25 August 2020: Clinical Research
The Relationship Between Corneal Hysteresis and Retinal Ganglion Cells – A Step Forward in Early Glaucoma Diagnosis
Vasile Potop1ABCDEF, Valeria Coviltir1DF, Speranţa Schmitzer1AF, Catalina Corbu1DE, Ioana Cătălina Ionescu2BC, Miruna Burcel2BC, Dana Dăscălescu1AE*DOI: 10.12659/MSM.924672
Med Sci Monit 2020; 26:e924672
Abstract
BACKGROUND: Glaucoma is a major cause of irreversible visual field (VF) loss across the world. Many studies have assessed the accuracy of glaucoma diagnostic tests for a more precise diagnosis to quickly identify patients with higher risk of progression.
MATERIAL AND METHODS: We conducted a study that included 214 eyes divided into 3 groups: 79 eyes from patients diagnosed with primary open-angle glaucoma (POAG), 68 eyes from patients diagnosed with ocular hypertension (OH), and 67 eyes from normal individuals (normal eyes, NE). All patients included in the study received a complete checkup.
RESULTS: In POAG patients, means of central corneal thickness (CCT), corneal hysteresis (CH), corneal resistance factor (CRF), mean defect (MD), visual field index (VFI), peripapillary retinal nerve fiber layer (pRNFL), and ganglion cell complex (GCC) are lower than in OH patients, and in NE are higher than in both groups. Also, we found a statistically significant direct correlation between CH and GCC thickness. Further statistical analysis revealed that both pRNFL thickness and GCC thickness are significantly influenced by CH value in a precise manner.
CONCLUSIONS: The first cell type affected in glaucoma is the retinal ganglion cell. We found a positive correlation between GCC thickness and CH, suggesting that CH might be a parameter to consider in the evaluation of all glaucoma patients from their first examination. Moreover, both pRNFL thickness and GCC thickness are influenced by CH, suggesting the utility of monitoring the value of CH at every checkup to detect its decrease in glaucoma patients.
Keywords: Corneal Pachymetry, Glaucoma, Open-Angle, Ocular Hypertension, Retinal Ganglion Cells, Case-Control Studies, Cornea, Early Diagnosis
Background
Glaucoma is an optic neuropathy that affects the ganglion cells in all their components (cell body, dendrite, and axon), resulting in VF loss.
Recent studies have increasingly focused on POAG and, by default, recognizing patients with a higher risk of disease progression, aiming to prevent VF defects [1].
Although risk factors in POAG are already well known, studies showed that, along with elevated intraocular pressure (IOP), thin cornea, or family history, we need to consider corneal biomechanical properties (CBP) as independent risk factors in glaucoma suspects or patients [2–14]. Recently, studies have revealed the importance of CH as a screening tool in glaucoma [7].
Moreover, the link between CH and lamina cribrosa has been increasingly studied [15–18]. We already know that both cornea and sclera have a common histological tissue, being composed of collagen fibers. Taking into consideration this common origin and the fact that the 2 connect to each other at 1 point, we can understand why corneal hysteresis and laminar response are related. In eyes with severe glaucoma, in response to increased IOP, the anterior lamina surface is displaced anteriorly and corneal hysteresis is decreased [19].
Lanzagorta-Aresti et al. demonstrated that CH and age were the only factors associated with the increase in laminar thickness in patients who received glaucoma treatment [20].
Considering the response of the eye in front of an elevated IOP, the anatomical considerations, and that death of ganglion cells is central to glaucoma physiopathology, GCC thickness and pRNFL thickness need to be evaluated the glaucoma suspects [21,22].
Because Ocular Response Analyzer (ORA) measurements are noninvasive and easy to perform, and as ganglion cell and pRNFL loss are among the first changes that appear in glaucoma, we decided to explore the relationship between pRNFL and GCC thickness and CBP in POAG patients, OH patients, and normal eyes.
Material and Methods
We conducted a retrospective study that included 214 eyes from 115 patients (58% women and 41% men) divided into 3 groups: the first group consisted of 79 eyes from patients diagnosed with POAG (open-angle, glaucomatous optic neuropathy, VF defect, and decreased pRNFL), regardless of the IOP and antiglaucomatous treatment; the second group consisted of 68 eyes from patients diagnosed with OH (open-angle, no sign of glaucomatous optic neuropathy, VF defect or decreased pRNFL in patients with raised IOP); and the third group consisted of 67 eyes from normal individuals (open-angle, no sign of glaucomatous optic neuropathy, VF defect or decreased pRNFL in patients with normal IOP).
Exclusion criteria for all 3 groups were closed-angle glaucoma, corneal scars, corneal dystrophy or degeneration, refractive surgery, pellucid marginal degeneration or keratoconus that could interfere with ORA and Goldmann IOP measurements, and macular or optic nerve pathology that could generate inaccurate GCC and pRNFL thickness values.
All patients received a complete ophthalmologic examination that included best corrected visual acuity measurement, Ocular Response Analyzer (Reichert Ophthalmic Instruments, Inc., Depew, NY) measurement, visual field examination (Humphrey Field Analyzer II, Carl Zeiss Meditec, Inc, Dublin, California strategy 24-4), Goldmann IOP measurement, pachymetry (Alcon® OcuScan® RxP Ophthalmic Ultrasound System), gonioscopy, fundus examination, and GCC and pRNFL thickness measurements (Optical Coherence Tomography 3D OCT-2000 Series).
SPSS 17.0 was used for statistical analyses. According to the diagnosis, we had 67 patients in the NE group, 68 patients in the OH group, and 79 patients in the POAG group.
The Ocular Response Analyzer (ORA) examination revealed 4 parameters: IOPcc (an IOP measurement compensated by CBP), IOPg (an IOP measurement similar to Goldmann IOP), CH (corneal hysteresis – a parameter that displays the viscoelastic behavior of the cornea), and CRF (corneal resistance factor – a parameter that exhibits the entire corneal resistance). Visual field examination provided 3 parameters: MD (mean defect – an overall view over the visual field loss), PSD (pattern standard deviation – best quantifies visual field loss in early stages), and VFI (visual field index – an index of the overall visual field loss). Pachymetry helped us determine central corneal thickness (CCT). Optical coherence tomography (OCT) provided pRNFL and GCC thickness.
Results
Among the whole group, 22% were POAG female patients (46 patients), 15% were male POAG patients (33 patients), 23% were female OH patients (49 patients), 9% were male OH patients (19 patients), 21% were normal female individuals (45 individuals), and 10% were normal male individuals (21 individuals) (Figure 1).
Means of IOP (Goldmann, IOPg, and IOPcc) were similar between POAG and OH groups and were significantly lower in NE. In addition, CCT was higher in NE than in OH and POAG groups. Means of both CH and CRF were higher in NE than in OH patients and were higher in OH higher than in POAG patients. The 3 groups had similar disc areas. Means of GCC and pRNFL thickness, MD, PSD, and VFI were higher in NE than in OH and POAG patients (Table 1).
In the POAG group, Pearson correlation coefficient analysis revealed a strong direct correlation between CH and pRNFL thickness (0.703 for global pRNFL thickness, 0.671 for superior pRNFL thickness, and 0.585 for inferior pRNFL thickness), and the differences were statistically significant (p<0.001). For the OH group, Pearson correlation coefficient analysis revealed a direct moderate correlation between CH and pRNFL thickness (0.564 for global pRNFL thickness, 0.472 for superior pRNFL thickness, and 0.420 for inferior pRNFL thickness. The results were statistically significant (p<0.001). For NE, Pearson correlation coefficient analysis showed a moderate-to-low statistically significant (p<0.001) direct correlation between CH and pRNFL thickness (0.495 for global pRNFL thickness, 0.444 for inferior pRNFL thickness) and 0.124, p<0.31 for superior pRNFL thickness (Figure 2–4).
Means of GCC thickness were lower in the POAG group than in the OH group, and were lower than in NE.
The correlation between CH and GCC thickness (globally, superior, and inferior) showed a statistically significant correlation, with a confidence interval higher than 99%, except for the global GCC thickness in normal individuals, where the correlation was statistically significant with a 95% confidence interval, and for the inferior GCC thickness in normal individuals, where the correlation had a confidence interval lower than 95%.
In the POAG group, Pearson correlation coefficient analysis showed a strong statistically significant (p<0.001) direct correlation between CH and GCC thickness (0.552 for global GCC thickness, 0.526 for superior GCC thickness, and 0.526 for inferior GCC thickness). For the OH group, Pearson correlation coefficient analysis showed a moderate statistically significant (p<0.005) direct correlation between CH and GCC thickness (0.373 for global GCC thickness, 0.379 for superior GCC thickness, and 0.335 for inferior GCC thickness. For NE, Pearson correlation coefficient analysis showed a moderate statistically significant (p<0.05) direct correlation between CH and GCC thickness (0.295 for global GCC thickness, 0.347 for superior GCC thickness), and 0.153 for inferior GCC thickness, p<215 (Figure 5–7).
Further statistical analysis revealed that both pRNFL thickness and GCC thickness were influenced by CH value in a precise manner. When we encountered a change in CH value by 1 unit (mmHG), both pRNFL thickness and GCC thickness varied. Therefore, any decrease in CH could have an impact on our patient outcomes (Tables 2, 3).
Discussion
Recently, there has been increased interest in glaucoma diagnosis and progression. Because glaucoma RNFL damage and visual field loss are irreversible, our purpose was to identify patients with a higher rate of progression who need to be monitored closely prior to visual field loss [13,23]. In addition, because ORA is a noninvasive, easy to perform, and not very expensive measurement, we studied CH and CRF to rapidly identify patients with faster progression of disease.
Risk factors that need to be taken into consideration in every glaucoma suspect are already known, but recent studies show that, in addition to IOP, genetics, and CCT, CBP might be a new risk factor independent of IOP [2–6,8,9,24–29]. Park et al. proved that in normal-tension glaucoma (NTG), low CH was associated with a thin pRNFL and a large cup/disc ratio, regardless of the IOP and CCT [30]. CH and CRF have been found to be lower than in normal eyes, even in patients with primary congenital glaucoma, and the biomechanical changes appear regardless of age [31].
We also need to consider the systemic status of the patient, because sometimes other diseases influence prognosis or treatment. In agreement with this, an important study conducted by Nicoleta Anton et al. revealed that in glaucoma patients with type II diabetes, CH may be altered due to diabetes and is not reliable [32].
Our study confirms once again that IOP and CCT are risk factors for glaucoma, showing that in both glaucoma and OH patients IOP values are higher than in normal individuals, while CCT values are lower [11–14,25,26,33–35]. In addition, some studies suggest the involvement of CBP in IOP measurement [8,25,33–37].
Our study revealed similar values between IOPg and Goldmann IOP, with higher values of IOPcc in glaucoma patients. The strong correlations between IOPg with Goldmann IOP and IOPcc in all 3 groups show that ORA generates a useful IOP value that is comparable to Goldmann IOP, and that ORA measurements are reliable in our patients.
Because IOP values can vary, Pensyl and Kotecha suggested the possibility of using both CCT and CH in the investigation of glaucoma suspects and patients [26–28,30,33,34]. Kida revealed that IOP values are higher in young healthy individuals during the night, but could not demonstrate a circadian variation of CBP [30–32,35].
As shown in other publications, in our study, both CH and CRF values were lower in POAG patients than in OH patients and in NE [10,24,25,30,31,33,36–40], and a study that included both eyes of POAG patients revealed that the most affected eye had a lower CH compared to the other eye [16]. Moreover, studies revealed that a high CH has a protective role in OH patients [38].
Also, we need to find the first changes that appear in order to prevent further structural damage. As the first cell type affected is the ganglion cell, and the typical way in which it dies (first the dendrite, followed by the cell body, and lastly the axon), the measurement of ganglion cell complex is a vital step in glaucoma management [7].
Our study revealed that means of GCC thickness were higher in NE than in OH eyes and higher than in POAG patients. Other studies also proved that in glaucoma patients, means of GCC correspond to means of pRNFL and are lower than in normal individuals [10,18,38,41].
Studies have found a statistically significant change in preperimetric glaucoma in patients compared to normal subjects, and that GCC scan gives similar results to pRNFL scan in patients with preperimetric glaucoma [10,33]. Since the cornea and sclera have a common histological base, corneal biomechanics could be linked to laminar changes in glaucoma patients. Also, because the first cell affected in glaucoma is the ganglion cell, if we want to prove the role of corneal biomechanics, we need to demonstrate the relationship between CH and GCC thickness. To the best of our knowledge, the present study is the first to show an association between CH and GCC thickness.
Conclusions
Our study revealed lower values for CH, CRF, and CCT in POAG patients in comparison to OH patients and lower than in normal individuals. This suggests that we could use these parameters to identify patients with a higher risk of progression. As ORA measurements are noninvasive and relatively easy to perform, the IOPcc and CH parameters could possibly be performed in all patients who are considered glaucoma suspects or are diagnosed with glaucoma. In addition, it could help identify patients who need to be monitored closely, but it is not recommended to base therapeutic decisions on a normal or modified value of CH.
Because the retinal ganglion cell is the first one affected in glaucoma, and given the positive correlation between corneal hysteresis and GCC thickness, it can be suggested that CH could be used to monitor glaucomatous patients from an early stage of the disease.
Rim area, MD, VFI, pRNFL (globally, superior and inferior), and GCC (globally, superior, and inferior) show lower values in POAG patients compared to OH patients and normal individuals, proving that the patients were divided properly into the 3 groups.
Measuring IOPcc and CH at the first checkup of a glaucomatous patient or suspect could help detect early patients with a faster rate of progression. It can also help evaluate and establish the monitoring frequency of a patient, but therapeutic decisions should never be based on CH alone.
Because pRNFL thickness and GCC thickness can be affected in a particular manner by the value of CH, any decrease in CH needs to be carefully monitored since it can affect the patient’s disease evolution.
Further randomized studies with larger groups of patients are necessary to confirm these results and to verify CH as an accepted parameter and independent risk factor in POAG.
Figures
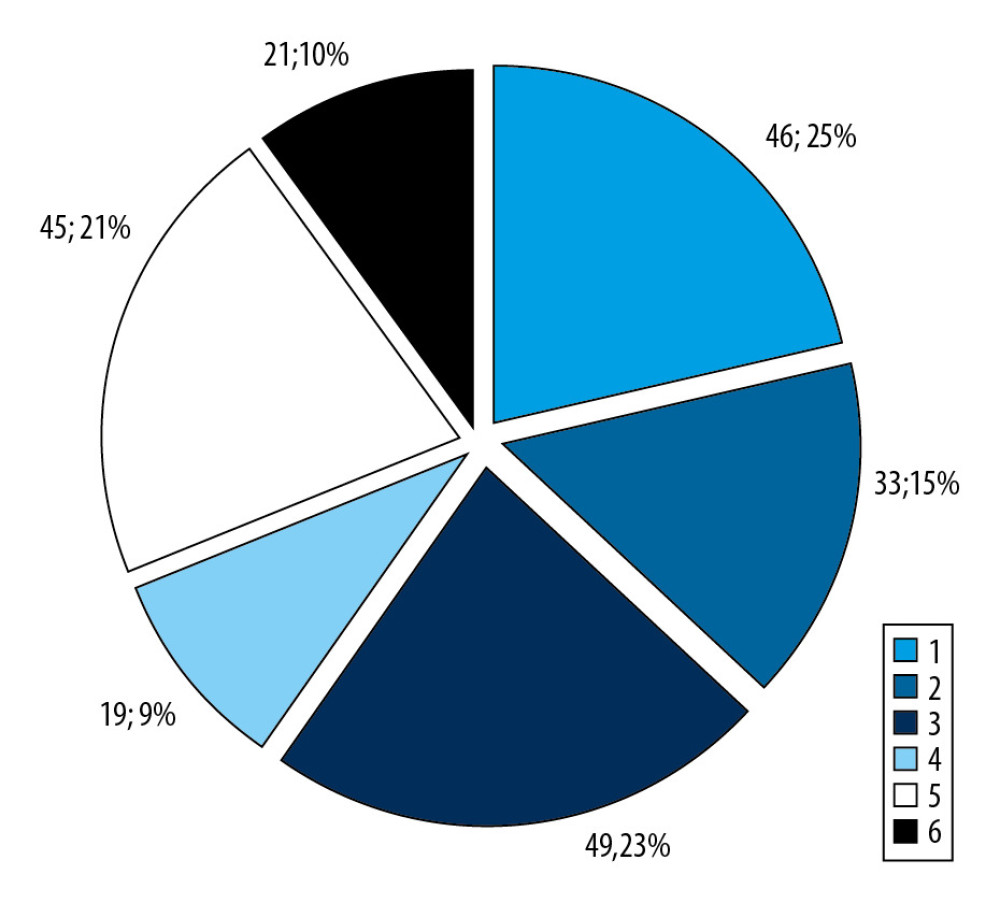 Figure 1. Patient distribution within the groups.
Figure 1. Patient distribution within the groups. 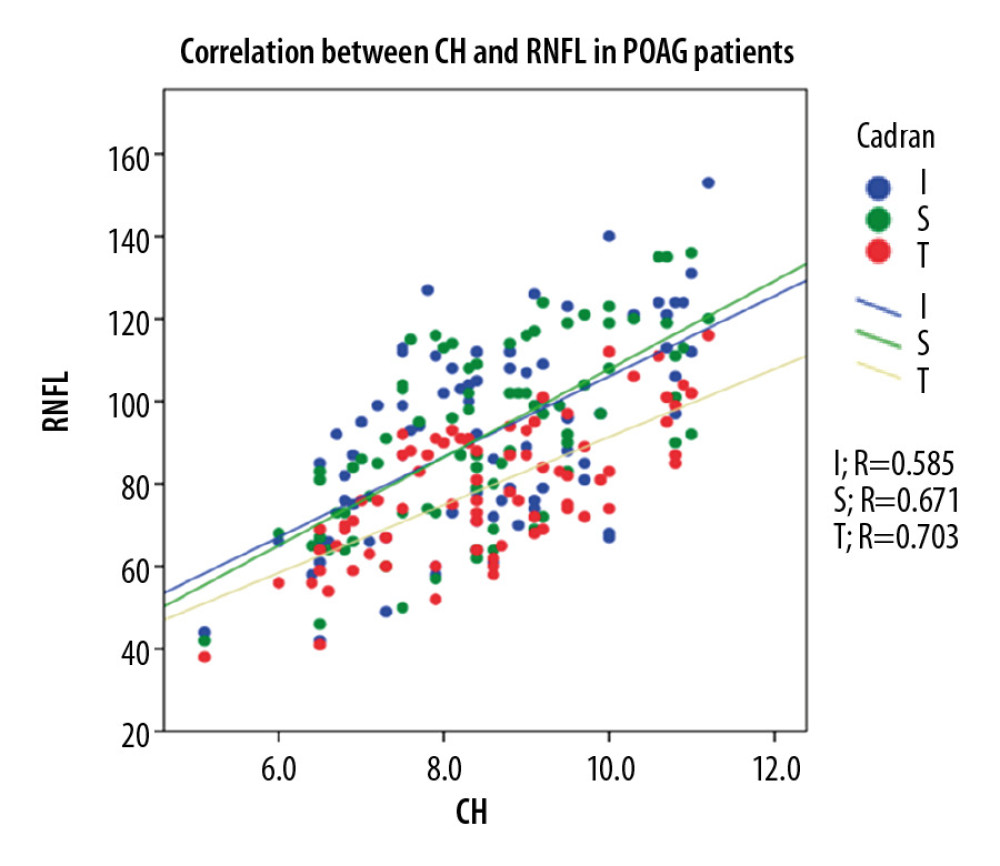 Figure 2. Correlation between CH and pRNFL thickness in POAG patients.
Figure 2. Correlation between CH and pRNFL thickness in POAG patients. 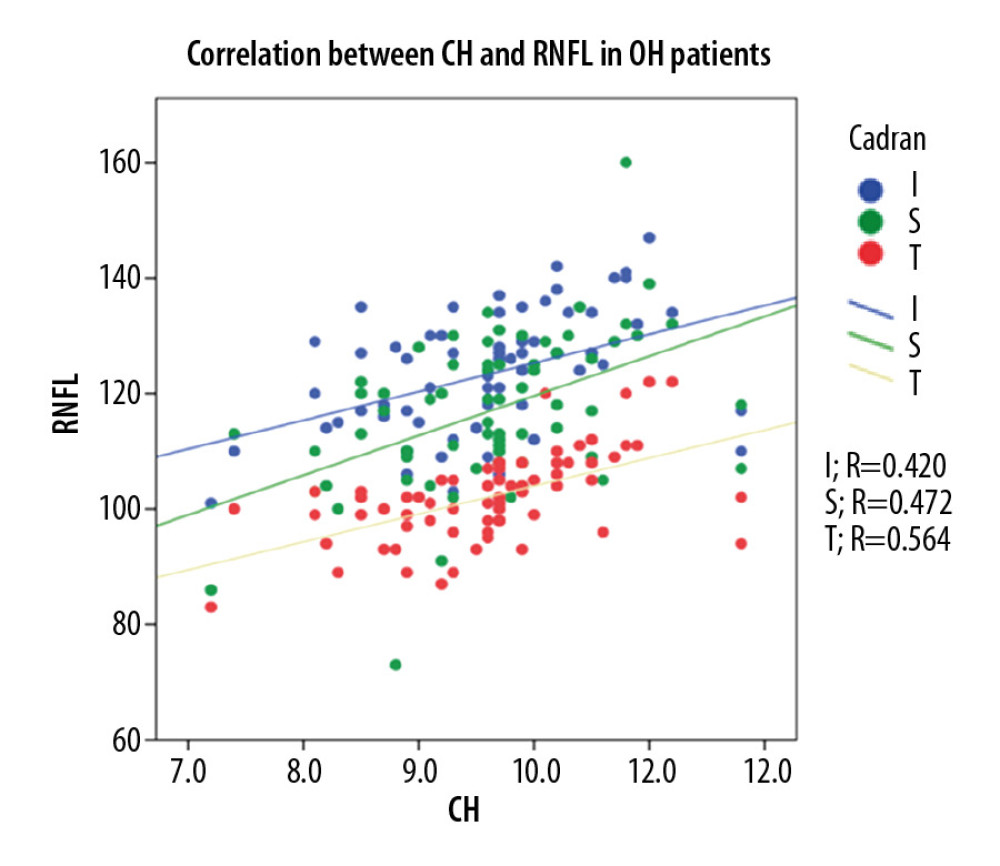 Figure 3. Correlation between CH and pRNFL thickness in OH patients.
Figure 3. Correlation between CH and pRNFL thickness in OH patients. 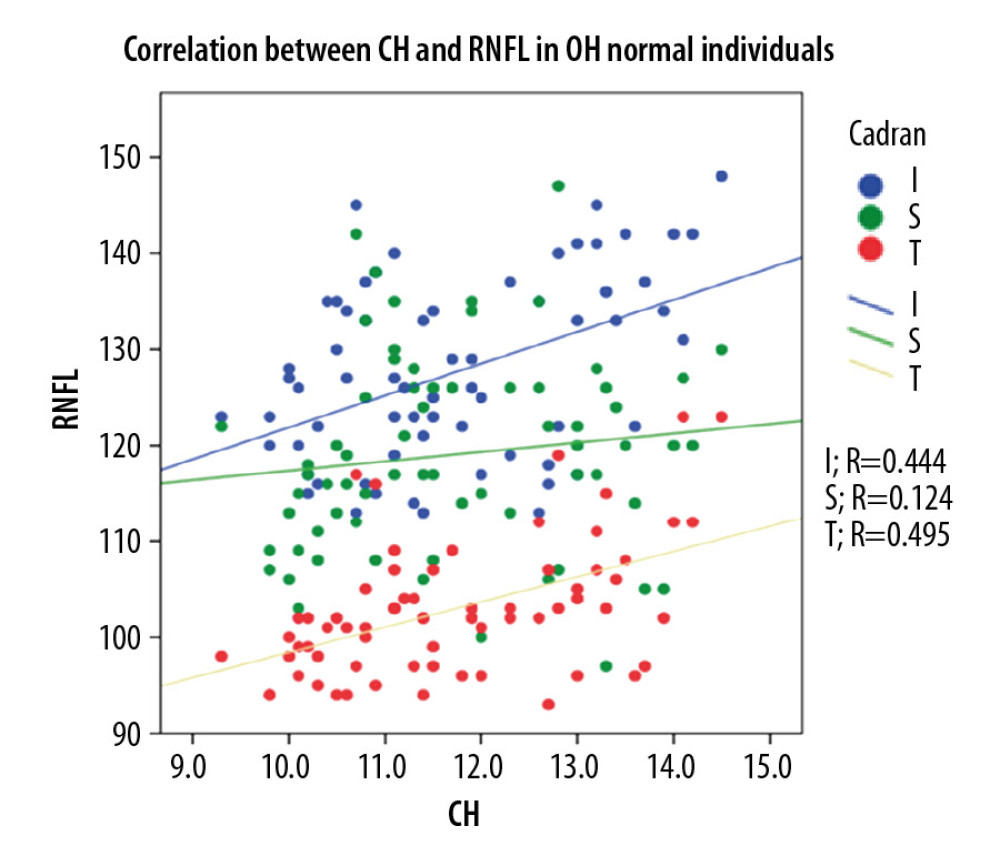 Figure 4. Correlation between CH and pRNFL thickness in normal individuals.
Figure 4. Correlation between CH and pRNFL thickness in normal individuals. 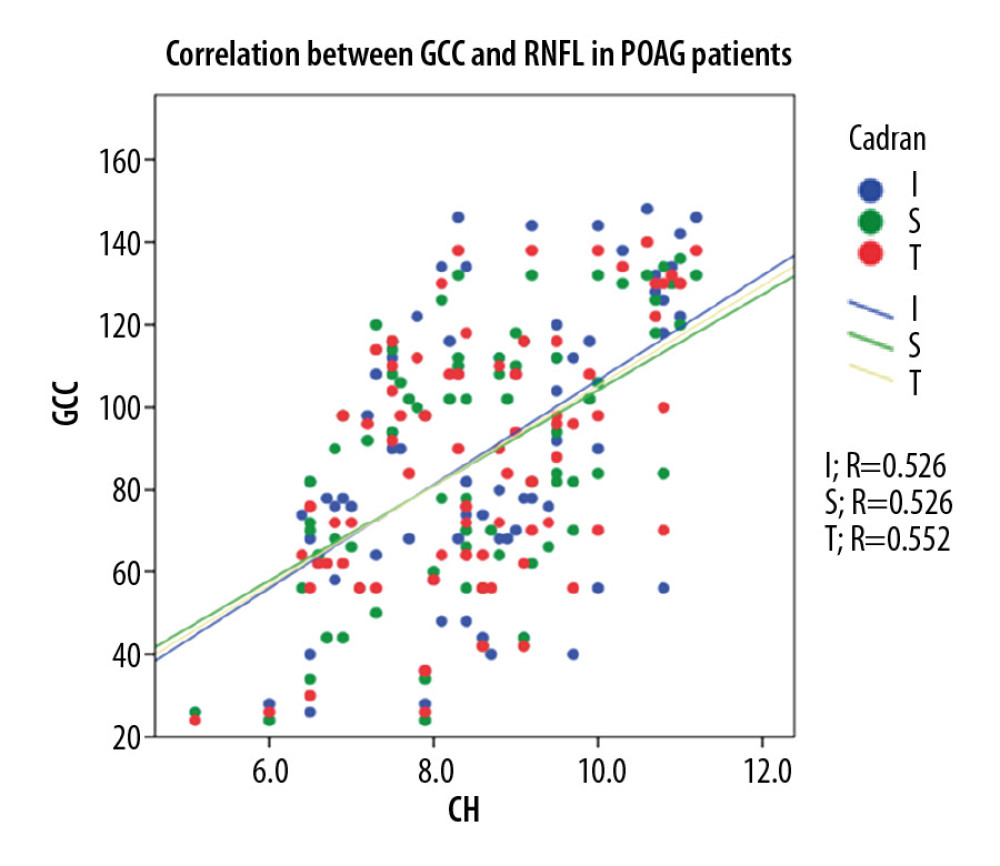 Figure 5. Correlation between CH and GCC thickness in POAG patients.
Figure 5. Correlation between CH and GCC thickness in POAG patients. 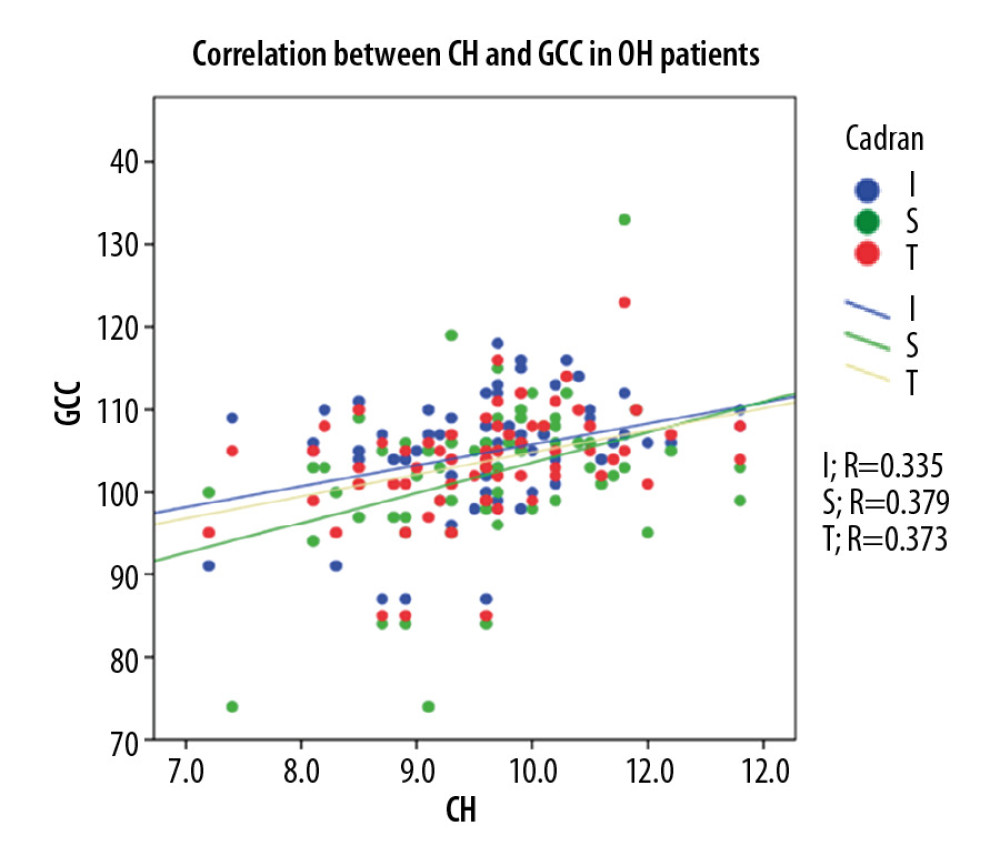 Figure 6. Correlation between CH and GCC thickness in OH patients.
Figure 6. Correlation between CH and GCC thickness in OH patients. 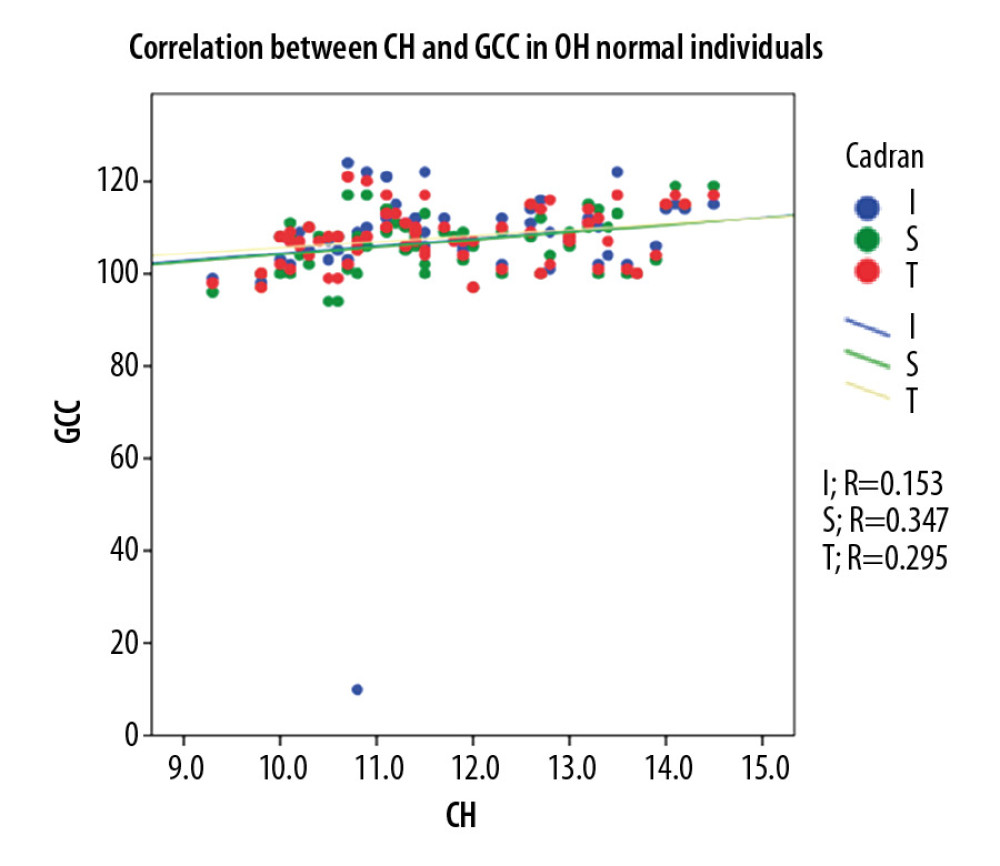 Figure 7. Correlation between CH and GCC thickness in normal individuals.
Figure 7. Correlation between CH and GCC thickness in normal individuals. References
1. Weinreb R, Garway-Heath D, Leung C: Progression of glaucoma, 2011, Kulger Publications
2. Gelaw Y, The impact of central corneal thickness on intraocular pressure among Ethiopian glaucoma patients a cross-sectional study: BMC Ophthalmol, 2012; 12; 58
3. Radcliffe N, Hysteresis: A powerful tool for glaucoma care: Review of Ophthalmology, 2014; 21; 50
4. De Moraes CG, Juthani V, Liebmann J, Risk factors for visual field progression in treated glaucoma: Arch Ophthalmol, 2010; 129; 562-68
5. Dascalescu D, Constantin M, Iancu R, Corneal hysteresis and primary open angle glaucoma: Rom J Ophthalmol, 2015; 59; 252-54
6. European Glaucoma Society: Terminology and guidelines for glaucoma, 2014
7. Schweitzer J, Ervin M, Berdahl J, Assessment of corneal hysteresis measured by the ocular response analyzer as a screening tool in patients with glaucoma: Clin Ophthalmol, 2018; 12; 1809-13
8. Dascalescu D, Corbu C, Cristea M, Corneal biomechanics involvement in primary open angle glaucoma: Proc Rom Acad Series B, 2015; 1; 55-57
9. Kaushik S, Pandav S, Banger A, Relationship between corneal biomechanical properties, central corneal thickness, and intraocular pressure across the spectrum of glaucoma: Am J Ophthalmol, 2012; 153; 840-49
10. Gaspar R, Pinto LA, Sousa D, Corneal properties and glaucoma: A review of the literature and meta-analysis: Arq Bras Oftalmol, 2017; 80; 202-6
11. Leske C, Heijl A, Hyman L, Predictors of long-term progression in the early manifest glaucoma trial: Ophthalmology, 2007; 114; 1965-72
12. Herndon L, Weizer J, Stinnett S, Central corneal thickness as a risk factor for advanced glaucoma damage: Arch Ophthalmol, 2004; 122; 17-21
13. Mansouri K, Leite M, Weinreb R, Association between corneal biomechanical properties and glaucoma severity: Am J Ophthalmol, 2012; 153; 419-27
14. Pensyl D, Sullivan-Mee M, Torres-Monte M, Combining corneal hysteresis with central corneal thickness and intraocular pressure for glaucoma risk assessment: Eye, 2012; 26; 1349-56
15. Wu Z, Xu G, Weinreb RN, Optic nerve head deformation in glaucoma: A prospective analysis of optic nerve head surface and lamina cribrosa surface displacement: Ophthalmology, 2015; 122; 1317-29
16. Tan N, Tham Q, Thakku S, Changes in the anterior lamina cribrosa morphology with glaucoma severity: Sci Rep, 2019; 9; 6612
17. Prata T, Lima V, Guedes L, Association between corneal biomechanical properties and optic nerve head morphology in newly diagnosed glaucoma patients: Clin Exp Ophthalmol, 2012; 40; 682-88
18. Gao E, Chen B, Yang J, Comparison of retinal thickness measurements between the topcon algorithm and a graph-based algorithm in normal and glaucoma eyes: PLoS One, 2015; 10(6); e0128925
19. Gizzi C, Cellini M, Campos E: Clin Ophthalmol, 2018; 12; 481-92
20. Lanzagorta-Aresti A, Perez-Lopez M, Palacios-Pozo E, Davo-Cabrera J, Relationship between corneal hysteresis and lamina cribrosa displacement after medical reduction of intraocular pressure: Br J Ophthalmol, 2017; 101; 290-94
21. Lakkis G, The ganglion cell complex and glaucoma: Pharma, 2014; 28-32
22. Kim YJ, Kang MH, Cho HY, Comparative study of macular ganglion cell complex thickness measured by spectral-domain optical coherence tomography in healthy eyes, eyes with preperimetric glaucoma, and eyes with early glaucoma: Jpn J Ophthalmol, 2014; 58; 244-51
23. Kanski J, Bowling B: Clinical ophthalmology: A systematic Approach, 2016, Saunders Ltd.
24. Hocaoğlu M, Kara C, Sen E, Relationships between corneal biomechanics and the structural and functional parameters of glaucoma damage: Arq Bras Oftalmol, 2020; 83; 132-40
25. Deol M, Taylor D, Radcliffec N, Corneal hysteresis and its relevance to glaucoma: Curr Opin Ophthalmol, 2015; 26; 96-102
26. Berdahl J, Medeiros F, Radcliffe N, Grover D, A New Glaucoma vital sign. How corneal hysteresis is improving glaucoma patient management: Review of Ophthalmology; 2019
27. Hučko B, Ferková SL, Ďuriš S, Rybář J: Journal of Mechanical Engineering, 2019; 69; 111-16
27. Dascalescu D, Corbu C, Potop V, The importance of assessing corneal biomechanical properties in glaucoma patients care – a review: Rom J Ophthalmol, 2016; 60; 219-25
28. Yanoff M, Duker J: Ophthalmology, 2018, Elsevier
29. Abitbol O, Bouden J, Doan S, Corneal hysteresis measured with the Ocular Response Analyzer in normal and glaucomatous eyes: Acta Ophthalmol, 2010; 88; 116-19
30. Park K, Shin J, Lee J, Relationship between corneal biomechanical properties and structural biomarkers in patients with normal-tension glaucoma: A retrospective study: BMC Ophthalmol, 2018; 18; 7
31. Zareei A, Razeghinejad MR, Salouti R, Corneal biomechanical properties and thickness in primary congenital glaucoma and normal eyes: A comparative study: Med Hypothesis Discov Innov Ophthalmol, 2018; 7; 68-72
32. Anton N, Ciuntu RE, Cantemir A, Clinical study on the evaluation of corneal biomechanical properties in patients with diabetes and glaucoma: E-Health and Bioengineering Conference (EHB), 2019; 1-3
33. Kotecha A, What biomechanical properties of the cornea are relevant for the clinician: Surv Ophthalmol, 2007; 109-14
34. Kotecha A, Crabb DP, Spratt A, Garway-Heath DF, The relationship between diurnal variations in intraocular pressure measurements and central corneal thickness and corneal hysteresis: Invest Ophthalmol Vis Sci, 2009; 50; 4229-36
35. Kida T, Liu JH, Weinreb RN, Effect of 24-hour corneal biomechanical changes on intraocular pressure measurement: Invest Ophthalmol Vis Sci, 2006; 47; 4422-26
36. Medeiros FA, Meira-Freitas D, Lisboa R, Corneal hysteresis as a risk factor for glaucoma progression: A prospective longitudinal study: Ophthalmology, 2013; 120; 1533-40
37. Medeiros FA, Weinreb RN, Evaluation of the influence of corneal biomechanical properties on intraocular pressure measurements using the ocular response analyzer: J Glaucoma, 2006; 15; 364-70
38. Murphy M, Pokrovskaya O, Galligan M, O’Brien C, Corneal hysteresis in patients with glaucoma-like optic discs, ocular hypertension and glaucoma: BMC Ophthalmol, 2017; 17; 1
39. Sullivan-Mee M, The role of ocular biomechanics in glaucoma management: Review of Ophthalmology, 2008; 145; 10
40. Pillunat KR, Hermann C, Spoerl E, Pillunat LE, Analyzing biomechanical parameters of the cornea with glaucoma severity in open-angle glaucoma: Graefes Arch Clin Exp Ophthalmol, 2016; 254; 1345-51
41. Dascalescu D, Corbu C, Coviltir V, The ganglion cell complex as a useful tool in glaucoma assessment: Rom J Ophthalmol, 2018; 62; 300-3
Figures
 Figure 1. Patient distribution within the groups.
Figure 1. Patient distribution within the groups. Figure 2. Correlation between CH and pRNFL thickness in POAG patients.
Figure 2. Correlation between CH and pRNFL thickness in POAG patients. Figure 3. Correlation between CH and pRNFL thickness in OH patients.
Figure 3. Correlation between CH and pRNFL thickness in OH patients. Figure 4. Correlation between CH and pRNFL thickness in normal individuals.
Figure 4. Correlation between CH and pRNFL thickness in normal individuals. Figure 5. Correlation between CH and GCC thickness in POAG patients.
Figure 5. Correlation between CH and GCC thickness in POAG patients. Figure 6. Correlation between CH and GCC thickness in OH patients.
Figure 6. Correlation between CH and GCC thickness in OH patients. Figure 7. Correlation between CH and GCC thickness in normal individuals.
Figure 7. Correlation between CH and GCC thickness in normal individuals. Tables
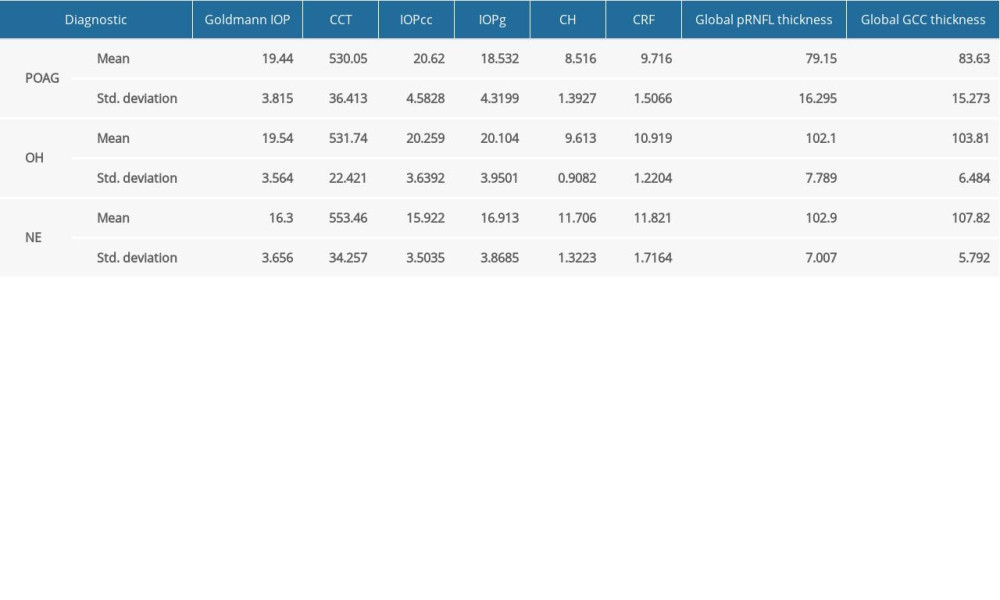 Table 1. Means of the main parameters measured in the study.
Table 1. Means of the main parameters measured in the study.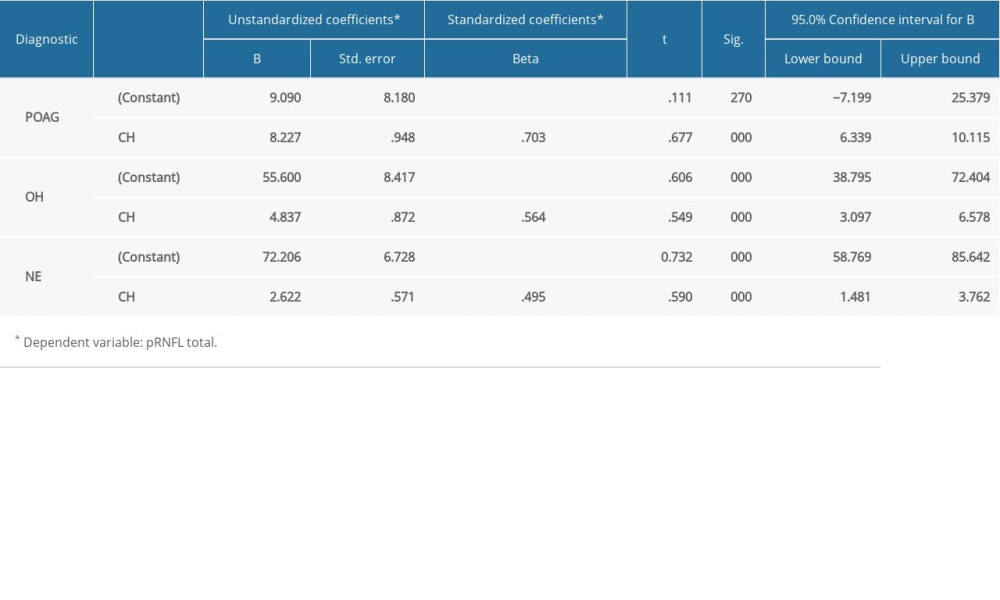 Table 2. The relationship between CH and pRNFL thickness in all 3 groups.
Table 2. The relationship between CH and pRNFL thickness in all 3 groups.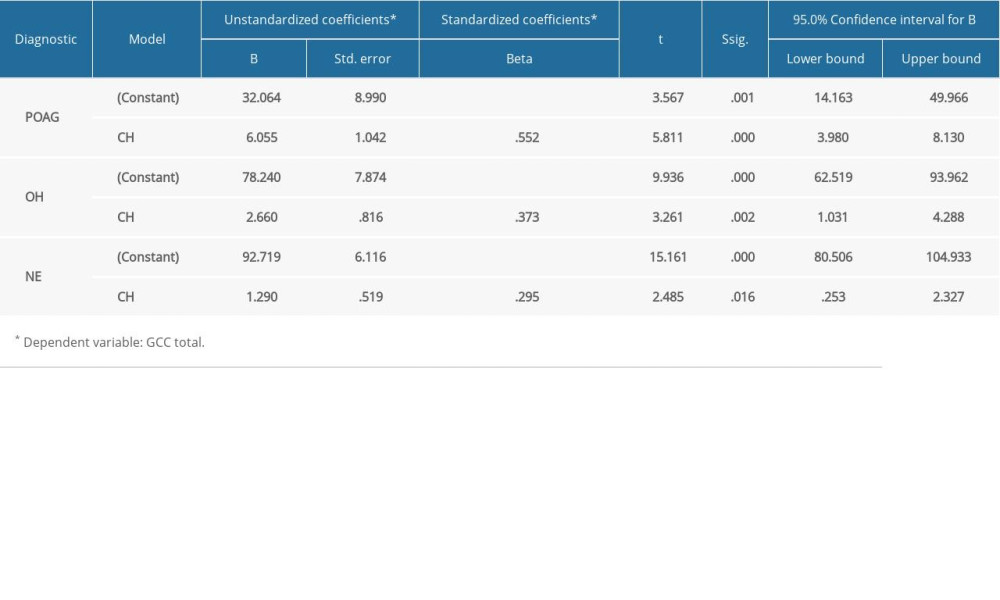 Table 3. The relationship between CH and GCC thickness in all 3 groups.
Table 3. The relationship between CH and GCC thickness in all 3 groups. Table 1. Means of the main parameters measured in the study.
Table 1. Means of the main parameters measured in the study. Table 2. The relationship between CH and pRNFL thickness in all 3 groups.
Table 2. The relationship between CH and pRNFL thickness in all 3 groups. Table 3. The relationship between CH and GCC thickness in all 3 groups.
Table 3. The relationship between CH and GCC thickness in all 3 groups. In Press
05 Mar 2024 : Clinical Research
Role of Critical Shoulder Angle in Degenerative Type Rotator Cuff Tears: A Turkish Cohort StudyMed Sci Monit In Press; DOI: 10.12659/MSM.943703
06 Mar 2024 : Clinical Research
Comparison of Outcomes between Single-Level and Double-Level Corpectomy in Thoracolumbar Reconstruction: A ...Med Sci Monit In Press; DOI: 10.12659/MSM.943797
21 Mar 2024 : Meta-Analysis
Economic Evaluation of COVID-19 Screening Tests and Surveillance Strategies in Low-Income, Middle-Income, a...Med Sci Monit In Press; DOI: 10.12659/MSM.943863
10 Apr 2024 : Clinical Research
Predicting Acute Cardiovascular Complications in COVID-19: Insights from a Specialized Cardiac Referral Dep...Med Sci Monit In Press; DOI: 10.12659/MSM.942612
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








