mechanisms underlying PC-AKI are complicated and multifactorial, it has been shown that active inflammation with an excessive response of cytokines and reactive oxygen radicals plays a key role in promoting the occurrence of PC-AKI [7–9]. Elevated systemic inflammatory status can enlarge local inflammation response and aggravate the renal injury. Emerging evidence suggests that systemic inflammatory biomarkers are associated with the occurrence of PC-AKI independent of the conventional risk factors [10,11]. Fibrinogen-to-albumin ratio (FAR), a novel inflammation-based risk index, has been found to be valuable in predicting the poor outcomes in cancers [12,13] and cardiovascular diseases [14–16]. Recently, studies have shown that preprocedural FAR levels are associated with PC-AKI occurrence in patients after carotid angiography [17] and emergency PCI [18]. However, to date, no study has investigated the potential association between the FAR and the risk for PC-AKI in non-acute patients undergoing elective PCI.
Mehran risk score is a classical model for predicting PC-AKI which has been proven useful in the clinical setting over a decade [19,20]. In this study, we aimed to evaluate the predictive value of FAR for PC-AKI, and investigate whether FAR can improve PC-AKI prediction over Mehran risk score in patients undergoing elective PCI.
Material and Methods
STUDY POPULATION:
We retrospectively enrolled 291 patients who underwent elective PCI in the First Affiliated Hospital of Guangxi Medical University from June 2017 to June 2019. Patients without sufficient baseline clinical data or repeat measurement of serum creatinine within 72 h after the procedure were excluded. In addition, patients with acute myocardial infarction, acute decompensated heart failure, autoimmune diseases, inflammatory or infectious diseases, end-stage renal disease, cancers, use of contrast media within 1 week, and AKI by other causes were also excluded. Standard medications were used at the time of hospitalization. All patients received drug-eluting stent placement, and the used dosage of contrast volume during the operation was recorded. The study protocol was reviewed and approved by the Human Research Ethics Committee of the First Affiliated Hospital of Guangxi Medical University (Approval number: 2020. KY-E-025). Given the retrospective design of our study, written informed consent was not required.
DATA COLLECTION AND RELATED DEFINITION:
The baseline clinical and laboratory data were acquired from the hospital’s database. Fasting blood samples were taken in the first morning at admission, and analyzed in the laboratory department of our institution. Plasma fibrinogen was measured using immune turbidity method. Serum albumin was measured using bromcresol green colorimetric method. Neutrophil to lymphocyte ratio was calculated as the ratio of neutrophil count to lymphocyte count. The FAR was calculated as the ratio of fibrinogen to the albumin level multiplied by 100. Mehran risk scores were calculated according to the corresponding algorithm [19].
PC-AKI was defined as an increase in serum creatinine ≥0.3 mg/dL (≥26.5 μmol/L), or ≥1.5 times baseline within 48 to 72 hours after PCI according to the 2018 guidelines of the ESUR [3]. Estimated glomerular filtration rate (eGFR) was calculated with CKD-EPI Equation [21]. Diabetes mellitus was defined as either a new diagnosis according to the latest guidelines [22] or previous diagnosis with antidiabetic medications. Hypertension was defined as a systolic blood pressure >140 mmHg, or diastolic blood pressure >90 mmHg, or a history of taking antihypertensive medications.
STATISTICAL ANALYSIS:
Continuous data are expressed as mean±SD or median (25th–75th percentiles), and were compared using the Student t test or the Mann-Whitney U test, as appropriate. Categorical data are expressed as number (percentage) and were compared by chi-square or Fisher exact test. To make comparison for PC-AKI prediction, the area under the receiver-operating characteristic (ROC) curve (AUC) was calculated and analyzed with DeLong’s test [23] using MedCalc, version 19.0.4. The optimal cutoff value was determined by maximal Youden’s index. Forward stepwise multivariable logistic regression, which included the variables that were statistically significant in the univariable analysis (P<0.05), was performed to assess the independent predictors for PC-AKI. To investigate the interaction effects of FAR levels and conventional risk factors on the PC-AKI prediction, we introduced interaction terms “FAR×relevant variables” into the multivariable logistic model. To investigate the incremental value of fibrinogen, albumin, and FAR for PC-AKI prediction over Mehran risk score, continuous net reclassification improvement (NRI), and integrated discrimination improvement (IDI) were calculated using R software, version 3.6.0. A two-tailed P value <0.05 was regarded as statistically significant. Statistical analysis was performed using SPSS, version 22, unless otherwise stated.
Results
PATIENTS’ CHARACTERISTICS:
From total 291 patients, PC-AKI occurred in 43 patients (14.8%). The clinical characteristics and baseline laboratory data are summarized in Table 1. Patients with PC-AKI were older and had a higher frequency of hypertension, diabetes, and history of chronic heart failure compared to non-PC-AKI patients. In addition, patients with PC-AKI had higher baseline levels of neutrophil to lymphocyte ratio, fibrinogen, and FAR, but lower levels of hemoglobin, albumin, eGFR, and left ventricular ejection fraction (LVEF) compared to non-PC-AKI patients. There was a trend toward higher hemoglobin A1c levels in patients with PC-AKI compared to those without, although that did not reach statistical difference (P=0.062). Notably, there was no difference in the contrast volume between PC-AKI and non-PC-AKI patients.
COMPARISON OF FAR, FIBRINOGEN, AND ALBUMIN FOR PREDICTING PC-AKI:
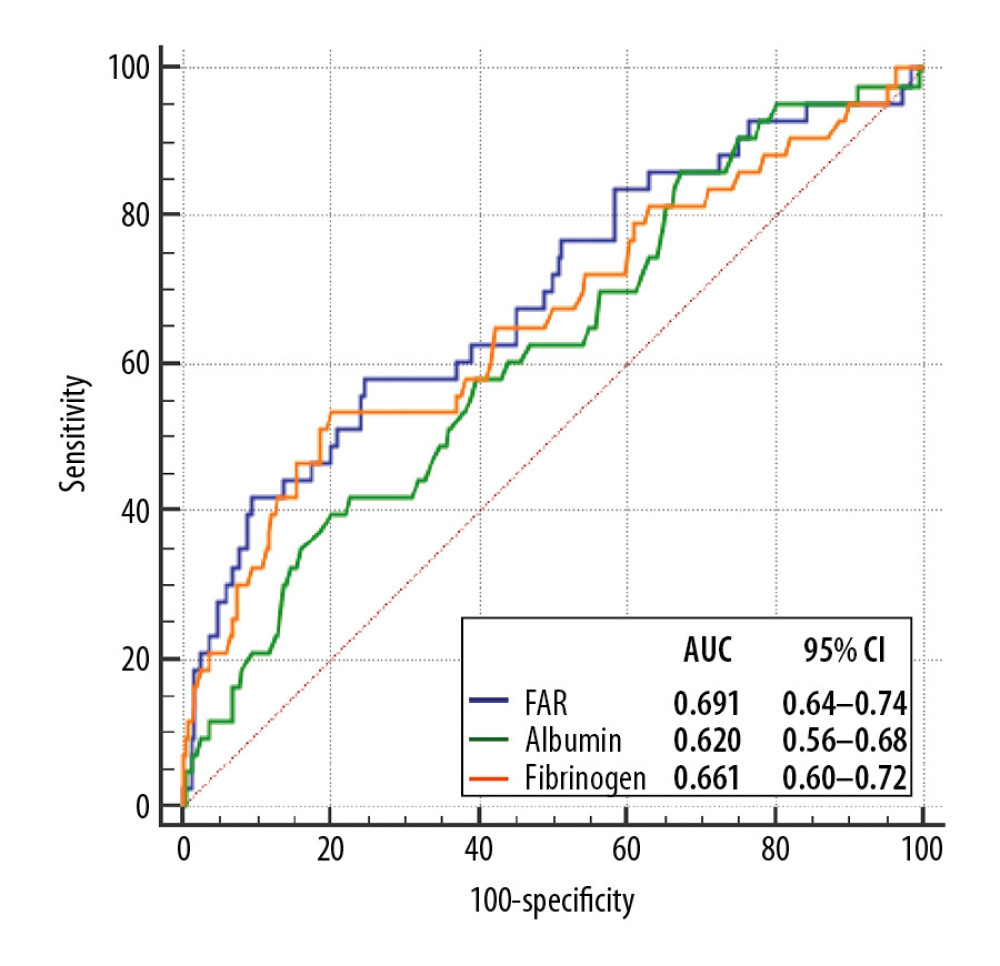
As shown in Figure 1, ROC analysis indicated that FAR can predict the occurrence of PC-AKI (optimal cutoff value: 9.83 with 58% sensitivity and 75% specificity, AUC, 0.691, 95% confidence interval [CI], 0.64–0.74; P<0.001). FAR displayed higher AUC value in comparison to fibrinogen (P=0.032), but not albumin (P=0.206), as a predictor of PC-AKI (Figure 1).
LOGISTIC REGRESSION ANALYSIS OF PREDICTIVE FACTORS FOR PC-AKI:
In univariable logistic regression analysis (Table 2), fibrinogen (per 1 g/L, OR: 2.00, 95% CI: 1.45–2.76; P<0.001), albumin (per 1 g/L, OR: 0.91, 95% CI: 0.85–0.98; P=0.015), and FAR (per 1 unit, OR: 1.27, 95% CI: 1.13–1.42; P<0.001) were significantly associated with the occurrence of PC-AKI. Stepwise multivariable logistic regression suggested that FAR (per 1 unit, OR: 1.15, 95% CI: 1.02–1.30; P=0.026) was an independent predictor of PC-AKI. In addition, hypertension (OR: 4.75, 95% CI: 1.04–21.59; P=0.044), diabetes (OR: 3.04, 95% CI: 1.41–6.57; P=0.005), hemoglobin (per 10 g/L, OR: 0.79, 95% CI: 0.63–0.99; P=0.039), eGFR (per 10 ml/min/1.73m2, OR: 0.79, 95% CI: 0.66–0.95; P=0.011), and LVEF (per 10%, OR: 0.62, 95% CI: 0.47–0.84; P=0.002) were also independently associated with the occurrence of PC-AKI (Table 2). In the subgroup analysis, no predictive interaction was observed between FAR and the conventional PC-AKI risk factors, including age (>75, ≤75), sex (male, female), diabetes, chronic heart failure, anemia, eGFR (eGFR <60, eGFR ≥60), and LVEF (LVEF ≤40, LVEF >40) (Data was not shown).
IMPROVEMENT OF PC-AKI PREDICTION BY FIBRINOGEN, ALBUMIN, AND FAR TO MEHRAN RISK SCORE:
We calculated Mehran risk scores for all patients. As shown in Table 3, addition of fibrinogen, albumin, and FAR to Mehran risk score was not associated with a significant improvement in the AUC for PC-AKI prediction. However, FAR significantly improved PC-AKI prediction in the continuous NRI (NRI: 0.400, 95%CI: 0.080–0.721, P=0.014) and IDI (IDI: 0.050, 95%CI: 0.009–0.091, P=0.017) over Mehran risk score, whereas fibrinogen had less predictive improvement, and albumin had no added value (Table 3).
Discussion
This study for the first time revealed that FAR was independently associated with PC-AKI occurrence in the setting of elective PCI, and significantly improved PC-AKI prediction over Mehran score.
Although not fully understood, the pathophysiological mechanisms of PC-AKI mainly include direct cytotoxic effects, renal vasoconstriction, endothelial dysfunction, renal tubular injury, and medullary hypoxia [4,7,8]. Fibrinogen and albumin are 2 proteins with inflammatory, thrombotic, and hemorheological properties [24–26], which are involved in the biological pathways predisposing to PC-AKI. Fibrinogen, an important coagulation factor, functions beyond blood clotting and regulates inflammation through facilitating leukocyte transmigration and enhancing the functions of leukocyte effector within damaged tissue [27–29]. Fibrinogen is significantly up-regulated in the kidney tissues after AKI [30], and urinary fibrinogen levels were increased significantly as early as 2 hours after coronary angiography [31]. Also, fibrinogen is increased in circulation during inflammatory state [28], and can promote platelet aggregation [32]. Elevated fibrinogen has an influence on blood viscosity, which can lead to endothelial shear-stress damage, tissue hypoperfusion, and medullary hypoxia [33,34]. It has been recently found that elevated serum fibrinogen level is independently associated with a higher risk of PC-AKI in patients undergoing emergency PCI [35].
Albumin is a major blood protein that regulates colloid osmotic pressure. Inflammation can reduce albumin level by decreasing its synthesis and enhancing its catabolism [36]. In addition, albumin possesses anti-inflammatory and antioxidant properties [37,38], and its serum levels are inversely correlated with systemic inflammatory status [36]. Albumin is a potent inhibitor of platelet activation and aggregation [39], and decreased albumin level can affect the blood viscosity and impair endothelial function [40,41]. Epidemiological evidence has linked low serum albumin levels with many cardiovascular diseases, such as coronary artery disease, heart failure, and stroke [42]. Furthermore, hypoalbuminemia is a well-established risk factor for PC-AKI [43].
FAR, a risk index integrating fibrinogen and albumin, allows for more sensitive reflection of the inflammatory status, blood viscosity, and thrombogenicity. FAR has been found to improve the clinical implication in multiple settings, including cardiovascular diseases. For example, FAR has been reported to be significantly correlated with the severity of coronary stenosis in patients with stable angina [44] and ST-elevation myocardial infarction [45], and can predict the occurrence of adverse cardiovascular events after PCI [14–16]. Furthermore, FAR is a useful biomarker for predicting the coronary slow-flow and no-reflow phenomenon [46,47]. Of note, studies have recently found that elevated FAR level is associated with the occurrence of PC-AKI. You et al. [18] found that elevated FAR can predict PC-AKI in acute myocardial infarction with an AUC of 0.721 (95% CI: 0.68–0.76). Ertas et al. [17] reported that FAR is an independent predictor of PC-AKI in patients undergoing carotid angiography, and they found that FAR can significantly improve risk reclassification for PC-AKI over fibrinogen and albumin alone. However, these studies did not conduct comprehensive discrimination analysis (AUC, continuous NRI, and IDI) to assess whether FAR provides incremental predictive information over the multivariable models, such as Mehran risk score.
Our study used the definition of PC-AKI according to the new guidelines of the ESUR, and we demonstrated that FAR was also an independent predictor of PC-AKI in the setting of elective PCI. In addition, FAR could significantly improve PC-AKI prediction in the continuous NRI and IDI over Mehran risk score. These findings indicated that FAR, a readily available laboratory index, is a useful biomarker in conjunction with the conventional risk factors for predicting PC-AKI. Prophylaxis strategies are needed to reduce PC-AKI for patients at high risk. Further large studies are required to explore the optimal risk threshold of FAR for PC-AKI prediction.
Several limitations of this study should be considered: First, it was limited by the retrospective design with small sample size, and thus there was a certain degree of bias. Second, as we had no information regarding the unmeasured hematological and biochemical parameters, such as blood viscosity status, C-reactive protein, and cystatin C, the independent value of fibrinogen in our multivariable logistic regression analysis might be misleading. Third, the prognostic information, including the long-term renal functions and overall mortality, was not considered in our study. More studies are needed in the future.
Conclusions
FAR is independently associated with the occurrence of PC-AKI, and can significantly improve PC-AKI prediction over Mehran risk score in patients undergoing elective PCI.
References
1. McCullough PA, Adam A, Becker CR, Epidemiology and prognostic implications of contrast-induced nephropathy: Am J Cardiol, 2006; 98(6A); 5K-13K
2. Rihal CS, Textor SC, Grill DE, Incidence and prognostic importance of acute renal failure after percutaneous coronary intervention: Circulation, 2002; 105(19); 2259-64
3. van der Molen AJ, Reimer P, Dekkers IA, Post-contrast acute kidney injury – Part 1: Definition, clinical features, incidence, role of contrast medium and risk factors: Recommendations for updated ESUR Contrast Medium Safety Committee guidelines: Eur Radiol, 2018; 28(7); 2845-55
4. Azzalini L, Spagnoli V, Ly HQ, Contrast-induced nephropathy: From pathophysiology to preventive strategies: Can J Cardiol, 2016; 32(2); 247-55
5. Powell SP, Chawla R, Bottinor W, Race, contrast-induced nephropathy and long-term outcomes after coronary and peripheral angiography and intervention: Cardiovasc Revasc Med, 2018; 19(6S); 31-35
6. Mody P, Wang T, McNamara R, Association of acute kidney injury and chronic kidney disease with processes of care and long-term outcomes in patients with acute myocardial infarction: Eur Heart J Qual Care Clin Outcomes, 2018; 4(1); 43-50
7. Jorgensen AL, Contrast-induced nephropathy: Pathophysiology and preventive strategies: Crit Care Nurse, 2013; 33(1); 37-46
8. Seeliger E, Sendeski M, Rihal CS, Persson PB, Contrast-induced kidney injury: Mechanisms, risk factors, and prevention: Eur Heart J, 2012; 33(16); 2007-15
9. Deng J, Wu G, Yang C, Rosuvastatin attenuates contrast-induced nephropathy through modulation of nitric oxide, inflammatory responses, oxidative stress and apoptosis in diabetic male rats: J Transl Med, 2015; 13; 53
10. Yuan Y, Qiu H, Hu X, Predictive value of inflammatory factors on contrast-induced acute kidney injury in patients who underwent an emergency percutaneous coronary intervention: Clin Cardiol, 2017; 40(9); 719-25
11. Gao F, Zhou YJ, Zhu X, C-reactive protein and the risk of contrast-induced acute kidney injury in patients undergoing percutaneous coronary intervention: Am J Nephrol, 2011; 34(3); 203-10
12. Wang YY, Liu ZZ, Xu D, Fibrinogen-albumin ratio index (FARI): A more promising inflammation-based prognostic marker for patients undergoing hepatectomy for colorectal liver metastases: Ann Surg Oncol, 2019; 26(11); 3682-92
13. Sun DW, An L, Lv GY, Albumin-fibrinogen ratio and fibrinogen-prealbumin ratio as promising prognostic markers for cancers: An updated meta-analysis: World J Surg Oncol, 2020; 18(1); 9
14. Cetin M, Erdogan T, Kiris T, Predictive value of fibrinogen-to-albumin ratio in acute coronary syndrome: Herz; 2019 [Epub ahead of print]
15. He D, Jiao Y, Yu T, Prognostic value of fibrinogen-to-albumin ratio in predicting 1-year clinical progression in patients with non-ST elevation acute coronary syndrome undergoing percutaneous coronary intervention: Exp Ther Med, 2019; 18(4); 2972-78
16. Xiao L, Jia Y, Wang X, Huang H, The impact of preoperative fibrinogen-albumin ratio on mortality in patients with acute ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention: Clin Chim Acta, 2019; 493; 8-13
17. Ertas F, Avci E, Kiris T, The ratio of fibrinogen to albumin as a predictor of contrast-induced nephropathy after carotid angiography: Angiology, 2019; 70(5); 458-64
18. You Z, Guo T, Lin F, Fibrinogen-to-albumin ratio predicts contrast-induced nephropathy in patients after emergency percutaneous coronary intervention: Cardiol Res Pract, 2019; 2019 8260583
19. Mehran R, Aymong ED, Nikolsky E, A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention: Development and initial validation: J Am Coll Cardiol, 2004; 44(7); 1393-99
20. Abellas-Sequeiros RA, Raposeiras-Roubin S, Abu-Assi E, Mehran contrast nephropathy risk score: Is it still useful 10 years later?: J Cardiol, 2016; 67(3); 262-67
21. Levey AS, Stevens LA, Schmid CH, A new equation to estimate glomerular filtration rate: Ann Intern Med, 2009; 150(9); 604-12
22. , 2. Classification and diagnosis of diabetes: Standards of medical care in siabetes – 2020: Diabetes Care, 2020; 43(Suppl 1); S14-31
23. DeLong ER, DeLong DM, Clarke-Pearson DL, Comparing the areas under two or more correlated receiver-operating characteristic curves: A nonparametric approach: Biometrics, 1988; 44(3); 837-45
24. Ozcan Cetin EH, Konte HC, Temizhan A, Blood viscosity should not be overlooked when evaluating the fibrinogen to albumin ratio: Angiology, 2019; 70(5); 465-66
25. Ertas F, Avci E, Kiris T, The ratio of fibrinogen to albumin as a predictor of contrast-induced nephropathy after carotid angiography: Reply: Angiology, 2019; 70(5); 467-68
26. Mikhailidis DP, Barradas MA, Maris A, Fibrinogen mediated activation of platelet aggregation and thromboxane A2 release: Pathological implications in vascular disease: J Clin Pathol, 1985; 38(10); 1166-71
27. Luyendyk JP, Schoenecker JG, Flick MJ, The multifaceted role of fibrinogen in tissue injury and inflammation: Blood, 2019; 133(6); 511-20
28. Davalos D, Akassoglou K, Fibrinogen as a key regulator of inflammation in disease: Semin Immunopathol, 2012; 34(1); 43-62
29. Szaba FM, Smiley ST: Blood, 2002; 99(3); 1053-59
30. Hoffmann D, Bijol V, Krishnamoorthy A, Fibrinogen excretion in the urine and immunoreactivity in the kidney serves as a translational biomarker for acute kidney injury: Am J Pathol, 2012; 181(3); 818-28
31. Du T, Dong H, Li C, Urinary fibrinogen is elevated in hospitalized patients undergoing angiography: Vascular, 2019; 27(1); 33-37
32. Weisel JW, Fibrinogen and fibrin: Adv Protein Chem, 2005; 70; 247-99
33. Lowe GD, Fowkes FG, Dawes J, Blood viscosity, fibrinogen, and activation of coagulation and leukocytes in peripheral arterial disease and the normal population in the Edinburgh Artery Study: Circulation, 1993; 87(6); 1915-20
34. Dhar P, Eadon M, Hallak P, Whole blood viscosity: Effect of hemodialysis treatment and implications for access patency and vascular disease: Clin Hemorheol Microcirc, 2012; 51(4); 265-75
35. Celik IE, Kurtul A, Duran M, Elevated serum fibrinogen levels and risk of contrast-induced acute kidney injury in patients undergoing a percutaneous coronary intervention for the treatment of acute coronary syndrome: Coron Artery Dis, 2016; 27(1); 13-18
36. Don BR, Kaysen G, Serum albumin: relationship to inflammation and nutrition: Semin Dial, 2004; 17(6); 432-37
37. Chen TA, Tsao YC, Chen A, Effect of intravenous albumin on endotoxin removal, cytokines, and nitric oxide production in patients with cirrhosis and spontaneous bacterial peritonitis: Scand J Gastroenterol, 2009; 44(5); 619-25
38. Roche M, Rondeau P, Singh NR, The antioxidant properties of serum albumin: FEBS Lett, 2008; 582(13); 1783-87
39. Gresele P, Deckmyn H, Huybrechts E, Vermylen J, Serum albumin enhances the impairment of platelet aggregation with thromboxane synthase inhibition by increasing the formation of prostaglandin D2: Biochem Pharmacol, 1984; 33(13); 2083-88
40. Dogra GK, Herrmann S, Irish AB, Insulin resistance, dyslipidaemia, inflammation and endothelial function in nephrotic syndrome: Nephrol Dial Transplant, 2002; 17(12); 2220-25
41. Joles JA, Willekes-Koolschijn N, Koomans HA, Hypoalbuminemia causes high blood viscosity by increasing red cell lysophosphatidylcholine: Kidney Int, 1997; 52(3); 761-70
42. Arques S, Human serum albumin in cardiovascular diseases: Eur J Intern Med, 2018; 52; 8-12
43. Zhang E, Lu Y, Chen G, Predictive value of hepatorenal status in contrast-induced nephropathy among patients receiving coronary angiography and/or intervention: A systematic review and meta-analysis: Angiology, 2019; 70(7); 633-41
44. Celebi S, Ozcan Celebi O, Berkalp B, Amasyali B, The association between the fibrinogen-to-albumin ratio and coronary artery disease severity in patients with stable coronary artery disease: Coron Artery Dis, 2020 Epub ahead of print]
45. Karahan O, Acet H, Ertas F, The relationship between fibrinogen to albumin ratio and severity of coronary artery disease in patients with STEMI: Am J Emerg Med, 2016; 34(6); 1037-42
46. Kayapinar O, Ozde C, Kaya A, Relationship between the reciprocal change in inflammation-related biomarkers (fibrinogen-to-albumin and hsCRP-to-albumin ratios) and the presence and severity of coronary slow flow: Clin Appl Thromb Hemost, 2019; 25; 1076029619835383
47. Zhao Y, Yang J, Ji Y, Usefulness of fibrinogen-to-albumin ratio to predict no-reflow and short-term prognosis in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention: Heart Vessels, 2019; 34(10); 1600-7
Tables
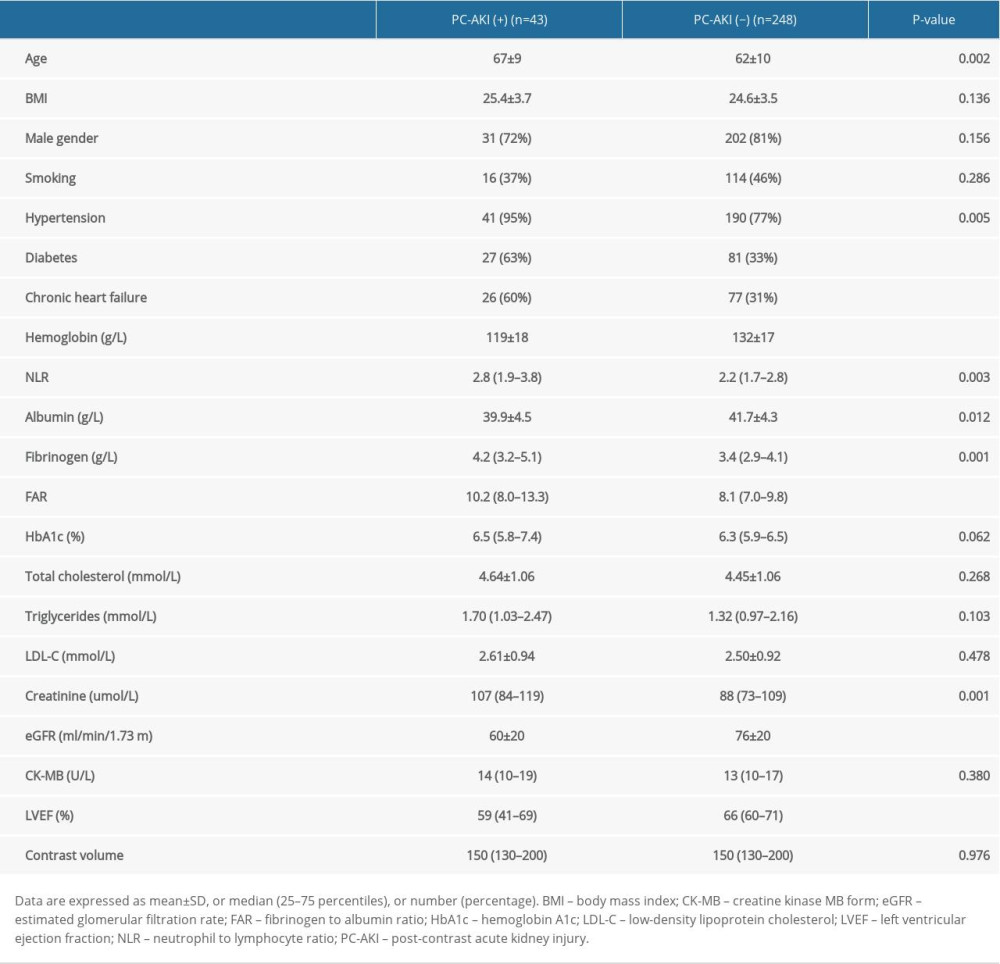 Table 1. Patients characteristics.
Table 1. Patients characteristics.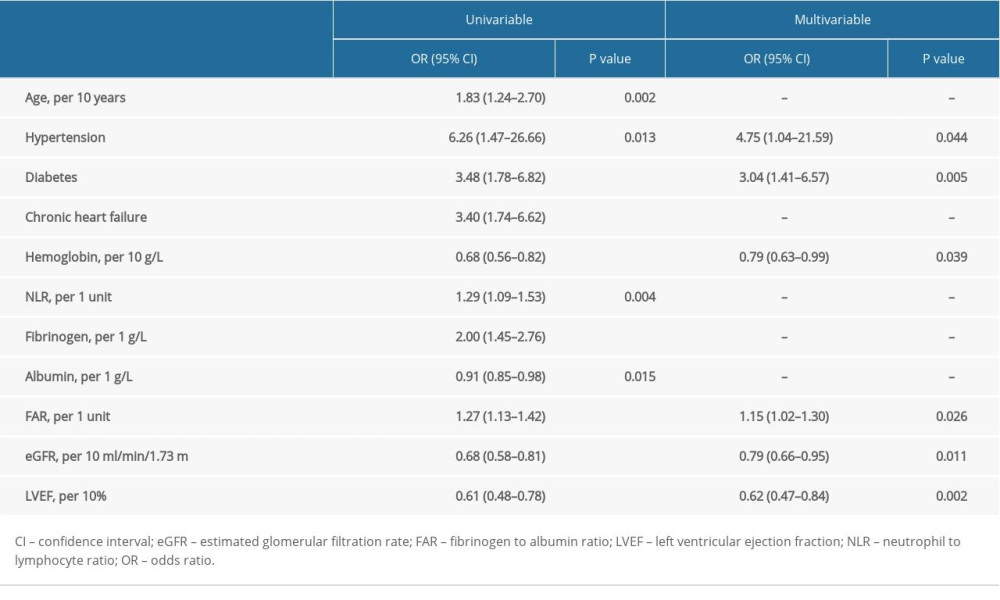 Table 2. Logistic regression analysis of the factors in predicting PC-AKI.
Table 2. Logistic regression analysis of the factors in predicting PC-AKI.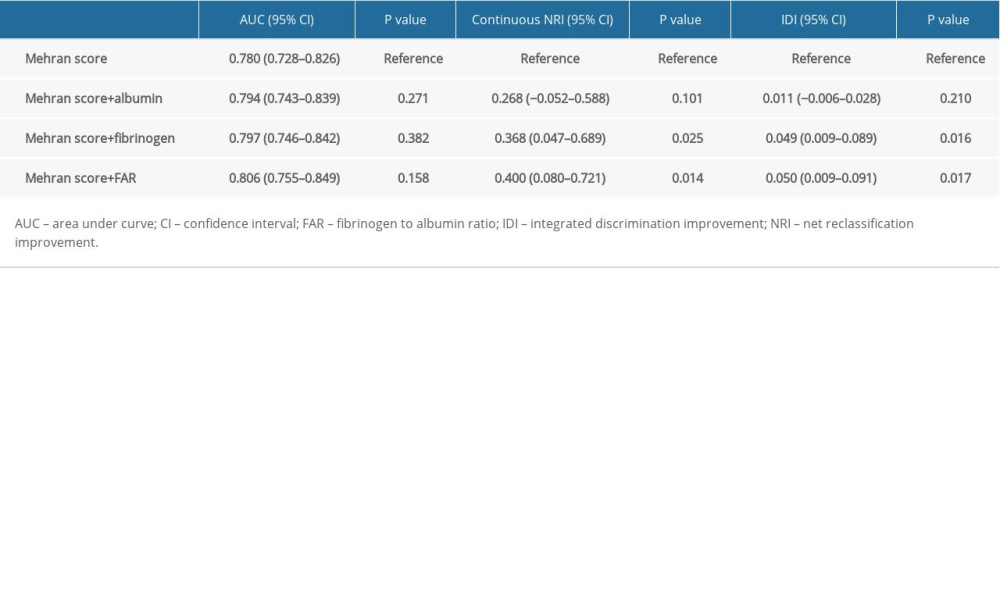 Table 3. Improvement of PC-AKI prediction by albumin, fibrinogen, and FAR over Mehran risk score.
Table 3. Improvement of PC-AKI prediction by albumin, fibrinogen, and FAR over Mehran risk score. Table 1. Patients characteristics.
Table 1. Patients characteristics. Table 2. Logistic regression analysis of the factors in predicting PC-AKI.
Table 2. Logistic regression analysis of the factors in predicting PC-AKI. Table 3. Improvement of PC-AKI prediction by albumin, fibrinogen, and FAR over Mehran risk score.
Table 3. Improvement of PC-AKI prediction by albumin, fibrinogen, and FAR over Mehran risk score. In Press
05 Mar 2024 : Clinical Research
Role of Critical Shoulder Angle in Degenerative Type Rotator Cuff Tears: A Turkish Cohort StudyMed Sci Monit In Press; DOI: 10.12659/MSM.943703
06 Mar 2024 : Clinical Research
Comparison of Outcomes between Single-Level and Double-Level Corpectomy in Thoracolumbar Reconstruction: A ...Med Sci Monit In Press; DOI: 10.12659/MSM.943797
21 Mar 2024 : Meta-Analysis
Economic Evaluation of COVID-19 Screening Tests and Surveillance Strategies in Low-Income, Middle-Income, a...Med Sci Monit In Press; DOI: 10.12659/MSM.943863
10 Apr 2024 : Clinical Research
Predicting Acute Cardiovascular Complications in COVID-19: Insights from a Specialized Cardiac Referral Dep...Med Sci Monit In Press; DOI: 10.12659/MSM.942612
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952