21 September 2020: Lab/In Vitro Research
P2X7 Receptor (P2X7R) of Microglia Mediates Neuroinflammation by Regulating (NOD)-Like Receptor Protein 3 (NLRP3) Inflammasome-Dependent Inflammation After Spinal Cord Injury
Xiao Fan12ABCEF, Wei Ma3ABCEF, Yingyu Zhang1BCD, Li Zhang24AFG*DOI: 10.12659/MSM.925491
Med Sci Monit 2020; 26:e925491
Abstract
BACKGROUND: Microglia participate in mediating neuroinflammation in which P2X7R triggered by adenosine triphosphate has a critical effect after spinal cord injury. However, how the P2X7R of microglia regulate neuroinflammation after spinal cord injury is still unclear. The aim of this study was to explore the mechanism by which the P2X7 receptor of microglia regulates neuroinflammation after spinal cord injury in NLRP3 inflammasome-dependent inflammation.
MATERIAL AND METHODS: Sixt rats were divided into 5 groups: a sham group, a model group, a BzATP group, an A-438079 group, and a BzATP+CY-09 group. Rats in the sham group were only subjected to laminectomy and rats in the other groups were subjected to spinal cord injury followed by treatment with physiological saline, BzATP, A-438079, and BzATP following CY-09, separately. Real-time polymerase chain reaction, Western blot, immunofluorescence staining, and enzyme-linked immunosorbent assay were used to analyze the scientific hypothesis.
RESULTS: (i) P2X7R of microglia was upregulated and downregulated by BzATP, and A-438079 was upregulated after spinal cord injury. (ii) Upregulation of P2X7R on microglia is coincident with increase of neuroinflammation after spinal cord injury. (iii) P2X7R of microglia participates in spinal cord-mediated neuroinflammation via regulating NLRP3 inflammasome-dependent inflammation.
CONCLUSIONS: P2X7R of microglia in spinal cord mediates neuroinflammation by regulating NLRP3 inflammasome-dependent inflammation after spinal cord injury.
Keywords: Inflammasomes, Microglia, neurogenic inflammation, Receptors, Purinergic P2X7, Spinal Cord Injuries, NLR Family, Pyrin Domain-Containing 3 Protein
Background
Spinal cord injury (SCI) is a central nervous system injury caused by trauma and violence to the spine. Fractures and dislocations of the spine injure the spinal cord and cauda equina nerve, resulting in motor, sensory, nerve reflex, and sphincter dysfunction of the limbs below the injury plane. According to studies [1,2], the incidence of spinal cord injury is about 27–83 cases per million people in the United States and 10–30 cases per million people in Europe. More than 2 million people worldwide have sequelae after SCI, including motor dysfunction, sensory dysfunction, neurogenic bladder, and gastrointestinal dysfunction, which brings enormous losses and heavy burden to patients, families, and society. Better understanding of the pathological mechanism of SCI will improve SCI sequelae therapy.
After SCI, there is a series of complex pathological responses, including neuroinflammation, edema, ion imbalance, spinal cord ischemia, electrolyte disorder, and excitatory amino acid poisoning, leading to neuron necrosis and apoptosis, myelin sheath rupture, deletion and demyelination changes, Wallerian degeneration, myelin sheath cavity, and glial scar formation [3]. Among these responses, neuroinflammation plays an important role in repair of the nervous system [4,5]. However, although neuroinflammation can help clear necrotic cells and tissue fragments, excessive neuroinflammation can further aggravate central nervous system injury, leading to failure of nerve repair after SCI [6].
Microglia (MG) are the innate immune cells in the central nervous system; they are immediately activated after SCI and play a key role in neuroinflammation [7]. When the body is in a normal state, microglia support, nourish, and protect neurons. When the central nervous system is damaged, microglia are activated and immediately proliferate to mediate neuroinflammation. During the neuroinflammation, the purinergic P2X7 receptor (P2X7R) – a subtype of ligand-gated ion channel receptors that conveys the ionotropic actions of extracellular ATP widely existing on different types of cell surfaces, including MG – regulates the expression of several cytokines and inflammation mediators like IL-1β and IL-18 to participant in mediating neuroinflammation [8]. In addition, P2X7R has the highest threshold for ATP-induced activation, triggering downstream mechanisms only when extracellular ATP reaches pathological concentrations. This makes P2X7R stand out among other purine receptors and it plays a central role in several pathological conditions, including inflammatory diseases and neuropathic pain [9]. However, how the P2X7R on MG mediates neuroinflammation by regulating the expression and secretion of cytokines and inflammation mediators after SCI is still unclear.
It is well known that nucleotide-binding oligomerization domain (NOD)-like receptor protein 3 (NLRP3) is an important member of the NOD-like receptor family, with a function of regulating innate immunity [10–12]. NLRP3 and other intracellular proteins oligomerize by protein–protein interactions to form a complex inflammasome to activate pro-caspase1, which cleaves the inactive IL-1β precursor to mediate inflammation [13,14]. P2X7R has been shown to be a sensor of cell injury and a trigger of the NLRP3 inflammasome, and the NLRP3/P2X7R interaction occurs at restricted subplasma membrane sites in microglia and macrophages [15]. However, most of these studies focussed on neurodegenerative diseases, liver fibrosis, and auto-immune diseases, including multiple sclerosis, Alzheimer disease, systemic lupus erythematosus, rheumatoid arthritis, inflammatory bowel disease, and multiple sclerosis. No study has specifically explored the NLRP3/P2X7R interaction in neuroinflammation after SCI [15,16]. It is important to know that the role that P2X7R exhibits depends both on the degree of ATP activation (depending on the concentration and duration of stimulation) and on the expression of the receptor itself, which may vary between different cells [17]. Therefore, in this study, we set out to assess whether spinal cord P2X7R on MG regulates NLRP3 inflammasome to mediate neuroinflammation after SCI.
Material and Methods
ANIMALS:
Sixty Sprague-Dawley rats (30 females, 30 males) weighing 200–220 g were purchased from Shanghai SLAC Laboratory Animal Co. and were housed at room temperature and 12/12-hour light/dark cycle and free to access the water and food. Before the experiment, the rats that were divided into 5 groups, with 12 rats randomly picked in each group, including a sham group, a model group, an A-438079 group, a BzATP group, and a BzATP+CY-09 group, then they were allowed to acclimate to the housing facilities and environment for 7 days.
INTERVENTION OF EACH GROUP:
The rats in the sham group were subjected to laminectomy without spinal cord injury and administered physiological saline by intraperitoneal injection. The rats in the model group were subjected to spinal cord injury and administered physiological saline by intraperitoneal injection. The rats in the inhibitor group were subjected to spinal cord injury and administered P2X7 receptor antagonist A-438079 (HY-15488, MCE, New Jersey, USA) at a dose of 35 μg/rat [18] by intraperitoneal injection. The rats in the BzATP group were subjected to spinal cord injury and administered 3′-O-(4-benzoylbenzoyl) adenosine 5′-triphosphate (BzATP) (ab120444, Abcam, Shanghai, China), a P2X7 receptor agonist, at a dose of 0.5μg/d [18] by intraperitoneal injection. The rats in the BzATP+CY-09 group were subjected to spinal cord injury and administered CY-09 (HY-103666, MCE, New Jersey, USA), a NLRP3 antagonist, at a dose of 10 mg/kg 1 h before administration of BzATP (ab120444, Abcam, Shanghai, China) at a dose of 0.5μg/d. All rats were killed on the third day after the intervention. The protocol for use of rats was approved by the Animal Care Committee of Qingdao Municipal Hospital.
SPINAL CORD CONTUSION INJURY ESTABLISHMENT:
Rat spinal cord contusion injury was established according to previous studies [19,20]. Briefly, rats were anesthetized by 1% pentobarbital solution (P-010, Sigma-Aldrich, Shanghai, China) at a dose of 40 mg/kg and fixed on the operating table in prone position. After depilation and sterilization, an approximately 4-cm incision was made along the T9 to T11 midline and paravertebral muscles were dissected bilaterally. Then, after exposing the spinal cord by laminectomy of total T9 to T11, the intact T10 spinal cord was contused using a MASCIS impactor device (Impactor M-III, W.M. Keck Center for Collaborative Neuroscience, New Jersey, USA), setting the parameter as 12.5 mm height and 10 g weight. After surgery, all rats were kept warm, housed singly, and defecated manually twice a day.
IMMUNOFLUORESCENCE STAINING:
After successful anesthesia, perfusion fixation was performed transcardially with physiological saline followed by 4% paraformaldehyde in 6 rats in each group. The T9 to T11 spinal cord segments were removed immediately and immersed in 4% paraformaldehyde for post-fixation for 48 h at 4°C and then embedded in paraffin. Tissue blocks were sectioned at 5 μm. Sections were subjected to antigen retrieval with citric acid buffer, blocked with 10% normal goat serum for 2 h at room temperature, and incubated with a mixture of rabbit P2X7R antibody (1: 100, APR-004, Alomone, Jerusalem, Israel) and goat IBA-1 antibody (1: 200, ab5076, Abcam, Shanghai, China) at 4°C overnight. Sections were further incubated with the mixture of donkey anti-goat Alexa Fluor 546 antibody (1: 200, A-11057, Thermo Fisher Scientific, Shanghai, China) and goat anti-rabbit Alexa Fluor 488 antibody (1: 200, A-11034, Thermo Fisher Scientific, Shanghai, China) at room temperature for 2 h in the dark and then incubated with DAPI (1: 1000, ab228549, Abcam, Shanghai, China) at room temperature for 3 min. Sections were observed under a confocal laser scanning microscope (710, CARL ZEISS, Germany) with wavelengths of 488 mm and 546 mm.
WESTERN BLOT ANALYSIS:
The T9 to T11 spinal cord segments from 6 rats in each group were removed immediately after surgery and washed with phosphate-buffered saline (pH 7.4) before extraction of proteins using the Protein Extraction Kit (89842, Thermo Fisher Scientific, Shanghai, China). BCA assay method was used to analyze the protein concentrations. Sodium dodecyl-polyacryl gradient gel electrophoresis with 10% Tris-Glycine gels at 80 V was used to separate proteins with 50 micrograms of protein per lane. Proteins were electrophoretically transferred onto polyvinylidene fluoride membranes. The membranes were incubated with rabbit P2X7R antibody (1: 1000, APR-004, Alomone, Jerusalem, Israel), goat NLRP3 antibody (1: 2000, ab214185, Abcam, Shanghai, China), rabbit cleaved-Caspase-1 (P20) antibody (BA2220, BOSTER, Wuhan, China), and β-actin antibody (1: 4000, ab8227, Abcam, Shanghai, China) at 4°C overnight, and then incubated with horseradish peroxidase-conjugated anti-rabbit or anti-goat antibody (1: 2000, ab6721, and ab6741, Abcam, Shanghai, China), respectively, at room temperature for 2 h. The chemiluminescence method (ECL system, GE Healthcare, Chalfont St. Giles, UK) was used to visualize the blots. Image J software (Bio-Rad, USA) was applied to analyze the immune activity. The specific bands were normalized against the loading control (β-actin).
ENZYME-LINKED IMMUNOSORBENT ASSAY (ELISA):
The T9 to T11 spinal cord segments from 6 rats in each group were removed immediately after surgery and washed with phosphate-buffered saline (pH 7.4). The spinal cord segments were ground to homogenate and centrifuged at 10 000 g for 10 min. The rat IL-1β ELISA kit (EK3711, BOSTER, Wuhan, China) and IL-18 ELISA kit (EK0592, BOSTER, Wuhan, China) were used to detect contents of IL-1β and IL-18 in the spinal cord segments according to the manufacturer’s instructions.
QUANTITATIVE REAL-TIME POLYMERASE CHAIN REACTION (QPCR):
The Quick-RNA™ MiniPrep kit was used to isolate the total RNA of the T9 to T11 spinal cord segments according to the manufacturer’s instructions (R223-01, Vazyme, Nanjing, China) and Thermo Nano Drop 2000 (2000, Thermo Fisher Scientific, Massachusetts, USA) was used to analyze the purity and concentration of the extracted RNA. To convert the complementary DNA (cDNA), the High-Capacity cDNA Reverse Transcription kit (Q111-02/03, Vazyme, Nanjing, China) was used to perform the reverse transcription. Primers of were designed as
The Real-Time PCR System (ABI 7500, Applied Biosystems, Massachusetts, USA) was used to perform qPCR with the cycling parameters set as denaturation at 95°C for 10 s, annealing at 60°C for 30 s, and extension at 72°C for 30 s. Each reaction was repeated 3 times and the relative quantification method of ΔΔCt was used to analyze the data, shown as relative gene expression levels compared to the control.
STATISTICAL ANALYSIS:
SPSS 22.0 software (Version 22.0, IBM, New York State, USA) was used to perform the statistical analysis. The data are expressed as mean±SEM and tested by normal distribution followed by one-way analysis of variance. LSD method was used if the variance was uniform; otherwise, the Games-Howell method was used. P<0.05 was considered to indicate a statistically significant difference and P<0.01 indicated a strongly significant difference.
Results
P2X7R OF MICROGLIA WAS UPREGULATED AND DOWNREGULATED BY BZATP, AND A-438079 SEPARATELY WAS UPREGULATED AFTER SCI:
As the innate immune cell in the central nervous system, microglia marked by IBA-1 play a key role in mediating neuroinflammation after injury. P2X7R, a subtype of purine receptor, is distributed on various types of cell surfaces, including microglia in the central nervous system, and has important effects on many pathological reactions of neuroinflammation [21,22]. To determine the expression of P2X7R on spinal cord microglia after SCI, immunofluorescent staining was carried out to examine if there was P2X7R and IBA-1 double-positivity (P2X7R+/IBA-1+) cells in the spinal cord after SCI. As showed in the study, there were some cells stained IBA-1-positive (IBA-1+) but almost none were stained P2X7R-positive (P2X7R+) in the sham group (Figure 1A). In contrast, P2X7R+/IBA-1+ cells were dramatically increased in the spinal cord of the model group (Figure 1A, 1B, ** P<0.01), exhibiting activated morphology of microglia and indicating that there was notable upregulation of P2X7R on microglia after SCI. In addition, when BzATP, the agonist of P2X7R, was applied by intraperitoneal injection, the number of P2X7R+/IBA-1+ cells were further increased in comparison with the model group (Figure 1A, 1B, * P<0.05), demonstrating that BzATP upregulated the P2X7R on microglia after SCI. We further applied A-438079, the antagonist of P2X7R, by intraperitoneal injection, and found it significantly decreased the number of P2X7R+/IBA-1+ in the spinal cord (Figure 1A, 1B, ** P<0.01). Compared to the sham group, whose P2X7R-positive microglia percentage was least, the P2X7R-positive microglia percentage of the model group, BzATP group, and A-438079 group were significantly increased (Figure 1C, ** P<0.01). Compared to the model group, the BzATP group accounted for a significantly increased proportion (Figure 1C, ** P<0.01) and the A-438079 group accounted for a significantly reduced proportion (Figure 1C, ** P<0.01). This result indicates that the most of microglia overexpressed P2X7R to participate in the pathological reaction as neuroinflammation after SCI, and the agonist BzATP increased the P2X7R-positive microglia proportion, which was inhibited by the antagonist A-438079.
To confirm the above results, we further performed qPCR and Western blotting for quantitative analysis of the expression of P2X7R in the spinal cord. The results showed remarkable upregulation of the expression of protein and mRNA of P2X7R in the spinal cord after SCI (Figure 2, ** P<0.01), which was promoted by BzATP (Figure 2, * P<0.05) and strongly inhibited by A-439079 (Figure 2, ** P<0.01), which was consistent with the results of immunofluorescent staining demonstrating, which showed upregulation of P2X7R on microglia in the spinal cord after SCI, regulated by BzATP and A-438079.
UPREGULATION OF P2X7R ON MICROGLIA IS COINCIDENT WITH INCREASE OF NEUROINFLAMMATION AFTER SCI:
IL-1β and IL-18, which are important inflammation factors, are regulated by microglia in the spinal cord to mediate neuroinflammation after SCI. As the downstream effectors of microglia mediating neuroinflammation, expression of IL-1β and IL-18 was detected by ELISA and qPCR, and the results showed that, compared to the low expression of IL-1β and IL-18 in the sham group, the expression of IL-1β and IL-18 in the model group was greatly increased (Figure 3, ** P<0.01), indicating that there was a higher level of neuroinflammation in the lesion after SCI, which agrees with previous studies [23,24]. To further investigate the effects of P2X7R of microglia in the spinal cord that were upregulated after SCI on neuroinflammation, we administered A438079, a selective P2X7R antagonist, and BzATP, a P2X7R agonist as control, to inhibit and increase the expression of P2X7R, separately. The results of ELISA and qPCR showed that compared to the model group, A438079 significantly decreased the expression of IL- 1β and IL-18 (Figure 3, ** P<0.01) and BzATP significantly increased the expression of IL- 1β and IL-18 (Figure 3, ** P<0.01), which wa inhibited by CY-09 (Figure 3, ** P<0.01), indicating that, along with the inhibition or activation of P2X7R on microglia in the spinal cord, the neuroinflammation after SCI was inhibited or promoted, revealing that upregulation of P2X7R on microglia participated in mediating neuroinflammation after SCI.
P2X7R OF MICROGLIA IN SPINAL CORD MEDIATED NEUROINFLAMMATION VIA REGULATING NLRP3 INFLAMMASOME-DEPENDENT INFLAMMATION:
The NLRP3 inflammasome is an important pathway regulating neuroinflammation in which the activated NLRP3 inflammasome has effects on converting pro-Caspase-1 to cleaved-Caspase-1 (P20), which can convert inactive precursor of IL-1β and IL-18 to active IL-1β and IL-18 with pro-inflammation [25]. To determine the mechanism of the P2X7R of microglia in spinal cord mediating neuroinflammation after SCI, and to determine whether the NLRP3 inflammasome-dependent inflammation is related to neuroinflammation mediated by P2X7R of microglia after SCI, we performed Western blot analysis of the expressions of NLRP3 and cleaved-Caspase-1 (P20) and used CY-09, the antagonist of NLRP3, as the control. The results showed that there was lower expression of NLRP3 and cleaved-Caspase-1 (P20) in the sham group (Figure 4). By comparison, the expressions of NLRP3 and cleaved-Caspase-1 (P20) were increased prominently in the model group (Figure 4, ** P<0.01), which were coincidence with the expression of IL-1β and IL-18 indicating that after SCI, NLRP3 inflammasome-dependent inflammation in the lesion was activated. Consistent with the expressions of IL-1β and IL-18 after SCI, BzATP, the agonist of P2X7R, promoted the expression of NLRP3 and cleaved-Caspase-1 (P20) (Figure 4, * P<0.05) while A-438079, the antagonist of P2X7R notably reduced the expression of NLRP3 and cleaved-Caspase-1 (P20) (Figure 4, ** P<0.01) compared to the sham group, revealing that P2X7R of microglia may be involved in regulating NLRP3 inflammasome-dependent inflammation in lesions after SCI. We then used CY-09, the selective and direct NLRP3 inhibitor directly binding to the ATP-binding motif of NLRP3 NACHT domain and inhibiting NLRP3 ATPase activity [26], resulting in the suppression of NLRP3 inflammasome assembly and activation in the study as the control. The results showed that CY-09 reduced the overexpression of NLRP3 and cleaved-Caspase-1 (P20) caused by BzATP (Figure 4, * P<0.05), indicating that the effects of upregulation of P2X7R of microglia caused by BzATP on promotion of neuroinflammation after SCI were inhibited by CY-09, the antagonist of NLRP3. Our results show P2X7R of microglia affects spinal cord-mediated neuroinflammation via regulating NLRP3 inflammasome-dependent inflammation.
Discussion
Neuroinflammation is one of the most important pathology responses, with a dual effect on the restoration and reconstruction of the nervous system after SCI. In the initial stage of injury, neuroinflammation helps to limit tissue damage in response to pathogens or injury to help the body detect and fight foreign antigens and restore tissue integrity [27,28]. With the development of injury, excessive neuroinflammation aggravates damage to the nervous system, leading to apoptosis and necrosis of neurons, as well as axonal demyelination, which can help reconstruct and repair the nervous system after SCI. Studies determined that the content of inflammatory factors, including IL-1β and IL-18, that were synthesized and secreted quickly to the lesion gradually increased to reach a peak at 72 h and then plateau. It is important to maintain and regulate neuroinflammation at an appropriate level after SCI in the initial stage of injury. For neuroinflammation, neuroinflammatory responses mediated by microglia, the resident immune cells in central nervous system are one of the important sources of neuroinflammation as the response to injury causing a variety of triggers [29–32].
As a crucial member of the purine receptor family, P2X7R is an ATP-gated nonselective cation channel regulating Na2+, K+, and Ca2+ intracellular and extracellular movement by triggering of adenosine triphosphate (ATP), which acts as a mediator of inflammation and immunity. Almost all immune and inflammatory cells express the P2X7 receptor, and its expression is upregulated during inflammation. By far the best-performing immune cell lineage for P2X7 activity are cells of the monocyte/macrophage axis, including dendritic cells, microglia, and osteoclasts. This receptor is also expressed by T lymphocytes, B lymphocytes, mast cells, and natural killer cells. Due to its well-established role in interleukin (IL)-1β and IL-18 release and in cell death and proliferation, P2X7R is thought to have an important role in chronic inflammatory disorders, in metabolic dysfunctions such as type 2 diabetes, in neurodegenerative diseases, and in cancer [33], especially in various chronic inflammatory neurological diseases. Several cell types in the nervous system express P2X7R, including astrocytes, oligodendrocytes, microglia, and Schwann cells [34]. A study clearly demonstrated the expression of P2X7R in myelinated Schwann cells and non-myelinated Schwann cells. Upregulation of P2X7R expression in Schwann cells of injured sciatic nerves was associated with proliferation of Schwann cells [35]. Furthermore, a study also showed that Schwann cell-specific ERK1/2 activation was sufficient to induce other responses associated with nerve injury and repair [36]. Enhanced and prolonged activation of astrocytes plays a detrimental role in many CNS disorders [37]. This has even led to a “gliocentric” concept of brain pathologies [38]. It is precisely because microglia-mediated neuroinflammatory response is one of the important sources of neuroinflammation that we conducted the present study to explore the role of P2X7R in microglia in this process.
After SCI, ATP increased release by endothelial cells during injury trigger of many cytokine and inflammatory factors released from inflammatory cells to activate P2X7R, which plays a central role in inflammation to aggravate damage and form a vicious circle. The immunofluorescence assay used in the study showed that, accompanied by activation of microglia, P2X7R-positive cells were mostly positive to Iba-1 and the number of P2X7R+/IBA-1+ cells was increased notably, indicating that the overexpression of P2X7R in the microglia in spinal cord significantly participates in pathology response after SCI, which agrees with previous studies [39–41]. Meanwhile, because purinergic receptors, especially P2X7R, contribute to regulating inflammation and immune responses, activated P2X7R in microglia has been thought to have an intimate relationship with neuroinflammation after SCI. In activated microglia, activation of P2X7R triggered by ATP after injury upregulated the neuroinflammation by promoting the release of inflammatory cytokines, including IL-1β and IL-18 [42–44]. We observed that the expression of inflammatory cytokines, including IL-1β and IL-18, around the lesion was greatly increased after SCI and can be up regulated by P2X7R agonist and downregulated by P2X7R antagonist, indicating that P2X7R in microglia participate in mediating and regulating neuroinflammation after SCI, which is supported by previous studies [45–47]. However, it is unknown how the P2X7R in microglia regulates neuroinflammation in the spinal cord after SCI.
To answer this question, we hypothesized that the NLRP3 inflammasome is closely related to inflammation after injury. In the damaged environment with ATP, cytokine, and inflammatory cytokines, NLRP3 oligomerizes with other intracellular proteins through protein-to-protein interaction to form a complex called the NLRP3 inflammasome. The assembled components of the NLRP3 inflammasome initiate the processing of inactive procaspase-1, resulting in formation of active cleaved-caspase-1 (P20), processing the precursor of IL-1β and IL-18 in the secretory lysosome, resulting in the generation and secretion of carboxy-terminal mature IL-1β and IL-18 [48–50]. We propose the scientific hypothesis that, triggered by ATP around the lesion, P2X7R in microglia in the spinal cord can regulate neuroinflammation by the NLRP3 inflammasome pathway after SCI. We observed that the expressions of NLRP3 inflammasome and cleaved-caspase-1 (P20) can be upregulated by P2X7R agonist and downregulated by P2X7R antagonist, accompanied by promotion and inhibition of neuroinflammation after SCI. However, we also discovered that CY-09, the antagonist of NLRP3 inflammasome, successfully inhibited the promotion of BzATP in neuroinflammation after SCI, revealing that P2X7R in microglia in the spinal cord can regulate neuroinflammation via the NLRP3 inflammasome pathway after SCI. The effect of the NLRP3 inflammasome on pro-IL-18 and pro-IL-1β processing places P2X7R at the center of neuroinflammation. In our opinion, P2X7R triggers the activation of the NLRP3 inflammasome, which is the main intracellular complex participating in transduction of danger signals and initiation of inflammation by ionic imbalance, considering that a large Ca2+ can be driven by P2X7R in microglia to influx from the extracellular space to play a role in activating the NLRP3 inflammasome after injury [51]. Therefore, it is important to be aware that P2X7R on microglia in the spinal cord can regulate neuroinflammation via NLRP3 inflammasome-dependent inflammation after SCI. In conclusion, this finding not only strengthens the understanding of the pathology of spinal cord injury, but also provides a possible new therapeutic strategy.
Our study has certain limitations: i) we only designed agonist and antagonist as a control without gene knockout; ii) we proposed a scientific hypothesis by animal trial without using microglia cells to perform the trial
Conclusions
The present study demonstrated that P2X7R of microglia mediates neuroinflammation by regulating NLRP3 inflammasome-dependent inflammation after SCI, revealing that P2X7R may be an effective target for treating SCI.
Figures
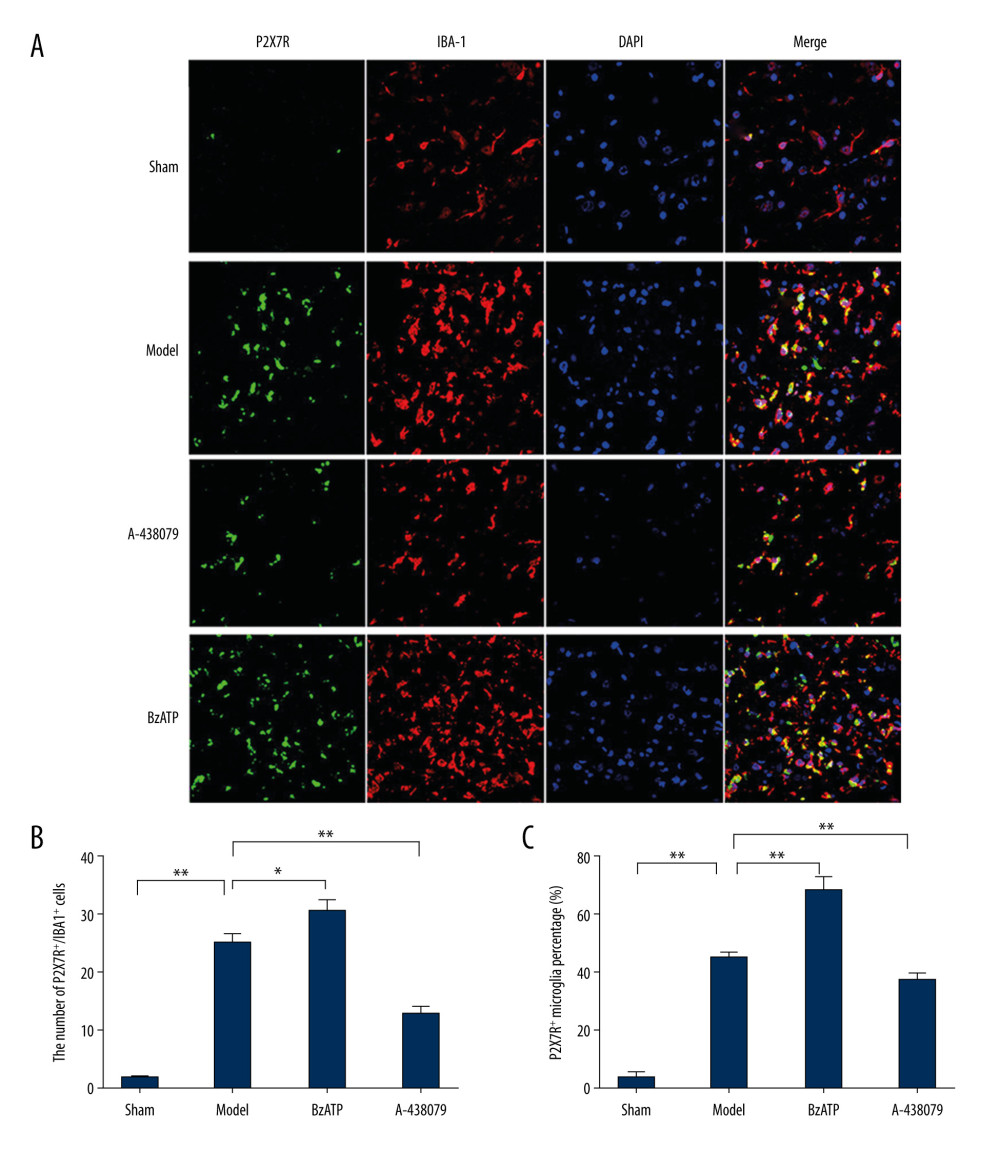 Figure 1. Immunofluorescence detected P2X7R of microglia in spinal cord. (A) P2X7R (P2X7R+, green) and IBA-1 (IBA-1+, red) co-expressed as P2X7R of microglia (P2X7R+/IBA-1+, yellow) that were overexpressed after spinal cord injury and BzATP intervention, and, compared to the morphology of microglia in the sham group, the morphology of activated microglia after injury changed to large soma and short irregular processes, revealing that many microglia in spinal cord became active after spinal cord injury. (B) The number of P2X7R of microglia (P2X7R+/IBA-1+) increased notably after spinal cord injury compared to the sham group (** P<0.01), and, compared to the model group, BzATP increased the number of P2X7R of microglia (* P<0.05) while A-438079 significantly decreased the number of P2X7R of microglia (** P<0.01). (C) The P2X7R-positive microglia percentage was clearly increased after spinal cord injury compared to the sham group (** P<0.01), and, compared to the model group, BzATP accounted for a significantly increased P2X7R-positive microglia proportion (** P<0.01) while A-438079 accounted for a significantly reduced P2X7R-positive microglia proportion (** P<0.01).
Figure 1. Immunofluorescence detected P2X7R of microglia in spinal cord. (A) P2X7R (P2X7R+, green) and IBA-1 (IBA-1+, red) co-expressed as P2X7R of microglia (P2X7R+/IBA-1+, yellow) that were overexpressed after spinal cord injury and BzATP intervention, and, compared to the morphology of microglia in the sham group, the morphology of activated microglia after injury changed to large soma and short irregular processes, revealing that many microglia in spinal cord became active after spinal cord injury. (B) The number of P2X7R of microglia (P2X7R+/IBA-1+) increased notably after spinal cord injury compared to the sham group (** P<0.01), and, compared to the model group, BzATP increased the number of P2X7R of microglia (* P<0.05) while A-438079 significantly decreased the number of P2X7R of microglia (** P<0.01). (C) The P2X7R-positive microglia percentage was clearly increased after spinal cord injury compared to the sham group (** P<0.01), and, compared to the model group, BzATP accounted for a significantly increased P2X7R-positive microglia proportion (** P<0.01) while A-438079 accounted for a significantly reduced P2X7R-positive microglia proportion (** P<0.01). 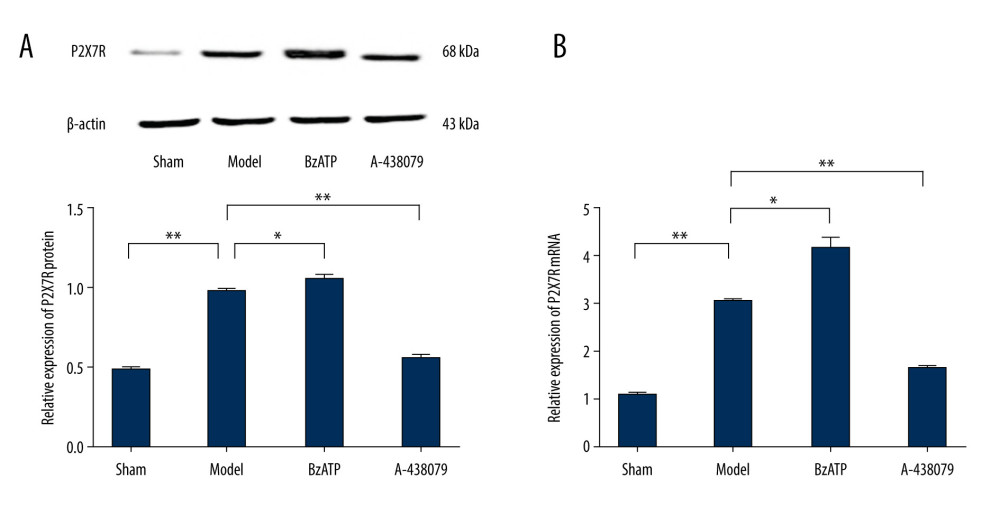 Figure 2. Western blot and qPCR assay were used to assess the expression of P2X7R protein and P2X7R mRNA in spinal cord. (A) Western blot assay showed that the expression of P2X7R protein in spinal cord was increased notably after spinal cord injury compared to the sham group (** P<0.01) and, compared to model group, BzATP increased the expression of P2X7R protein in spinal cord (* P<0.05) while A-438079 significantly decreased the expression of P2X7R protein in spinal cord (** P<0.01). (B) qPCR assay showed that the expression of P2X7R mRNA in spinal cord increased notably after spinal cord injury compared to the sham group (** P<0.01), and compared to the model group, BzATP increased the expression of P2X7R mRNA in spinal cord (* P<0.05) while A-438079 significantly decreased the expression of P2X7R mRNA in spinal cord (** P<0.01).
Figure 2. Western blot and qPCR assay were used to assess the expression of P2X7R protein and P2X7R mRNA in spinal cord. (A) Western blot assay showed that the expression of P2X7R protein in spinal cord was increased notably after spinal cord injury compared to the sham group (** P<0.01) and, compared to model group, BzATP increased the expression of P2X7R protein in spinal cord (* P<0.05) while A-438079 significantly decreased the expression of P2X7R protein in spinal cord (** P<0.01). (B) qPCR assay showed that the expression of P2X7R mRNA in spinal cord increased notably after spinal cord injury compared to the sham group (** P<0.01), and compared to the model group, BzATP increased the expression of P2X7R mRNA in spinal cord (* P<0.05) while A-438079 significantly decreased the expression of P2X7R mRNA in spinal cord (** P<0.01). 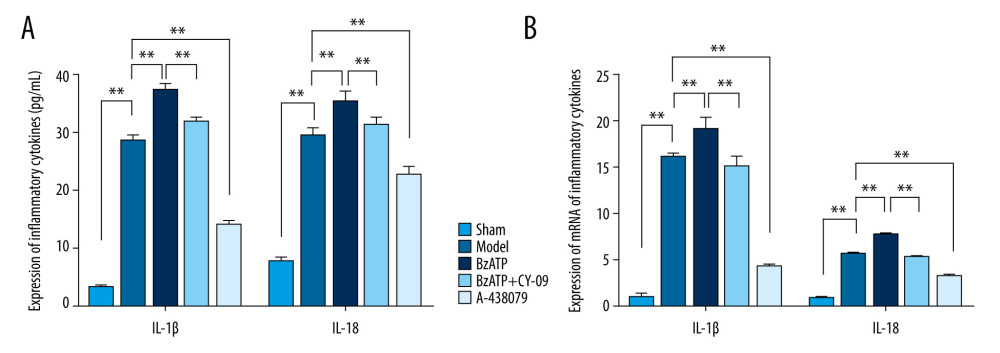 Figure 3. P2X7R of microglia in spinal cord-mediated neuroinflammation after spinal cord injury. (A) ELISA showed that, compared to the sham group, the expression of IL-1β and IL-18 in the spinal cord were significantly increased after spinal cord injury (** P<0.01) and, compared to the model group, A438079 significantly inhibited the expression of IL- 1β and IL-18 (** P<0.01) and BzATP significantly increased the expression of IL- 1β and IL-18 (** P<0.01), which was strongly inhibited by CY-09 (** P<0.01). (B) qPCR assay showed that, compared to the sham group, the expression of IL-1β mRNA and IL-18 mRNA in the spinal cord was significantly increased after spinal cord injury (** P<0.01) and, compared to the model group, A438079 significantly inhibited the expression of IL-1β mRNA and IL-18 mRNA (** P<0.01), and BzATP significantly increased the expression of IL-1β mRNA and IL-18 mRNA (** P<0.01), which was strongly inhibited by CY-09 (** P<0.01).
Figure 3. P2X7R of microglia in spinal cord-mediated neuroinflammation after spinal cord injury. (A) ELISA showed that, compared to the sham group, the expression of IL-1β and IL-18 in the spinal cord were significantly increased after spinal cord injury (** P<0.01) and, compared to the model group, A438079 significantly inhibited the expression of IL- 1β and IL-18 (** P<0.01) and BzATP significantly increased the expression of IL- 1β and IL-18 (** P<0.01), which was strongly inhibited by CY-09 (** P<0.01). (B) qPCR assay showed that, compared to the sham group, the expression of IL-1β mRNA and IL-18 mRNA in the spinal cord was significantly increased after spinal cord injury (** P<0.01) and, compared to the model group, A438079 significantly inhibited the expression of IL-1β mRNA and IL-18 mRNA (** P<0.01), and BzATP significantly increased the expression of IL-1β mRNA and IL-18 mRNA (** P<0.01), which was strongly inhibited by CY-09 (** P<0.01). 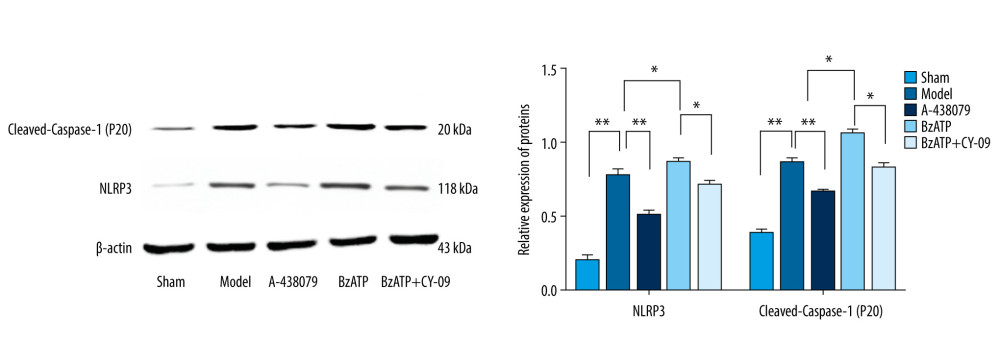 Figure 4. P2X7R of microglia in spinal cord regulated the expression of NLRP3 and cleaved-Caspase-1 (P20). Compared to the sham group, the expression of NLRP3 and cleaved-Caspase-1 (P20) in the spinal cord significantly increased after spinal cord injury (** P<0.01) and, compared to the model group, BzATP promoted the expression of NLRP3 and cleaved-Caspase-1 (P20) (* P<0.05), which was inhibited by CY-09 (* P<0.05), while A-438079 notably reduced the expression of NLRP3 and cleaved-Caspase-1 (P20) (** P<0.01).
Figure 4. P2X7R of microglia in spinal cord regulated the expression of NLRP3 and cleaved-Caspase-1 (P20). Compared to the sham group, the expression of NLRP3 and cleaved-Caspase-1 (P20) in the spinal cord significantly increased after spinal cord injury (** P<0.01) and, compared to the model group, BzATP promoted the expression of NLRP3 and cleaved-Caspase-1 (P20) (* P<0.05), which was inhibited by CY-09 (* P<0.05), while A-438079 notably reduced the expression of NLRP3 and cleaved-Caspase-1 (P20) (** P<0.01). References
1. Wong S, Santullo P, Saif M, Use of antibiotic and incidence of antibiotic associated diarrhoea in patients with spinal cord injuries: A UK National Spinal Injury Centre experience: Spine J, 2016; 16; S58
2. Wilson J, Tetreault L, Aarabi B, Guidelines for the management of patients with spinal cord injury: The optimal timing of decompression: Spine J, 2016; 16; S213-14
3. Hachem LD, Ahuja CS, Fehlings MG, Assessment and management of acute spinal cord injury: From point of injury to rehabilitation: J Spinal Cord Med, 2017; 40; 665-75
4. McMahill BG, Borjesson DL, Sieber-Blum M, Stem cells in canine spinal cord injury – promise for regenerative therapy in a large animal model of human disease: Stem Cell Rev Rep, 2015; 11; 180-93
5. Ung RV, Lapointe NP, Rouleau P, Non-assisted treadmill training does not improve motor recovery and body composition in spinal cord-transected mice: Spinal Cord, 2010; 48; 750-55
6. Eickmeier O, Seki H, Haworth O, Aspirin-triggered resolvin D1 reduces mucosal inflammation and promotes resolution in a murine model of acute lung injury: Mucosal Immunol, 2013; 6; 256-66
7. Windelborn JA, Mitchell GS, Glial activation in the spinal ventral horn caudal to cervical injury: Respir Physiol Neurobiol, 2012; 180; 61-68
8. Illes P, Verkhratsky A, Burnstock G, P2X receptors and their roles in astroglia in the central and peripheral nervous system: Neuroscientist, 2012; 18; 422-38
9. Di Virgilio F, Dal Ben D, Sarti AC, The P2X7 receptor in infection and inflammation: Immunity, 2017; 471; 15-31
10. Aksentijevich I, Nowak M, Mallah M: Arthritis Rheum, 2002; 46; 3340-48
11. Agostini L, Martinon F, Burns K, NALP3 forms an IL-1beta-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder: Immunity, 2004; 20; 319-25
12. Leissinger M, Kulkarni R, Zemans RL, Investigating the role of nucleotide-binding oligomerization domain-like receptors in bacterial lung infection: Am J Respir Crit Care Med, 2014; 189; 1461-68
13. Lavelle EC, McNaughton A, McNeela E, NLRP3 in protective immunity and vaccination against respiratory infection: Expert Rev Vaccines, 2011; 10; 255-57
14. Chen M, Wang H, Chen W, Regulation of adaptive immunity by the NLRP3 inflammasome: Int Immunopharmacol, 2011; 11; 549-54
15. Adinolfi E, Giuliani AL, De Marchi E, The P2X7 receptor: A main player in inflammation: Biochem Pharmacol, 2018; 151; 234-44
16. Cao F, Hu LQ, Yao SR, P2X7 receptor: A potential therapeutic target for autoimmune diseases: Autoimmun Rev, 2019; 188; 767-77
17. Furini F, Giuliani AL, Parlati ME, P2X7 receptor expression in patients with serositis related to systemic lupus erythematosus: Front Pharmacol, 2019; 10; 435
18. Ito G, Suekawa Y, Watanabe M, P2X7 receptor in the trigeminal sensory nuclear complex contributes to tactile allodynia/hyperalgesia following trigeminal nerve injury: Eur J Pain (London, England), 2013; 17; 185-99
19. Chen WF, Chen CH, Chen NF, Neuroprotective effects of direct intrathecal administration of granulocyte colony-stimulating factor in rats with spinal cord injury: CNS Neurosci Ther, 2015; 21; 698-707
20. Young W, Spinal cord contusion models: Prog Brain Res, 2002; 137; 231-55
21. Jiang K, Zhuang Y, Yan M, Effects of riluzole on P2X7R expression in the spinal cord in rat model of neuropathic pain: Neurosci Lett, 2016; 618; 127-33
22. McLarnon JG, Roles of purinergic P2X7 receptor in glioma and microglia in brain tumors: Cancer Lett, 2017; 402; 93-99
23. Huang YH, Yen JC, Lee JJ, P2X7 is involved in the anti-inflammation effects of levobupivacaine: J Surg Res, 2015; 193; 407-14
24. Pena-Altamira LE, Polazzi E, Giuliani P, Release of soluble and vesicular purine nucleoside phosphorylase from rat astrocytes and microglia induced by pro-inflammatory stimulation with extracellular ATP via P2X7 receptors: Neurochem Int, 2018; 115; 37-49
25. Dinarello CA, Overview of the IL-1 family in innate inflammation and acquired immunity: Immunol Rev, 2018; 281; 8-27
26. Jiang H, He H, Chen Y, Identification of a selective and direct NLRP3 inhibitor to treat inflammatory disorders: J Exp Med, 2017; 214; 3219-38
27. Burnstock G, P2X ion channel receptors and inflammation: Purinergic Signal, 2016; 12; 59-67
28. Gicquel T, Victoni T, Fautrel A, Involvement of purinergic receptors and NOD-like receptor-family protein 3-inflammasome pathway in the adenosine triphosphate-induced cytokine release from macrophages: Clin Exp Pharmacol Physiol, 2014; 41; 279-86
29. Lewis CA, Manning J, Rossi F, The neuroinflammatory response in ALS: The roles of microglia and T cells: Neurol Res Int, 2012; 2012 803701
30. Streit WJ, Mrak RE, Griffin WS, Microglia and neuroinflammation: A pathological perspective: J Neuroinflammation, 2004; 1; 14
31. Bagla S, Dombkowski AA, Neuroinflammatory nexus of pediatric epilepsy: J Pediatr Epilepsy, 2018; 7; 32-39
32. Gao F, Shen J, Zhao L, Curcumin alleviates lipopolysaccharide (LPS)-activated neuroinflammation via modulation of miR-199b-5p/IκB kinase β (IKKβ)/nuclear factor kappa B (NF-κB) pathway in microglia: Med Sci Monit, 2019; 25; 9801-10
33. Kan LK, Seneviratne S, Drummond KJ, P2X7 receptor antagonism inhibits tumour growth in human high-grade gliomas: Purinergic Signal, 2020 [Online ahead of print]
34. Sperlágh B, Vizi ES, Wirkner K, P2X7 receptors in the nervous system: Prog Neurobiol, 2006; 786; 327-46
35. Song XM, Xu XH, Zhu J, Up-regulation of P2X7 receptors mediating proliferation of Schwann cells after sciatic nerve injury: Purinergic Signal, 2015; 112; 203-13
36. Napoli I, Noon LA, Ribeiro S: Neuron, 2012; 734; 729-42
37. Giaume C, Kirchhoff F, Matute C, Glia: The fulcrum of brain diseases: Cell Death Differ, 2007; 147; 1324-35
38. De Keyser J, Mostert JP, Koch MW, Dysfunctional astrocytes as key players in the pathogenesis of central nervous system disorders: J Neurol Sci, 2008; 267; 3-16
39. Mehta N, Kaur M, Singh M, Purinergic receptor P2X7: A novel target for anti-inflammatory therapy: Bioorg Med Chem, 2013; 22; 54-88
40. Collo G, Neidhart S, Kawashima E, Tissue distribution of the P2X7 receptor: Neuropharmacology, 1997; 36; 1277-83
41. Melani A, Amadio S, Gianfriddo M, P2X7 receptor modulation on microglial cells and reduction of brain infarct caused by middle cerebral artery occlusion in rat: J Cereb Blood Flow Metab, 2006; 26; 974-82
42. Kanellopoulos JM, Delarasse C, Pleiotropic roles of P2X7 in the central nervous system: Front Cell Neurosci, 2019; 13; 401
43. Di Virgilio F, Liaisons dangereuses: P2X(7) and the inflammasome: Trends Pharmacol Sci, 2007; 28; 465-72
44. Iwata M, Ota KT, Li XY, Psychological stress activates the inflammasome via release of adenosine triphosphate and stimulation of the purinergic type 2X7 receptor: Biol Psychiatry, 2016; 80; 12-22
45. Idzko M, Ferrari D, Eltzschig HK, Nucleotide signalling during inflammation: Nature, 2014; 509; 310-17
46. Di Virgilio F, P2X receptors and inflammation: Curr Med Chem, 2015; 22; 866-77
47. Zhang XM, Zhu J, Kainic Acid-induced neurotoxicity: Targeting glial responses and glia-derived cytokines: Curr Neuropharmacol, 2011; 9; 388-98
48. Martinon F, Burns K, Tschopp J, The inflammasome: A molecular platform triggering activation of inflammatory caspases and processing of proIL-beta: Mol Cell, 2002; 10; 417-26
49. Amer AO, The inflammasome: Front Microbiol, 2011; 2; 4
50. Yang Y, Wang H, Kouadir M, Recent advances in the mechanisms of NLRP3 inflammasome activation and its inhibitors: Cell Death Dis, 2019; 10; 128
51. Lee BH, Hwang DM, Palaniyar N, Activation of P2X(7) receptor by ATP plays an important role in regulating inflammatory responses during acute viral infection: PLoS One, 2012; 7; e35812
Figures
 Figure 1. Immunofluorescence detected P2X7R of microglia in spinal cord. (A) P2X7R (P2X7R+, green) and IBA-1 (IBA-1+, red) co-expressed as P2X7R of microglia (P2X7R+/IBA-1+, yellow) that were overexpressed after spinal cord injury and BzATP intervention, and, compared to the morphology of microglia in the sham group, the morphology of activated microglia after injury changed to large soma and short irregular processes, revealing that many microglia in spinal cord became active after spinal cord injury. (B) The number of P2X7R of microglia (P2X7R+/IBA-1+) increased notably after spinal cord injury compared to the sham group (** P<0.01), and, compared to the model group, BzATP increased the number of P2X7R of microglia (* P<0.05) while A-438079 significantly decreased the number of P2X7R of microglia (** P<0.01). (C) The P2X7R-positive microglia percentage was clearly increased after spinal cord injury compared to the sham group (** P<0.01), and, compared to the model group, BzATP accounted for a significantly increased P2X7R-positive microglia proportion (** P<0.01) while A-438079 accounted for a significantly reduced P2X7R-positive microglia proportion (** P<0.01).
Figure 1. Immunofluorescence detected P2X7R of microglia in spinal cord. (A) P2X7R (P2X7R+, green) and IBA-1 (IBA-1+, red) co-expressed as P2X7R of microglia (P2X7R+/IBA-1+, yellow) that were overexpressed after spinal cord injury and BzATP intervention, and, compared to the morphology of microglia in the sham group, the morphology of activated microglia after injury changed to large soma and short irregular processes, revealing that many microglia in spinal cord became active after spinal cord injury. (B) The number of P2X7R of microglia (P2X7R+/IBA-1+) increased notably after spinal cord injury compared to the sham group (** P<0.01), and, compared to the model group, BzATP increased the number of P2X7R of microglia (* P<0.05) while A-438079 significantly decreased the number of P2X7R of microglia (** P<0.01). (C) The P2X7R-positive microglia percentage was clearly increased after spinal cord injury compared to the sham group (** P<0.01), and, compared to the model group, BzATP accounted for a significantly increased P2X7R-positive microglia proportion (** P<0.01) while A-438079 accounted for a significantly reduced P2X7R-positive microglia proportion (** P<0.01). Figure 2. Western blot and qPCR assay were used to assess the expression of P2X7R protein and P2X7R mRNA in spinal cord. (A) Western blot assay showed that the expression of P2X7R protein in spinal cord was increased notably after spinal cord injury compared to the sham group (** P<0.01) and, compared to model group, BzATP increased the expression of P2X7R protein in spinal cord (* P<0.05) while A-438079 significantly decreased the expression of P2X7R protein in spinal cord (** P<0.01). (B) qPCR assay showed that the expression of P2X7R mRNA in spinal cord increased notably after spinal cord injury compared to the sham group (** P<0.01), and compared to the model group, BzATP increased the expression of P2X7R mRNA in spinal cord (* P<0.05) while A-438079 significantly decreased the expression of P2X7R mRNA in spinal cord (** P<0.01).
Figure 2. Western blot and qPCR assay were used to assess the expression of P2X7R protein and P2X7R mRNA in spinal cord. (A) Western blot assay showed that the expression of P2X7R protein in spinal cord was increased notably after spinal cord injury compared to the sham group (** P<0.01) and, compared to model group, BzATP increased the expression of P2X7R protein in spinal cord (* P<0.05) while A-438079 significantly decreased the expression of P2X7R protein in spinal cord (** P<0.01). (B) qPCR assay showed that the expression of P2X7R mRNA in spinal cord increased notably after spinal cord injury compared to the sham group (** P<0.01), and compared to the model group, BzATP increased the expression of P2X7R mRNA in spinal cord (* P<0.05) while A-438079 significantly decreased the expression of P2X7R mRNA in spinal cord (** P<0.01). Figure 3. P2X7R of microglia in spinal cord-mediated neuroinflammation after spinal cord injury. (A) ELISA showed that, compared to the sham group, the expression of IL-1β and IL-18 in the spinal cord were significantly increased after spinal cord injury (** P<0.01) and, compared to the model group, A438079 significantly inhibited the expression of IL- 1β and IL-18 (** P<0.01) and BzATP significantly increased the expression of IL- 1β and IL-18 (** P<0.01), which was strongly inhibited by CY-09 (** P<0.01). (B) qPCR assay showed that, compared to the sham group, the expression of IL-1β mRNA and IL-18 mRNA in the spinal cord was significantly increased after spinal cord injury (** P<0.01) and, compared to the model group, A438079 significantly inhibited the expression of IL-1β mRNA and IL-18 mRNA (** P<0.01), and BzATP significantly increased the expression of IL-1β mRNA and IL-18 mRNA (** P<0.01), which was strongly inhibited by CY-09 (** P<0.01).
Figure 3. P2X7R of microglia in spinal cord-mediated neuroinflammation after spinal cord injury. (A) ELISA showed that, compared to the sham group, the expression of IL-1β and IL-18 in the spinal cord were significantly increased after spinal cord injury (** P<0.01) and, compared to the model group, A438079 significantly inhibited the expression of IL- 1β and IL-18 (** P<0.01) and BzATP significantly increased the expression of IL- 1β and IL-18 (** P<0.01), which was strongly inhibited by CY-09 (** P<0.01). (B) qPCR assay showed that, compared to the sham group, the expression of IL-1β mRNA and IL-18 mRNA in the spinal cord was significantly increased after spinal cord injury (** P<0.01) and, compared to the model group, A438079 significantly inhibited the expression of IL-1β mRNA and IL-18 mRNA (** P<0.01), and BzATP significantly increased the expression of IL-1β mRNA and IL-18 mRNA (** P<0.01), which was strongly inhibited by CY-09 (** P<0.01). Figure 4. P2X7R of microglia in spinal cord regulated the expression of NLRP3 and cleaved-Caspase-1 (P20). Compared to the sham group, the expression of NLRP3 and cleaved-Caspase-1 (P20) in the spinal cord significantly increased after spinal cord injury (** P<0.01) and, compared to the model group, BzATP promoted the expression of NLRP3 and cleaved-Caspase-1 (P20) (* P<0.05), which was inhibited by CY-09 (* P<0.05), while A-438079 notably reduced the expression of NLRP3 and cleaved-Caspase-1 (P20) (** P<0.01).
Figure 4. P2X7R of microglia in spinal cord regulated the expression of NLRP3 and cleaved-Caspase-1 (P20). Compared to the sham group, the expression of NLRP3 and cleaved-Caspase-1 (P20) in the spinal cord significantly increased after spinal cord injury (** P<0.01) and, compared to the model group, BzATP promoted the expression of NLRP3 and cleaved-Caspase-1 (P20) (* P<0.05), which was inhibited by CY-09 (* P<0.05), while A-438079 notably reduced the expression of NLRP3 and cleaved-Caspase-1 (P20) (** P<0.01). In Press
05 Mar 2024 : Clinical Research
Muscular Function Recovery from General Anesthesia in 132 Patients Undergoing Surgery with Acceleromyograph...Med Sci Monit In Press; DOI: 10.12659/MSM.942780
05 Mar 2024 : Clinical Research
Effects of Thermal Insulation on Recovery and Comfort of Patients Undergoing Holmium Laser LithotripsyMed Sci Monit In Press; DOI: 10.12659/MSM.942836
05 Mar 2024 : Clinical Research
Role of Critical Shoulder Angle in Degenerative Type Rotator Cuff Tears: A Turkish Cohort StudyMed Sci Monit In Press; DOI: 10.12659/MSM.943703
06 Mar 2024 : Clinical Research
Comparison of Outcomes between Single-Level and Double-Level Corpectomy in Thoracolumbar Reconstruction: A ...Med Sci Monit In Press; DOI: 10.12659/MSM.943797
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








