03 October 2020: Clinical Research
Effects and Mechanisms of Dapagliflozin Treatment on Ambulatory Blood Pressure in Diabetic Patients with Hypertension
Zirao Hao1BCDE, Yue Sun1BF, Yingzhen Wen1BF, Lijuan Cui1BF, Guiping Li1DF*, Yan Liu2AGDOI: 10.12659/MSM.925987
Med Sci Monit 2020; 26:e925987
Abstract
BACKGROUND: Studies have shown that dapagliflozin has antihypertensive effects. However, the effects and mechanisms of dapagliflozin on ambulatory blood pressure (ABP) have not been fully evaluated. In this study, we aimed to evaluate the effects of dapagliflozin treatment on ABP in patients with type 2 diabetes and hypertension.
MATERIAL AND METHODS: Patients were prospectively enrolled and divided into 2 groups: dapagliflozin treatment group (n=182) and no dapagliflozin treatment group (n=304). Clinical characteristics and measures of treatment, serum uric acid (SUA), 24-h urinary UA (UUA) excretion, and 24-h ABP were collected. The effects and mechanisms of dapagliflozin on 24-h ABP were evaluated.
RESULTS: After 3 months, the patients without dapagliflozin treatment had higher SUA, lower 24-h UUA excretion, and higher 24-h and daytime systolic blood pressure (SBP) (P<0.05) compared to patients with dapagliflozin treatment. After adjusting for covariates, results showed that dapagliflozin treatment was significantly associated with reduced 24-h SBP (β=–0.29 and P=0.02) and reduced daytime SBP (β=–0.33 and P=0.009). After additionally adjusting for SUA and 24-h UUA excretion, there were no significant relationships found between dapagliflozin treatment and 24-h (β=–012, P=0.10) and daytime SBP (β=–0.20, P=0.06).
CONCLUSIONS: In patients with diabetes and hypertension, dapagliflozin treatment was associated with reduced 24-h and daytime SBP, which could be related to the drug’s effect of increasing 24-h UUA excretion.
Keywords: arterial pressure, Diabetes Complications, Uric Acid, Antihypertensive Agents, Benzhydryl Compounds, Blood Pressure, Blood Pressure Monitoring, Ambulatory, Diabetes Mellitus, Type 2, Glucosides, Hypertension
Background
Diabetes mellitus is a major risk factor for cardiovascular and renal diseases [1,2]. In the past decade, several novel antidiabetic medications have been developed, and among these, sodium-glucose cotransporter-2 inhibitors (SGLT2i) have consistently shown promising cardio- and renoprotective effects [3–7]. For example, the DAPA-HF (Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction) trial showed that dapagliflozin therapy is beneficial in reducing cardiovascular and all-cause mortality in diabetic and nondiabetic patients with heart failure [4]. In patients with diabetes and chronic kidney disease, canagliflozin significantly reduces major adverse cardiovascular events and kidney failure [6]. In patients with diabetes who have increased cardiovascular risk, empagliflozin therapy is associated with better renal function and a lower risk of renal events compared to that of placebo [7]. Unfortunately, most participants in these major clinical trials were white, and the cardiovascular and renal benefits of SGLT2i in a Chinese patient population with diabetes have not been as thoroughly investigated. Recognizing the increasing prevalence and incidence of diabetes mellitus in Chinese populations [8,9] and the racial and ethnic differences in the clinical characteristics and prognosis of diabetes mellitus, it is imperative to carefully evaluate the safety and efficacy of SGLT2i in Chinese diabetic populations.
Hypertension is a common comorbidity in patients with diabetes [10]. Numerous studies have shown that the presence of hypertension was associated with a higher cardiovascular and renal risk in diabetic patients than in their counterparts without hypertension [11–13]. Importantly, some studies have reported that in addition to lowering blood glucose levels, SGLT2i lower blood pressure (BP), which might be due to their natriuretic and diuretic effects [14,15]. However, whether these benefits also exist in the Chinese population are unknown. In addition, the effects and mechanisms of SGLT2i on 24-h ambulatory blood pressure (ABP) have not been fully investigated.
Herein, leveraging data from our ongoing prospective diabetic and hypertensive cohort study, we evaluated the effects of 3 months of dapagliflozin treatment on 24-h ABP in patients with diabetes and hypertension. In addition, because few studies have reported that SGLT2i increase urinary uric acid (UUA) excretion [16,17], we evaluated whether a change in 24-h ABP was mediated by an increase in UUA excretion and the resultant reduction in serum UA (SUA) with dapagliflozin treatment.
Material and Methods
STUDY PARTICIPANTS:
This study was approved by the Institutional Review Board of the Third People’s Hospital of Huizhou (approval no. TPHHZ-20180627301), and written inform consent was obtained before participants’ enrollment. The inclusion criteria were as follows: >18 years old; type 2 diabetes mellitus; primary hypertension; 24-h ABP measurement at baseline and 3 months after dapagliflozin treatment; and baseline estimated glomerular filtration rate (eGFR) ≥60 mL/min/1.73 m2. The exclusion criteria were as follows: prior history of diabetic ketone acidosis or hyperosmolar coma; documented secondary hypertension; treatment with UA-lowering medications; treatment with a diuretic at baseline or follow-up; any cardiovascular or renal event from baseline to the 3-month follow-up; missing 24-h urine collection, SUA, or blood glucose measurements.
DATA COLLECTION:
A structured questionnaire was used for prospective data collection by 2 independent investigators. Baseline data included demographics (age and sex), anthropometrics (office BP, heart rate, weight, height, and risk factors and comorbidities (obesity, cigarette smoking, hyperuricemia, dyslipidemia, atrial fibrillation, congestive heart failure, coronary heart disease, ischemic stroke/transient ischemic attack, peripheral arterial disease, and obstructive sleep apnea). At baseline and the 3-month follow-up, fasting venous plasma was drawn to evaluate fasting plasma glucose, glycated hemoglobin (HbA1c), total cholesterol, triglycerides, SUA, serum creatinine, and C-reactive protein levels; a 24-h urinary specimen was collected for the analysis of 24-h urine volume, UUA, and urinary creatinine and albumin excretion. The 24-h UAA was indexed to body surface area, and the urinary albumin to creatinine ratio (UACR) was calculated as urinary albumin/urinary creatinine. Body mass index (BMI) was calculated by weight in kg divided by height in squared meters, and BMI ≥28 kg/m2 was defined as obesity based on the WHO recommendation for Asian populations [18]. Hyperuricemia was defined as SUA ≥420 umol/L in men and ≥360 umol/L in women. Creatinine was used to calculate eGFR based on the Modification of Diet in Renal Disease formula as previously described [19]. The 24-h ABP measurements were performed based on a prior description [20], whereby ABP was recorded from the non-dominant arm for 24 h at 20-min intervals during the day (06:00–22:00) and at 30-min intervals during the night (22:00–06:00) using a portable BP monitoring device (model 90207, Space-Labs Medical Inc, Redmond, WA, USA). The medications used were at physicians’ discretion and were collected from the electronic health records. All data were entered into encrypted Excel datasets.
STATISTICAL ANALYSIS:
Study participants were divided into 2 groups: with and without dapagliflozin treatment. Continuous variables were presented as mean±standard deviation (SD) or median (interquartile range), and between-group differences were evaluated using a
Results
COMPARISON OF BASELINE CHARACTERISTICS:
From January 2019 to December 2019, a total of 486 participants were enrolled in our study (Figure 1). Participants were separated into 2 groups: with dapagliflozin treatment and without dapagliflozin treatment. In brief, patients in the dapagliflozin treatment group received a dose of 5 mg of dapagliflozin per day. Comparisons of baseline characteristics are presented in Table 1. Compared to patients with dapagliflozin treatment, patients without dapagliflozin treatment were younger, had a higher BMI and triglyceride levels, and were more likely to be female and obese (P<0.05).
COMPARISONS OF UA AND ABP AT BASELINE AND 3-MONTH FOLLOW-UP:
As presented in Table 2, there were no significant differences in SUA, 24-h UUA, UACR, and 24-h ABP at baseline between the 2 groups. At the 3-month follow-up, compared to patients with dapagliflozin treatment, patients without dapagliflozin treatment had higher SUA and UACR, and a lower 24-h urine volume and UUA excretion (P<0.05). In addition, the 24-h and daytime systolic BP (SBP) were higher in patients without dapagliflozin than in patients with dapagliflozin treatment (P<0.05).
COMPARISONS OF MEDICATIONS USED AT BASELINE AND 3-MONTH FOLLOW-UP:
The lists of medications used at baseline and at the 3-month follow-up are shown in Table 3. At baseline, the use of angiotensin-converting enzyme inhibitor/angiotensin receptor blockers (ACEI/ARB) was higher and the use of calcium channel blockers (CCB) was lower in patients without dapagliflozin treatment than in patients with dapagliflozin treatment (P<0.05). The use of antidiabetic drugs at baseline was similar in both groups. At the 3-month follow-up, the use of beta-blockers and CCB was decreased in the dapagliflozin group, and the use of ACEI/ARB and CCB was increased in patients without dapagliflozin treatment. The between-group difference in ACEI/ARB use remained the same from baseline to follow-up. In addition, the use of metformin, sulfonylureas, and alpha-glucoside inhibitors increased in patients without dapagliflozin treatment, and was higher than in patients with dapagliflozin treatment. Compared to baseline, the use of metformin, sulfonylureas, alpha-glucoside inhibitors and other antidiabetic medications decreased at the 3-month follow-up in patients with dapagliflozin treatment. In both groups, fasting plasma glucose and HbA1c levels were reduced similarly at the 3-month follow-up compared to the levels at baseline. No adverse effects including hypoglycemia, hypotension, hyponatremia, and acute kidney injury were reported in either group.
RELATIONSHIP BETWEEN 24-H ABP AND DAPAGLIFLOZIN TREATMENT:
As presented in Table 4, before adjusting for covariates, dapagliflozin treatment was associated with a lower 24-h SBP, daytime SBP, and nighttime SBP, and there was no significant relationship between dapagliflozin treatment and ambulatory diastolic BP. After adjustment for potential covariates, dapagliflozin treatment remained significantly associated with 24-h SBP (β=−0.29, R2=0.25, and P=0.02) and daytime SBP (β=−0.33, R2=0.32, and P-value=0.009). However, after further adjusting for SUA and 24-h UUA excretion at follow-up, there were no significant associations between dapagliflozin treatment and 24-h SBP (β=−012, R2=0.14, and P=0.10) and daytime SBP (β=−0.20, R2=0.1, and P=0.06).
Discussion
The present study has 3 main findings. First, dapagliflozin treatment increased 24-h UUA secretion; second, dapagliflozin treatment was associated with a reduced 24-h SBP and daytime SBP after 3 months of treatment; and third, the BP-lowering benefits of dapagliflozin treatment were at least partly attributable to its increasing 24-h UUA excretion. To the best of our knowledge, this is the first study to evaluate the effects of dapagliflozin on 24-h ABP in Chinese patients with diabetes and hypertension. This study’s findings provide novel insights into the effects and mechanisms of dapagliflozin on 24-h ABP reduction. Further studies with larger sample sizes and longer follow-up periods are needed to evaluate whether these findings can benefit diabetic patients with hypertension.
Hypertension is prevalent in patients with diabetes [21–23]. Kabakov et al. reported that among patients with type 2 diabetes, the prevalence of hypertension is 60.2% [22]; and a systematic review reported this prevalence is as high as 75% [23]. Notably, the presence of hypertension is associated with poor prognosis in patients with diabetes [10]. Dapagliflozin is a novel antidiabetic medication. Through inhibiting SGLT2 in the renal proximal tubule, dapagliflozin increases urinary glucose excretion. In addition, experimental and human studies have shown that dapagliflozin could increase urinary sodium excretion and result in diuresis and natriuresis. Compared to placebo, SGLT2i were associated with a significant reduction of office BP. Some studies have also reported that among patients with type 2 diabetes, empagliflozin, taken with antihypertensive medications, significantly reduces 24-h, daytime, and nighttime SBP [24]. Additionally, a meta-analysis showed that SGLT2i significantly reduce 24-h ambulatory systolic and diastolic BP by 3.76 mmHg and 1.83 mmHg, respectively [25]. Consistent with the previous research, the present study demonstrated that mean 24-h and daytime SBP were reduced by 4 mmHg and 5 mmHg, respectively, after 3 months of dapagliflozin treatment. Notably, the use of antihypertensive medications (beta-blockers and CCB) was reduced in the dapagliflozin treatment group, suggesting that the observed 24-h SBP and daytime SBP reductions were attributable to dapagliflozin treatment rather than to an increased use of antihypertensive medications.
Uric acid has been considered an independent risk factor for hypertension development and BP progression. For example, Perlstein et al. reported that SUA independently predicted hypertension development after adjustment for risk factors [26]. Also, Krupp et al. reported that patients with hyperuricemia were at an increased risk of hypertension, regardless of age [27]. Despite convincing evidence demonstrating that increased SUA was associated with BP elevation and hypertension development, no randomized clinical trials have been conducted to determine if lowering SUA can decrease BP. The present findings suggest that 3 months of dapagliflozin treatment significantly reduced SUA through increasing 24-h UUA excretion, which was consistent with previous reports [16,17]. Based on our present linear regression analysis, we postulate that the effects of SGLT2i on BP reduction are more likely due to SUA reduction and less likely due to 24-h urine volume change. Indeed, after adjusting for 24-h urine volume, dapagliflozin treatment remained significantly associated with 24-h and daytime SBP. However, after making further adjustments for SUA and 24-h UUA excretion, dapagliflozin treatment was no longer significantly associated with 24-h and daytime SBP. In addition, several potential mechanisms of the antihypertensive effects of SGLT2i have been postulated. For example, SGLT2 is co-expressed with the Na+/H+ exchanger-3 membrane protein in the early proximal renal tubule. SGLT2 inhibition with dapagliflozin can enhance natriuresis, which can in turn increase sodium excretion and lower BP [28]. Furthermore, via osmotic diuresis induced by urinary glucose and sodium excretion, SGLT2i reduced BP in diabetic patients [15]. Weight loss associated with SGLT2i treatment can also contribute to BP reduction, as was shown in a pooled analysis of 2 randomized placebo-control trials [29]. Moreover, SGLT2i have been shown to improve arterial stiffness [30] and suppress the renal renin-angiotensin system [31], which together might reduce BP.
It is worth mentioning that, as shown in Table 1, several factors associated with BP, including age, sex, BMI, and obesity, were significantly different between the dapagliflozin treatment and no dapagliflozin treatment groups. One can speculate that these differences contribute to the observed between-group differences in 24-h and daytime SBP. Nonetheless, after adjustment for the potential confounding factors (Table 4), dapagliflozin treatment was still significantly associated with reduced 24-h and daytime SBP, further supporting the benefits of dapagliflozin for BP reduction. Interestingly, some studies have shown that aspirin treatment can reduce BP because of its anti-inflammatory effects [32], while other studies did not show any antihypertensive effects of aspirin treatment [33]. In the present study, the use of aspirin in the 2 groups was comparable at baseline and 3-month follow-up. The inclusion of aspirin use in the linear regression model (Table 4) did not influence the relationship between dapagliflozin and 24-h or daytime ABP, suggesting that aspirin did not have a major effect on BP change in the current study.
This study has some limitations. First, this is an observational study and findings from the analysis cannot be used to make conclusions about causal relationships. Second, this study was conducted in a Chinese population, and it is not known whether these findings can be extrapolated to other racial and ethnic groups. Third, this was a short-term study and further studies are needed to evaluate the long-term safety and efficacy of dapagliflozin treatment in this population. Fourth, it is important to also evaluate whether BP-lowering dapagliflozin treatment can reduce cardiovascular events. Lastly, although we have adjusted for potential covariates, undetected and unknown biases might have influenced the results that indicated the relationship between dapagliflozin treatment and 24-h and daytime SBP.
Conclusions
In conclusion, our results showed that dapagliflozin treatment was associated with 24-h and daytime SBP reduction in patients with diabetes and hypertension, and this benefit might be due to its effect on increasing 24-h UUA excretion.
Figures
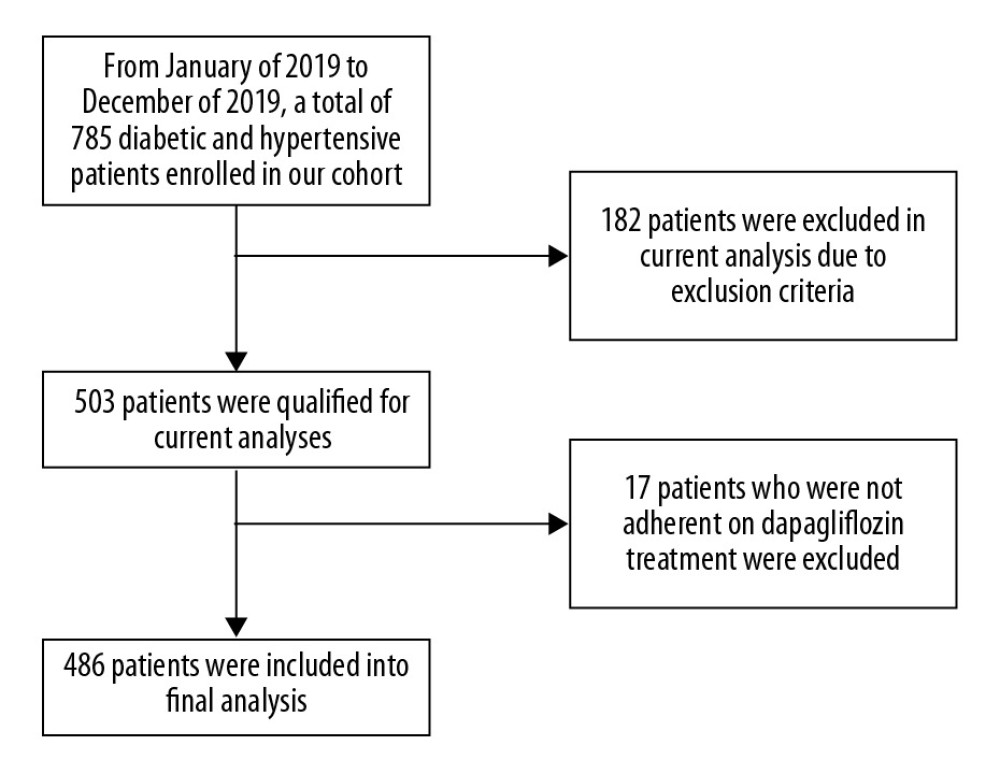 Figure 1. Study flowchart.
Figure 1. Study flowchart. References
1. Mayer-Davis EJ, Lawrence JM, Dabelea D, Incidence trends of type 1 and type 2 diabetes among youths, 2002–2012: New Engl J Med, 2017; 376(15); 1419-29
2. Arnold SV, Bhatt DL, Barsness GW, Clinical management of stable coronary artery disease in patients with type 2 diabetes mellitus: A scientific statement from the American Heart Association: Circulation, 2020; 141; e779-e806
3. Sun Y, Yan D, Hao Z, Effects of dapagliflozin and sitagliptin on insulin resistant and body fat distribution in newly diagnosed type 2 diabetic patients: Med Sci Monit, 2020; 26; e921891
4. McMurray JJV, Solomon SD, Inzucchi SE, Dapagliflozin in patients with heart failure and reduced ejection fraction: N Engl J Med, 2019; 381(21); 1995-2008
5. Neal B, Perkovic V, Mahaffey KW, Canagliflozin and cardiovascular and renal events in type 2 diabetes: N Engl J Med, 2017; 377(7); 644-57
6. Mahaffey KW, Jardine MJ, Bompoint S, Canagliflozin and cardiovascular and renal outcomes in type 2 diabetes mellitus and chronic kidney disease in primary and secondary cardiovascular prevention groups: Circulation, 2019; 40(9); 739-50
7. Wanner C, Inzucchi SE, Lachin JM, Empagliflozin and progression of kidney disease in type 2 diabetes: Ne Engl J Med, 2016; 375(4); 323-34
8. Yang W, Lu J, Weng J, Prevalence of diabetes among men and women in China: N Engl J Med, 2010; 362(12); 1090-101
9. Wang L, Gao P, Zhang M, Prevalence and ethnic pattern of diabetes and prediabetes in China in 2013: JAMA, 2017; 317(24); 2515-23
10. Tsimihodimos V, Gonzalez-Villalpando C, Meigs JB, Ferrannini E, Hypertension and diabetes mellitus: Coprediction and time trajectories: Hypertension, 2018; 71(3); 422-28
11. Cai A, Liu C, Zhou D, Ambulatory blood pressure is superior to clinic blood pressure in relation to ischemic stroke in both diabetic and nondiabetic patients: Blood Press Monit, 2017; 22(6); 314-21
12. Khangura D, Kurukulasuriya LR, Whaley-Connell A, Sowers JR, Diabetes and hypertension: clinical update: Am J Hypertens, 2018; 31(5); 515-21
13. Petrie JR, Guzik TJ, Touyz RM, Diabetes, hypertension, and cardiovascular disease: Clinical insights and vascular mechanisms: Can J Cardiol, 2018; 34(5); 575-84
14. Davidson JA, SGLT2 inhibitors in patients with type 2 diabetes and renal disease: Overview of current evidence: Postgrad Med J, 2019; 131(4); 251-60
15. Kawasoe S, Maruguchi Y, Kajiya S, Mechanism of the blood pressure-lowering effect of sodium-glucose cotransporter 2 inhibitors in obese patients with type 2 diabetes: BMC Pharmacol Toxicol, 2017; 18(1); 23
16. Chino Y, Samukawa Y, Sakai S, SGLT2 inhibitor lowers serum uric acid through alteration of uric acid transport activity in renal tubule by increased glycosuria: Biopharm Drug Dispos, 2014; 35(7); 391-404
17. Bailey CJ, Uric acid and the cardio-renal effects of SGLT2 inhibitors: Diabetes Obes Metab, 2019; 21(6); 1291-98
18. WHO Expert Consultation, Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies: Lancet, 2004; 363(9403); 157-63
19. Collard D, Brouwer TF, Olde Engberink RHG, Initial estimated glomerular filtration rate decline and long-term renal function during intensive antihypertensive therapy: A post hoc analysis of the SPRINT and ACCORD-BP randomized controlled trials: Hypertension, 2020; 75(5); 1205-12
20. Cai A, Zhong Q, Liu C, Associations of systolic and diastolic blood pressure night-to-day ratios with atherosclerotic cardiovascular diseases: Hypertens Res, 2016; 39(12); 874-78
21. Hao Z, Li G, Sun Y, Liu Y, Relationship and associated mechanisms between ambulatory blood pressure and clinic blood pressure with prevalent cardiovascular disease in diabetic hypertensive patients: Medicine, 2017; 96(16); e6756
22. Kabakov E, Norymberg C, Osher E, Prevalence of hypertension in type 2 diabetes mellitus: impact of the tightening definition of high blood pressure and association with confounding risk factors: J Cardiomet Syndr, 2006; 1(2); 95-101
23. Colosia AD, Palencia R, Khan S, Prevalence of hypertension and obesity in patients with type 2 diabetes mellitus in observational studies: A systematic literature review: Diabetes Metab Syndr Obes, 2013; 6; 327-38
24. Kario K, Okada K, Kato M, 24-hour blood pressure-lowering effect of an SGLT-2 inhibitor in patients with diabetes and uncontrolled nocturnal hypertension: Results from the randomized, placebo-controlled SACRA study: Circulation, 2018; 139(18); 2089-97
25. Baker WL, Buckley LF, Kelly MS, Effects of sodium-glucose cotransporter 2 inhibitors on 24-hour ambulatory blood pressure: A systematic review and meta-analysis: J Am Heart Assoc, 2017; 6(5); e005686
26. Perlstein TS, Gumieniak O, Williams GH, Uric acid and the development of hypertension: The normative aging study: Hypertension, 2006; 48(6); 1031-36
27. Krupp D, Esche J, Mensink GB, Diet-independent relevance of serum uric acid for blood pressure in a representative population sample: J Clin Hypertens, 2017; 19(10); 1042-50
28. Vallon V, Thomson SC, Targeting renal glucose reabsorption to treat hyperglycaemia: The pleiotropic effects of SGLT2 inhibition: Diabetologia, 2017; 60(2); 215-25
29. Briasoulis A, Al Dhaybi O, Bakris GL, SGLT2 inhibitors and mechanisms of hypertension: Curr Cardiol Rep, 2018; 20(1); 1
30. Pfeifer M, Townsend RR, Davies MJ, Effects of canagliflozin, a sodium glucose co-transporter 2 inhibitor, on blood pressure and markers of arterial stiffness in patients with type 2 diabetes mellitus: A post hoc analysis: Cardiovasc Diabetol, 2017; 16(1); 29
31. Griebeler EM, Werner J, Formal comment on: Myhrvold (2016) Dinosaur metabolism and the allometry of maximum growth rate. PLoS One, 11(11): e0163205: PLoS One, 2018; 13(2); e0184756
32. Bautista LE, Vera LM, Antihypertensive effects of aspirin: What is the evidence?: Curr Hypertens Rep, 2010; 12(4); 282-89
33. Nawarskas JJ, Townsend RR, Cirigliano MD, Spinler SA, Effect of aspirin on blood pressure in hypertensive patients taking enalapril or losartan: Am J Hypertens, 1999; 12(8 Pt 1); 784-89
Figures
Tables
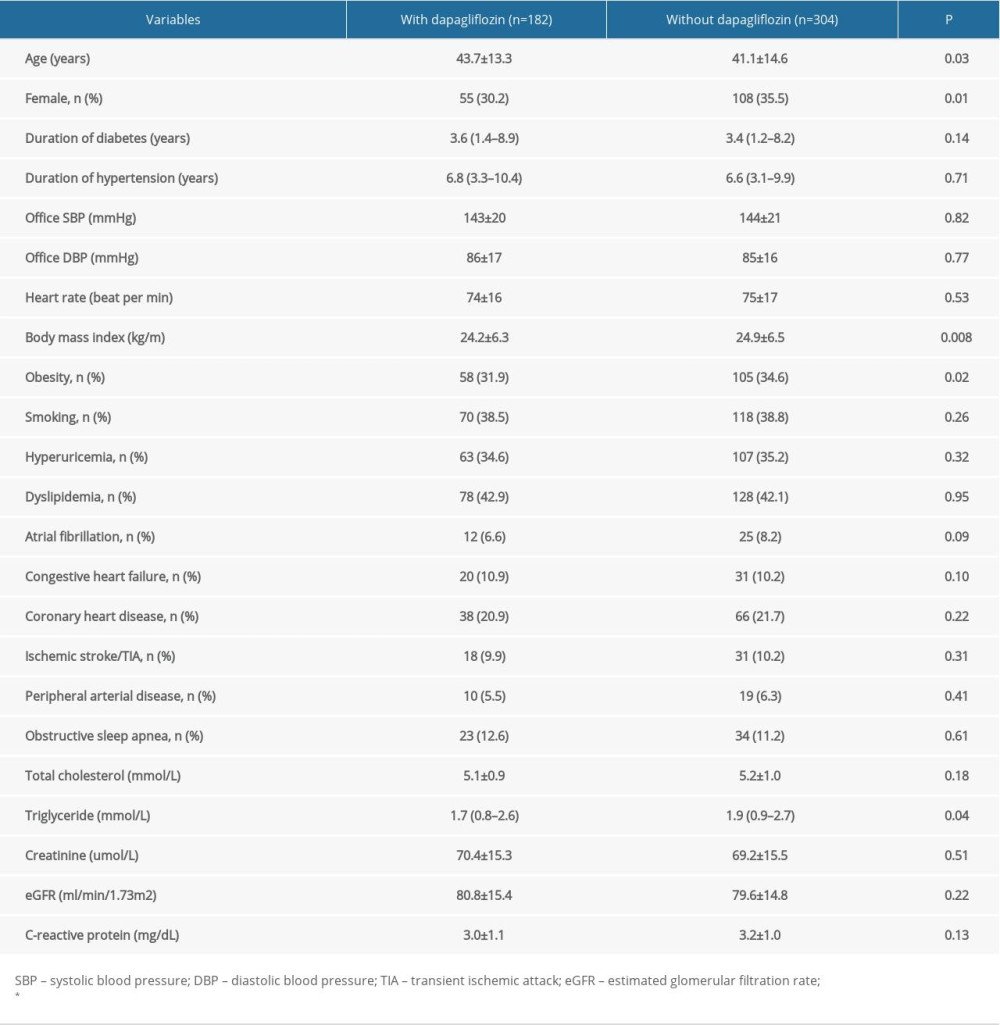 Table 1. Comparison of patient baseline characteristics.
Table 1. Comparison of patient baseline characteristics.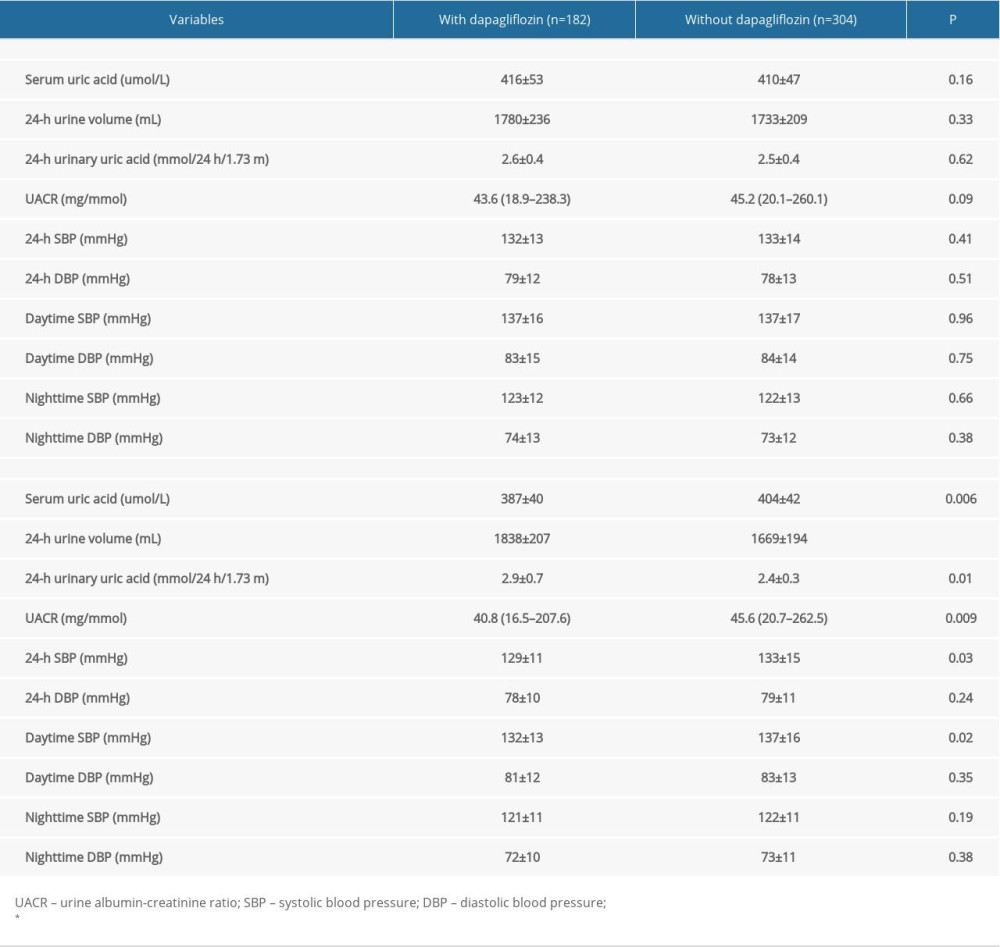 Table 2. Uric acid and ambulatory blood pressure at baseline and 3-month follow-up.
Table 2. Uric acid and ambulatory blood pressure at baseline and 3-month follow-up.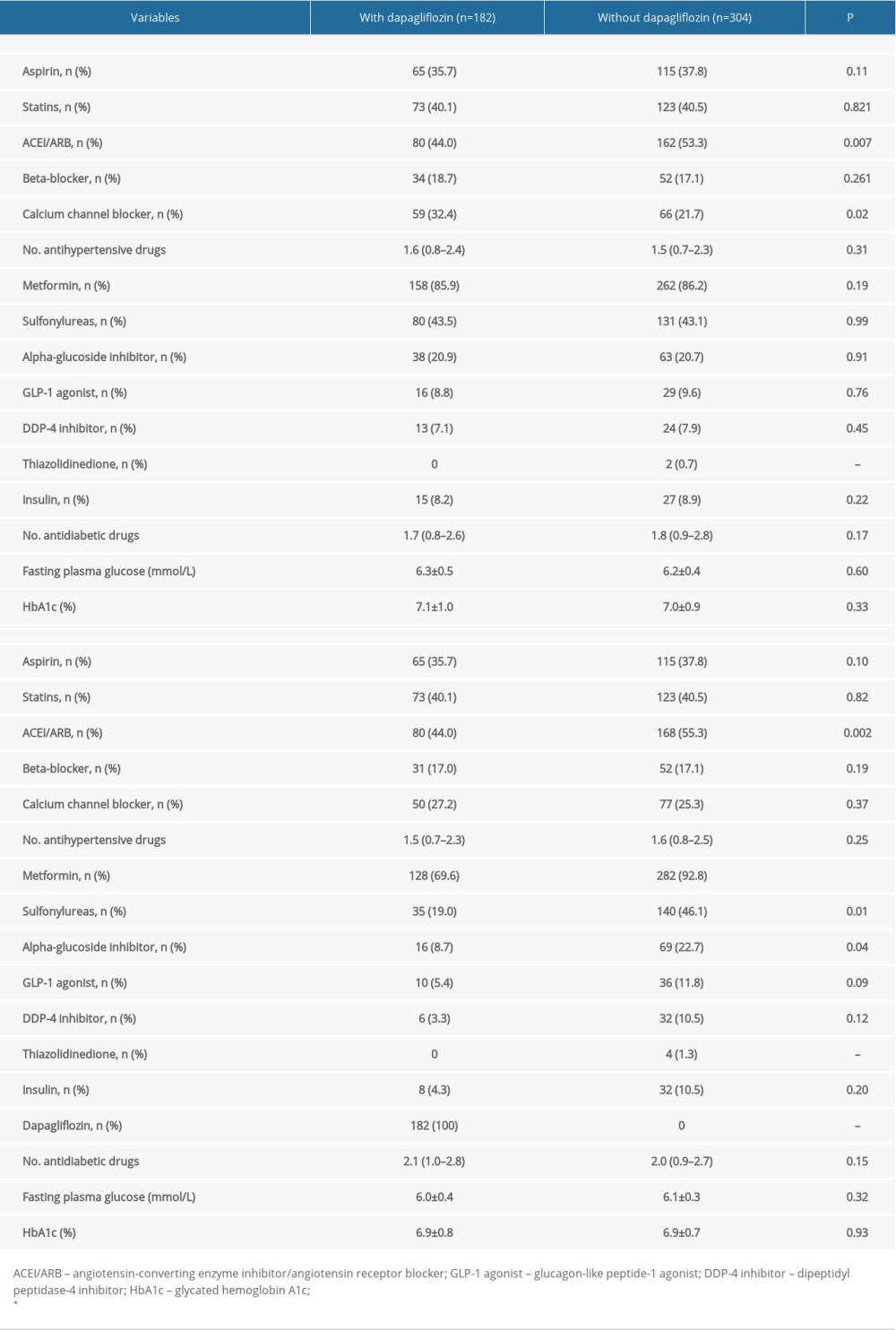 Table 3. Medications use at admission and 3-month follow-up.
Table 3. Medications use at admission and 3-month follow-up.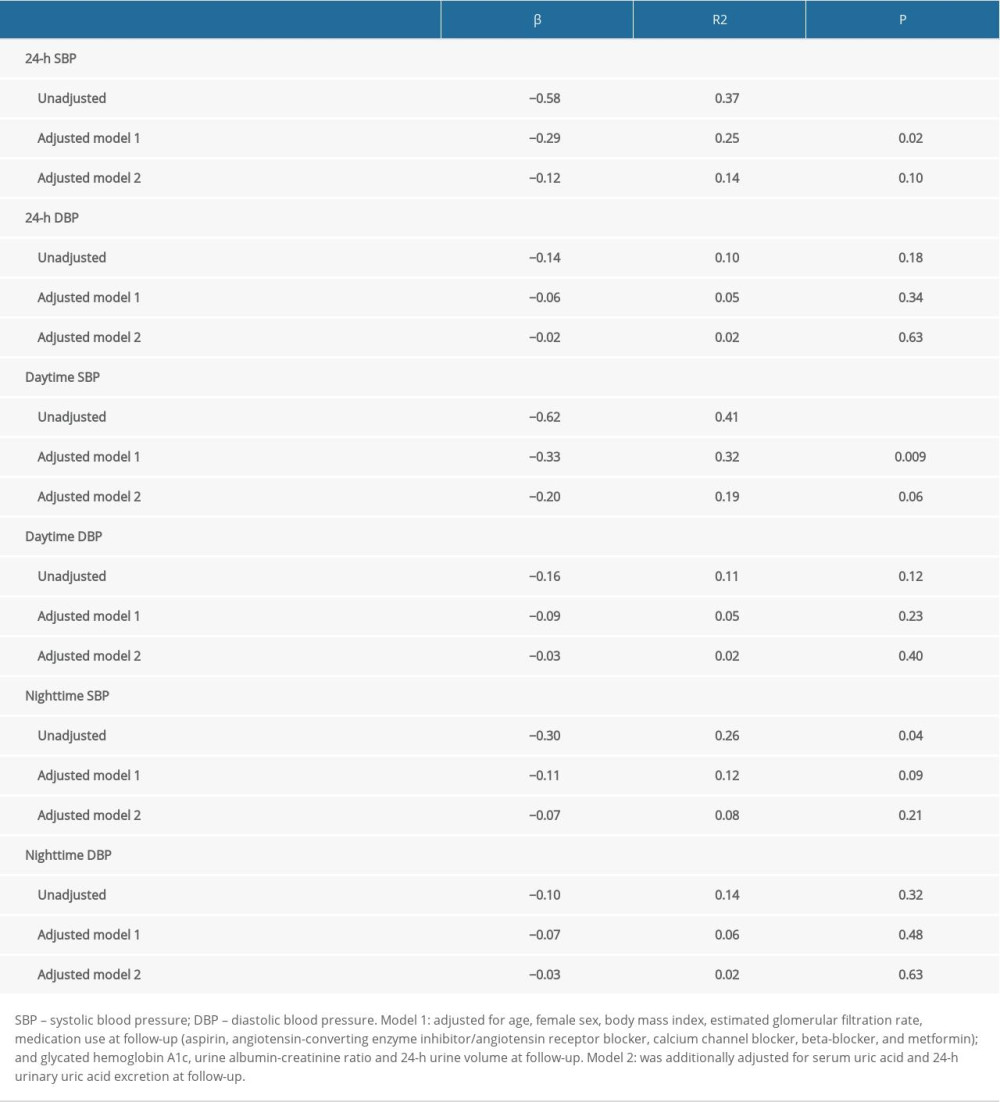 Table 4. Relationship between 24-h ABP and dapagliflozin treatment.
Table 4. Relationship between 24-h ABP and dapagliflozin treatment. Table 1. Comparison of patient baseline characteristics.
Table 1. Comparison of patient baseline characteristics. Table 2. Uric acid and ambulatory blood pressure at baseline and 3-month follow-up.
Table 2. Uric acid and ambulatory blood pressure at baseline and 3-month follow-up. Table 3. Medications use at admission and 3-month follow-up.
Table 3. Medications use at admission and 3-month follow-up. Table 4. Relationship between 24-h ABP and dapagliflozin treatment.
Table 4. Relationship between 24-h ABP and dapagliflozin treatment. In Press
05 Mar 2024 : Clinical Research
Role of Critical Shoulder Angle in Degenerative Type Rotator Cuff Tears: A Turkish Cohort StudyMed Sci Monit In Press; DOI: 10.12659/MSM.943703
06 Mar 2024 : Clinical Research
Comparison of Outcomes between Single-Level and Double-Level Corpectomy in Thoracolumbar Reconstruction: A ...Med Sci Monit In Press; DOI: 10.12659/MSM.943797
21 Mar 2024 : Meta-Analysis
Economic Evaluation of COVID-19 Screening Tests and Surveillance Strategies in Low-Income, Middle-Income, a...Med Sci Monit In Press; DOI: 10.12659/MSM.943863
10 Apr 2024 : Clinical Research
Predicting Acute Cardiovascular Complications in COVID-19: Insights from a Specialized Cardiac Referral Dep...Med Sci Monit In Press; DOI: 10.12659/MSM.942612
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








