24 September 2020: Clinical Research
A Retrospective Study from 2 Centers in China on the Effects of Continued Use of Angiotensin-Converting Enzyme Inhibitors and Angiotensin II Receptor Blockers in Patients with Hypertension and COVID-19
Zhongchao Wang1ABCDEFG, Dewei Zhang2BC, Shengming Wang3BC, Yanhua Jin2BC, Jianbo Huan4BC, Yue Wu5CDF, Cheng Xia2CD, Zhe Li6B, Xingshun Qi7CF, Duanzhen Zhang1DF, Xiumin Han1DF, Xianyang Zhu1DEF, Ying Qu8ABCDEFG*, Qiguang Wang9ACDEFGDOI: 10.12659/MSM.926651
Med Sci Monit 2020; 26:e926651
Abstract
BACKGROUND: Use of renin-angiotensin-aldosterone system inhibitors in coronavirus disease 2019 (COVID-19) patients lacks evidence and is still controversial. This study was designed to investigate effects of angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin II receptor blockers (ARBs) on clinical outcomes of COVID-19 patients and to assess the safety of ACEIs/ARBs medication.
MATERIAL AND METHODS: COVID-19 patients with hypertension from 2 hospitals in Wuhan, China, from 17 Feb to 18 Mar 2020 were retrospectively screened and grouped according to in-hospital medication. We performed 1: 1 propensity score matching (PSM) analysis to adjust for confounding factors.
RESULTS: We included 210 patients and allocated them to ACEIs/ARBs (n=81; 46.91% males) or non-ACEIs/ARBs (n=129; 48.06% males) groups. The median age was 68 [interquartile range (IQR) 61.5–76] and 66 (IQR 59–72.5) years, respectively. General comparison showed mortality in the ACEIs/ARBs group was higher (8.64% vs. 3.88%) but the difference was not significant (P=0.148). ACEIs/ARBs was associated with significantly more cases 7-categorical ordinal scale >2 at discharge, more cases requiring Intensive Care Unit (ICU) stay, and increased values and ratio of days that blood pressure (BP) was above normal range (P<0.05). PSM analysis showed no significant difference in mortality, cumulative survival rate, or other clinical outcomes such as length of in-hospital/ICU stay, BP fluctuations, or ratio of adverse events between groups after adjustment for confounding parameters on admission.
CONCLUSIONS: We found no association between ACEIs/ARBs and clinical outcomes or adverse events, thus indicating no evidence for discontinuing use of ACEIs/ARBs in the COVID-19 pandemic.
Keywords: angiotensin receptor antagonists, Angiotensin-Converting Enzyme Inhibitors, Blood Pressure, COVID-19, Hypertension, Angiotensin-Converting Enzyme 2, Antihypertensive Agents, Betacoronavirus, COVID-19, Comorbidity, Coronavirus Infections, Hospital Mortality, Intensive Care Units, Length of Stay, Pandemics, Peptidyl-Dipeptidase A, Pneumonia, Viral, propensity score, SARS-CoV-2, Survival Rate
Background
Coronavirus disease 2019 (COVID-19) has rapidly spread to the whole world and been officially declared a global pandemic by the World Health Organization (WHO) on 11 Mar 2020 [1,2]. A novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has been revealed to be responsible for COVID-19 infection [3,4]. Similar to SARS-CoV and Middle Eastern respiratory syndrome coronavirus (MERS), the viruses inducing the SARS and MERS epidemics, respectively, SARS-CoV-2 has a high affinity to angiotensin-converting enzyme 2 (ACE2) receptors, by which this virus can be transported into the lungs [2–5].
With the continuously increasing number of COVID-19 patients, a high prevalence of concomitant complications such as hypertension and diabetes has been characterized and relevant disease severity and mortality has also been reported [4,6]. Given the fact that renin-angiotensin-aldosterone system (RAAS)-inhibiting drugs are among the main guideline-recommended first-line medications for blood pressure control, it is extremely important to determine if these drugs are still suitable for patients with hypertension in the context of COVID-19 [4,7].
Regarding to this issue, the main controversy originates from 2 perspectives. One perspective is supported by studies showing ACE inhibitors (ACEIs) or angiotensin II receptor blockers (ARBs) can upregulate ACE2 receptors as a feedback response of angiotensin I (Ang I) downregulation, rendering more binding sites for SARS-CoV-2 and thus aggravating infection [8,9]. The other perspective is supported by investigations demonstrating ACEIs/ARBs can exert beneficial effects through upregulating ACE2 expression, which can activate the ACE2/Ang 1-7/MAS (MAS-related G protein-coupled receptor) pathway, thus ameliorating oxidative stress and inflammation responses in acute lung injury models [2,10].
Therefore, as proposed by several recent published authoritative reviews and research, studies on the association between RAAS inhibition therapy and treatment outcomes in COVID-19 patients are urgently needed [1,2,4]. For example, Li et al. [11] conducted a single-center retrospective study showing no association between ACEIs/ARBs intake and severity or mortality of COVID-19 patients. A recent multi-center study by Zhang et al. [12] demonstrated a positive relationship between inpatient use of ACEIs/ARBs with lower risk of all-cause mortality among hospitalized COVID-19 patients. Another study [13] even showed a negative role of ACEIs/ARBs in the clinical outcomes of patients with COVID-19 and hypertension. Therefore, the present retrospective study was designed to share our multi-center experience and further investigate the efficacy and safety of ACEIs/ARBs in COVID-19 patients with hypertension.
Material and Methods
PATIENTS AND STUDY DESIGN:
The medical records of COVID-19 patients with a hypertension history admitted to 2 local hospitals supported by military medical teams in Wuhan, China, including Taikang Tongji (Wuhan) Hospital and Guanggu District, Maternity and Childcare Hospital of Hubei Province, from 17 Feb 2020 to 18 Mar 2020 were carefully reviewed. A total of 210 patients were finally included in this retrospective multi-center cohort study. Patients were assessed for eligibility on the basis of positive SARS-CoV-2 nuclei acid testing result by reverse transcription-polymerase chain reaction (RT-PCR) with nasopharyngeal swab samples at a designated testing center on admission. RT-PCR utilized primers matching for
DATA COLLECTION:
Patients’ baseline information, previous medical history, admission evaluation, blood pressure (BP) measurement during hospitalization, laboratory data, imagological examination, and clinical outcomes were collected. Baseline information included age, sex, height (H), body weight (BW), and the calculated body mass index (BMI) using the formula BMI=BW (kg)/H(m)2. Previous medical history, especially comorbidities such as hypertension, was extracted from the hospitalization records. Admission evaluation parameters included vital signs (body temperature, BP, heart rate, respiratory rate, and oxyhemoglobin saturation) and severity assessment. BP fluctuation was acquired from nursing records. Laboratory data including ion concentration, hepatic and renal function parameters, blood cell count, C-reactive protein (CRP), and interleukin-6 (IL-6) were collected from the laboratory information system. CT image and reports were obtained from the radiographic system. Data on symptom relief or clinical improvement were carefully extracted from the progress note files.
ADMISSION EVALUATION AND OUTCOME MEASURES:
As released in the New Coronavirus Pneumonia Prevention and Control Program (7th trial edition) published by the National Health Commission of China, the clinical classification of COVID-19 consisted of 4 grades: mild (grade 1), ordinary (grade 2), severe (grade 3), and critical severe (grade 4) [14]. According to this classification system, patients were diagnosed with explicit severity on admission. In the present study, we defined patients in grade 1 and 2 as mild cases and patients in grade 3 and 4 as severe cases. To better differentiate patient state and disease severity before hospitalization, a 7-category ordinal scale was also applied, consisting of the following categories: 1 no need for hospitalization, not affecting normal activities; 2 no need for hospitalization, affecting normal activities; 3 hospitalization needed, not requiring supplemental oxygen; 4 hospitalization needed, requiring supplemental oxygen; 5 hospitalization needed, requiring nasal high-flow oxygen therapy, noninvasive mechanical ventilation, or both; 6 hospitalization needed, requiring extracorporeal membrane oxygenation (ECMO), invasive mechanical ventilation, or both; and 7 death [15].
The primary outcomes we observed included mortality and 7-category ordinal scale at discharge; the latter is commonly used in studies investigating severe influenza and COVID-19. Other outcomes included duration of hospital stay, duration of Intensive Care Unit (ICU) stay, BP control during hospitalization, cases and ratio of days required from treatment initiation to symptom relief, first negative SARS-CoV-2 nuclei acid testing result, and definite absorption of pulmonary infection as shown by CT images. BP control parameters consisted of ratio of days and absolute BP values above the normal range. The upper limit of the BP normal range was set as 139/89 mmHg.
DEFINITIONS:
According to in-hospital antihypertensive drug application, patients were divided into 2 groups. Patients receiving ACEIs/ARBs with or without other antihypertensive drugs during hospital stay were defined as the ACEIs/ARBs group. Patients receiving other types of antihypertensive drugs but not ACEIs/ARBs were defined as the non- ACEIs/ARBs group. Other antihypertensive drugs included the commonly used calcium channel blockers (CCBs), β-blockers, and other drugs with antihypertensive effects, such as diuretics and nitrates. Hypertension was defined as systolic blood pressure (SBP) ≥140 mmHg or diastolic blood pressure ≥90 mmHg, in accordance with the 2018 European Society of Cardiology and European Society of Hypertension (ESC/ESH) Guidelines for the management of arterial hypertension and the 2019 National Institute for Health and Care Excellence (NICE) guidelines for hypertension in adults [16,17].
STATISTICAL ANALYSIS:
Continuous variables were expressed as median and interquartile range (IQR) and were compared by the nonparametric Mann-Whitney U test. Categorial variables were expressed as frequency and percentage and compared by the chi-square test or Fisher’s exact test. Propensity score matching (PSM) with a ratio of 1: 1 was conducted to adjust the effects of age, sex, BMI, previous comorbidities, vital signs, disease severity, ion concentration, hepatic and renal function, blood cell count, CRP, and IL-6 on the clinical outcomes. For better comparability, a caliper size of 0.02 was used. Cumulative survival analysis was performed using the Kaplan-Meier method. All statistical analysis was performed using SPSS 22.0 (SPSS, Inc.) and Prism 8.0 (GraphPad). A two-tailed value of
Results
GENERAL INFORMATION:
Following strict exclusion criteria, 232 COVID-19 patients with a history of hypertension were selected through preliminary screening, including 154 patients from Taikang Tongji (Wuhan) Hospital and 78 patients from Guanggu District, Maternity and Childcare Hospital of Hubei Province. After a second-round screening, 22 patients were further excluded, including 17 cases switching from ACEIs/ARBs to other types of antihypertensive drugs and 5 cases discontinuing antihypertensive drugs during hospitalization.
Finally, a total of 210 patients were included in the present study, with 100 males (47.62%) and 110 females (52.38%), and the median age was 67 (IQR 59.75–74) years (Table 1). As recorded in the medical files, 65 (30.95%) patients were diagnosed as severe cases on admission, including 52 cases in grade 3 (severe) and 13 cases in grade 4 (critical severe). The 7-categorical ordinal scale data on admission showed that 99 (47.14%) patients needed oxygen therapy (scale >3), including nasal low-flow oxygen therapy (89 cases, scale 4) and nasal high-flow or noninvasive mechanical ventilation oxygen therapy (10 cases, scale 5). Regarding comorbidities, besides hypertension, 136 (64.76%) patients were complicated with other diseases (Table 1). Since some patients were complicated with more than 1 other disease, the total number of other comorbidities was not equal to sum of the specific diseases listed. Other general informations of the included patients, such as vital signs and laboratory tests on admission, are also listed in Table 1. The main in-hospital medications are summarized in Table 2.
As shown in Table 3, the mortality rate in this study population was 5.71% (12/210). The 7-categorical ordinal scale data at discharge showed that 190 (90.48%) patients remained at low scale (159 cases at scale 1 and 31 cases at scale 2), while. 8 (3.81%, scale >2) patients still needed to be hospitalized in other specialized hospitals and 5 of them (2.38%, scale 4 and 5) needed oxygen therapy. The median length of in-hospital stay was 17 (IQR 13–21) days. Sixteen (7.62%) patients needed ICU stay, and the percentage of days in the ICU during hospitalization was 73.86% (IQR 53.64–100%). Symptom relief was reported by 180 (85.71%) patients, as recorded in progress notes. In 203 (96.67%) patients, SARS-CoV-2 nuclei acid testing turned negative and 198 (94.29%) patients CT showed significant absorption of infection. Eleven (5.24%) adverse events were recorded during hospitalization, including 3 general events (pain, rash and pruritus), 4 digestive system events (nausea, vomiting and gaseous distension), 2 nervous system events (dizzy and headache), and 2 respiratory system events (chest distress).
GROUPING AND COMPARISONS:
According to in-hospital use of antihypertensive drugs (Table 2), of the 210 enrolled patients, 81 patients were allocated to the ACEIs/ARBs group and 129 patients were allocated to the non-ACEIs/ARBs group. In the ACEIs/ARBs group, 16 (19.75%) patients received ACEIs and the other 65 (80.25%) used ARBs. No significant difference was observed in the application of other antihypertensive drugs, including CCBs, diuretics, β-blockers, and nitrates between groups, as well as other in-hospital medications such as antiviral drugs and antibiotics (Table 2). Regarding parameters on admission (Table 1), SBP [144 (IQR 130–155) mmHg vs. 140 (128–147) mmHg] and CREA [61.23 (IQR 51.35–76.45) μmol/L vs. 58.35 (IQR 47.76–71.28) μmol/L] of the ACEIs/ARBs group was significantly higher than that of the non-ACEIs/ARBs group (P<0.05). We found no significant difference in age, sex, BMI, other vital signs (body temperature, SpO2, DBP, HR and RR), or other laboratory test results (e.g., WBC, CRP, IL-6, and other liver/renal function parameters) was detected between groups. Classification analysis showed no significant difference in the distribution of mild (grade 1 and 2) or severe (grade 3 and 4) cases between the 2 groups (Table 1). However, as shown by 7-categorical ordinal scale data on admission, there were more cases with scale >3 in the ACEIs/ARBs group than in the non-ACEIs/ARBs group [49 (60.49%) vs. 50 (38.76%), P=0.002], and this difference was mainly due to patients in scale 4 [42 (51.85%) vs. 47 (36.43%), P=0.028], indicating more patients needed oxygen therapy, and thus showing a worse state of illness in the ACEIs/ARBs group. Besides hypertension, a second or more comorbidities were more prevalent in the ACEIs/ARBs group [62 (76.54%) vs. 74 (57.36%), P=0.005].
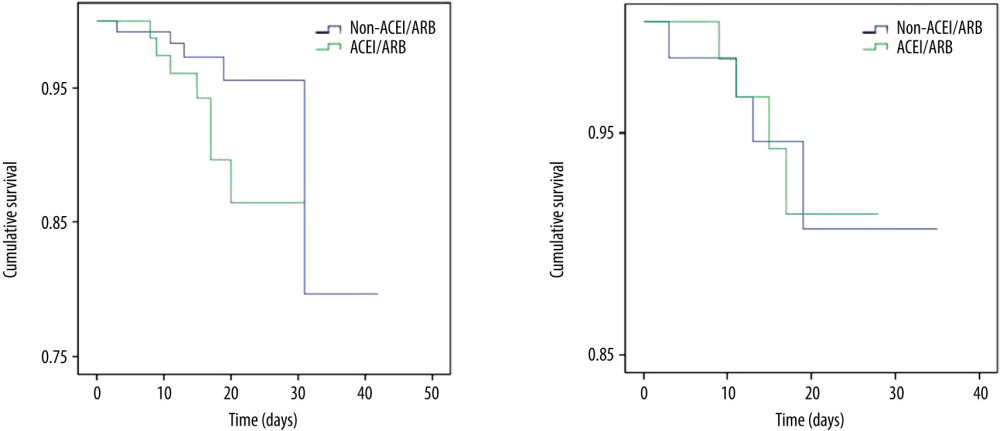
As shown by Table 3, mortality in the ACEIs/ARBs group was higher than in the non-ACEIs/ARBs group [7 (8.64%) vs. 5 (3.88%)], but the difference was not significant (P=0.148). Survival analysis showed that the difference in cumulative survival rates between groups was not significant (Figure 1A, χ2=2.552, P=0.11). The 7-category ordinal scale data at discharge showed more patients still needed to be hospitalized in other specialized hospitals (scale >2) in ACEIs/ARBs group [12 (14.81%) vs. 8 (6.20%), P=0.038]. As compared with the non-ACEIs/ARBs group, in the ACEIs/ARBs group there were significantly more cases that needed ICU stay [10 (12.35%) vs. 6 (4.65%), P=0.041], higher percentage of days of BP above normal range [46.15% (IQR 27.89–76.70%) vs. 38.46% (13.96–61.39%), P=0.012], and greater mSBP [7 (IQR 3–11.72) mmHg vs. 5 (0–10.85) mmHg, P=0.025] and eSBP [8.79 (IQR 4–13.12) mmHg vs. 6 (1.25–10.88) mmHg, P=0.003] fluctuation. However, the percentage of days required from treatment initiation to definite CT-shown absorption of pulmonary infection was significantly increased in the non-ACEIs/ARBs group [63.64% (45–78.57%) vs. 75% (58.33–84.62%), P=0.004]. No significant differences in other clinical outcomes were observed between groups, including length of in-hospital stay and number of cases with symptom relief, SARS-CoV-2 nuclei acid testing turning negative, or CT-shown absorption of pulmonary infection, as well as percentage of days required from treatment initiation to symptom relief or first negative SARS-CoV-2 nuclei acid testing result. The difference in ratio of adverse events between the 2 groups was not significant (Table 3).
PROPENSITY SCORE MATCHING:
To increase the comparability of the 2 groups and better reveal effects of ACEIs/ARBs on the clinical outcomes of COVID-19 patients with hypertension, a 1: 1 PSM analysis was applied to adjust for potential confounding factors, including age, sex, BMI, vital signs, 7-categorical ordinal scale, classification, comorbidities, and laboratory tests on admission. There were 124 patients (62 patients from each group) who were 1: 1 matched, and the median age was 68 (IQR 59.25–75) years. Sixty-three (50.81%) patients were males, 40 (32.26%) were severe cases, and 91 (73.39%) were complicated with other comorbidities. Other parameters, such as BMI, vital signs, and laboratory tests on admission, are described in Table 4. Among PSM selected patients, 8 (6.45%) patients died and 17 (13.71%) patients had 7-categorical ordinal scale >2 at discharge (Table 5). The median length of in-hospital stay was 16.5 (IQR 13–21) days, and 6 (4.84%) adverse events were observed during hospitalization. Other clinical outcomes were also listed in Table 5.
As shown by group comparison results after PSM adjustment (Table 4), all parameters before treatment initiation and patient allocation were equalized, including age, sex, BMI, vital signs and laboratory tests, other comorbidities, 7-categorical ordinal scale, and classification on admission. Table 5 shows that the mortality rate was not significantly different between the 2 groups [6.45% (4/62)] and no significant difference was detected between groups in 7-categorical ordinal scale data at discharge, percentage of days of BP above normal range and BP fluctuation during hospitalization, or percentage of days required from treatment initiation to definite CT-shown absorption of pulmonary infection (P>0.05). We did not find any significant difference in cumulative survival rate (Figure 1B, χ2=0.001, P=0.969) or other clinical outcomes (Table 5), including length of in-hospital stay, number of cases and duration of ICU stay, number of cases with symptom relief, SARS-CoV-2 nuclei acid testing turning negative, or CT-shown absorption of infection, as well as percentage of days required from treatment initiation to symptom relief, first negative SARS-CoV-2 nuclei acid testing result, and CT amelioration (P>0.05).
Discussion
Although it has not been directly demonstrated that RAAS inhibitors can upregulate ACE2 expression in human lung tissues, some previous studies did report increased expression of ACE2 in rat models and human cells
Because of the continuous heated debate about the role of ACEIs/ARBs in COVID-19 patients with hypertension, relevant studies, especially clinical prospective trials and retrospective analysis, are urgently needed to help answer this question in the setting of the still growing pandemic of COVID-19 [1,4,18]. Due to the lack of clinical data and evidence, recently published specialist statements and comments strongly recommended the continuous use of ACEIs/ARBs in COVID-19 patients complicated with hypertension [6,19]. The experts also called for studies investigating the effect of ACEIs/ARBs medication on clinical outcomes of COVID-19 patients [6,19].
To date, limited data has aggravated the controversy about the advantage/disadvantage of ACEIs/ARBs application in the context of COVID-19. Guo et al. reported that prior use of ACEIs/ARBs could indirectly negatively affect the clinical outcomes of COVID-19 patients through the elevation of troponin levels [13]. However, more studies found a positive role of these RAAS inhibitors [12,20]. A recent retrospective study by Zhang et al. [12] demonstrated that the inpatient use of ACEIs/ARBs was associated with lower risk of all-cause mortality. Another study also gave support to this positive conclusion [20]. In a newly published retrospective study reviewed 18 472 patients taking ACEIs/ARBs at the time of COVID-19 testing, PSM analysis showed no association between ACEIs/ARBs intake and SARS-CoV-2 nuclei acid test positivity [21].
Our present study retrospectively reviewed 210 COVID-19 patients with history of hypertension from multiple centers, analyzed more parameters other than mortality, and observed the efficacy and safety of ACEIs/ARBs medication. A general comparison showed use of ACEIs/ARBs was associated with worse clinical outcomes, including more cases in high 7-categorical ordinal scale (>2) at discharge, indicating more patients still needed to be hospitalized or receive oxygen therapy in other specialized hospitals, more cases required ICU stay, a higher ratio of days of BP above normal range, and more fluctuations of mSBP and eSBP during hospitalization. However, ACEIs/ARBs were also associated with a lower ratio of days required for CT-shown absorption of pulmonary infection from treatment initiation. Since more patients with 7-categorical ordinal scale >3 and other comorbidities were allocated to the ACEIs/ARBs group and their SBP on admission was also significantly higher, the disease severity in the 2 groups might be imbalanced, thus interfering with the final statistical comparison. Therefore, we performed PSM analysis to adjust for these confounding factors. As compared with the recently published study by Zhang et al. [12], which also adjusted for potential confounding factors such as age, sex, and comorbidities with a mixed-effects Cox model and PSM analysis, our study considered more factors directly or indirectly related with disease severity, making group comparison more accurate. After a 1: 1 match process, 62 patients from each group were retained with equalized baseline characteristics and disease severity. Further statistical analysis showed ACEIs/ARBs use did not affect in-hospital mortality, cumulative survival rate, or other clinical outcomes. The ratio of adverse events was also similar in patients taking ACEIs/ARBs and those taking non-ACEIs/ARBs.
Recently published observational and case-control studies showed no association between RAAS inhibitors with inpatient mortality, hospitalization rate, or risk of infection during the COVID-19 pandemic [22–25]. For instance, Li et al. [11] analyzed 1178 hospitalized patients with COVID-19 infections and found that ACEIs/ARBs were not associated with the severity or mortality rate in these patients. Consistent with these viewpoints, the present study found inpatient mortality and cumulative survival rate was not changed by the use of ACEIs/ARBs. Besides, ACEIs/ARBs did not affect other clinical outcomes, such as length of in-hospital and ICU stay, ratio of patients with symptom relief and negative SARS-CoV-2 nuclei acid test, and BP control. Meanwhile, the ratio of adverse events was not different with and without using ACEIs/ARBs, indicating good safety of ACEIs/ARBs intake by COVID-19 patients with hypertension.
The retrospective design of the present study limits the strength of our findings. Future randomized controlled trials with larger sample sizes are still urgently needed. Since the outbreak of COVID-19 has severely affected the normal medical service and consumed medical resources, some clinical parameters are not available or are incomplete. As a global pandemic, COVID-19 has shown ubiquitous infectivity and virulence around the world, future studies investigating the difference between races and districts are badly needed to better understand the pathogenesis and propagation characteristics of this new virus.
Conclusions
Use of ACEIs/ARBs was not different in COVID-19 patients complicated with hypertension. ACEIs/ARBs have no impact on mortality, length of in-hospital and ICU stay, BP control, and ratio of adverse events in the context of the COVID-19 pandemic. Therefore, ACEIs/ARBs should not be discontinued or switched to other types of antihypertensive drugs unless necessary for long-term therapy of hypertension.
Tables
Table 1. Baseline characteristics and disease severity of all included COVID-19 patients complicated with hypertension.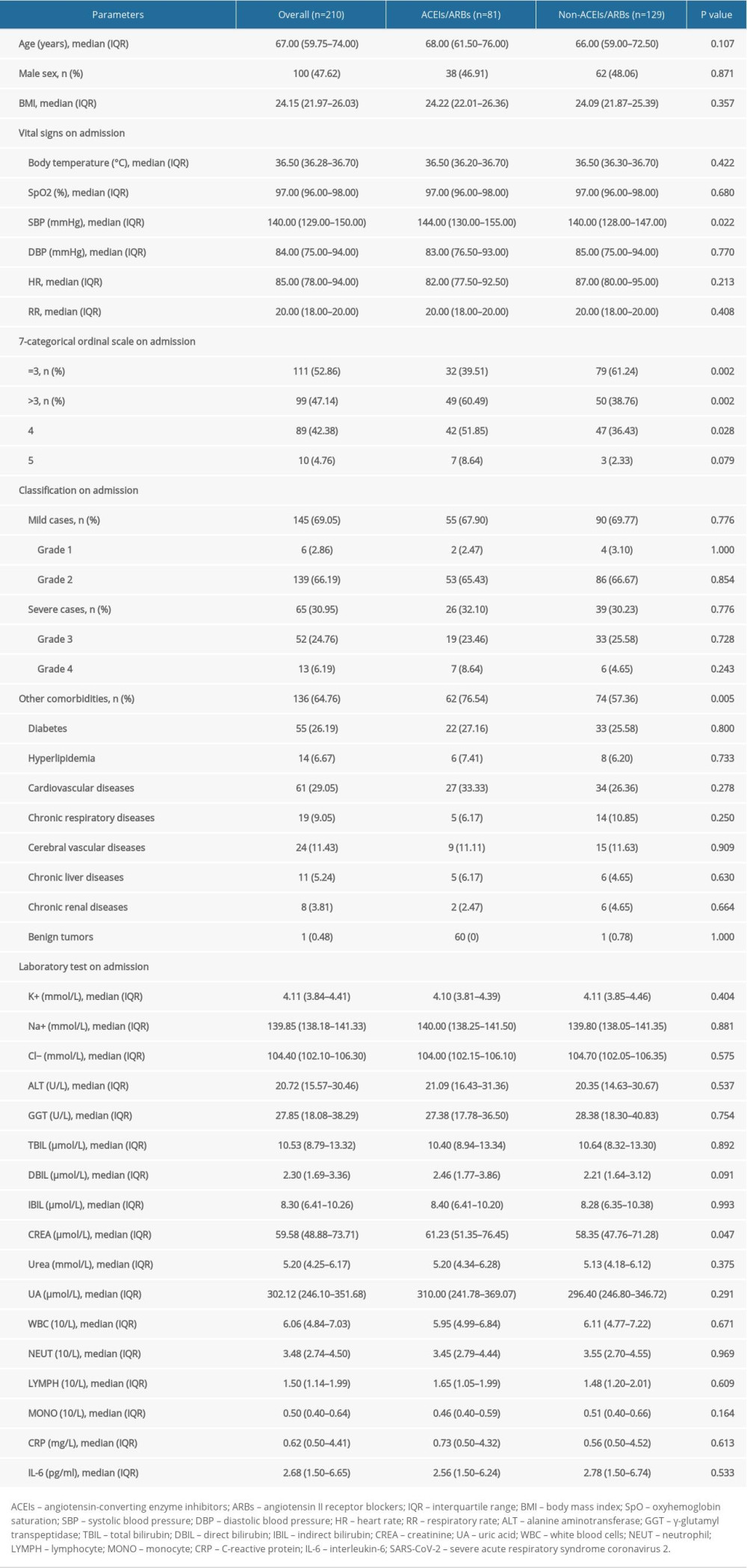 Table 2. Main in-hospital medications of all included COVID-19 patients complicated with hypertension.
Table 2. Main in-hospital medications of all included COVID-19 patients complicated with hypertension.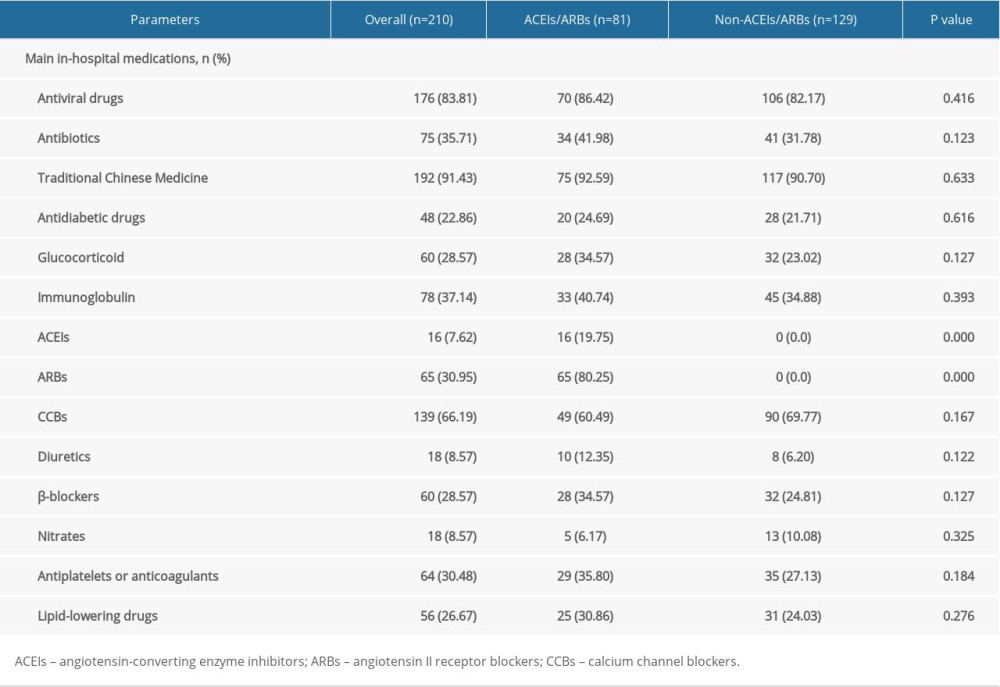 Table 3. Clinical outcomes and adverse events of all included COVID-19 patients complicated with hypertension.
Table 3. Clinical outcomes and adverse events of all included COVID-19 patients complicated with hypertension.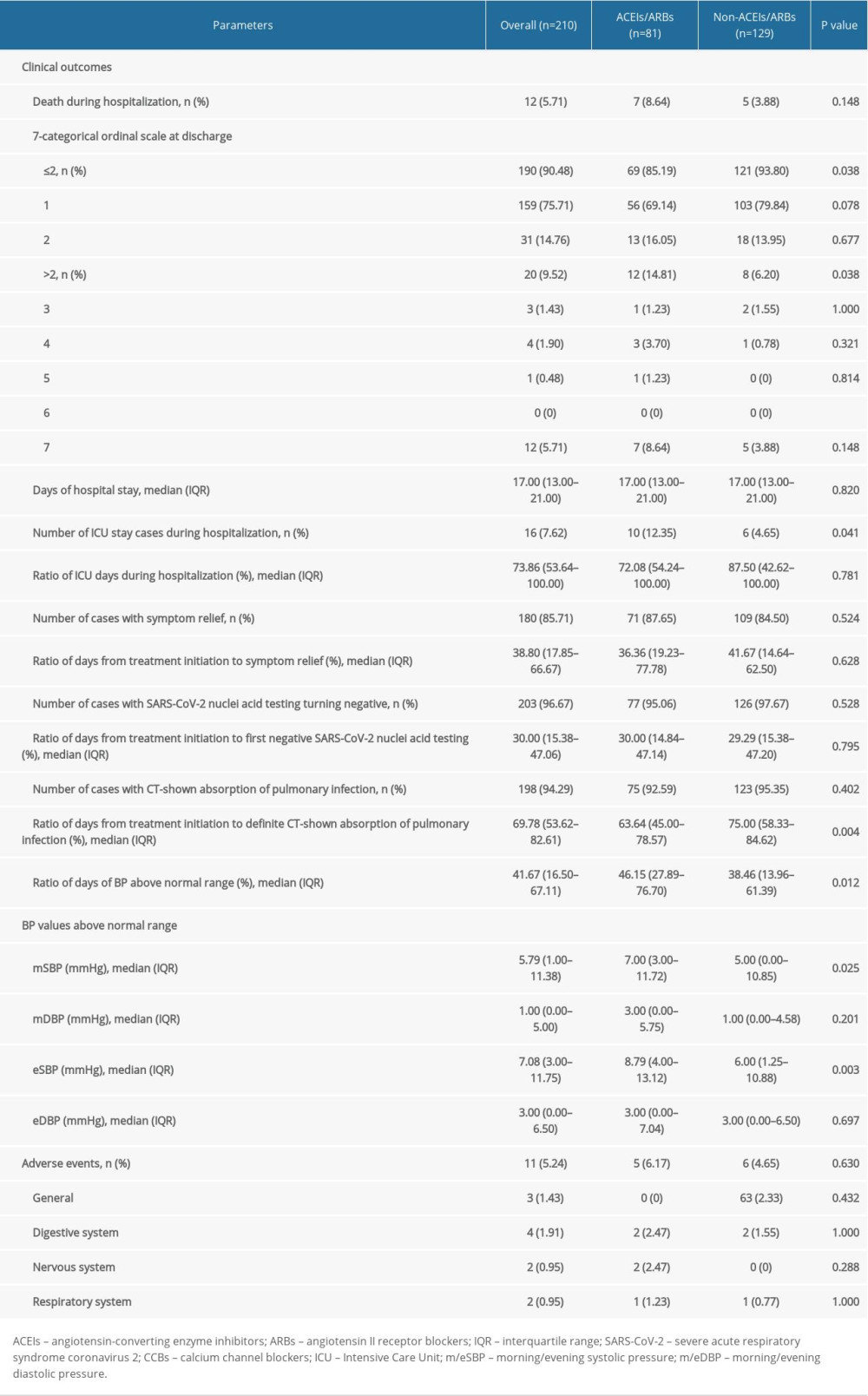 Table 4. Baseline characteristics and disease severity of COVID-19 patients complicated with hypertension after propensity score matching.
Table 4. Baseline characteristics and disease severity of COVID-19 patients complicated with hypertension after propensity score matching.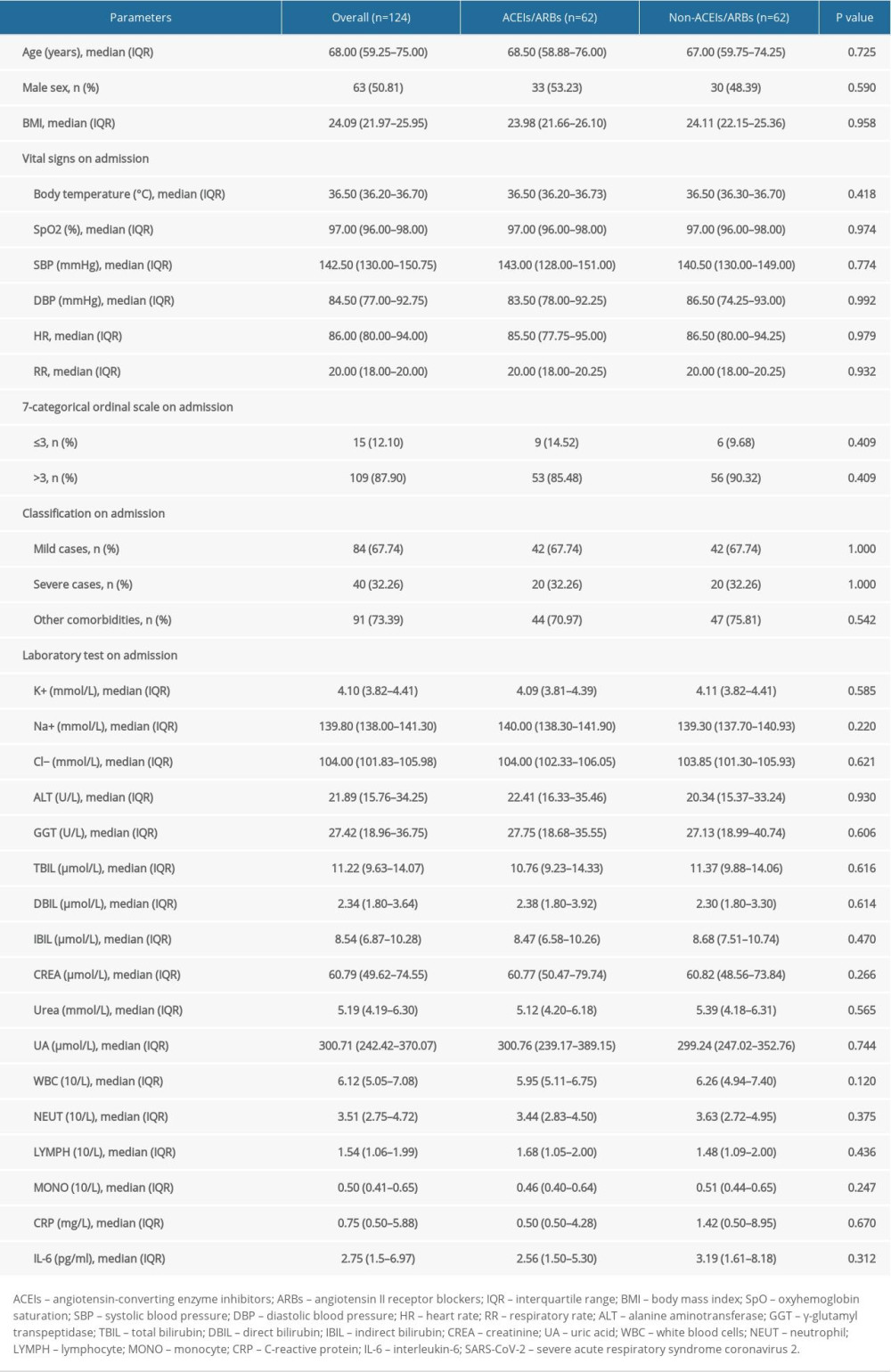 Table 5. Clinical outcomes and adverse events of COVID-19 patients complicated with hypertension after propensity score matching.
Table 5. Clinical outcomes and adverse events of COVID-19 patients complicated with hypertension after propensity score matching.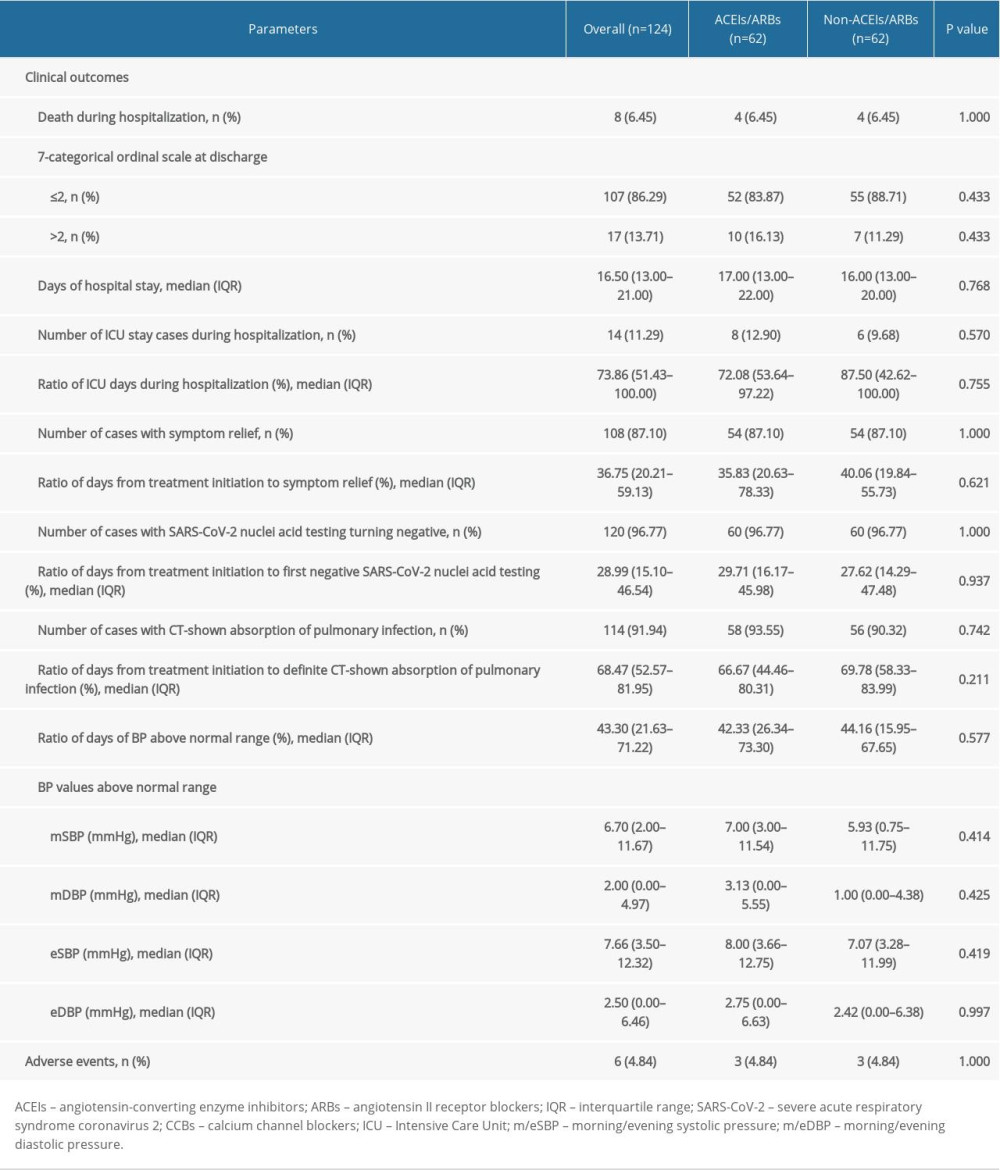
References
1. Kreutz R, Algharably E, Azizi M, Hypertension, the renin-angiotensin system, and the risk of lower respiratory tract infections and lung injury: implications for COVID-19: Cardiovasc Res, 2020; 116(10); 1688-99
2. Danser A, Epstein M, Batlle D, Renin-angiotensin system blockers and the COVID-19 pandemic: At present there is no evidence to abandon renin-angiotensin system blockers: Hypertension, 2020; 75(6); 1382-85
3. Zhou P, Yang XL, Wang XG, A pneumonia outbreak associated with a new coronavirus of probable bat origin: Nature 5, 2020; 79(7798); 270-73
4. Zheng YY, Ma YT, Zhang JY, Xie X, COVID-19 and the cardiovascular system: Nat Rev Cardiol, 2020; 17(5); 259-60
5. Hoffmann M, Kleine-Weber H, Schroeder S, SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor: Cell, 2020; 181(2); 271-80
6. Williams B, Zhang Y, Hypertension, renin-angiotensin-aldosterone system inhibition, and COVID-19: Lancet, 2020; 395(10238); 1671-73
7. Unger T, Borghi C, Charchar F, 2020 International Society of Hypertension Global Hypertension Practice Guidelines: Hypertension, 2020; 75(6); 1334-57
8. Igase M, Kohara K, Nagai T, Increased expression of angiotensin converting enzyme 2 in conjunction with reduction of neointima by angiotensin II type 1 receptor blockade: Hypertens Res, 2008; 31(3); 553-59
9. Ferrario CM, Jessup J, Chappell MC, Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2: Circulation 1, 2005; 11(20); 2605-10
10. Kuba K, Imai Y, Rao S, A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury: Nat Med, 2005; 11(8); 875-79
11. Li J, Wang X, Chen J, Association of renin-angiotensin system inhibitors with severity or risk of death in patients with hypertension hospitalized for coronavirus disease 2019 (COVID-19) infection in Wuhan, China: JAMA Cardiol, 2020 [Online ahead of print]
12. Zhang P, Zhu L, Cai J, Association of inpatient use of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers with mortality among patients with hypertension hospitalized with COVID-19: Circ Res, 2020; 126(12); 1671-81
13. Guo T, Fan Y, Chen M, Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19): JAMA Cardiol, 2020 [Online ahead of print]
14. Xie LX: Chronic Dis Transl Med, 2020; 6(2); 75-78
15. Wang Y, Fan G, Salam A, Comparative effectiveness of combined favipiravir and oseltamivir therapy versus oseltamivir monotherapy in critically ill patients with influenza virus infection: J Infect Dis, 2020; 221(10); 1688-98
16. Williams B, Mancia G, Spiering W, 2018 ESC/ESH Guidelines for the management of arterial hypertension: Eue Heart J, 2018; 39(33); 3021-104
17. McCormack T, Boffa RJ, Jones NR, The 2018 ESC/ESH hypertension guideline and the 2019 NICE hypertension guideline, how and why they differ: Eur Heart J, 2019; 40(42); 3456-58
18. Rico-Mesa JS, White A, Anderson AS, Outcomes in patients with COVID-19 infection taking ACEI/ARB: Curr Cardiol Rep, 2020; 22(5); 31
19. Pechere-Bertschi A, Ponte B, Wuerzner GRenin-angiotensin-aldosterone blockers and Covic-19 infection: friends or enemies ?: Rev Med Suisse, 2020; 16(693); 1003-7
20. Yang G, Tan Z, Zhou L, Effects of angiotensin II receptor blockers and ACE (angiotensin-converting enzyme) inhibitors on virus infection, inflammatory status, and clinical outcomes in patients with COVID-19 and hypertension: A single-center retrospective study: Hypertension 7, 2020; 6(1); 51-58
21. Mehta N, Kalra A, Nowacki AS, Association of use of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers with testing positive for coronavirus disease 2019 (COVID-19): JAMA Cardiol, 2020 [Online ahead of print]
22. de Abajo FJ, Rodriguez-Martin S, Lerma V, Use of renin-angiotensin-aldosterone system inhibitors and risk of COVID-19 requiring admission to hospital: A case-population study: Lancet, 2020; 395(10238); 1705-14
23. Reynolds HR, Adhikari S, Pulgarin C, Renin-angiotensin-aldosterone system inhibitors and risk of Covid-19: N Engl J Med, 2020; 382(25); 2441-48
24. Mancia G, Rea F, Ludergnani M, Renin-angiotensin-aldosterone system blockers and the risk of Covid-19: N Engl J Med, 2020; 382(25); 2431-40
25. Mehra MR, Desai SS, Kuy S, Cardiovascular disease, drug therapy, and mortality in Covid-19: N Engl J Med, 2020; 382(25); e102
Tables
 Table 1. Baseline characteristics and disease severity of all included COVID-19 patients complicated with hypertension.
Table 1. Baseline characteristics and disease severity of all included COVID-19 patients complicated with hypertension. Table 2. Main in-hospital medications of all included COVID-19 patients complicated with hypertension.
Table 2. Main in-hospital medications of all included COVID-19 patients complicated with hypertension. Table 3. Clinical outcomes and adverse events of all included COVID-19 patients complicated with hypertension.
Table 3. Clinical outcomes and adverse events of all included COVID-19 patients complicated with hypertension. Table 4. Baseline characteristics and disease severity of COVID-19 patients complicated with hypertension after propensity score matching.
Table 4. Baseline characteristics and disease severity of COVID-19 patients complicated with hypertension after propensity score matching. Table 5. Clinical outcomes and adverse events of COVID-19 patients complicated with hypertension after propensity score matching.
Table 5. Clinical outcomes and adverse events of COVID-19 patients complicated with hypertension after propensity score matching. Table 1. Baseline characteristics and disease severity of all included COVID-19 patients complicated with hypertension.
Table 1. Baseline characteristics and disease severity of all included COVID-19 patients complicated with hypertension. Table 2. Main in-hospital medications of all included COVID-19 patients complicated with hypertension.
Table 2. Main in-hospital medications of all included COVID-19 patients complicated with hypertension. Table 3. Clinical outcomes and adverse events of all included COVID-19 patients complicated with hypertension.
Table 3. Clinical outcomes and adverse events of all included COVID-19 patients complicated with hypertension. Table 4. Baseline characteristics and disease severity of COVID-19 patients complicated with hypertension after propensity score matching.
Table 4. Baseline characteristics and disease severity of COVID-19 patients complicated with hypertension after propensity score matching. Table 5. Clinical outcomes and adverse events of COVID-19 patients complicated with hypertension after propensity score matching.
Table 5. Clinical outcomes and adverse events of COVID-19 patients complicated with hypertension after propensity score matching. In Press
15 Apr 2024 : Laboratory Research
The Role of Copper-Induced M2 Macrophage Polarization in Protecting Cartilage Matrix in OsteoarthritisMed Sci Monit In Press; DOI: 10.12659/MSM.943738
07 Mar 2024 : Clinical Research
Knowledge of and Attitudes Toward Clinical Trials: A Questionnaire-Based Study of 179 Male Third- and Fourt...Med Sci Monit In Press; DOI: 10.12659/MSM.943468
08 Mar 2024 : Animal Research
Modification of Experimental Model of Necrotizing Enterocolitis (NEC) in Rat Pups by Single Exposure to Hyp...Med Sci Monit In Press; DOI: 10.12659/MSM.943443
18 Apr 2024 : Clinical Research
Comparative Analysis of Open and Closed Sphincterotomy for the Treatment of Chronic Anal Fissure: Safety an...Med Sci Monit In Press; DOI: 10.12659/MSM.944127
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952