21 June 2020: Database Analysis
Development and Validation of Prognostic Nomograms for Patients with Duodenal Neuroendocrine Neoplasms
Shenghong Sun1BDEF, Wei Wang1ACDFG*, Chiyi He1ABGDOI: 10.12659/MSM.922613
Med Sci Monit 2020; 26:e922613
Abstract
BACKGROUND: This study was designed to predict prognosis of patients with primary duodenal neuroendocrine neoplasms (D-NENs) by developing nomograms.
MATERIAL AND METHODS: Patients diagnosed with D-NENs between 1988 and 2015 were queried from the SEER database and a total of 965 appropriate cases were randomly separated into the training and validation sets. Kaplan-Meier analysis was used to generated survival curves, and the difference among the groups was assessed by the log-rank test. Independent prognostic indicators were acquired by Cox regression analysis, and were used to develop predictive overall survival (OS) and cancer-specific survival (CSS) nomograms. Harrell’s concordance index (C-index), area under the curve (AUC), calibration curves, and decision curve analysis (DCA) were used to assess the efficacy of nomograms. Tumor stage was regarded as a benchmark in predicting prognostic compared with the nomograms built in this study.
RESULTS: The C-index was 0.739 (0.690–0.788) and 0.859 (0.802–0.916) for OS and CSS nomograms, respectively. Calibration curves exhibited obvious consistency between the nomograms and the actual observations. In addition, C-index, AUC, and DCA were better than tumor stage in the evaluative performance of nomograms.
CONCLUSIONS: The nomograms were able to predict the 1-, 5-, and 10-year OS and CSS for D-NENs patients. The good performance of these nomograms suggest that they can be used for evaluating the prognosis of patients with D-NENs and can facilitate individualized treatment in clinical practice.
Keywords: Duodenal Neoplasms, nomograms, Adolescent, African Americans, Age Factors, Aged, 80 and over, Carcinoid Tumor, Carcinoma, Neuroendocrine, Digestive System Surgical Procedures, ethnicity, Gastrinoma, Marital Status, Neoplasm Grading, neuroendocrine tumors, Proportional Hazards Models, SEER Program, Sex Factors, Whites, young adult
Background
Duodenal neuroendocrine neoplasms (D-NENs) are rare and indolent, making up 3% of all primary duodenal malignancies and comprising about 2–3% of all gastrointestinal neuroendocrine neoplasms (GI-NENs) [1–3]. The incidence of D-NENs has been dramatically increasing in the United States, from 0.027/100 000 in 1983 to 1.1/100 000 in 2010 [4,5]. Recent studies showed that the increased incidence of D-NENs reflects increased physician awareness and the widespread use of diagnostic endoscopy [6,7]. D-NENs have been anatomically classified as ampullary and non-ampullary D-NENs (NADNENs). Previous reports have indicated that ampullary NENs have more aggressive clinical features and worse prognosis than NADNENs [8–11]. Specifically, ampullary NENs tends to metastasize at a smaller size or at a lower mitotic rate than NADNENs. Additionally, the mortality rate of ampullary NENs is higher than that of NADNENs. In this study, we only focused on the NADNENs.
The American Joint Committee on Cancer (AJCC) staging system is by far the most widely used prognostic tool for many neoplasms. The pathological variables of the tumor stage for D-NENs are tumor characteristics (T), lymph node status (N), and distant metastases (M) of neoplasms. However, many other vital clinicopathological features, such as age at diagnosis, tumor stage, surgery, sex, marital status, and tumor grade, can also influence the outcomes of individual D-NENs patients. Accordingly, research has focused on developing more effective prognostic models for D-NENs patients.
As a decision-making tool, nomograms can accurately and discriminately predict the outcomes of patients with neoplasms in clinical practice [12]. To draw the best conclusion, the construction of a nomogram not only considers the prognostic weight of each variable, but also integrates multiple independent variables. There have been no reports on use of nomograms for predicting the prognosis of D-NENs patients using population-based data. Therefore, this research aimed to construct nomograms to predict overall survival (OS) and cancer-specific survival (CSS) of D-NENs patients.
Material and Methods
PATIENTS:
Data on patients diagnosed with primary D-NENs between 1988 and 2015 were retrieved from the Surveillance, Epidemiology and End Results (SEER) database using the SEER*Stat software (version 8.3.5) and were categorized according to the third edition for histological type of International Classification of Diseases for Oncology (ICD-O-3) as 8153 gastrinoma, 8240 carcinoid not otherwise specified (NOS), 8241 enterochromaffin, 8246 neuroendocrine carcinoma, and 8249 atypical carcinoid. The site code used was C17.0. Demographic and clinical variables were sex, marital status, age at diagnosis, tumor grade, race, tumor size, year of diagnosis, TNM stage, surgery, and tumor stage (AJCC 7th). Patients with multiple primary carcinomas, age <18 years old, no microscopic confirmation, diagnosis at autopsy or death certificate, survival times <1 month or unknown, and variables (race, tumor size, tumor grade, tumor stage, and cause of death) with incomplete data were excluded (Figure 1). The cases were divided into well-differentiated (G1); moderately differentiated (G2); poorly differentiated (G3), and undifferentiated (G4) by histologic grade information. G3 and G4 were combined into 1 category for all analyses because of their limited sample sizes. In addition, the NX stage were restaged into N0 (lymph node-negative) or N1 (lymph node-positive) according to regional nodes-positive status. The tumor stage of all eligible cases was converted into the AJCC 7th staging system for subsequent analysis.
DEFINITION:
We mainly focused on 2 indicators – OS and CSS – in which OS is the time from diagnosis to death given any reason or the last day of survival information available in the SEER database, while CSS refers the time from diagnosis to death resulting from the D-NENs. Eligible patients were randomly assigned to either the validation cohort or the training cohort, with a ratio of 3: 7. The nomograms were drawn based on the results of the multivariate analyses in the training cohort and the prediction model were validated by the internal validation cohort.
STATISTICAL ANALYSIS:
Categorical variables were transferred from continuous variables and are shown as numbers (percent). The survival curves were developed by Kaplan-Meier (KM) analysis and the differences among groups were compared by log-rank test. The independent predictive factors of OS and CSS are evaluated by Cox proportional hazards (PH) regression analyses. The outcomes of the Cox analysis generated nomograms for 1-, 5-, and 10-year OS, as well as CSS. Harrell’s concordance index (C-index) was used for assessment of discrimination between observed and predicted outcomes [13]. Calibration plots were formulated based on the constancy between predicted survival and actual survival. The area under the receiver operator characteristic (ROC) curve (AUC) shows the accuracy of the survival predictions. Evaluation of the clinical effectiveness to the constructed nomograms was assessed by decision curve analyses (DCA) [14]. R (version 3.6.1) was used to conduct data analyses. Two-tailed p-value <0.05 signified statistical significance.
Results
PATIENT CHARACTERISTICS:
Among the 965 qualified patients with D-NENs, 677 patients were allocated to the OS training cohort and 288 patients were allocated to the validation cohort, while 593 patients were allocated to the CSS training cohort and 252 patients were allocated to the validation cohort. The results showed that the median age at diagnosis was 63 (18–95) years and the median follow-up time was 40 (1–229) months. Histologic types for D-NENs were mainly carcinoid NOS (71.6%) and neuroendocrine carcinoma (25.6%), and less common types were gastrinoma and atypical carcinoid, with fewer than 20 patients per type. The majority of patients were white (67.3%) and had stage I disease (54.7%). The proportion of female patients (484 cases, 50.2%) was similar to that of male patients (481 cases, 49.8%). There were 818 (84.8%) patients who underwent surgery and 801 (83%) patients had well-differentiated neoplasms. About 83% of patients had neoplasms ≤2 cm in size. Patients with D-NENs tended to have T1 (57.5%), T2 (30.9%), N0 (83.7%), and M0 disease (95.2%). Table 1 illustrated the baseline characteristics of D-NENs patients.
PROGNOSIS OF D-NENS PATIENTS:
The 1-, 5-, and 10-year survival rates were 94.8%, 94.7%, and 79.3% for OS, and 97.4%, 92.0%, and 89.1% for CSS, respectively. Further, KM analysis by log-rank test showed that tumor grade, age at diagnosis, marital status, tumor size, year of diagnosis, T stage, surgery, and tumor stage were associated with the prognosis of D-NENs patients, and this result was statistically significant. M stage and N stage were associated with CSS but not with OS (Figures 2, 3).
The results of Cox analyses revealed that clinicopathological factors, including surgery, marital status, year of diagnosis, age at diagnosis, tumor grade, and tumor size, were significantly correlated with OS prognosis (Table 2). Factors of tumor grade, M stage, surgery, age at diagnosis, and year of diagnosis were independently correlated with CSS prognosis (Table 3).
CONSTRUCTION AND VALIDATION OF NOMOGRAMS:
All independent indicators by multivariate Cox analysis in the training group were involved in the nomograms for predicting OS and CSS. In addition, although the parameter of tumor size was associated with CSS on univariate analysis, this finding did not exist in multivariate analysis. However, it was still incorporated into the construction of the nomogram as an essential clinical factor in our study (Figure 4). The calibration curves indicated the predicted values of OS and CSS agreed with the observed ones for 1, 5, and 10 years (Figure 5). The C-index of OS and CSS were 0.739 and 0.859 in the training group, respectively (Table 4). The AUC values of the nomogram were expected to be 0.740, 0.716, and 0.716, and 0.828, 0.836, and 0.819, for 1-, 5-, and 10-year OS and CSS, respectively, which were all greater than those in the AJCC 7th staging system. The validation cohort and total cohort had similar results (Figure 6, Table 4). In addition, DCA showed that the nomogram performed better for D-NENs patients more for tumor stage (Figure 7). These results show that the nomogram is superior to tumor stage in terms of discriminatory capacity and accuracy of survival prediction.
SUBGROUP ANALYSIS:
The result of multivariate Cox analysis illustrated that surgery was a critical independent factor for the prognosis of OS and CSS, and the KM analysis also showed that surgery had a strong effect on OS and CSS of D-NENs patients. Therefore, we stratified the cohort according to different clinicopathologic features to further analyze whether surgery could make any difference in patient prognosis.
KM analysis indicated that D-NENs patients with longer OS was associated with age (age at diagnosis <65 years, p<0.005; age at diagnosis ≥65 years, p<0.001), sex (female, p<0.001; male, p<0.005), race (white, p<0.001; black, p<0.001), marital status (married, p<0.001; unmarried, p<0.001), year of diagnosis (1988–2009, p<0.001; 2010–2015, p<0.001), tumor grade (G1, p<0.001), tumor size (<1 cm, p<0.05; 1–2 cm, p<0.001; >2 cm, p<0.001), tumor stage (I, p<0.01; II, p<0.001; III, p<0.005; IV, p<0.005), and surgery (Figure 8). Longer CSS was associated with age (age at diagnosis ≥65 years, p<0.001), sex (female, p<0.001), race (White, p<0.05; Black, p<0.005), marital status (married, p<0.001), year of diagnosis (1988–2009, p<0.05; 2010–2015, p<0.001), tumor grade (G1, p<0.001), tumor size (>2 cm, p<0.001), tumor stage (III, p<0.001; IV, p<0.005), and surgery (Figure 9).
Discussion
D-NENs are rare neoplasms originating from cells of the neuroendocrine system [15]. Given the increasing burden and the heterogeneity of D-NENs, it is important to improve individualized predictions of survival risks and determine the optimal individual therapies. In this study, we identified critical prognostic factors for D-NENs patients. Importantly, we constructed and authenticated nomograms to predict the prognosis of D-NENs patients. This model has been assessed by different methods, and the results show that the model has good performance.
The results of KM analysis with log-rank test showed that age at diagnosis, marital status, year of diagnosis, tumor grade, tumor size, T stage, tumor stage, and surgery were strongly associated with the OS and CSS in D-NENs patients, while M stage and N stage were associated with CSS but not with OS. Multivariate Cox analyses indicated that age at diagnosis, year of diagnosis, tumor grade, and surgery were independent predictors for both CSS and OS when marital status and tumor size were incorporated into OS and M stage was incorporated into CSS. Similar to a previous study, sex and race were not associated with OS and CSS in D-NENs patients, and patients who were older than 65 years tended to have shorter survival than younger patients, both in OS and CSS [5]. We found that tumor grade was an independent predictor of OS and CSS, while tumor grade across the full range of the point axis was a better predictor of OS and CSS more than were the other variables in the constructed nomograms.
Patients with malignancies show are likely to have psychological distress, which can prolong the function of cortisol and disturb normal immune function. This phenomenon is more common in unmarried patients [16–18]. Compared with unmarried patients, married patients are more likely to feel cared for and encouraged and supported physically and spiritually, which contributes to a better prognosis [19]. Our study found that marital status had a statistically significant effect on OS, but not on CSS. Research in a larger cohort might help to explain this phenomenon.
Multivariate Cox regression analyses showed that surgery is an independent predictor of OS and CSS. By stratifying the total cohort by different clinicopathological characteristics, we found improvements in OS and CSS in patients who underwent surgical resection, regardless of race, G1, or year of diagnosis. Surgery also remarkably improved the OS in patients in different subgroups stratified by age at diagnosis, marital status, sex, and tumor stage, and CSS in patients with age at diagnosis ≥65 years, female, married, and stage III and IV. Our study also revealed that patients with tumor size >2 cm who received surgery tended to have better CSS, similar to a previous study in which patients who received surgery had longer OS, regardless of tumor size [20].
The AJCC staging system, ENETS staging system, and WHO grading system are widely used in clinical practice to predict the prognosis of D-NENs patients. The nomograms presented here performed better than the AJCC 7th staging system in predicting patient prognosis, allowing physicians to more accurately predict individual clinical outcomes and to provide personalized treatment.
The Ki-67 index or mitotic rate, a marker of cell division and measure of proliferation, has been shown to provide significant prognostic information for GEP-NENs [21–25]. Khan et al. concluded that the Ki-67 index is a better prognostic marker than mitotic rate in metastatic pancreatic and ‘midgut’ NENs [25]. However, some investigators have questioned the Ki-67 index as an independent prognostic variable in GEP-NENs [26,27]. Previous studies demonstrated that WHO grade based on the Ki-67 index was independently correlated with the prognosis of OS in D-NENs patients [28,29]. In another study, Alessandro et al. revealed that CSS of G1 versus G2 (with Ki-67 index of 2% as threshold) was significantly different only in univariate analysis but not in multivariate analysis after adjusting for age and stage in D-NENs patients [30]. These results indicate that Ki-67 index or mitotic rate as an independent prognostic parameter in neuroendocrine neoplasms remains controversial. Therefore, it is necessary to further analyze the prognostic role of Ki-67 index and/or mitotic rate in patients with D-NENs.
Limitations exist in our study. First, we did not have important information on mitotic rate and Ki-67 index, as this information is not available in the SEER database. Second, this was a retrospective study, which might have led to potential selection bias in the participants. Our results still need to be validated by larger randomized controlled studies.
Conclusions
We found that the nomograms are effective in predicting the prognosis of patients with D-NENs, and they have consistent reliability and wide clinical application.
Figures
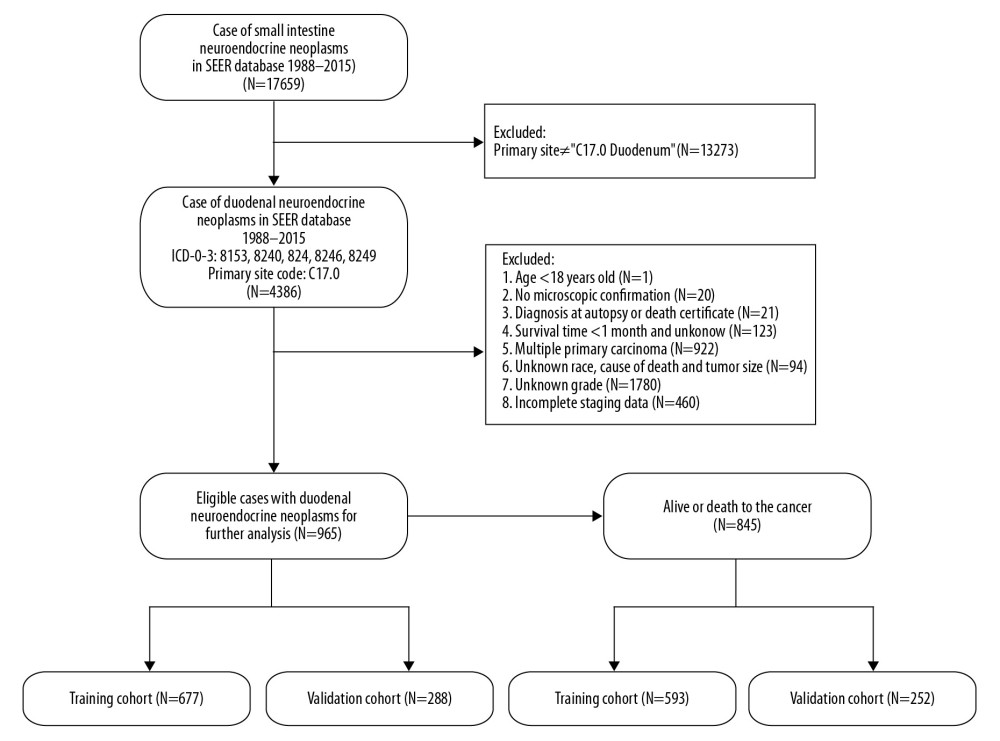 Figure 1. Flowchart of the enrolled patients according to inclusion and exclusion criteria.
Figure 1. Flowchart of the enrolled patients according to inclusion and exclusion criteria. 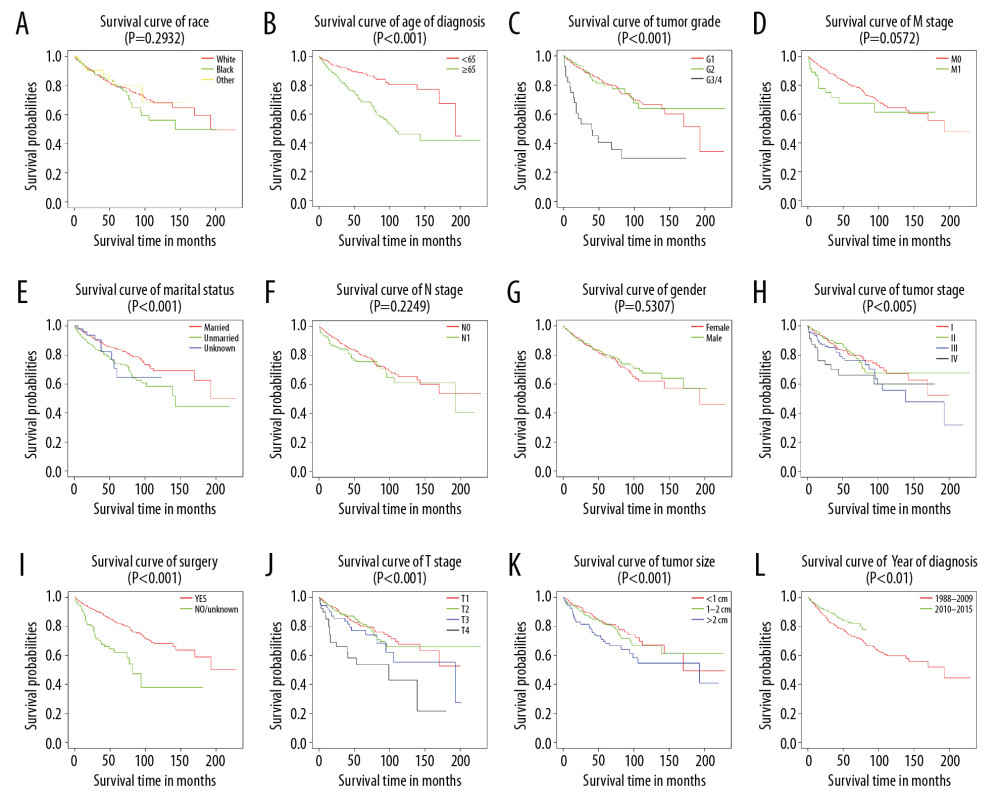 Figure 2. Kaplan-Meier analysis of overall survival in primary duodenal neuroendocrine neoplasm patients stratified by (A) race, (B) age of diagnosis, (C) tumor grade, (D) M stage, (E) marital status, (F) N stage, (G) sex, (H) tumor stage, (I) surgery, (J) T stage, (K) tumor size, and (L) year of diagnosis.
Figure 2. Kaplan-Meier analysis of overall survival in primary duodenal neuroendocrine neoplasm patients stratified by (A) race, (B) age of diagnosis, (C) tumor grade, (D) M stage, (E) marital status, (F) N stage, (G) sex, (H) tumor stage, (I) surgery, (J) T stage, (K) tumor size, and (L) year of diagnosis. 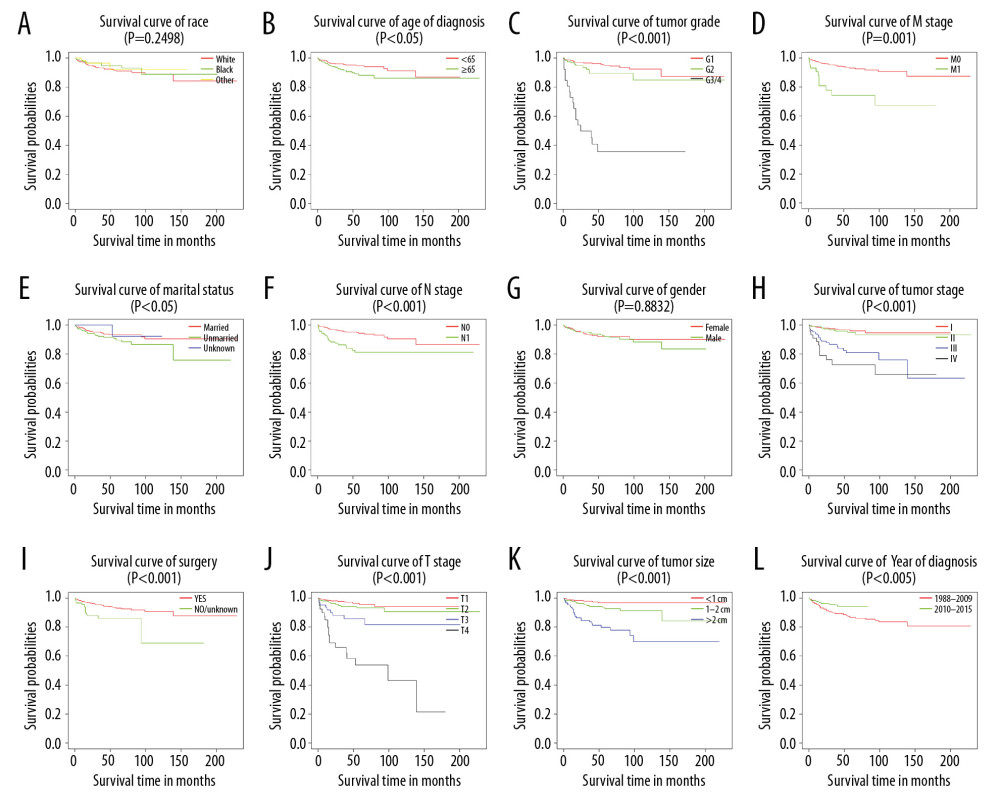 Figure 3. Kaplan-Meier analysis of cancer-specific survival in primary duodenal neuroendocrine neoplasms patients stratified by (A) race, (B) age of diagnosis, (C) tumor grade, (D) M stage, (E) marital status, (F) N stage, (G) sex, (H) tumor stage, (I) surgery, (J) T stage, (K) tumor size, and (L) year of diagnosis.
Figure 3. Kaplan-Meier analysis of cancer-specific survival in primary duodenal neuroendocrine neoplasms patients stratified by (A) race, (B) age of diagnosis, (C) tumor grade, (D) M stage, (E) marital status, (F) N stage, (G) sex, (H) tumor stage, (I) surgery, (J) T stage, (K) tumor size, and (L) year of diagnosis. 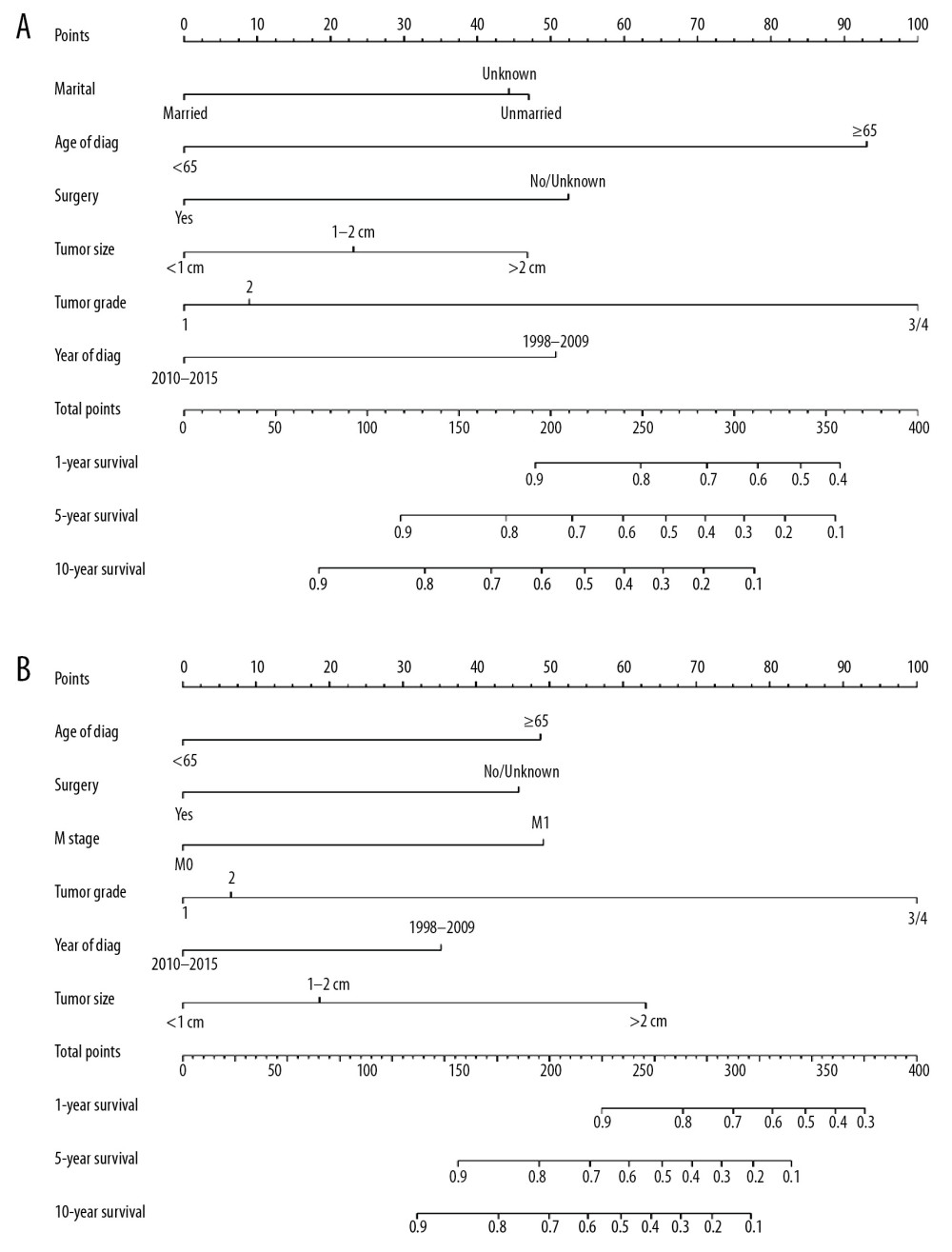 Figure 4. Nomograms predicting 1-, 5-, and 10-year overall survival (A) and cancer-specific survival (B) of patients with PGINHL.
Figure 4. Nomograms predicting 1-, 5-, and 10-year overall survival (A) and cancer-specific survival (B) of patients with PGINHL. 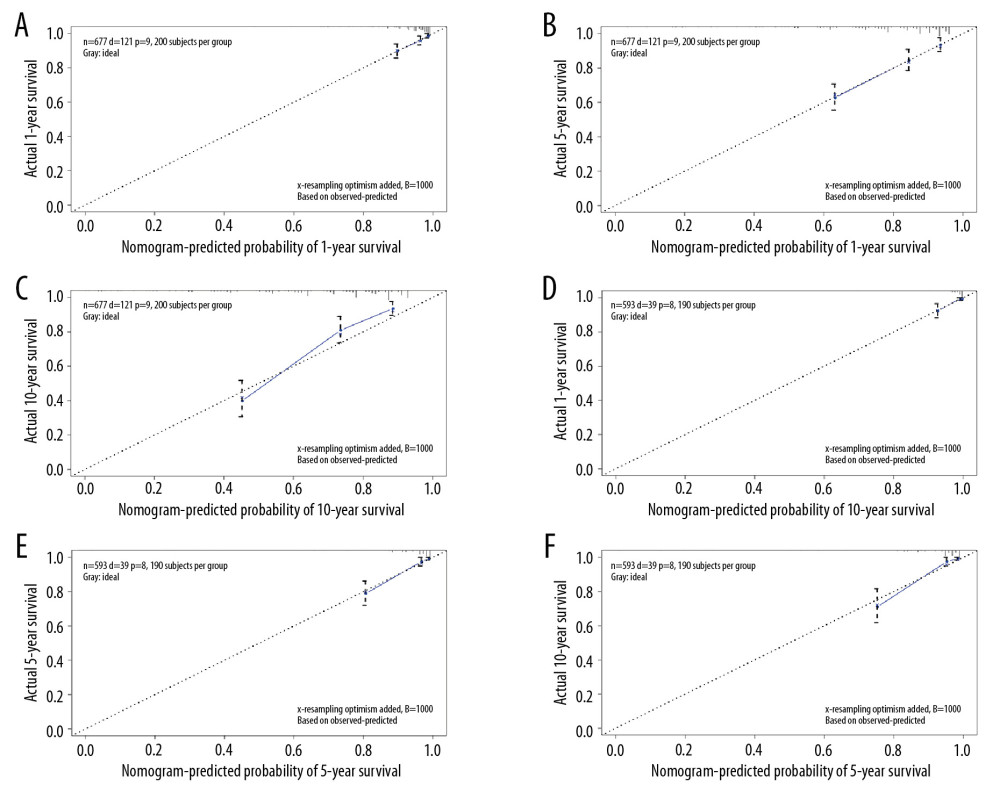 Figure 5. Calibration plots for predicting patient overall survival (A–C) and cancer-specific survival (D–F) at 1, 5, and 10 years.
Figure 5. Calibration plots for predicting patient overall survival (A–C) and cancer-specific survival (D–F) at 1, 5, and 10 years. 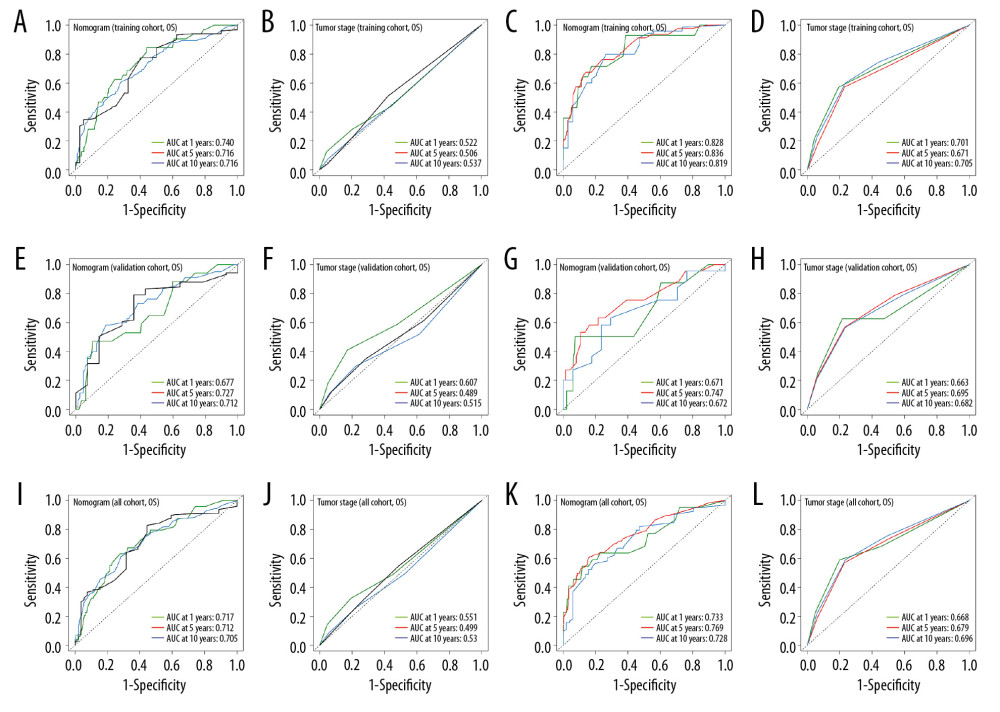 Figure 6. ROC curves of the nomograms and AJCC 7th staging system for 1-, 5-, and 10-year overall survival and cancer-specific survival prediction in the training cohort (A–D), validation cohort (E–H) and whole cohort (I–L).
Figure 6. ROC curves of the nomograms and AJCC 7th staging system for 1-, 5-, and 10-year overall survival and cancer-specific survival prediction in the training cohort (A–D), validation cohort (E–H) and whole cohort (I–L). 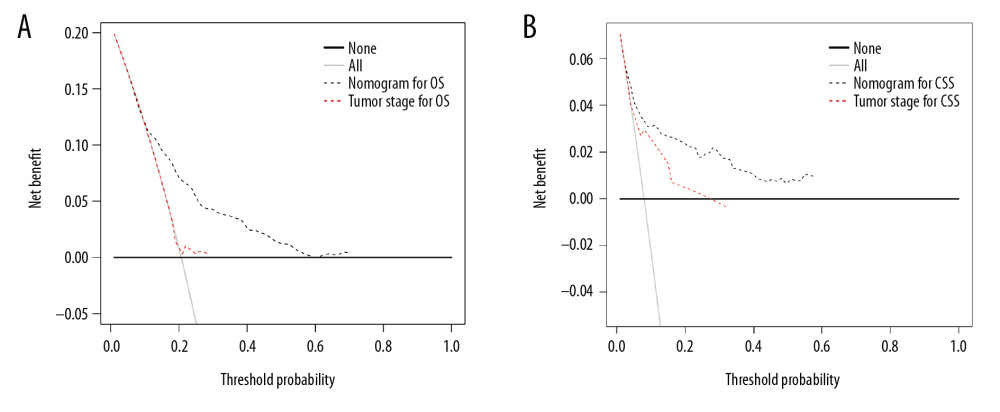 Figure 7. Decision curve analysis of the nomograms and AJCC 7th staging system for predicting overall survival (A) and cancer-specific survival (B) in patients with primary duodenal neuroendocrine neoplasms.
Figure 7. Decision curve analysis of the nomograms and AJCC 7th staging system for predicting overall survival (A) and cancer-specific survival (B) in patients with primary duodenal neuroendocrine neoplasms. 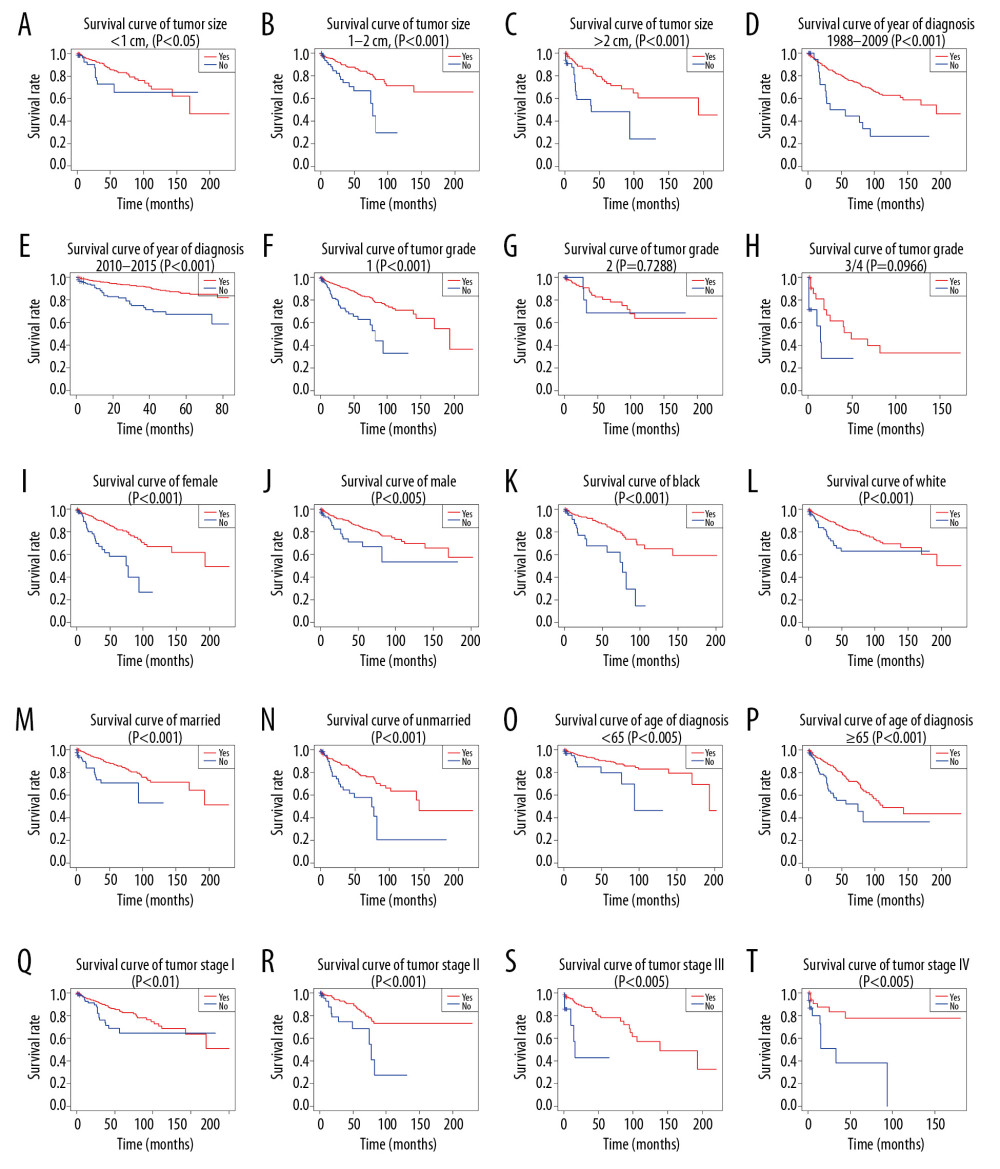 Figure 8. (A–T) Kaplan-Meier analysis of overall survival in primary duodenal neuroendocrine neoplasms patients with or without surgery.
Figure 8. (A–T) Kaplan-Meier analysis of overall survival in primary duodenal neuroendocrine neoplasms patients with or without surgery. 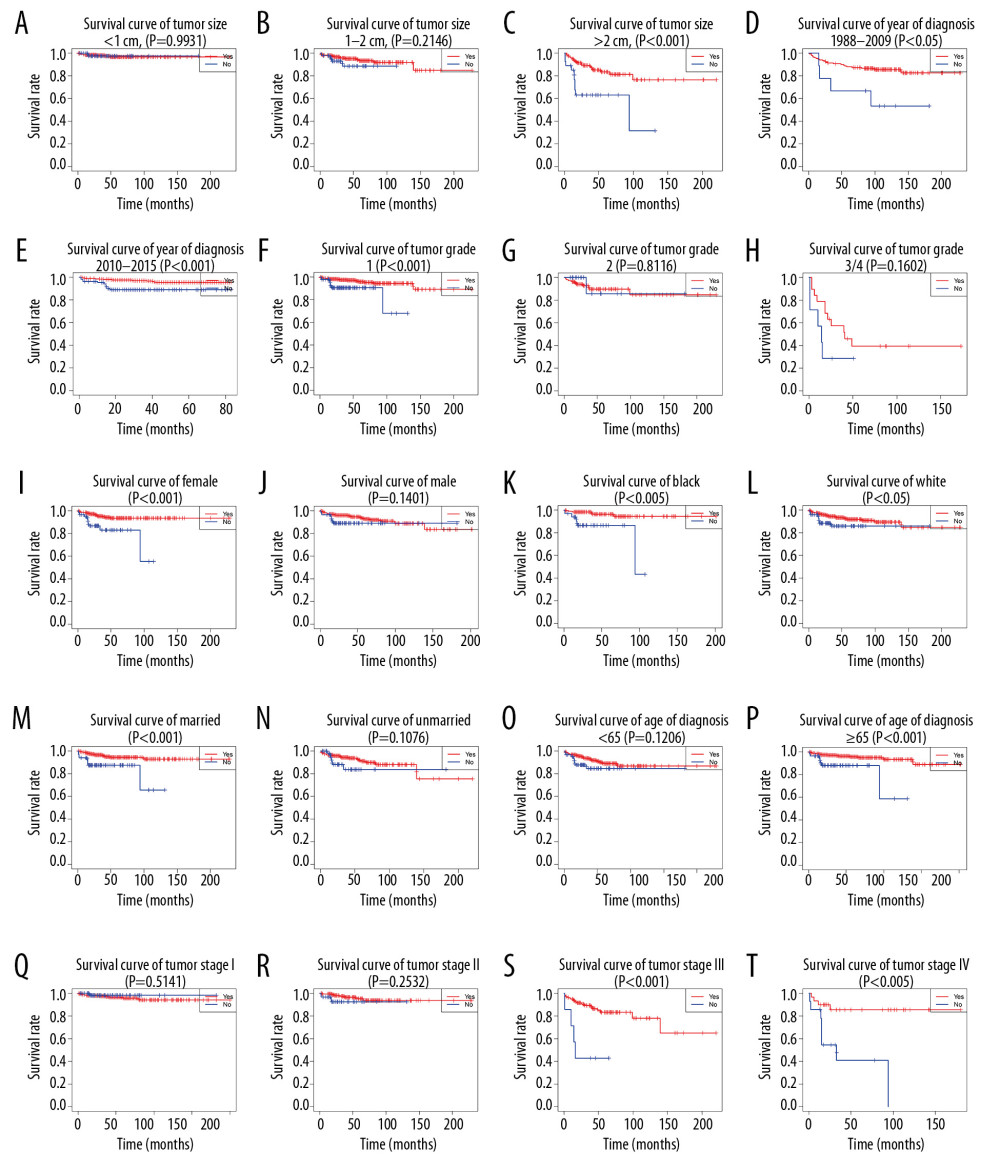 Figure 9. (A–T) Kaplan-Meier analysis of cancer-specific survival in primary duodenal neuroendocrine neoplasms patients with or without surgery.
Figure 9. (A–T) Kaplan-Meier analysis of cancer-specific survival in primary duodenal neuroendocrine neoplasms patients with or without surgery. Tables
Table 1. Clinical features and demographics of patients with D-NENs.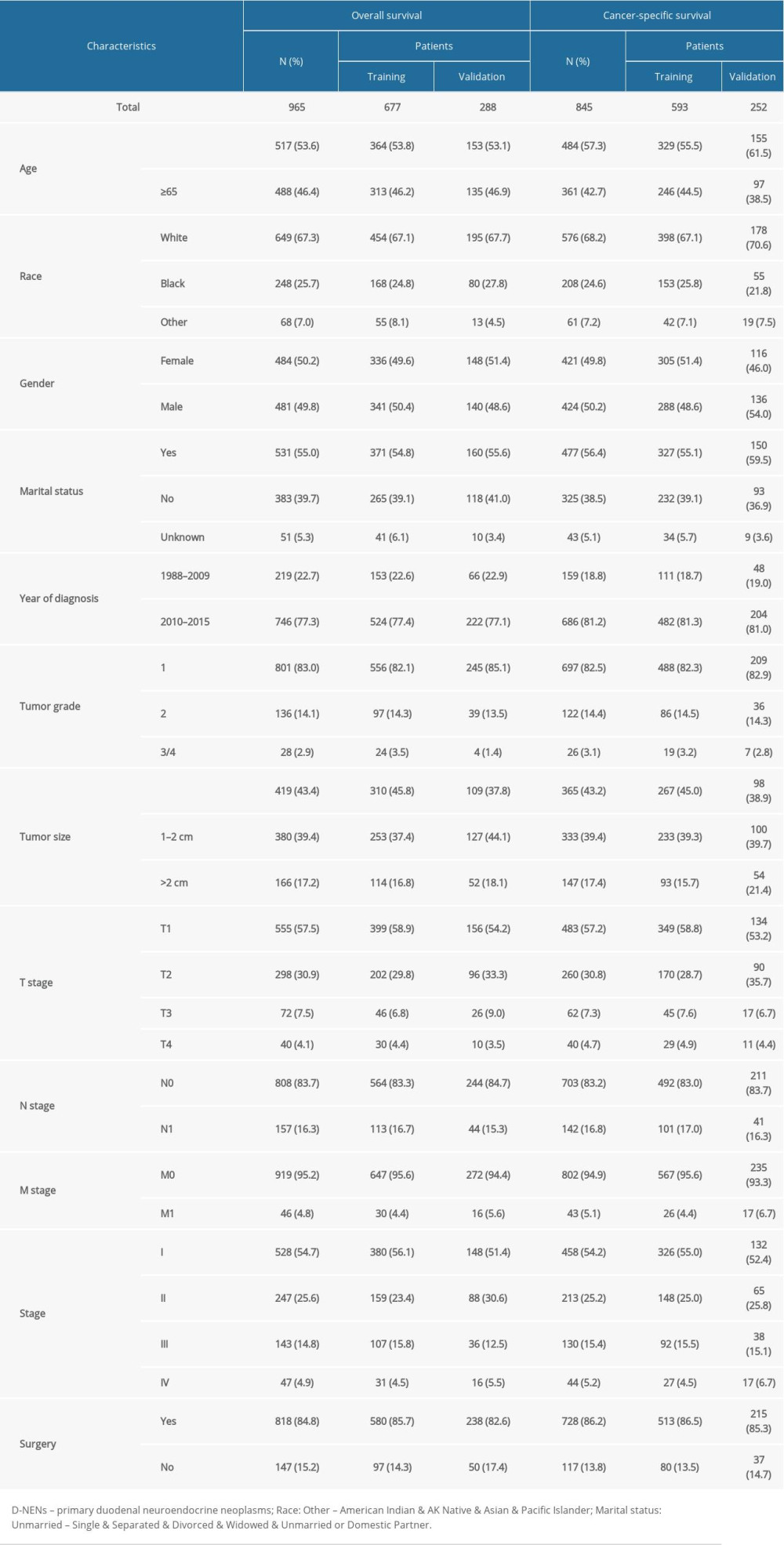 Table 2. Univariate and multivariate analyses of OS in patients with D-NENs.
Table 2. Univariate and multivariate analyses of OS in patients with D-NENs.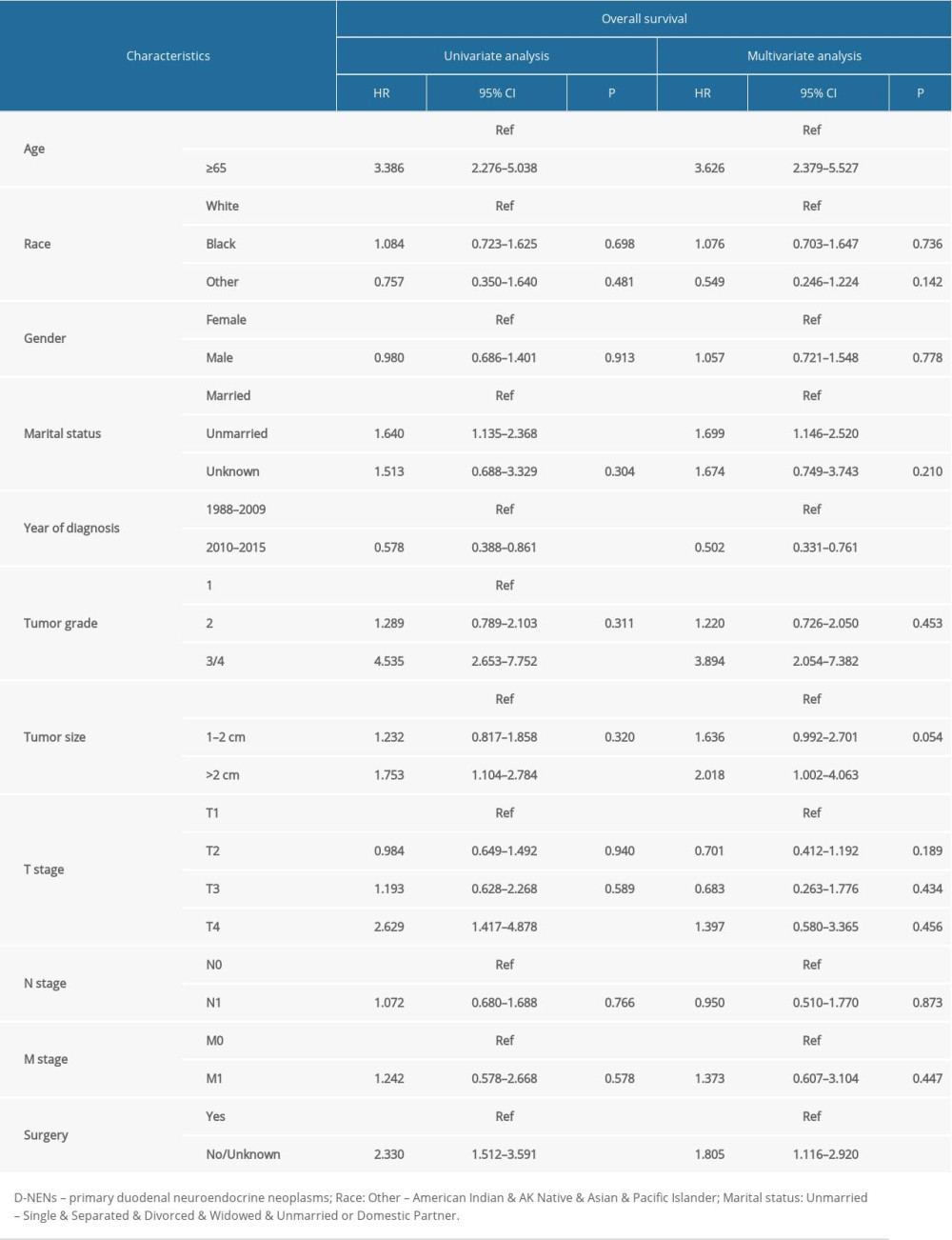 Table 3. Univariate and multivariate analyses of CSS in patients with D-NENs.
Table 3. Univariate and multivariate analyses of CSS in patients with D-NENs.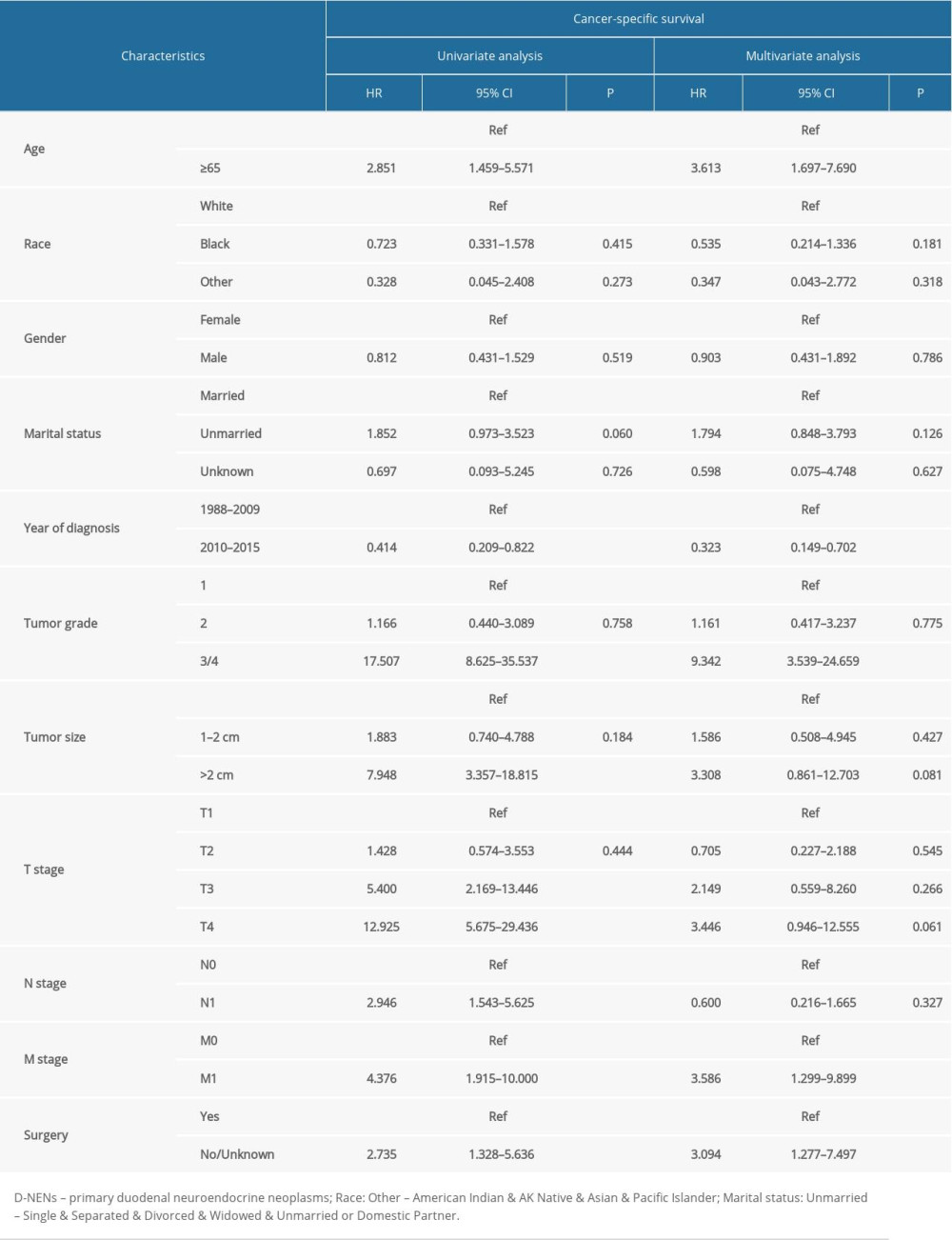 Table 4. The C-index for nomograms and the AJCC 7th staging system in patients with D-NENs.
Table 4. The C-index for nomograms and the AJCC 7th staging system in patients with D-NENs.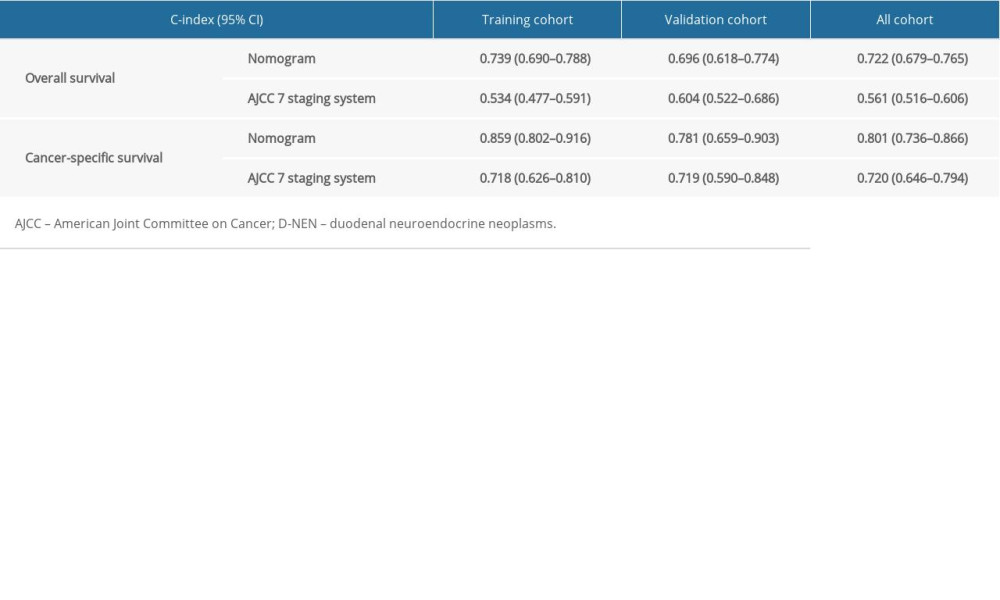
References
1. O’Toole D, Delle Fave G, Jensen RT, Gastric and duodenal neuroendocrine tumours: Best Pract Res Clin Gastroenterol, 2012; 26; 719-35
2. Jensen RT, Rindi G, Arnold R, European Neuroendocrine Tumor, Well-differentiated duodenal tumor/carcinoma (excluding gastrinomas): Neuroendocrinology, 2006; 84; 165-72
3. Modlin IM, Lye KD, Kidd M, Carcinoid tumors of the stomach: Surg Oncol, 2003; 12; 153-72
4. Modlin IM, Lye KD, Kidd M, A 5-decade analysis of 13,715 carcinoid tumors: Cancer, 2003; 97; 934-59
5. Fitzgerald TL, Dennis SO, Kachare SD, Increasing incidence of duodenal neuroendocrine tumors: Incidental discovery of indolent disease?: Surgery, 2015; 158; 466-71
6. Fraenkel M, Kim MK, Faggiano A, Epidemiology of gastroenteropancreatic neuroendocrine tumours: Best Pract Res Clin Gastroenterol, 2012; 26; 691-703
7. Scherübl H, Streller B, Stabenow R, Clinically detected gastroenteropancreatic neuroendocrine tumors are on the rise: Epidemiological changes in Germany: World J Gastroenterol, 2013; 19; 9012-19
8. Randle RW, Ahmed S, Newman NA, Clinical outcomes for neuroendocrine tumors of the duodenum and ampulla of Vater: A population-based study: J Gastrointest Surg, 2014; 18; 354-62
9. Makhlouf HR, Burke AP, Sobin LH, Carcinoid tumors of the ampulla of Vater: A comparison with duodenal carcinoid tumors: Cancer, 1999; 85; 1241-49
10. Untch BR, Bonner KP, Roggin KK, Pathologic grade and tumor size are associated with recurrence-free survival in patients with duodenal neuroendocrine tumors: J Gastrointest Surg, 2014; 18; 457-63
11. Bornstein-Quevedo L, Gamboa-Dominguez A, Carcinoid tumors of the duodenum and ampulla of vater: A clinicomorphologic, immunohistochemical, and cell kinetic comparison: Hum Pathol, 2001; 32; 1252-56
12. Shariat SF, Karakiewicz PI, Suardi N, Comparison of nomograms with other methods for predicting outcomes in prostate cancer: A critical analysis of the literature: Clin Cancer Res, 2008; 14; 4400-7
13. Wolbers M, Koller MT, Witteman JC, Prognostic models with competing risks: Methods and application to coronary risk prediction: Epidemiology, 2009; 20; 555-61
14. Vickers AJ, Cronin AM, Elkin EB, Extensions to decision curve analysis, a novel method for evaluating diagnostic tests, prediction models and molecular markers: BMC Med Inform Decis Mak, 2008; 8; 53
15. Kim SH, Park CH, Ki HS, Endoscopic treatment of duodenal neuroendocrine tumors: Clin Endosc, 2013; 46; 656-61
16. Goldzweig G, Andritsch E, Hubert A, Psychological distress among male patients and male spouses: What do oncologists need to know?: Ann Oncol, 2010; 21; 877-83
17. Nipp RD, El-Jawahri A, Fishbein JN, The relationship between coping strategies, quality of life, and mood in patients with incurable cancer: Cancer, 2016; 122; 2110-16
18. Miller GE, Cohen S, Ritchey AK, Chronic psychological stress and the regulation of pro-inflammatory cytokines: A glucocorticoid-resistance model: Health Psychol, 2002; 21; 531-41
19. Woods LM, Rachet B, Coleman MP, Origins of socio-economic inequalities in cancer survival: A review: Ann Oncol, 2006; 17; 5-19
20. Gamboa AC, Liu Y, Lee RM, Duodenal neuroendocrine tumors: Somewhere between the pancreas and small bowel: J Surg Oncol, 2019; 120; 1293-301
21. Strosberg J, Nasir A, Coppola D, Correlation between grade and prognosis in metastatic gastroenteropancreatic neuroendocrine tumors: Hum Pathol, 2009; 40; 1262-68
22. Lin Z, Wang H, Zhang Y, Development and validation of a prognostic nomogram to guide decision-making for high-grade digestive neuroendocrine neoplasms: Oncologist, 2019 [Epub ahead of print]
23. Martin-Perez E, Capdevila J, Castellano D, Prognostic factors and long-term outcome of pancreatic neuroendocrine neoplasms: Ki-67 index shows a greater impacton survival than disease stage. The large experience of the Spanish National Tumor Registry (RGETNE): Neuroendocrinology, 2013; 98; 156-68
24. Panzuto F, Merola E, Rinzivill M, Advanced digestive neuroendocrine tumors: Metastatic pattern is an independent factor affecting clinical outcome: Pancreas, 2014; 43; 212-18
25. Khan MS, Luong TV, Watkins J, A comparison of Ki-67 and mitotic count as prognostic markers for metastatic pancreatic and midgut neuroendocrine neoplasms: Br J Cancer, 2013; 108; 1838-45
26. Zimmermann ME, Bosman FT, Proliferative activity of well differentiated neuroendocrine tumours of the gut: Histol Histopathol, 2003; 18; 353-58
27. Hochwald SN, Zee S, Conlon KC, Prognostic factors in pancreatic endocrine neoplasms: An analysis of 136 cases with a proposal for low-grade and intermediate-grade groups: J Clin Oncol, 2002; 20; 2633-42
28. Zhang XF, Wu XN, Tsilimigras DI, Duodenal neuroendocrine tumors: Impact of tumor size and total number of lymph nodes examined: J Surg Oncol, 2019; 120; 1302-10
29. Massironi S, Campana D, Partelli S, Heterogeneity of duodenal neuroendocrine tumors: An Italian multi-center experience: Ann Surg Oncol, 2018; 25; 3200-6
30. Vanoli A, La Rosa S, Klersy C, Four neuroendocrine tumor types and neuroendocrine carcinoma of the duodenum: Analysis of 203 cases: Neuroendocrinology, 2017; 104; 112-25
Figures
 Figure 1. Flowchart of the enrolled patients according to inclusion and exclusion criteria.
Figure 1. Flowchart of the enrolled patients according to inclusion and exclusion criteria. Figure 2. Kaplan-Meier analysis of overall survival in primary duodenal neuroendocrine neoplasm patients stratified by (A) race, (B) age of diagnosis, (C) tumor grade, (D) M stage, (E) marital status, (F) N stage, (G) sex, (H) tumor stage, (I) surgery, (J) T stage, (K) tumor size, and (L) year of diagnosis.
Figure 2. Kaplan-Meier analysis of overall survival in primary duodenal neuroendocrine neoplasm patients stratified by (A) race, (B) age of diagnosis, (C) tumor grade, (D) M stage, (E) marital status, (F) N stage, (G) sex, (H) tumor stage, (I) surgery, (J) T stage, (K) tumor size, and (L) year of diagnosis. Figure 3. Kaplan-Meier analysis of cancer-specific survival in primary duodenal neuroendocrine neoplasms patients stratified by (A) race, (B) age of diagnosis, (C) tumor grade, (D) M stage, (E) marital status, (F) N stage, (G) sex, (H) tumor stage, (I) surgery, (J) T stage, (K) tumor size, and (L) year of diagnosis.
Figure 3. Kaplan-Meier analysis of cancer-specific survival in primary duodenal neuroendocrine neoplasms patients stratified by (A) race, (B) age of diagnosis, (C) tumor grade, (D) M stage, (E) marital status, (F) N stage, (G) sex, (H) tumor stage, (I) surgery, (J) T stage, (K) tumor size, and (L) year of diagnosis. Figure 4. Nomograms predicting 1-, 5-, and 10-year overall survival (A) and cancer-specific survival (B) of patients with PGINHL.
Figure 4. Nomograms predicting 1-, 5-, and 10-year overall survival (A) and cancer-specific survival (B) of patients with PGINHL. Figure 5. Calibration plots for predicting patient overall survival (A–C) and cancer-specific survival (D–F) at 1, 5, and 10 years.
Figure 5. Calibration plots for predicting patient overall survival (A–C) and cancer-specific survival (D–F) at 1, 5, and 10 years. Figure 6. ROC curves of the nomograms and AJCC 7th staging system for 1-, 5-, and 10-year overall survival and cancer-specific survival prediction in the training cohort (A–D), validation cohort (E–H) and whole cohort (I–L).
Figure 6. ROC curves of the nomograms and AJCC 7th staging system for 1-, 5-, and 10-year overall survival and cancer-specific survival prediction in the training cohort (A–D), validation cohort (E–H) and whole cohort (I–L). Figure 7. Decision curve analysis of the nomograms and AJCC 7th staging system for predicting overall survival (A) and cancer-specific survival (B) in patients with primary duodenal neuroendocrine neoplasms.
Figure 7. Decision curve analysis of the nomograms and AJCC 7th staging system for predicting overall survival (A) and cancer-specific survival (B) in patients with primary duodenal neuroendocrine neoplasms. Figure 8. (A–T) Kaplan-Meier analysis of overall survival in primary duodenal neuroendocrine neoplasms patients with or without surgery.
Figure 8. (A–T) Kaplan-Meier analysis of overall survival in primary duodenal neuroendocrine neoplasms patients with or without surgery. Figure 9. (A–T) Kaplan-Meier analysis of cancer-specific survival in primary duodenal neuroendocrine neoplasms patients with or without surgery.
Figure 9. (A–T) Kaplan-Meier analysis of cancer-specific survival in primary duodenal neuroendocrine neoplasms patients with or without surgery. Tables
 Table 1. Clinical features and demographics of patients with D-NENs.
Table 1. Clinical features and demographics of patients with D-NENs. Table 2. Univariate and multivariate analyses of OS in patients with D-NENs.
Table 2. Univariate and multivariate analyses of OS in patients with D-NENs. Table 3. Univariate and multivariate analyses of CSS in patients with D-NENs.
Table 3. Univariate and multivariate analyses of CSS in patients with D-NENs. Table 4. The C-index for nomograms and the AJCC 7th staging system in patients with D-NENs.
Table 4. The C-index for nomograms and the AJCC 7th staging system in patients with D-NENs. Table 1. Clinical features and demographics of patients with D-NENs.
Table 1. Clinical features and demographics of patients with D-NENs. Table 2. Univariate and multivariate analyses of OS in patients with D-NENs.
Table 2. Univariate and multivariate analyses of OS in patients with D-NENs. Table 3. Univariate and multivariate analyses of CSS in patients with D-NENs.
Table 3. Univariate and multivariate analyses of CSS in patients with D-NENs. Table 4. The C-index for nomograms and the AJCC 7th staging system in patients with D-NENs.
Table 4. The C-index for nomograms and the AJCC 7th staging system in patients with D-NENs. In Press
21 Mar 2024 : Meta-Analysis
Economic Evaluation of COVID-19 Screening Tests and Surveillance Strategies in Low-Income, Middle-Income, a...Med Sci Monit In Press; DOI: 10.12659/MSM.943863
10 Apr 2024 : Clinical Research
Predicting Acute Cardiovascular Complications in COVID-19: Insights from a Specialized Cardiac Referral Dep...Med Sci Monit In Press; DOI: 10.12659/MSM.942612
06 Mar 2024 : Clinical Research
Enhanced Surgical Outcomes of Popliteal Cyst Excision: A Retrospective Study Comparing Arthroscopic Debride...Med Sci Monit In Press; DOI: 10.12659/MSM.941102
06 Mar 2024 : Clinical Research
Prevalence and Variation of Medical Comorbidities in Oral Surgery Patients: A Retrospective Study at Jazan ...Med Sci Monit In Press; DOI: 10.12659/MSM.943884
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








