07 July 2022: Clinical Research
Optimal Timing and Outcomes of Minimally Invasive Approach in Acute Biliary Pancreatitis
Mihai Faur12ABCE, Sorin Radu FleacaDOI: 10.12659/MSM.937016
Med Sci Monit 2022; 28:e937016
Abstract
BACKGROUND: We analyzed the outcomes of early biliary decompression by a minimally invasive approach in acute biliary pancreatitis (ABP).
MATERIAL AND METHODS: A retrospective study was conducted on 143 patients with ABP who underwent biliary decompression by laparoscopic or endoscopic approach between January 2015 and March 2022. Data from the observation sheets and surgical protocols were analyzed in terms of demographic characteristics, clinical and paraclinical features at admission, comorbidities, therapeutic management, and outcomes.
RESULTS: The mean patient age was 62.3±11.4 years. Mild ABP had a higher frequency in men (75.5%) and urban areas (70.4%). The comorbidities associated with a higher risk of severe forms were diabetes mellitus (odds ratio [OR]: 11.250), chronic bronchopneumopathy (OR: 29.297), and ischemic coronary disease (OR: 2.784). The mean hospital stay was 7.6±3.8 days and was significantly higher in severe forms (10±2.4 days, P<0.001). The time from onset to presentation was significantly higher in severe vs mild forms (5.6 vs 1.8 days, P<0.001) and was associated with systemic and local complications. Creatinine over 2 mg/dL (OR: 4.821) and leukocytes >15 000/mmc at admission (OR: 3.533) were risk factors for systemic complications, while obesity was associated with increased local complications (OR: 5.179). None of the patients with an early presentation developed severe ABP.
CONCLUSIONS: Early biliary decompression, as soon as possible after onset, either by an endoscopic or minimally invasive approach, is a safe and effective procedure in ABP. The type of procedure and optimal timing should be individualized, according to the patient’s local and general features.
Keywords: Biliary Tract Surgical Procedures, Laparoscopy, Pancreatitis, Aged, Humans, Length of Stay, Male, Middle Aged, Risk Factors
Background
Acute pancreatitis is an inflammatory condition of the pancreas and is one of the most common causes of gastrointestinal hospitalization worldwide [1,2]. Although in most cases the disease is mild and self-limited, approximately 20% of patients develop life-threatening complications, leading to multiple organ failure and death. A recent multicentric study by Matta et al [1] showed that the causes of acute pancreatitis are most frequently related to biliary obstruction (42%), followed by alcohol abuse (25%). Less common causes are related to disturbances in lipid metabolism, trauma, and toxicity or are idiopathic.
The current guideline for the diagnosis of acute pancreatitis is the revised Atlanta classification, with diagnosis requiring at least the first 2 out of 3 of the following findings: (1) characteristic upper abdominal pain, (2) elevated levels of serum amylases of more than 3 times above the normal value, and (3) features on ultrasound or computed tomography (CT) examination suggesting acute pancreatitis [3,4].
According to the revised Atlanta classification, the severity of the disease is based on the presence and persistence of organ insufficiency or the onset of pancreatic and peripancreatic collections. The patients are categorized as having mild acute pancreatitis if no organ failure was present at admission; moderate-severe pancreatitis if transient organ dysfunction was present initially but resolved within <48 h or a sterile peripancreatic collection is documented; and severe acute pancreatitis if it is associated with persistent single or multiple organ failure or an infected pancreatic collection [4].
The revised Atlanta classification discriminates the evolution of acute pancreatitis, with an early phase (during the first week from the onset of symptoms) characterized by local pancreatic and peripancreatic inflammation with systemic involvement, in which the diagnosis and monitoring should be based on biochemical and laboratory data more than on imaging data [3]. The late phase is characterized by organic changes at the level of the pancreas that can be documented on CT and magnetic resonance imaging examinations.
Acute biliary pancreatitis occurs most frequently as a result of increased pressure in the main and intracanalicular bile ducts, resulting in the reflux of enzymes from the Wirsung canal. This hypertension occurs through the following mechanisms of production: papillary obstruction due to the inclusion of a stone or papillary edema caused by lesions provoked by the passage of a stone through the papilla, a pathogenic mechanism that occurs in biliary pancreatitis, and reflux of duodenal contents due to the relaxation of the Oddi sphincter or bile reflux through a spasm of the Oddi sphincter in the pancreatic canalicular system, a mechanism found in alcoholic pancreatitis.
The evolution toward severe acute pancreatitis is the result of maintaining hypertension in the main and intracanalicular bile ducts and is influenced by the duration of its persistence. Early decompression of the bile-pancreatic tree is necessary to prevent progression to severe acute pancreatitis.
Norman et al introduced the term “therapeutic window” in the treatment of acute pancreatitis to indicate the first 7 days after the onset of the disease [5,6]. Their study focused on cytokines and their role in the pathogenesis of acute pancreatitis, knowing that cytokines are responsible for the systemic complications of acute pancreatitis, up to multisystem organ failure, as evidenced by experimental animal studies [5,6].
The proposed drug therapy of Normal et al includes [6–8] rapid ductal decompression performed by oddian spasmolysis by contact papillary anesthesia (xylin 1%) and general lysis of the oddian spasm (coronary dilator-nitroglycerin patch), blockage of secretion by digestive emptying (gastric tube), and pharmacological therapy through specific antisecretory (Sandostatin) and general (atropine and gastric antisecretory) analgesics (and sometimes epidural analgesia in patients with severe pancreatitis). Antibiotic therapy, by administration of Meronem, has been given to all patients with severe pancreatitis at a dose of 3 g/day for at least 10 days [6,7,9]. A central venous catheter was placed routinely for hydro-electrolytic rebalancing through the administration of crystalloid and colloidal solutions and the administration of parenteral feeding [9,10].
In our department, we applied a new concept regarding the therapeutic window in acute pancreatitis, namely the introduction of minimally invasive surgical therapy in the first 7 days after the onset of pancreatitis, in addition to drug therapy.
The primary objective of the minimally invasive pathogenic therapeutic approach in acute biliary pancreatitis is to interrupt the pathogenic chain through early biliopancreatic decompression through a laparoscopic approach in order to improve the evolution of acute pancreatitis. It also aims to resolve symptoms of pain, dyspeptic syndrome, vomiting, and dynamic ileus and prevent local and systemic complications.
In this study, we analyzed the outcomes of early biliary decompression by a minimally invasive approach in acute biliary pancreatitis in terms of postoperative morbidity and mortality. The preoperative data that correlated best with the surgical decision and postoperative outcomes were analyzed by binary logistic regression.
Material and Methods
STUDY DESIGN:
We conducted a retrospective study on a group of 143 patients who were admitted in an emergency setting with acute biliary pancreatitis between January 2015 and March 2022 to the Surgical Department of Sibiu County Clinical Emergency Hospital, Romania. The data from the observation sheets and the surgical protocols were analyzed in terms of demographic characteristics, time from onset to presentation, clinical and paraclinical data, comorbidities, therapeutic management, and outcomes.
The diagnosis of acute pancreatitis at admission was performed according to the revised Atlanta classification. The biliary etiology was documented based on imagistic findings.
The inclusion criteria were early phase acute pancreatitis, within a maximum of 7 days from the onset of symptoms to admission to the hospital, as defined by the revised Atlanta classification; proven biliary origin by imagistic examinations (CT and/or abdominal ultrasound); and biliary decompression procedure, by laparoscopic or endoscopic approach performed during hospitalization.
The exclusion criteria were the onset of pancreatitis of more than 7 to 10 days, pancreatic abscess, pancreatic pseudocyst, acute pancreatitis of other etiology than biliary, and patients that did not undergo surgery, either because of a personal decision or severe general condition.
The severity of acute pancreatitis was evaluated according to the revised Atlanta classification [3].
DATA COMPARISON AND STATISTICAL ANALYSIS:
Three categories of patients were defined in the study: those with mild ABP, moderate-severe ABP, and severe ABP, according to the revised Atlanta classification. Descriptive statistics were reported as percentages or means. The nonparametric Kruskal-Wallis test was used to compare the distributions of scale variables (continuous or discrete). Fisher’s exact test was used for contingency tables. The odds ratio (OR) was estimated with a binary logistic regression model. A
Results
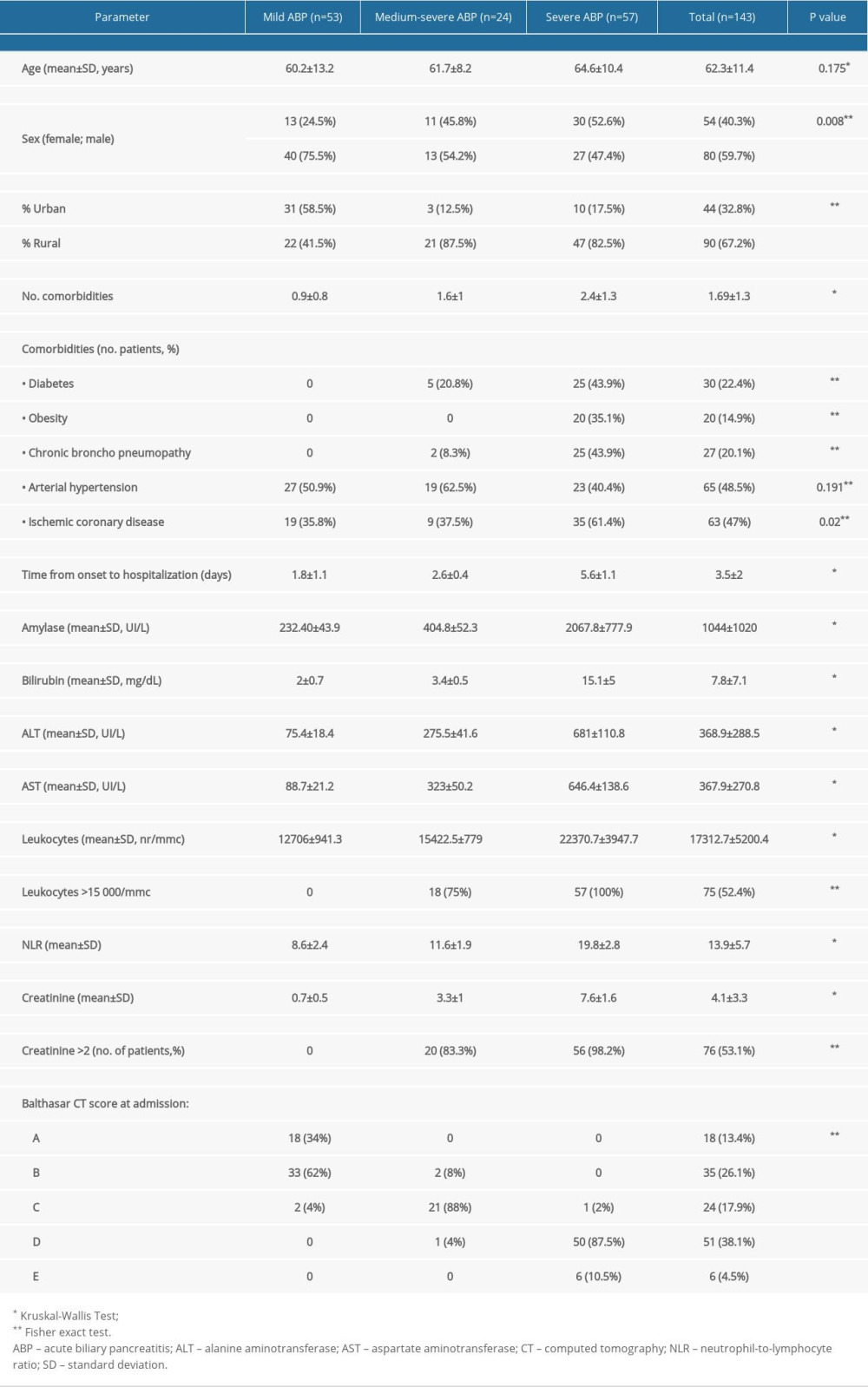
Of the total 143 patients, 53 patients had mild ABP, 24 patients presented with moderate-severe ABP, and 57 presented with severe ABP (Table 1).
The mean age of the patients included in the study was of 62.3±11.4 years. The statistical analysis showed a trend of increasing age with the severity of ABP. However, the differences between the 3 categories were not statistically significant (
There was a slight male predominance (59.7%) in the total study population. The sex distribution according to the severity of the forms of ABP showed significant differences between male and female patients. Mild ABP had a higher frequency in men (75.5% vs 25.5%), while most women that presented with ABP had a severe form of ABP (30 of 54 patients, 55.5%).
The severe forms of ABP were most frequent in patients from rural areas (47 of 90 patients, 52.2%), while patients from urban areas presented most often with mild ABP (31 patients, 70.4%). The difference was statistically significant (
The distribution of the number of comorbidities per patient was different across categories of severity of ABP (
The univariate logistic regression analysis showed that the comorbidities associated with an increased risk of developing a severe form of ABP in the study group were diabetes mellitus (OR: 11.250; CI: 3.950–32.039), chronic bronchopneumopathy (OR: 29.297; CI: 6.547–131.109), and ischemic coronary disease (OR: 2.784; CI: 1.373–5.646).
The time from onset of symptoms from admission was significantly different among the 3 groups of ABP (
The paraclinical evaluation showed that there were statistically significant differences in the mean values for amylases, transaminases, leukocytes, neutrophil-to-lymphocyte ratio, and creatinine among the 3 groups of ABP, with a trend of increasing values from the mild to severe forms. However, these clear differences could also be explained by the delayed presentation in severe forms, in which, in the absence of appropriate therapy, the pathologic conditions in and around the pancreas could have progressed to systemic inflammatory response syndrome. Leukocytes >15 000/mmc and creatinine >2 mg/dL were significantly associated with moderate-severe and severe forms of ABP (
The therapeutic protocol in our department recommends early biliary decompression in all cases of acute pancreatitis in which a biliary origin is suspected. At admission, all patients underwent medical therapy, according to the following protocol: rapid canal decompression performed by oddian spasmolysis by papillary contact anesthesia (xylin 1% administered orally, 2 ampoules every 6 h) and lysis of oddian spasm in general (coronary dilator-nitroglycerin patch, 1 patch every 3 days); blockade of secretion by gastric emptying (gastric tube) and pharmacology by specific antisecretory Sandostatin (3 ampules/day subcutaneously), general antisecretory atropine (1 ampoule/day subcutaneously), and gastric antisecretory medications (Controloc/pantoprazole 2 ampoules/day i.v.). Analgesia was performed by administration of pethidine hydrochloride, with 2 mL diluted every 4 h, Dynastat 3 ampoules/day, and in selected cases, epidural analgesia in patients with severe pancreatitis with xyline on the injection pump, 5 ampoules of 1% diluted in 50 mL saline, with administration of 4 to 6 mL/h, with external local refrigeration. Antibiotic therapy included the administration of Meronem at a dose of 3 g/day for at least 10 days. Hydro-electrolytic rebalancing was done via a central venous catheter with crystalloid and colloidal solutions, up to 4000 mL of liquids, according to patients’ cardiovascular comorbidities, and parenteral nutrition (Kabiven buffered with 8 U insulin, depending on glycemia, administered in 72 h). For the patients admitted during the Covid-19 pandemic, RT-PCR testing at admission and strict adherence to Covid-19 prevention measures were respected, including wearing full protective personal equipment, admission of the patients following special design circuits, and isolation until the results were obtained [11].
In mild cases of ABP with an early presentation, less than 48 h from the onset, endoscopic retrograde cholangiopancreatography (ERCP) was performed in the emergency setting, followed the next day by laparoscopic cholecystectomy. When ERCP service was not available, laparoscopic cholecystectomy with exploration and drainage of the main bile duct was performed as soon as possible after admission.
In moderate-severe and severe cases, the laparoscopic approach was preferred for biliary decompression. If dissection of the Calot triangle could be performed safely, laparoscopic cholecystectomy and drainage of the main bile duct were performed. In patients with severe local inflammatory changes, a laparoscopic cholecystostomy was placed initially, followed by laparoscopic cholecystectomy after an interval of 5 to 7 days. The early surgical-laparoscopic treatment performed in the surgical therapeutic window had exploratory, pathogenic, and radical indications. Laparoscopy allowed an exploration of the peritoneal cavity, with the staging of pancreatitis, sampling of pancreatic ascites, evaluation of cytosteatonecrosis, and continuous capsular analgesia, by administration of contact anesthetics (xyline, lidocaine).
The technical procedure consisted of the celioscopic approach of the upper abdominal region by inserting the trocars after performing the pneumoperitoneum, with the first trocar, the optical one, being inserted in the supraumbilical area, and the following trocars being introduced under optical control.
Peritoneal cavity exploration was performed, with the confirmation of the diagnosis of acute pancreatitis and the establishment of the intra-abdominal lesion balance. Biliary decompression was performed by cholecystostomy and transcystic or Kehr drainage. To allow the approach and exploration of the whole pancreas, the alternative insertion of the working channel telescope could be performed in all three 15-mm trocars (supraumbilical, epigastric, and on the right flank), facilitating the direct access to the necrotic corporeo-caudal areas.
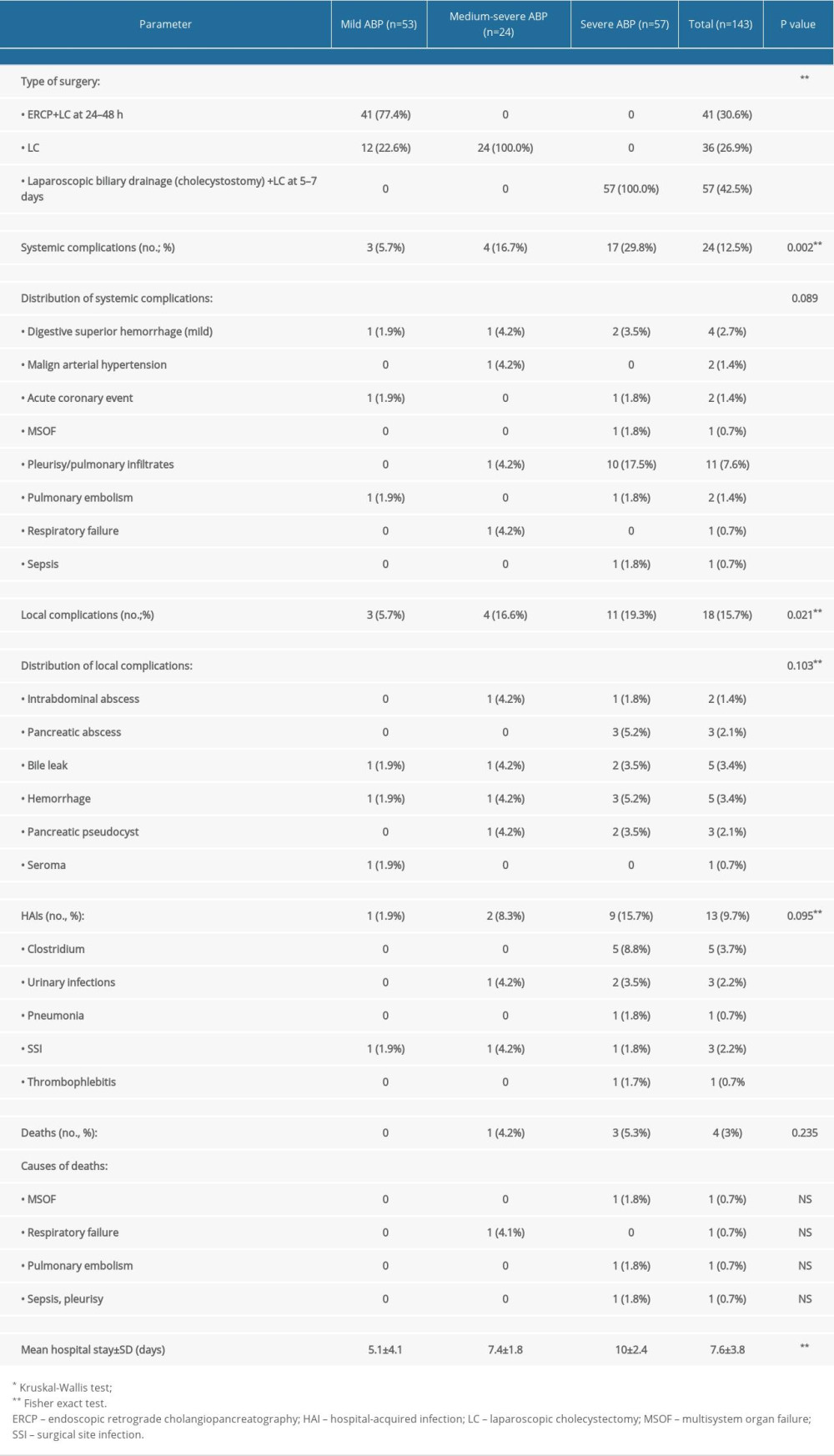
The types of surgical interventions and the outcomes in the study groups are presented in Table 2.
Complications were classified as local (pancreatic abscess, pseudocyst, biliary leak, hemorrhage, and sepsis requiring surgical or endoscopic intervention), systemic (renal failure, respiratory failure, shock, coagulopathy, pulmonary embolism, and acute cardiac events), and hospital-acquired infections.
Local postoperative complications were present in 18 patients (15.7%). Most of the complications were managed conservatively, requiring no further intervention. Pancreatic abscesses (3 patients, 2.1%), were resolved by percutaneous drainage under CT guidance. Hemorrhages from the omental bursa in 2 patients (3.3%) were resolved by laparoscopic hemostasis. Pancreatic pseudocysts were documented in 3 patients (2.1%), of which there were 2 patients who had external drainage performed laparoscopically and the other had a favorable outcome by conservative treatment, with no surgical treatment being necessary.
The incidence of systemic and local complications increased significantly with the severity of ABD, with values of
In the severe ABP group, the most frequent systemic complications encountered were pleuropulmonary, specifically, pleurisy and pulmonary infiltrates (10 patients, 17.5%), while the most frequent local complications were pancreatic abscess (3 patients, 5.2%), pseudocysts (2 patients, 3.5%), hemorrhage (3 patients, 5.2%), and bile leak (2 patients, 3.5%).
Nosocomial infections were higher in the severe forms of ABP (9 patients, 15.7%) but there was not a statistically significant difference among groups (
Overall mortality in the study was low (3%, 4 patients). Only 1 patient’s case progressed to death, with multisystem organ failure, the patient having had severe pancreatitis since admission.
The mean hospital stay was 5.1±4.1 days in mild cases and increased to 10±2.4 days in patients with severe forms of ABP (
Binary logistic regression analysis was conducted to investigate the factors associated with adverse outcomes in the 3 groups. The factors associated with increased risk of systemic complications were creatinine >2 mg/dL (OR: 4.821; CI: 1.547–15.026), leukocytes >15 000/mmc (OR: 3.533; CI: 1.232–10.135), and time from onset to presentation (OR: 1.432; CI: 1.137–1.805). For the local complications, the statistically significant risk factors were obesity (OR: 5.179; CI: 1.786–15.022) and the time from onset to presentation (OR: 1.268; CI: 1.007–1.597).
Discussion
The pathophysiological processes in acute pancreatitis are systematized chronologically as the pre-enzymatic, enzymatic, enzymatic and cytotoxic septicemia, and post-enzymatic stages [15].
The
The
The fundamental local manifestations are edema and inflammatory processes, hemorrhage, and necrosis, with anatomical-clinical forms, from mild, edematous to extremely severe, with extensive necrosis. Exocrine secretion of the pancreas is paradoxically reduced or absent in the first 3 to 5 days after the onset of acute pancreatitis [16,23–25].
The
The
Complications of acute pancreatitis are common during and at the end of the enzymatic stage but can also occur in the post-enzymatic stage, characterized by bacterial contamination of areas of pancreatic necrosis, with repercussions, such as several local and systemic secondary complications that cause and maintain toxic-septic shock [25]. Pancreatic aggression in the early stages leads to the release and activation of proteolytic and lipolytic enzymes responsible for glandular and periglandular autodigestion. The damage caused is of an inflammatory type in the early stages (edema, leukocyte infiltrates), followed by the hemorrhagic and necrotic stage (through ischemic vascular disorders and/or thrombosis). To these are added the effects of the systemic response to the products, resulting in the enzymatic-toxic stage and multiple visceral lesions [26,27].
Currently, there is no clinical or paraclinical variable that can predict the severity of acute pancreatitis in individuals. C-reactive protein, inerleukin-1, interleukin-6, serum tumor necrosis factor alpha, and procalcitonin were evaluated for the possible prognostic utility in acute pancreatitis; however, the results still need to be validated by further analysis [28–30]. Arabul et al found that hepcidin, a circulating peptide hormone that regulates the entry of iron into plasma, which increases during inflammation, may be a better prognostic tool than C-reactive protein for the severity of acute pancreatitis [30]. In a recent study, Silva-Vaz et al [31] found that the neutrophil-to-lymphocyte ratio and systemic inflammation response index, defined as neutrophil count×monocyte/lymphocyte count, has a good predictive value for the severity of inflammation in acute pancreatitis [31,32].
In the present study, we found significant differences among the groups of mild, moderate-severe, and severe ABP in the mean value of amylases, transaminases, creatinine, bilirubin, and leukocytes.
However, one of the limitations of this study was related to the relatively wide differences from onset to presentation (1–7 days) in the patients. The increase of these biomarkers in patients with severe forms must be interpreted as a dynamic process of increasing intensity of inflammatory changes during ABP. Binary logistic regression showed correlations only with creatine >2 mg/mL and leukocytes >15 000/mmc at admission and the risk of systemic complications. Although only up to 20% of the patients developed severe ABP, in these cases the mortality rate was high due to a systemic inflammatory response and multisystem organ failure [33].
Any patient with acute pancreatitis should be hospitalized [34,35]. Given the possibility of progression to general or local complications, the patient should be referred to a unit with multidisciplinary care possibilities: gastroenterology with the possibility of biliopancreatic endoscopy, endoscopic ultrasound, intensive care, imaging radiology (CT) and interventional radiology, and surgery. Patients should be monitored clinically, with repeated examinations for identifying any possible worsening. Repeating the dosage of pancreatic enzymes is worthless. Also, in the absence of signs of aggravation, it is not advisable to repeat the CT examination if it has already been performed [36,37].
The minimally invasive pathogenic therapeutic approach in ABP is based on laparoscopic decompression of the bile tree by cholecystostomy, transcystic drainage, and case-appropriate Kehr drainage. According to a recent metanalysis of Zhong et al [38], early laparoscopic cholecystectomy within 48 to 72 h from onset can be performed safely and effectively in patients with mild ABP. The comparative results between early and delayed laparoscopic cholecystectomy in these cases show the benefit of early surgical intervention in terms of decreased hospital stay, number of complications, and need for subsequent ERCP, taking into account that up to 60% of patients with acute gallstone pancreatitis will have recurrent episodes [38]. Similar findings were reported in a randomized clinical study of acute moderate biliary pancreatitis by Davoodabadi et al [39], who compared early to delayed laparoscopic cholecystectomy. In the present study, we also showed the efficiency of early laparoscopic cholecystectomy in mild and moderate cases of ABP. If cholecystectomy cannot be performed safely due to local inflammation, a cholecystostomy is an efficient drainage method, and laparoscopic cholecystectomy may be performed in a second step.
The proposed objectives are disrupting the links of the pathogenic chain from acute pancreatitis through early biliopancreatic decompression by the laparoscopic approach, aiming to improve the evolution of acute pancreatitis and relieving pain, dyspeptic syndrome, vomiting, and dynamic ileus and preventing local and systemic complications.
The minimally invasive approach to acute pancreatitis has multiple benefits. By laparoscopy, we can explore, irrigate, and perform decompression of the biliopancreatic tree and drain the pancreatic lodge; we can also perform postoperative lavage through drainage tubes. The extrahepatic biliary decompression in ABP allows for the elimination of biliary stasis, avoids the development of acute enzymatic cholecystitis or secondary angiocholitis, reduces pancreatic edema, especially the cephalic type, and prevents the evolution of pancreatitis to a severe form [13]. The laparoscopic approach creates less trauma in the early stages of acute pancreatitis, and postoperative lavage and drainage eliminate the harmful, irritating autodigestive effect of pancreatic ascites and prevent the absorption of toxic products into the systemic circulation. Drainage tubes are also used for continuous peritoneal lavage, which allows for the removal of sphaceli and necrotic debris or for the introduction of lactic acid to cut off the harmful effect of intraperitoneal extravasated pancreatic juice. The pancreatic abscess can be drained by the transcutaneous approach under ultrasound control or CT, which was also performed by us in 2 patients, with subsequent favorable evolution.
Currently, less invasive alternatives to laparoscopic biliary decompression are considered the endoscopic approach by ERCP or, in selected cases, abdominal external drainage. In severe forms, with increased local inflammation and high anesthetico-surgical risk, cholecystostomy under local anesthesia can be performed by abdominal external drainage, either by CT or echocardiogram guidance [40].
The 2019, the World Society of Emergency Surgery guidelines for the management of severe acute pancreatitis [4] recommend ERCP in patients with ABP with proven biliary obstruction and cholangitis. In these patients, early routine ERCP was documented to significantly decrease mortality and complications. However, the routine use of ERCP in patients with gallstone pancreatitis is still a subject of controversy. A systematic review and metanalysis of Tse et al [41] showed no proven benefits of routine ERCP in decreasing the severity of acute pancreatitis. Moreover, according to the current guidelines, in severe ABP with no signs of biliary obstruction or angiocholitis, ERCP cannot be recommended, based on the previously published evidence [4,42]. Similar results were confirmed by the recently published APEC study, which showed that in patients with predicted severe gallstone pancreatitis but without cholangitis, urgent ERCP with sphincterotomy did not reduce the composite endpoint of major complications or mortality, compared with conservative treatment [43].
The present study had some limitations. The relatively small number of patients and the differences in the time from onset to presentations between the mild and severe ABP groups could not allow the assessment of predictive factors of biological data upon severity. Although the differences found between study groups were statistically significant for most paraclinical data, they could be strongly influenced by the time factor from the onset of symptoms. We found good results of early surgery in ABP, with a therapeutic approach depending upon the severity and local conditions. However, owing to the protocol of practice in our surgical department, a comparative group treated only medically was not available for statistical analysis. ABP is still a topic for future research, as consensus was not reached on several issues. A future study direction could include the identification of biomarkers with a more accurate prediction of severity and the role of early ERCP in patients expected to develop severe forms. Another question still to be answered is whether ERCP alone may be sufficient in preventing recurrences of ABP, especially in patients with high anesthetic-surgical risk.
The therapeutic management of ABP is complex through medical and surgical conduct, both having the same common denominator of biliopancreatic decompression through the administration of antisecretory medicine, spasmolytic medicine, and nasogastric tube assembly associated with cholecystostomy. In addition to surgical treatment, medical therapy is equally important in the patient’s recovery and consists of hydro-electrolytic rebalancing to compensate for the loss of space III, gastric decompression by mounting a nasogastric tube, introduction of anti-enzymatic therapy, treatment of organ failure if it occurs, antibiotic therapy, and parenteral nutrition, following as much as possible the proposed medical protocol.
Laparoscopic treatment in acute pancreatitis has multiple advantages that allow exploration of the peritoneal cavity, biliopancreatic decompression, drainage of harmful secretions from the abdominal cavity, and continuous postoperative washing with betadine solution or lactic acid, eliminating the enzymatic toxic effect in the abdominal cavity and preventing the occurrence of systemic inflammatory response syndrome and multiple organ dysfunction syndrome.
Conclusions
The surgical therapeutic window protocol in ABP consists of pathogenic medication administration of oddian spasmolytics, inhibitors of gastric and pancreatic secretion, antibiotic therapy, and hydro-electrolytic rebalancing and a minimally invasive procedure for the decompression of the biliary tree (cholecystostomy, transcystic drainage, or Kehr drainage, adapted to the patient). It showed a favorable evolution of the cases, with minimum mortality (3%) and reduced morbidity and hospital stay. Creatinine >2 mg/dL and leukocytes >15 000/mmc at admission were associated with an increased risk for systemic complications. The delayed presentation was associated with increased risk both for systemic and local complications.
Early biliary decompression, either by an endoscopic or minimally invasive approach is a safe and effective procedure in ABP. It allows disrupting the pathogenic chain that leads to systemic inflammation and multisystem organ failure. The type of procedure and optimal timing (either 1-step or 2-step surgery) should be individualized, according to the patient’s local and general features.
References
1. Matta B, Gougol A, Gao X, Worldwide variations in demographics, management, and outcomes of acute pancreatitis: Clin Gastroenterol Hepatol, 2020; 18(7); 1567-75e2
2. Chatila AT, Bilal M, Guturu P, Evaluation and management of acute pancreatitis: World J Clin Cases, 2019; 7(9); 1006-20
3. Thoeni RF, The revised Atlanta classification of acute pancreatitis: Its importance for the radiologist and its effect on treatment: Radiology, 2012; 262(3); 751-64
4. Leppäniemi A, Tolonen M, Tarasconi A, 2019 WSES guidelines for the management of severe acute pancreatitis: World J Emerg Surg, 2019; 14; 27
5. Sternby H, Hartman H, Thorlacius H, Regnér S, The initial course of IL1β, IL-6, IL-8, IL-10, IL-12, IFN-γ and TNF-α with regard to severity grade in acute pancreatitis: Biomolecules, 2021; 11(4); 591
6. Norman J, The role of cytokines in the pathogenesis of acute pancreatitis: Am J Surg, 1998; 175; 76-83
7. Phillip V, Steiner JM, Algül H, Early phase of acute pancreatitis: Assessment and management: World J Gastrointest Pathophysiol, 2014; 5(3); 158-68
8. Gülen B, Dur A, Serinken M, Pain treatment in patients with acute pancreatitis: A randomized controlled trial: Turk J Gastroenterol, 2016; 27(2); 192-96
9. Nakaharai K, Morita K, Jo T, Early prophylactic antibiotics for severe acute pancreatitis: A population-based cohort study using a nationwide database in Japan: J Infect Chemother, 2018; 24(9); 753-758
10. Annane D, Siami S, Jaber SCRISTAL Investigators, Effects of fluid resuscitation with colloids vs crystalloids on mortality in critically ill patients presenting with hypovolemic shock: the CRISTAL randomized trial: JAMA, 2013; 310(17); 1809-17
11. Serban D, Socea B, Badiu CD, Acute surgical abdomen during the COVID-19 pandemic: Clinical and therapeutic challenges: Exp Ther Med, 2021; 21(5); 519
12. Mathew MJ, Parmar AK, Sahu D, Reddy PK, Laparoscopic necrosectomy in acute necrotizing pancreatitis: Our experience: J Minim Access Surg, 2014; 10(3); 126-31
13. Raraty MG, Halloran CM, Dodd S, Minimal access retroperitoneal pancreatic necrosectomy: Improvement in morbidity and mortality with a less invasive approach: Ann Surg, 2010; 251(5); 787-93
14. Freeman ML, Werner J, van Santvoort HCInternational Multidisciplinary Panel of Speakers and Moderators, Interventions for necrotizing pancreatitis: Summary of a multidisciplinary consensus conference: Pancreas, 2021; 41(8); 1176-94
15. Al Mofleh IA, Severe acute pancreatitis: pathogenetic aspects and prognostic factors: World J Gastroenterol, 2008; 14; 675-84
16. Mounzer R, Whitcomb DC, Genetics of acute and chronic pancreatitis: Curr Opin Gastroenterol, 2013; 29(5); 544-51
17. Lankisch PG, Apte M, Banks PA, Acute pancreatitis: Lancet, 2015; 386(9988); 85-96
18. Dixit A, Dawra RK, Dudeja VS, Ashok K: Role of trypsinogen activation in genesis of pancreatitis, 2016, Pancreapedia, Exocrine Pancreas Knowledge Base
19. Xiao B, Xu HB, Jiang ZQ, Current concepts for the diagnosis of acute pancreatitis by multiparametric magnetic resonance imaging: Quant Imaging Med Surg, 2019; 9(12); 1973-85
20. Dawra R, Sah RP, Dudeja V, Intra-acinar trypsinogen activation mediates early stages of pancreatic injury but not inflammation in mice with acute pancreatitis: Gastroenterology, 2011; 141(6); 2210-17e2
21. Braha J, Tenner S, Fluid collections and pseudocysts as a complication of acute pancreatitis: Gastrointest Endosc Clin N Am, 2018; 28(2); 123-30
22. Cicalese L, Sahai A, Sileri P, Acute pancreatitis and bacterial translocation: Dig Dis Sci, 2001; 46(5); 1127-32
23. Sargent S, Pathophysiology, diagnosis and management of acute pancreatitis: Br J Nurs, 2006; 15(18); 999-1005
24. Dugernier T, Starkel P, Laterre PF, Reynaert MS, Severe acute pancreatitis: pathophysiologic mechanisms underlying pancreatic necrosis and remote organ damage: Acta Gastroenterol Belg, 1996; 59(3); 178-85
25. Louhimo J, Steer ML, Perides G, Necroptosis is an important severity determinant and potential therapeutic target in experimental severe pancreatitis: Cell Mol Gastroenterol Hepatol, 2016; 2(4); 519-35
26. Schmidt PN, Roug S, Hansen EF, Spectrum of microorganisms in infected walled-off pancreatic necrosis – impact on organ failure and mortality: Pancreatology, 2014; 14(6); 444-49
27. Rau BM, Bothe A, Kron M, Beger HG, Role of early multisystem organ failure as major risk factor for pancreatic infections and death in severe acute pancreatitis: Clin Gastroenterol Hepatol, 2006; 4(8); 1053-61
28. Rao SA, Kunte AR, Interleukin-6: An early predictive marker for severity of acute pancreatitis: Indian J Crit Care Med, 2017; 21(7); 424-28
29. Ahmad R, Bhatti KM, Ahmed M, C-reactive protein as a predictor of complicated acute pancreatitis: Reality or a myth?: Cureus, 2021; 13(11); e19265
30. Arabul M, Celik M, Aslan O, Hepcidin as a predictor of disease severity in acute pancreatitis: A single center prospective study: Hepatogastroenterology, 2013; 60(123); 595-600
31. Silva-Vaz P, Abrantes AM, Morgado-Nunes S, Evaluation of prognostic factors of severity in acute biliary pancreatitis: Int J Mol Sci, 2020; 21(12); 4300
32. Gu L, Ma X, Wang L, Prognostic value of a systemic inflammatory response index in metastatic renal cell carcinoma and construction of a predictive model: Oncotarget, 2017; 8; 52094-103
33. Sarri G, Guo Y, Iheanacho I, Puelles J, Moderately severe and severe acute pancreatitis: A systematic review of the outcomes in the USA and European Union-5: BMJ Open Gastroenterol, 2019; 6(1); e000248
34. Triantopoulou C, Delis S, Dervenis C, Imaging evaluation of post-pancreatitis infection: Infect Disord Drug Targets, 2010; 10(1); 15-20
35. Hirota M, Ohmuraya M, Baba H, The role of trypsin, trypsin inhibitor, and trypsin receptor in the onset and aggravation of pancreatitis: J Gastroenterol, 2006; 41; 832-36
36. Working Group IAP/APA Acute Pancreatitis Guidelines, IAP/APA evidence-based guidelines for the management of acute pancreatitis: Pancreatology, 2013; 13(4 Suppl 2); e1-15
37. Bugiantella W, Rondelli F, Boni M, Necrotizing pancreatitis: A review of the interventions: Int J Surg, 2016; 28(Suppl 1); S163-71
38. Zhong FP, Wang K, Tan XQ, The optimal timing of laparoscopic cholecystectomy in patients with mild gallstone pancreatitis: A meta-analysis: Medicine (Baltimore), 2019; 98(40); e17429
39. Davoodabadi A, Beigmohammadi E, Gilasi H, Optimizing cholecystectomy time in moderate acute biliary pancreatitis: A randomized clinical trial study: Heliyon, 2020; 6(2); e03388
40. Gliem N, Ammer-Herrmenau C, Ellenrieder V, Neesse A, Management of severe acute pancreatitis: An update: Digestion, 2021; 102(4); 503-7
41. Tse F, Yuan Y, Early routine endoscopic retrograde cholangiopancreatography strategy versus early conservative management strategy in acute gallstone pancreatitis: Cochrane Database Syst Rev, 2012; 39; CD009779
42. van Geenen E-JM, van Santvoort HC, Lack of consensus on the role of endoscopic retrograde cholangiography in acute biliary pancreatitis in published meta-analyses and guidelines: A systematic review: Pancreas, 2013; 42; 774-80
43. Schepers NJ, Hallensleben NDL, Besselink MGDutch Pancreatitis Study Group, Urgent endoscopic retrograde cholangiopancreatography with sphincterotomy versus conservative treatment in predicted severe acute gallstone pancreatitis (APEC): A multicentre randomised controlled trial: Lancet, 2020; 396(10245); 167-76
In Press
06 Mar 2024 : Clinical Research
Comparison of Outcomes between Single-Level and Double-Level Corpectomy in Thoracolumbar Reconstruction: A ...Med Sci Monit In Press; DOI: 10.12659/MSM.943797
21 Mar 2024 : Meta-Analysis
Economic Evaluation of COVID-19 Screening Tests and Surveillance Strategies in Low-Income, Middle-Income, a...Med Sci Monit In Press; DOI: 10.12659/MSM.943863
10 Apr 2024 : Clinical Research
Predicting Acute Cardiovascular Complications in COVID-19: Insights from a Specialized Cardiac Referral Dep...Med Sci Monit In Press; DOI: 10.12659/MSM.942612
06 Mar 2024 : Clinical Research
Enhanced Surgical Outcomes of Popliteal Cyst Excision: A Retrospective Study Comparing Arthroscopic Debride...Med Sci Monit In Press; DOI: 10.12659/MSM.941102
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952