10 August 2020: Lab/In Vitro Research
Preparation and Characterization of a Novel Triple Composite Scaffold Containing Silk Fiborin, Chitosan, and Alginate for 3D Culture of Colonic Carcinoma Cells
Xianhao Su1ACE, Liang Chen1AD, Shanliang Han1B, Gengming Niu1F, Jun Ren1C, Chongwei Ke1AG*DOI: 10.12659/MSM.922935
Med Sci Monit 2020; 26:e922935
Abstract
BACKGROUND: Three-dimensional (3D) cell-culture scaffolds are ideal in vitro models to bridge the gap between two-dimensional cell culture in vitro and in vivo cancer models. Construction of 3D scaffolds using two kinds of biomaterials has been reported, but there are still many defects. To improve the performance of the scaffolds for 3D cell culture of colonic carcinoma (CC) cells in vitro, we attempted to construct triple composite scaffolds using silk fibroin (SF), chitosan (Cs), and alginate (Alg).
MATERIAL AND METHODS: We explored the suitability of triple composite scaffolds of SF/Cs/Alg at ratios of 1: 1: 0.5, 1: 1: 1, and 1: 1: 2 for 3D culture of CC cells, and used the dual composite scaffold of SF/Cs (1: 1) as a control group. We analyzed the physicochemical characteristics of these scaffolds and studied cell adhesion, cell proliferation, migration, colony-forming ability, microstructure and ultrastructure, and spheroid-forming capacity of the commercially available CC cell line HCT-116 on the prepared scaffolds.
RESULTS: Our results show that SF/Cs/Alg (1: 1: 1) scaffolds demonstrated the best profile, the highest uniform porosity and connectivity, and excellent hydroscopicity, and also exhibited appropriate and controlled swelling and degradation characteristics. The adhesion, proliferation, colony-forming, and wound-healing assays, green fluorescent protein-labeled HCT116 cell imaging, 4’,6-diamidino-2-phenylindole and DY-554-phalloidin staining, scanning electron microscopy, and haematoxylin and eosin staining revealed that the triple composite scaffolds of SF/CS/Alg (1: 1: 1) supported cell adhesion, proliferation, migration, colony-forming ability, and spheroid formation far better than the dual composite scaffold of SF/CS (1: 1).
CONCLUSIONS: This study successfully demonstrated the potential of SF/Cs/Alg (1: 1: 1) scaffold as an alternative for the 3D in vitro culture of CC cells.
Keywords: Alginates, Chitosan, Colorectal Neoplasms, Hereditary Nonpolyposis, Fibroins, Tissue Scaffolds, Cell Adhesion, Colonic Neoplasms, HCT116 Cells
Background
Colonic carcinoma (CC) is the world’s second-deadliest malignancy after lung cancer, with almost 881 000 deaths every year [1]. The disease can be considered a marker of socioeconomic development, as the highest rates of incidence are in developed countries [2]. As more people become richer and adopt the Western diet and lifestyle, the incidence of CC is likely to increase. The classical two-dimensional (2D) cell culture provides researchers a convenient
The scientific literature has demonstrated that three-dimensional (3D) cell culture systems are ideal
To overcome the shortcomings of the scaffolds made exclusively from a single polymer, researchers have been exploring a composite system that includes more than one polymer. Multiple polymers in combination are expected to impart their individual properties and result in a scaffold that might facilitate cell adhesion, proliferation, and differentiation [16]. To date, SF/Cs (1: 1) scaffolds have been studied in sciatic nerve gap repair [17], cartilage repair [18], and reconstruction of bone defects [19]. Further, Cs/Alg scaffolds have been shown to provide an ideal growth environment for cartilage and bone regeneration [20] and stem cell renewal [21]. There are few reports on triple composite scaffolds in the literature, especially those using SF, Cs, and Alg as raw materials.
In this paper, a novel study on
Material and Methods
MATERIAL AND ANIMALS:
Cocoons of
SCAFFOLD SYNTHESIS AND BLOCK DESIGN:
The scaffolds for the experiments were produced via the freeze-drying technique and chemical cross-linking method reported previously [10]. Briefly, SF scaffolds were produced with 3% SF solution alone, Cs scaffolds were fabricated with 3% Cs solution alone, and Alg scaffolds were constructed with a pure 3% Alg solution. SF/Cs (1: 1) scaffolds were synthesized with a 1: 1 ratio (w/w) of SF solution and Cs solution. SF/Cs/Alg scaffolds were produced with different ratios of 1: 1: 0.5, 1: 1: 1, and 1: 1: 2 (w/w). Subsequently, 95% aqueous ethanol solution containing 50 mmol/L EDC and 18 mmol/L NHS were added to the mixtures with continuous magnetic stirring for 15 min to obtain a homogeneous solution. Next, the solution was added to wells of 24- and 96-well plates and left to cross-link at 4°C for 24 h. Subsequently, the samples were frozen at −20°C for 12 h before being placed in a −80°C freezer for another 12 h. The samples were maintained in a freeze-drying machine for 24 h to produce the scaffolds. Before initiating the cell culture, the scaffolds were sterilized with Cobalt 60 radiation for 72 h.
MACROSCOPIC APPEARANCE:
The 3D scaffolds were removed from the matrix (24-well plate) and compared using anterior-, posterior-, and lateral-view photography.
INTERNAL MORPHOLOGY:
The scaffolds were removed from the 24-well plates. The thin membrane with cross-sectional structure of the scaffold was left at the bottom of the well, and the structure of the thin membrane was viewed with an inverted microscope and photographed. Scanning electron microscopy (SEM, Hitachi, Washington, D.C.; S-3400N, serial no. 341352-08, accelerating voltage 10 000 V, working distance=6600 μm) was used to detect the surface morphology of the scaffold prepared. We cut the scaffold into thin slices with a surgical blade, stuck the slices on the sample table with conductive adhesive, used gold plate under vacuum for 20 s, observed the internal structure of the gold coating the scaffold and pore size distribution under SEM, and took pictures (5 images for each group, 100 pores per image for pore size measurement).
POROSITY EVALUATION: As previously reported, the porosity of the scaffolds was measured by the liquid-displacement method described previously [22]. Briefly, the scaffolds were immersed in absolute ethanol until they were saturated, and they were weighed before and after immersion in alcohol. The porosity was calculated using the equation:
where
WATER ABSORPTION RATE:
Water absorption rate (%) was calculated as per the equation:
where
SWELLING RATE:
According to the change of the volume of the scaffolds in phosphate-buffered saline (PBS), the swelling rate (%) was obtained. The swelling rate was calculated as per the equation:
where
DEGRADATION PROPERTY:
The degradation ratio (%) was calculated as per the equation:
where
CELL INCORPORATION INTO SCAFFOLDS:
Scaffolds were dampened with culture media and a total of 50 000cells in 50 μL of complete media was seeded onto them. The scaffolds were placed in 24-well plates and shaken on a vibrator for 5 min to settle the cells on the scaffold. Cells were allowed to infiltrate the scaffold in an incubator for 1 h, followed by the addition of 1 mL of fully supplemented media to each well. For samples precultured on 2D matrix, 50 000 cells in 1 mL of fully supplemented media were added to 24-well plate wells. The culture medium was replaced every other day.
CELL ADHESION IN SCAFFOLDS:
Adhesion rate (%) was calculated as per the formula:
Briefly, HCT-116 cells were detached, centrifuged, counted, and prepared into a final cell suspension of 2×105 cells/mL per group (
CELL PROLIFERATION IN SCAFFOLD AND THE EXTRACTING LIQUID OF SCAFFOLD:
The proliferation of cells cultured on a 2D plate, SF/Cs (1: 1) scaffolds, and SF/Cs/Alg (1: 1: 1) scaffolds was determined using CCK-8. Briefly, 1 mL of cell suspension containing 5×104 cells was seeded on the prewetted scaffolds. Cell proliferation was checked on days 1, 3, and 5 following the manufacturer’s protocol. Briefly, we added 20 μL of CCK-8 reagent to each well in the dark and the plates were incubated for 3 h. Subsequently, the plates were agitated for 15 min before removing the scaffolds and shifted to a 96-well plate to measure the absorbance at 450 nm. For cell proliferation in the extracting liquid of the scaffold, the scaffolds were placed into Dulbecco’s modified Eagle medium (DMEM) complete culture medium and soaked for 3 d in a humidified incubator (37°C, 5% CO2) to prepare the extracting liquid of the scaffold that was used for cell culture. Cell proliferation in the extracting liquid of the scaffold was detected using the CCK-8 kit as per the manufacturer’s protocol.
COLONY-FORMATION ASSAY:
A total of 1500 cells was seeded per 60-mm Petri dish and incubated using the extracting liquid of the scaffold for 12 d. Subsequently, the colonies were stained with crystal violet and counted (>50 cells under a microscope was the criterion to define a colony).
WOUND-HEALING ASSAYS:
Approximately 5×104 cells/well were plated and incubated using the extracting liquid of the scaffold in a 6-well plate. After overnight incubation, wounds were created by scratching the monolayer surface using a sterile 10-μL pipette tip. Cells were cultured in different kinds of serum-free media (DMEM complete culture medium, the extracting liquid of the SF/Cs [1: 1] scaffold, and the extracting liquid of the SF/Cs/Alg scaffold). The closure and gaps were measured using Image J software and the plates were photographed using an inverted microscope (Nikon ECLIPSE Ts2R).
4′,6-DIAMIDINO-2-PHENYLINDOLE (DAPI) AND DY-554-PHALLOIDIN STAINING:
HCT-116 cells (5×105 cells) were cultured in the extracting liquid of a scaffold prepared in advance. For cytoskeleton staining, the cells were treated with 4% formaldehyde in PBS for 20 min, followed by dehydration with ethanol and permeabilization with 0.1% Triton X-100 for 5 min. The cytoskeleton was stained with 50 μg/mL DY-554-phalloidin–fluorescein isothiocyanate (FITC) solution for 30 min at room temperature and subsequently washed with PBS to remove unreacted DY-554-phalloidin conjugates. Nuclear staining was done with DAPI. The images were recorded under fluorescent microscope Leica DM2500.
SCANNING ELECTRON MICROSCOPY:
The culture medium was aspirated on days 3 and 7, and the scaffolds were rinsed with PBS three times. This was followed by the addition of 1 mL of 4% paraformaldehyde solution to fix the cells. Next, the plates were frozen at −20°C for 12 h before being placed in a −80°C freezer for 12 h. The plates were finally put into a freeze-drying machine for 48 h, and the morphology of cells on the scaffolds was imaged with a scanning electron microscope.
HEMATOXYLIN AND EOSIN (HE) STAINING:
HE staining was performed to observe the growth state of cells in the scaffold over time and compare the morphology of the subcutaneous tumorigenesis model of nude mice with the morphology of multicellular spheroids in the scaffold under light microscopy (oil immersion). Two weeks after the HCT116 cells were inoculated under the skin, the nude mice were sacrificed and the tumor was excised. On days 3 and 7, SF/Cs (1: 1) and SF/Cs/Alg (1: 1: 1) scaffolds and excised tumors containing HCT-116 cells were fixed using 10% paraformaldehyde, embedded in paraffin, and cut into sections of 3-μm thickness. The sections were stained with HE and imaged under the oil-immersion lens using a Leica DM2500 microscope.
STATISTICAL ANALYSIS:
Graph Pad Prism 8 software (Graph Pad, San Diego, CA) was used to perform all statistical analyses. All experiments were performed in triplicate. Quantitative data were presented as the mean±standard deviation. Statistical significance was determined by analysis of variance and Student’s
Results
Characterization of the 3D porous scaffolds
MACROSCOPIC APPEARANCE: The macroscopic appearance of SF, Cs, Alg, SF/Cs (1: 1), SF/Cs/Alg (1: 1: 0.5), SF/Cs/Alg (1: 1: 1), and SF/Cs/Alg (1: 1: 2) scaffolds is shown in Figure 3. We observe that the SF and Alg scaffolds are pure white in color, whereas other scaffolds are yellowish-white. The SF/Cs/Alg (1: 1: 1) scaffold is smoother and more symmetrical than other scaffolds.
INTERNAL STRUCTURE: We used an optical microscope and a scanning electron microscope to elucidate the internal structure of the scaffolds. As shown in Figure 4, all scaffolds have structures with porous network featuring different pore size and homogeneity and good connectivity between the pores except for the SF and Alg scaffolds. The Cs alone, SF/Cs (1: 1), SF/Cs/Alg (1: 1: 0.5), SF/Cs/Alg (1: 1: 1), and SF/Cs/Alg (1: 1: 2) scaffolds have pores of different sizes and homogeneity. The SF/CS/Alg (1: 1: 1) scaffold has the largest pore size and good uniformity among all scaffolds (mean=400 μm, ranking first; coefficient of variation=39%, ranking second.) (Table 1).
WATER ABSORPTION RATES:
The water absorption rates of the scaffolds are illustrated in Figure 5B. Water uptake rates of all the scaffolds are above 2413.99% in deionized water. The SF/Cs/Alg (1: 1: 2) scaffold has the highest water uptake rate (5261.70±69.95%), followed by the SF/Cs/Alg (1: 1: 1) scaffold (3872.42±53.39%). On the other hand, the SF/Cs (1: 1) scaffold has the lowest water uptake rate (2473.38±60.00%). Our results show that the difference in the water absorption rates between SF/Cs (1: 1) and SF/Cs/Alg (1: 1: 1) scaffolds was statistically significant (P<0.001).
SWELLING RATES:
The swelling rates of the samples are determined by placing them in buffers mimicking physiological fluids. Figure 5C describes the results from the swelling tests of the SF/Cs (1: 1), SF/Cs/Alg (1: 1: 0.5), SF/Cs/Alg (1: 1: 1), and SF/Cs/Alg (1: 1: 2) scaffolds at 37°C, where gels attained equilibrium swelling state at 24 h. We observe that the swelling rate of SF/Cs/Alg (1: 1: 2) scaffold is the highest among all the scaffolds, followed by SF/Cs/Alg (1: 1: 1), SF/Cs/Alg (1: 1: 0.5), and SF/Cs (1: 1). Our results show that the difference between the swelling rates of SF/Cs (1: 1) and SF/Cs/Alg (1: 1: 1) scaffolds was not statistically significant (P=0.071).
DEGRADATION RATES:
The degradation rates of the scaffolds immersed in the culture medium are shown in Figure 5D. The addition of Alg enhanced the degradation properties of scaffolds, and the degree of enhancement was positively correlated with the amount of Alg added. The SF/Cs/Alg (1: 1: 2) scaffold has the highest degradation rate, followed by that of SF/Cs/Alg (1: 1: 1) scaffold. The SF/Cs (1: 1) scaffold has minimum degradation properties. Statistical analysis shows that pairwise comparisons among all groups present statistical differences (P<0.05). All four groups of scaffolds have different degrees of degradation within 30 d, but the general trend is similar. The sixth day is the inflection point; the first 6 d is a rapid degradation period; after 6 d is a slow degradation period; day 18 to day 30 is the plateau period.
CELL ADHESION IN SCAFFOLDS: Analysis of cell adhesion on the scaffold was performed by cell counting using a cell counting board at 1, 3, and 6 h after cell inoculation on the prewetted scaffolds. The cell adhesion rates are presented in Figure 5E, showing that the SF/Cs/Alg (1: 1: 1) scaffold has the highest cell adhesion rate (79.96±1.77% at 1 h to 91.96±2.43% at 6 h), followed by that of the SF/Cs (1: 1) scaffold (66.46±2.51% at 1 h to 84.49±2.33% at 6 h). The SF/Cs/Alg (1: 1: 1) scaffold shows significantly higher cell adhesion (P=0.0016, 0.0010, and 0.0185 for 1, 3, and 6 h, respectively).
CELL PROLIFERATION IN SCAFFOLD AND THE EXTRACTING LIQUID OF SCAFFOLD: We used a commercially available CCK-8 kit to measure cell proliferation on the scaffolds (Figure 5F) and the extracting liquid of the scaffold (Figure 5G). Our results show that the highest number of cells proliferated on the SF/Cs/Alg (1: 1: 1) scaffold at all time points. From the statistical results we know that the cell proliferation shows no significant difference between the two scaffolds on day 1 (P=0.143), whereas significantly higher cell proliferation is observed on the triple scaffold on day 3 (P=0.0075) and day 5 (P=0.0044). Results from cell proliferation in the extracting liquid of the scaffold show that the triple scaffold registered a higher cell proliferation (P=0.0032 at day 1, 0.0003 at day 3, and 0.0012 at day 5).
COLONY-FORMING ASSAY AND WOUND-HEALING ASSAYS OF CELLS IN THE EXTRACTING LIQUID OF SCAFFOLD: To investigate whether the extracting liquid of the scaffold facilitated the migration, population dependence, and proliferation of HCT-116 cells, we performed a wound-healing assay (Figure 6A1–A3, B1–B3, C1–C3) and colony-forming assay (Figure 6D1–D3). We observed that the cell mobility is significantly increased in the extracting liquid of the scaffold compared with the control (Figure 6E, 6F). At 96 h, the wound-healing percentage of the SF/Cs/Alg (1: 1: 1) group (46.44±1.17%) is greater than that of the SF/Cs (1: 1) group (63.60±1.15%) (P=0.00005). At 192 h, the wound-healing percentage of SF/Cs/Alg (1: 1: 1) (69.42±1.13%) is greater than that of SF/Cs (1: 1) (78.75±1.29%) (P=0.0007). Similarly, the results from the colony-forming assay demonstrated considerable augmentation in colony-forming efficiency in the extracting liquid of the scaffold when compared with the control (Figure 6G). The cloning efficiency of SF/Cs/Alg (1: 1: 1) is greater than that of SF/Cs (1: 1) (P=0.005).
DAPI AND DY-554-PHALLOIDIN STAINING:
We used DAPI to stain the cell nuclei to confirm cell division, and it is evident that there is statistically significant difference in the number of cells per field among the three groups (2D, SF/Cs [1: 1], SF/Cs/Alg [1: 1: 1]) at all time points (P<0.001) (Figure 8A1, A2; B1, B2; C1, C2). SF/Cs/Alg (1: 1: 1) has the highest cell proliferation capacity.
The cytoskeleton plays an important role in maintaining cell morphology, movement, and cell division. To observe the organization of the cytoskeleton, we used DY-554-phalloidin-FITC to stain actin filaments; the optical microscope Leica DM2500 was used to image the cells. Our results show that cells in the 2D group are rich in actin, with a decreasing and regular organization from the nuclear periphery to the membrane. However, our findings are consistent with an earlier report that shows that after treatment with the extracting liquid of the scaffold, actin organization changed dramatically, with an abundant but disordered local concentration in the cytoplasm, a contractile ring around the nucleus, and a contraction ring at the location of the cell mitosis groove. Consistent with these findings, we also observe that the cells grown in 2D culture are mainly long and spindle shaped, whereas the cells grown on the scaffold of SF/Cs (1: 1) and SF/Cs/Alg (1: 1: 1) are mainly round, indicating cells undergoing cell division. More cells in the dividing phase are observed in the extracting liquid of the SF/Cs/Alg (1: 1: 1) scaffold (Figure 8D1, D2; E1, E2; F1, F2).
SCANNING ELECTRON MICROSCOPE:
The SEM images showing the morphology of the cells on the scaffolds are presented in Figure 9. We observe that the diameter of the channel in SF/Cs/Alg (1: 1: 1) is larger than that in SF/Cs (1: 1), which provides a broader space for cell division and proliferation. More cells proliferated on the Alg-containing scaffolds; the SF/Cs/Alg (1: 1: 1) scaffold supported a greater cell proliferation under the same conditions than the SF/Cs (1: 1) scaffold. The cellular morphology in the 3D scaffolds (SF/Cs/Alg [1: 1: 1] and SF/Cs [1: 1]) is quite different from that in the common culture dish. In the 3D scaffold, the cells do not adhere to the scaffold completely and maintained their shape as in vivo. Moreover, though the cells grew in number over time, they did not spread over the internal surface of scaffolds. It is clear from these results that the 3D scaffolds provide a better culture system than a regular culture dish. We also observed that more cells proliferated on the Alg-containing scaffolds; the SF/Cs/Alg (1: 1: 1) scaffold supported a greater cell proliferation under the same conditions.
HE STAINING:
The images of HE-stained scaffolds are shown in Figure 10. Consistent with our SEM results, these results show that the diameter of the channel in SF/Cs/Alg (1: 1: 1) is larger than that in SF/Cs (1: 1), which provides a broader space for cell division and proliferation. More cells proliferated on the Alg-containing scaffolds; the SF/Cs/Alg (1: 1: 1) scaffold supported a greater cell proliferation under the same conditions than the SF/Cs (1: 1) scaffold. However, the cells on the scaffold of SF/Cs (1: 1) and SF/Cs/Alg (1: 1: 1) tend to be spherical and cling to each other rather spreading out, which is more obvious in the SF/Cs/Alg (1: 1: 1) group than in the SF/Cs (1: 1) group. The multicellular globules formed on the scaffold are morphologically similar to the subcutaneous tumor seen in nude mice. In tumors and multicellular spheroids, cells maintain close contact with each other.
Discussion
The classical 2D cell cultures provide researchers a convenient
SF, Cs, and Alg are thought to interact with each other via OH− and COO− groups, resulting in the formation of hydrogen bonds and electrostatic interactions that improve the tensile strength of the polymers made from them. The addition of Alg to the SF/CS (1: 1) blend scaffold enhanced its overall porosity and average pore diameter. Adequate pore size and porosity provide physical space, improved oxygen and nutrient availability to cells, and facilitate efficient metabolic waste removal, thereby remarkably influencing attachment, proliferation, migration, distribution, differentiation, mitosis, and metabolic activity of the cells [25]. An optimal scaffold pore size has been reported to depend on the specific cell type [26]. In our study, the SF/Cs/Alg (1: 1: 1) scaffold with the biggest average pore size (~400 μm) is best suited for HCT-116 cell culture and growth. Scaffolds with high porosity facilitate enhanced cellular infiltration and mechanical interlocking [27]. In our study, SF/Cs/Alg (1: 1: 1) scaffold has the highest porosity (93.73±1.12%) and supported the maximum cell growth. A higher water uptake ratio is preferable for the cells to infiltrate the scaffold. A lower swelling ratio helps the scaffold to maintain its structural stability. In our case, the increase in the proportion of Alg polymer led to a gradual increase in the water uptake ratio, swelling ratio, and degradation rate. Our results show that the SF/Cs/Alg (1: 1: 1) scaffold has a high water absorption rate (3872.42±53.39%), low swelling rate (60.18±3.05%), and appropriate degradation rate.
Our results from the characterization of these scaffolds show that the highest cell adhesion and proliferation rates are observed for cells on SF/Cs/Alg (1: 1: 1), and these results are supported by the cell growth and morphological features shown by SEM. The combined results from the biological characterization indicated that the SF/Cs/Alg (1: 1: 1) scaffold may be the best one for
We observe that, unlike the 2D monolayer model, HCT-116 cells implanted on 3D scaffolds tend to aggregate into multicellular spheroids. These 3D multicellular aggregates (spheroids) recapitulate the natural tumor microenvironment and thus have emerged as an important alternative for cancer research [34]. Our findings show that the SF/Cs/Alg (1: 1: 1) scaffold supported the growth of larger spheroids than that seen in SF/Cs (1: 1). There is increasing evidence that multicellular structures respond to mechanical cues, such as the confinement and compression exerted by the surrounding environment. An earlier study compared the response of multicellular spheroids and individual cells to the same osmotic perturbation, and their results show a more significant decrease in the volume of multicellular spheroids than the individual cells when subjected to the same pressure [36]. In light of these results and on the basis of our observation, we opine that SF/Cs/Alg (1: 1: 1) provides a broader surrounding environment for HCT-116 cells to grow and thus facilitates the formation of larger spheroids. Many techniques have been reported to construct 3D spheroids, among which the formation of substrate-derived spheroids and the mechanism of their formation is unique [36]. Our results, on the basis of formation of biomaterial substrate-mediated multicellular spheroids, are a testimony to this observation.
Though our study provides some interesting insights into the development and potential of composite scaffolds synthesized from compatible biopolymers, many questions remain unanswered. For instance, the underlying mechanism of our observations remains unknown. Although our SF/Cs/Alg (1: 1: 1) scaffold shows the best results related to cell adhesion, proliferation, migration, and spheroid formation, it ranked second in water absorption and degradation rate. We could not draw a conclusion regarding the relationships among water absorption rate, degradation rate, and cell growth. Last but not least, although our results show the best results with the SF/Cs/Alg (1: 1: 1) scaffold, which has an average pore size of 400 μm, we are unable to draw a conclusion about the optimal pore size. This warrants further research to elucidate the underlying mechanism of these scaffolds supporting cell growth and proliferation, the best pore size of composite scaffolds to support the same, and to validate these findings before these 3D models can be optimized and made available to clinical and basic science researchers worldwide.
Conclusions
Our comparative study of HCT-116 cell culture using three different scaffolds (2D, 3D SF/Cs [1: 1] scaffold, and 3D SF/Cs/Alg [1: 1: 1] triple scaffold) show that the biological behavior of CC cells varied with the types of materials used. Our results show that the SF/Cs/Alg (1: 1: 1) scaffold holds potential for developing into a promising experimental model for CC cell culture experiments and mimicking the 3D cell growth environment
Figures
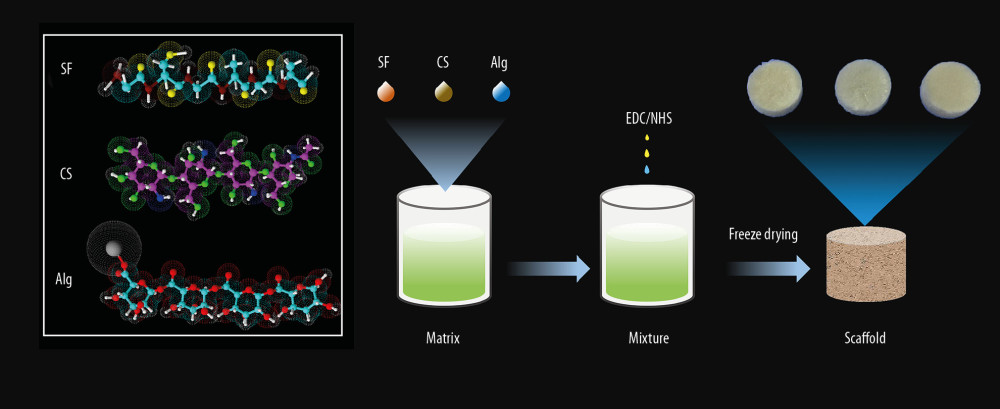 Figure 1. Schematic illustration of constructing a porous scaffold based on silk fibroin, chitosan, and alginate via freeze-drying technique and chemical cross-linking method.
Figure 1. Schematic illustration of constructing a porous scaffold based on silk fibroin, chitosan, and alginate via freeze-drying technique and chemical cross-linking method. 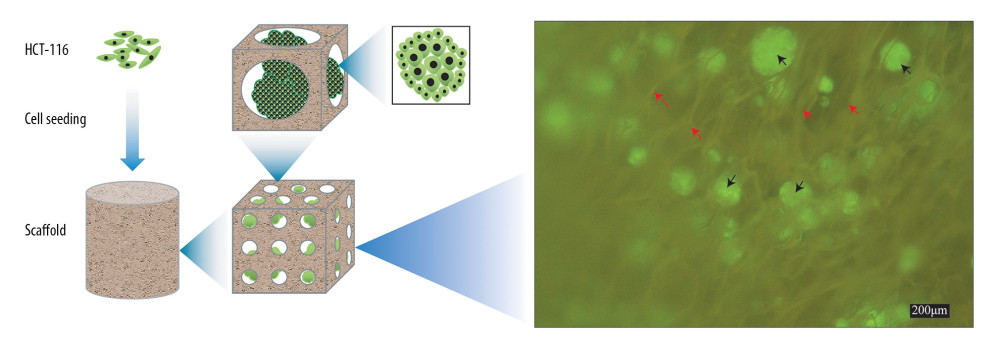 Figure 2. Schematic illustration of cells seeded on the scaffold to form biomaterial substrate-mediated multicellular spheroids. Red arrows represent the scaffold. Black arrows indicate the multicellular spheroids.
Figure 2. Schematic illustration of cells seeded on the scaffold to form biomaterial substrate-mediated multicellular spheroids. Red arrows represent the scaffold. Black arrows indicate the multicellular spheroids. 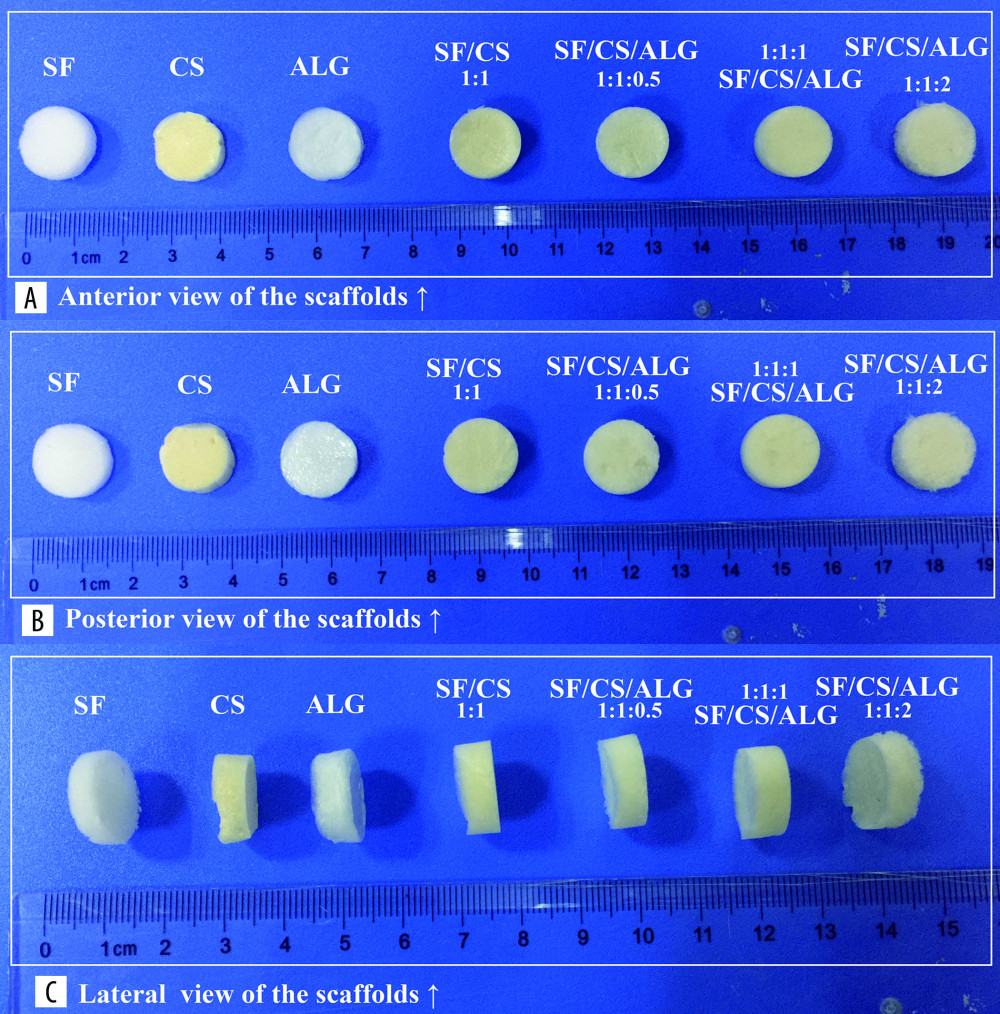 Figure 3. (A–C) Macroscopic appearance of silk fibroin (SF), chitosan (Cs), alginate (Alg), SF/Cs (1: 1), SF/Cs/Alg (1: 1: 0.5), SF/Cs/Alg (1: 1: 1), and SF/Cs/Alg (1: 1: 2) scaffolds is shown.
Figure 3. (A–C) Macroscopic appearance of silk fibroin (SF), chitosan (Cs), alginate (Alg), SF/Cs (1: 1), SF/Cs/Alg (1: 1: 0.5), SF/Cs/Alg (1: 1: 1), and SF/Cs/Alg (1: 1: 2) scaffolds is shown. 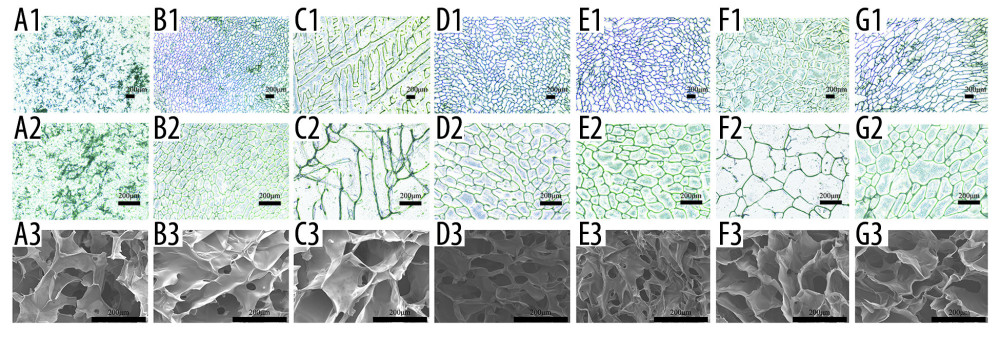 Figure 4. Optical microscope photographs (A1–G1, A2–G2) and scanning electron microscopy images (A3–G3) of the scaffolds made from silk fibroin (SF; A1–A3), chitosan (Cs; B1–B3), alginate (Alg; C1–C3), SF/Cs (1: 1) (D1–D3), SF/Cs/Alg (1: 1: 0.5) (E1–E3), SF/Cs/Alg (1: 1: 1) (F1–F3), and SF/Cs/Alg (1: 1: 2) (G1–G3), are presented.
Figure 4. Optical microscope photographs (A1–G1, A2–G2) and scanning electron microscopy images (A3–G3) of the scaffolds made from silk fibroin (SF; A1–A3), chitosan (Cs; B1–B3), alginate (Alg; C1–C3), SF/Cs (1: 1) (D1–D3), SF/Cs/Alg (1: 1: 0.5) (E1–E3), SF/Cs/Alg (1: 1: 1) (F1–F3), and SF/Cs/Alg (1: 1: 2) (G1–G3), are presented. 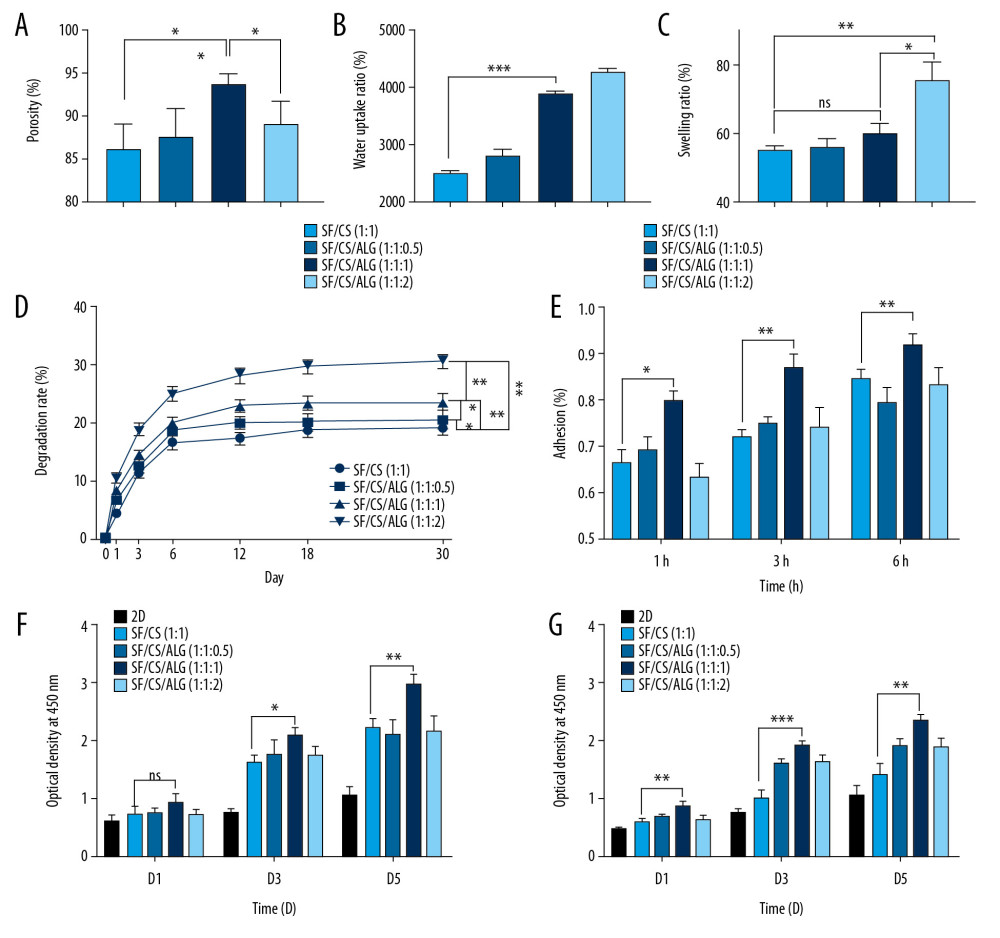 Figure 5. Results of porosity (A), water uptake rate (B), swelling rate (C) and degradation rate (D) of the scaffolds, and cell adhesion (E) and cell proliferation (F, G).
Figure 5. Results of porosity (A), water uptake rate (B), swelling rate (C) and degradation rate (D) of the scaffolds, and cell adhesion (E) and cell proliferation (F, G). 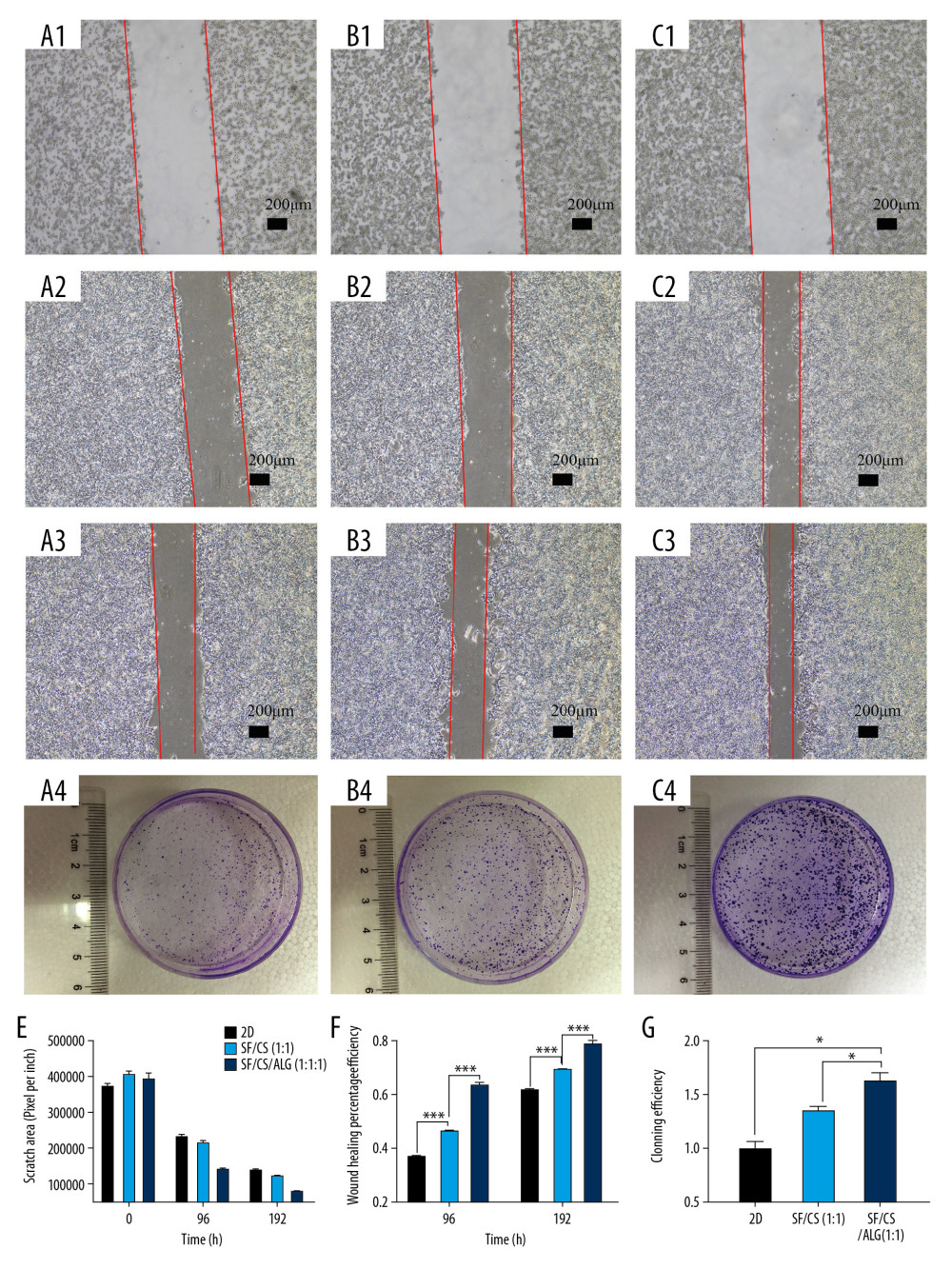 Figure 6. Results of wound-healing assays (A1–A3, B1–B3, C1–C3, E, F) and colony-forming assay (D1–D3, G) of cells in the extracting liquid of the scaffold are represented.
Figure 6. Results of wound-healing assays (A1–A3, B1–B3, C1–C3, E, F) and colony-forming assay (D1–D3, G) of cells in the extracting liquid of the scaffold are represented. 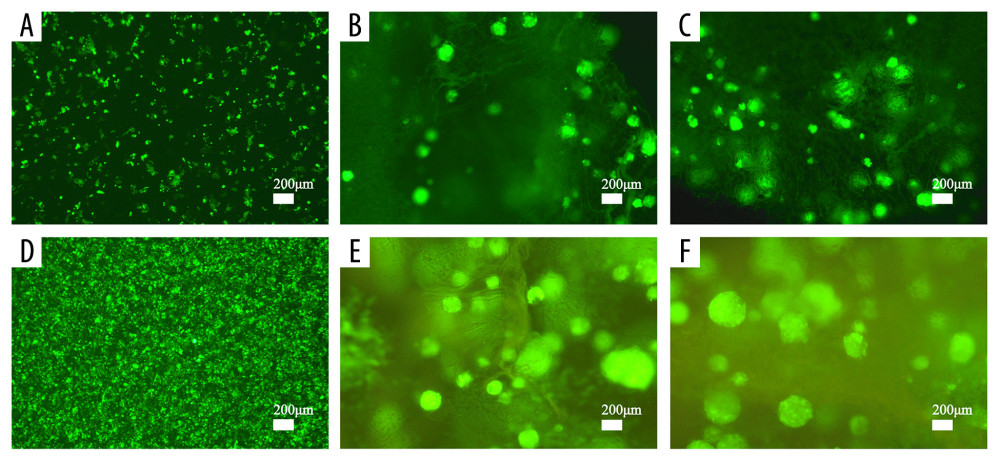 Figure 7. Cell proliferation analyses are accomplished by green fluorescent protein (GFP)-labeled HCT116 cell imaging. HCT-116 cells transfected with pCDH-CMV-MCS-EF1-copGFP-Puro lentivirus (5×109 transduction units/mL) were cultured in the two-dimensional (2D) plate, silk fibroin (SF)/chitosan (Cs) (1: 1) scaffolds, and SF/Cs/alginate (Alg) (1: 1: 1) scaffolds. The scaffolds and the cells are observed under Nikon ECLIPSE Ts2R microscope and images are taken on day 3 (A–C) and day 7 (D–F). 2D group (A, D), SF/Cs (1: 1) group (B, E), SF/Cs/Alg (1: 1: 1) group (C, F).
Figure 7. Cell proliferation analyses are accomplished by green fluorescent protein (GFP)-labeled HCT116 cell imaging. HCT-116 cells transfected with pCDH-CMV-MCS-EF1-copGFP-Puro lentivirus (5×109 transduction units/mL) were cultured in the two-dimensional (2D) plate, silk fibroin (SF)/chitosan (Cs) (1: 1) scaffolds, and SF/Cs/alginate (Alg) (1: 1: 1) scaffolds. The scaffolds and the cells are observed under Nikon ECLIPSE Ts2R microscope and images are taken on day 3 (A–C) and day 7 (D–F). 2D group (A, D), SF/Cs (1: 1) group (B, E), SF/Cs/Alg (1: 1: 1) group (C, F). 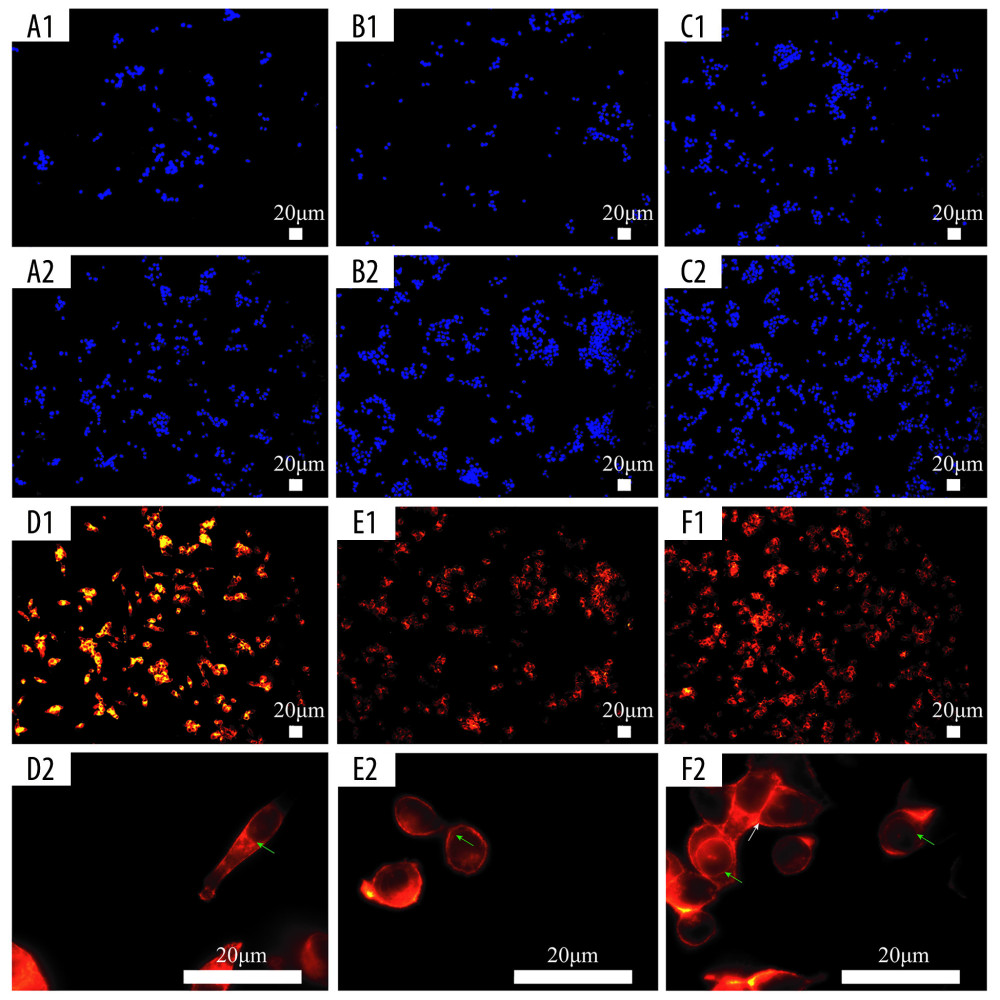 Figure 8. Fluorescence staining of actin in HCT-116 cells cultured in the extracting liquid of scaffold. F-Actin is marked with fluorescein isothiocyanate (FITC)-DY-554-phalloidin (red). Nuclear counterstain with 4′,6-diamidino-2-phenylindole (DAPI; blue). Two-dimensional (2D) culture system on day 3 (A1), silk fibroin (SF)/chitosan (Cs) (1: 1) scaffold on day 3 (B1), SF/Cs/alginate (Alg) (1: 1: 1) scaffold on day 3 (C1), 2D culture system on day 7 (A2, D1, D2), SF/Cs (1: 1) scaffold on day 7 (B2, E1, E2), SF/Cs/Alg (1: 1: 1) scaffold on day 7 (C2, F1, F2). The green arrow indicates the contractile ring around the nucleus, and the white arrow indicates the contraction ring at the location of the cell mitosis groove.
Figure 8. Fluorescence staining of actin in HCT-116 cells cultured in the extracting liquid of scaffold. F-Actin is marked with fluorescein isothiocyanate (FITC)-DY-554-phalloidin (red). Nuclear counterstain with 4′,6-diamidino-2-phenylindole (DAPI; blue). Two-dimensional (2D) culture system on day 3 (A1), silk fibroin (SF)/chitosan (Cs) (1: 1) scaffold on day 3 (B1), SF/Cs/alginate (Alg) (1: 1: 1) scaffold on day 3 (C1), 2D culture system on day 7 (A2, D1, D2), SF/Cs (1: 1) scaffold on day 7 (B2, E1, E2), SF/Cs/Alg (1: 1: 1) scaffold on day 7 (C2, F1, F2). The green arrow indicates the contractile ring around the nucleus, and the white arrow indicates the contraction ring at the location of the cell mitosis groove. 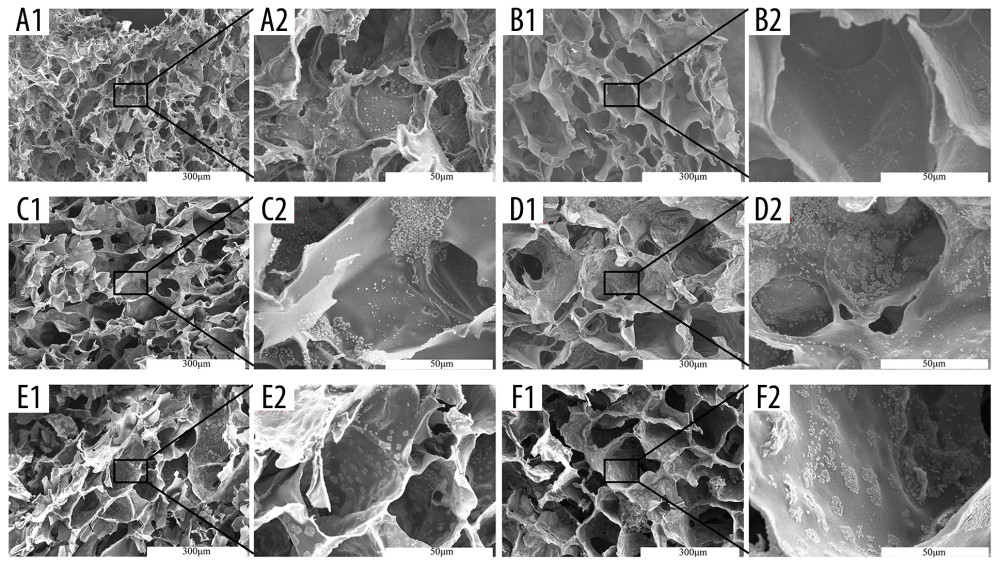 Figure 9. Scanning electron microscopy images of cell morphology on the scaffolds. Silk fibroin (SF)/chitosan (Cs) (1: 1) scaffold on day 1 (A1, A2), SF/Cs (1: 1) scaffold on day 3 (C1, C2), SF/Cs (1: 1) scaffold on day 7 (E1, E2), SF/Cs/alginate (Alg) (1: 1: 1) scaffold on day 1 (B1, B2), SF/Cs/Alg (1: 1: 1) scaffold on day 3 (D1, D2), SF/Cs/Alg (1: 1: 1) scaffold on day 7 (F1, F2).
Figure 9. Scanning electron microscopy images of cell morphology on the scaffolds. Silk fibroin (SF)/chitosan (Cs) (1: 1) scaffold on day 1 (A1, A2), SF/Cs (1: 1) scaffold on day 3 (C1, C2), SF/Cs (1: 1) scaffold on day 7 (E1, E2), SF/Cs/alginate (Alg) (1: 1: 1) scaffold on day 1 (B1, B2), SF/Cs/Alg (1: 1: 1) scaffold on day 3 (D1, D2), SF/Cs/Alg (1: 1: 1) scaffold on day 7 (F1, F2). 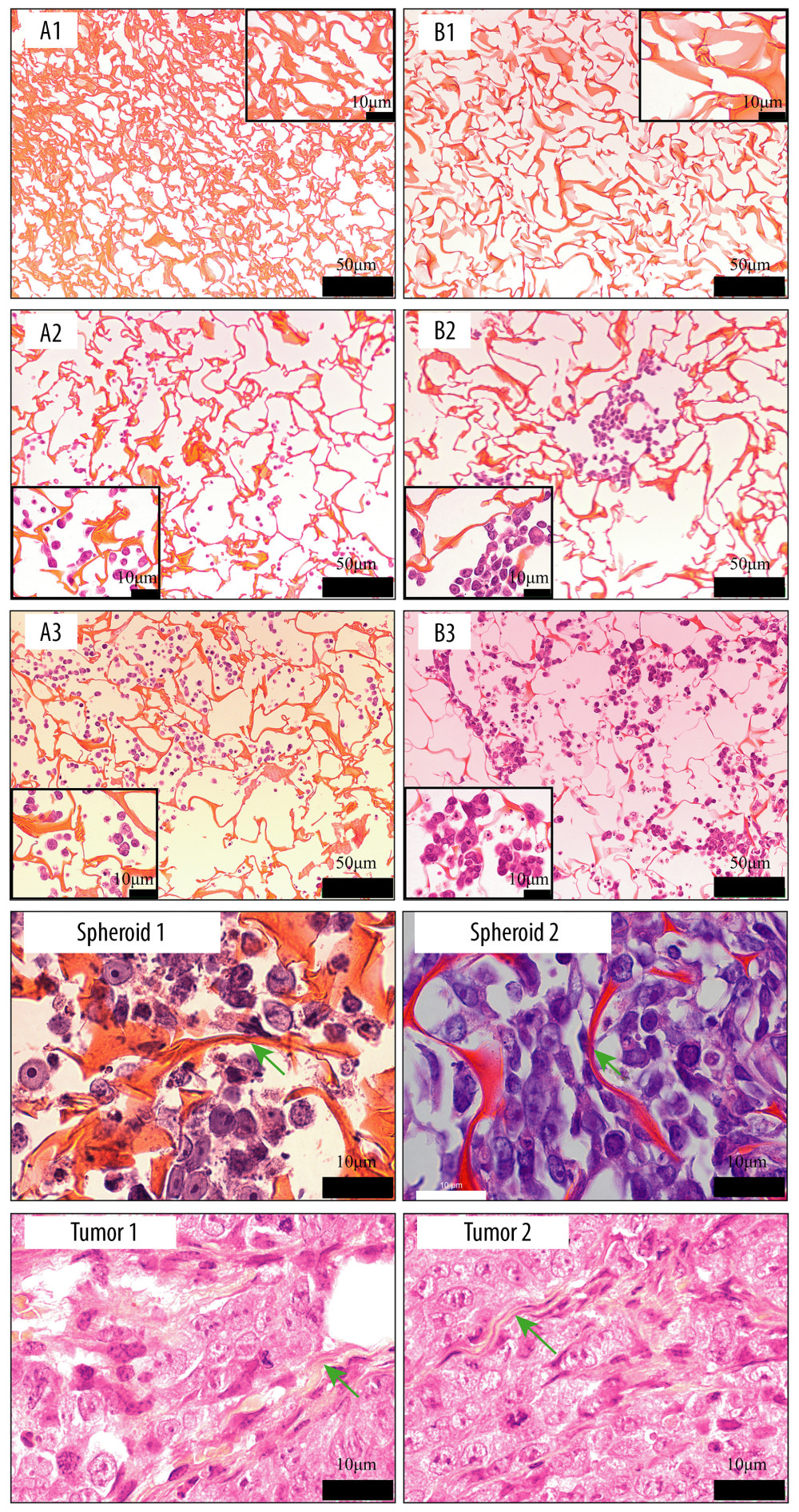 Figure 10. Images of hematoxylin and eosin staining of scaffold. Silk fibroin (SF)/chitosan (Cs) (1: 1) scaffold without cells (A1), SF/Cs/alginate (Alg) (1: 1: 1) scaffold without cells (B1), SF/Cs (1: 1) scaffold with cells on day 3 (A2), SF/Cs/Alg (1: 1: 1) scaffold with cells on day 3 (B2), SF/Cs (1: 1) scaffold with cells on day 7 (A3), SF/Cs/Alg (1: 1: 1) scaffold with cells on day 7 (B3). Spheroids formed in the SF/Cs (1: 1) scaffold are observed under oil lens of Leica DM2500 (spheroid 1). Spheroids formed in the SF/Cs/Alg (1: 1: 1) scaffold are observed under oil lens of Leica DM2500 (spheroid 2). Tumor formed subcutaneously in nude mice after inoculation with HCT-116 cells, observed under oil lens of Leica DM2500 (tumor 1, 2). Green arrows indicate the fiber band.
Figure 10. Images of hematoxylin and eosin staining of scaffold. Silk fibroin (SF)/chitosan (Cs) (1: 1) scaffold without cells (A1), SF/Cs/alginate (Alg) (1: 1: 1) scaffold without cells (B1), SF/Cs (1: 1) scaffold with cells on day 3 (A2), SF/Cs/Alg (1: 1: 1) scaffold with cells on day 3 (B2), SF/Cs (1: 1) scaffold with cells on day 7 (A3), SF/Cs/Alg (1: 1: 1) scaffold with cells on day 7 (B3). Spheroids formed in the SF/Cs (1: 1) scaffold are observed under oil lens of Leica DM2500 (spheroid 1). Spheroids formed in the SF/Cs/Alg (1: 1: 1) scaffold are observed under oil lens of Leica DM2500 (spheroid 2). Tumor formed subcutaneously in nude mice after inoculation with HCT-116 cells, observed under oil lens of Leica DM2500 (tumor 1, 2). Green arrows indicate the fiber band. References
1. Bray F, Ferlay J, Soerjomataram I, Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries: Cancer J Clin, 2018; 68(6); 394-424
2. Brody H, Colorectal cancer: Nature, 2015; 521; S1
3. Smalley KS, Lioni M, Herlyn M, Life isn’t flat: Taking cancer biology to the next dimension: In Vitro Cell Dev Biol Anim, 2006; 42(8–9); 242-47
4. Ravi M, Paramesh V, Kaviya SR, 3D cell culture systems: Advantages and applications: J Cell Physiol, 2015; 230(1); 16-26
5. Jensen C, Teng Y, Is it time to start transitioning from 2D to 3D cell culture?: Front Mol Biosci, 2020; 7; 33
6. Ravi M, Ramesh A, Pattabhi A, Contributions of 3D cell cultures for cancer research: J Cell Physiol, 2017; 232(10); 2679-97
7. Salerno A, Zeppetelli S, Di Maio E, Processing/structure/property relationship of multi-scaled PCL and PCL-HA composite scaffolds prepared via gas foaming and NaCl reverse templating: Biotechnol Bioeng, 2011; 108(4); 963-76
8. Scaglione S, Lazzarini E, Ilengo C, Quarto R, A composite material model for improved bone formation: J Tissue Eng Regen Med, 2010; 4(7); 505-13
9. Du M, Gu J, Wang J, Silk fibroin/poly(L-lactic acid-co-epsilon-caprolactone) electrospun nanofibrous scaffolds exert a protective effect following myocardial infarction: Exp Ther Med, 2019; 17(5); 3989-98
10. Li J, Wang Q, Gu Y, Production of composite scaffold containing silk fibroin, chitosan, and gelatin for 3D cell culture and bone tissue regeneration: Med Sci Monit, 2017; 23; 5311-20
11. Keane TJ, Badylak SF, The host response to allogeneic and xenogeneic biological scaffold materials: J Tissue Eng Regen Med, 2015; 9(5); 504-11
12. Reddy R, Reddy N, Biomimetic approaches for tissue engineering: J Biomater Sci, 2018; 29(14); 1667-85
13. Chomchalao P, Pongcharoen S, Sutheerawattananonda M, Tiyaboonchai W, Fibroin and fibroin blended three-dimensional scaffolds for rat chondrocyte culture: Biomed Eng Online, 2013; 12(1); 28
14. Patrulea V, Ostafe V, Borchard G, Jordan O, Chitosan as a starting material for wound healing applications: Eur J Pharm Biopharm, 2015; 97(Pt B); 417-26
15. Augst AD, Kong HJ, Mooney DJ, Alginate hydrogels as biomaterials: Macromol Biosci, 2006; 6(8); 623-33
16. Ghosh M, Halperin-Sternfeld M, Adler-Abramovich L, Bio mimicking of extracellular matrix: Adv Exp Med Biol, 2019; 1174; 371-99
17. Gu Y, Zhu J, Xue C, Chitosan/silk fibroin-based, Schwann cell-derived extracellular matrix-modified scaffolds for bridging rat sciatic nerve gaps: Biomaterials, 2014; 35(7); 2253-63
18. Bhardwaj N, Nguyen QT, Chen AC: Biomaterials, 2011; 32(25); 5773-81
19. Rios CN, Skoracki RJ, Miller MJ: Tissue Eng Part A, 2009; 15(9); 2717-25
20. Muzzarelli R, El Mehtedi M, Bottegoni C, Genipin-crosslinked chitosan gels and scaffolds for tissue engineering and regeneration of cartilage and bone: Mar Drugs, 2015; 13(12); 7314-38
21. Li Z, Leung M, Hopper R, Feeder-free self-renewal of human embryonic stem cells in 3D porous natural polymer scaffolds: Biomaterials, 2010; 31(3); 404-12
22. Serra IR, Fradique R, Vallejo MCS, Production and characterization of chitosan/gelatin/β-TCP scaffolds for improved bone tissue regeneration: Mater Sci Eng C, 2015; 55; 592-604
23. Gupta V, Aseh A, Ríos CN, Fabrication and characterization of silk fibroin-derived curcumin nanoparticles for cancer therapy: Int J Nanomedicine, 2009; 4; 115-22
24. Backer A, Erhardt O, Wietbrock L, Silk scaffolds connected with different naturally occurring biomaterials for prostate cancer cell cultivation in 3D: Biopolymers, 2017; 107(2); 70-79
25. Nava MM, Draghi L, Giordano C, Pietrabissa R, The effect of scaffold pore size in cartilage tissue engineering: J Appl Biomater Funct Mater, 2016; 14(3); e223-29
26. Murphy CM, Duffy GP, Schindeler A, O’Brien FJ, Effect of collagen-glycosaminoglycan scaffold pore size on matrix mineralization and cellular behavior in different cell types: J Biomed Mater Res A, 2016; 104(1); 291-304
27. Ionescu LC, Mauck RL: Tissue Eng Part A, 2013; 19(3–4); 538-47
28. Jin H, Pi J, Huang X, BMP2 promotes migration and invasion of breast cancer cells via cytoskeletal reorganization and adhesion decrease: an AFM investigation: Appl Microbiol Biotechnol, 2012; 93(4); 1715-23
29. Ma L, Song B, Jin H, Cinobufacini induced MDA-MB-231 cell apoptosis-associated cell cycle arrest and cytoskeleton function: Bioorg Med Chem Lett, 2012; 22(3); 1459-63
30. Jiang J, Jin H, Liu L, Curcumin disturbed cell-cycle distribution of HepG2 cells via cytoskeletal arrangement: Scanning, 2013; 35(4); 253-60
31. Strube F, Infanger M, Wehland M, Alteration of cytoskeleton morphology and gene expression in human breast cancer cells under simulated microgravity: Cell J, 2020; 22(1); 106-14
32. Terriac E, Schütz S, Lautenschläger F, Vimentin intermediate filament rings deform the nucleus during the first steps of adhesion: Front Cell Dev Biol, 2019; 7; 106
33. Kamei H, Relationship of nuclear invaginations to perinuclear rings composed of intermediate filaments in MIA PaCa-2 and some other cells: Cell Struct Funct, 1994; 19(3); 123-32
34. Hirschhaeuser F, Menne H, Dittfeld C, Multicellular tumor spheroids: An underestimated tool is catching up again: J Biotechnol, 2010; 148(1); 3-15
35. Monnier S, Delarue M, Brunel B, Effect of an osmotic stress on multicellular aggregates: Methods, 2016; 94; 114-19
36. McMahon KM, Volpato M, Chi HY, Characterization of changes in the proteome in different regions of 3D multicell tumor spheroids: J Proteome Res, 2012; 11(5); 2863-75
Figures
 Figure 1. Schematic illustration of constructing a porous scaffold based on silk fibroin, chitosan, and alginate via freeze-drying technique and chemical cross-linking method.
Figure 1. Schematic illustration of constructing a porous scaffold based on silk fibroin, chitosan, and alginate via freeze-drying technique and chemical cross-linking method. Figure 2. Schematic illustration of cells seeded on the scaffold to form biomaterial substrate-mediated multicellular spheroids. Red arrows represent the scaffold. Black arrows indicate the multicellular spheroids.
Figure 2. Schematic illustration of cells seeded on the scaffold to form biomaterial substrate-mediated multicellular spheroids. Red arrows represent the scaffold. Black arrows indicate the multicellular spheroids. Figure 3. (A–C) Macroscopic appearance of silk fibroin (SF), chitosan (Cs), alginate (Alg), SF/Cs (1: 1), SF/Cs/Alg (1: 1: 0.5), SF/Cs/Alg (1: 1: 1), and SF/Cs/Alg (1: 1: 2) scaffolds is shown.
Figure 3. (A–C) Macroscopic appearance of silk fibroin (SF), chitosan (Cs), alginate (Alg), SF/Cs (1: 1), SF/Cs/Alg (1: 1: 0.5), SF/Cs/Alg (1: 1: 1), and SF/Cs/Alg (1: 1: 2) scaffolds is shown. Figure 4. Optical microscope photographs (A1–G1, A2–G2) and scanning electron microscopy images (A3–G3) of the scaffolds made from silk fibroin (SF; A1–A3), chitosan (Cs; B1–B3), alginate (Alg; C1–C3), SF/Cs (1: 1) (D1–D3), SF/Cs/Alg (1: 1: 0.5) (E1–E3), SF/Cs/Alg (1: 1: 1) (F1–F3), and SF/Cs/Alg (1: 1: 2) (G1–G3), are presented.
Figure 4. Optical microscope photographs (A1–G1, A2–G2) and scanning electron microscopy images (A3–G3) of the scaffolds made from silk fibroin (SF; A1–A3), chitosan (Cs; B1–B3), alginate (Alg; C1–C3), SF/Cs (1: 1) (D1–D3), SF/Cs/Alg (1: 1: 0.5) (E1–E3), SF/Cs/Alg (1: 1: 1) (F1–F3), and SF/Cs/Alg (1: 1: 2) (G1–G3), are presented. Figure 5. Results of porosity (A), water uptake rate (B), swelling rate (C) and degradation rate (D) of the scaffolds, and cell adhesion (E) and cell proliferation (F, G).
Figure 5. Results of porosity (A), water uptake rate (B), swelling rate (C) and degradation rate (D) of the scaffolds, and cell adhesion (E) and cell proliferation (F, G). Figure 6. Results of wound-healing assays (A1–A3, B1–B3, C1–C3, E, F) and colony-forming assay (D1–D3, G) of cells in the extracting liquid of the scaffold are represented.
Figure 6. Results of wound-healing assays (A1–A3, B1–B3, C1–C3, E, F) and colony-forming assay (D1–D3, G) of cells in the extracting liquid of the scaffold are represented. Figure 7. Cell proliferation analyses are accomplished by green fluorescent protein (GFP)-labeled HCT116 cell imaging. HCT-116 cells transfected with pCDH-CMV-MCS-EF1-copGFP-Puro lentivirus (5×109 transduction units/mL) were cultured in the two-dimensional (2D) plate, silk fibroin (SF)/chitosan (Cs) (1: 1) scaffolds, and SF/Cs/alginate (Alg) (1: 1: 1) scaffolds. The scaffolds and the cells are observed under Nikon ECLIPSE Ts2R microscope and images are taken on day 3 (A–C) and day 7 (D–F). 2D group (A, D), SF/Cs (1: 1) group (B, E), SF/Cs/Alg (1: 1: 1) group (C, F).
Figure 7. Cell proliferation analyses are accomplished by green fluorescent protein (GFP)-labeled HCT116 cell imaging. HCT-116 cells transfected with pCDH-CMV-MCS-EF1-copGFP-Puro lentivirus (5×109 transduction units/mL) were cultured in the two-dimensional (2D) plate, silk fibroin (SF)/chitosan (Cs) (1: 1) scaffolds, and SF/Cs/alginate (Alg) (1: 1: 1) scaffolds. The scaffolds and the cells are observed under Nikon ECLIPSE Ts2R microscope and images are taken on day 3 (A–C) and day 7 (D–F). 2D group (A, D), SF/Cs (1: 1) group (B, E), SF/Cs/Alg (1: 1: 1) group (C, F). Figure 8. Fluorescence staining of actin in HCT-116 cells cultured in the extracting liquid of scaffold. F-Actin is marked with fluorescein isothiocyanate (FITC)-DY-554-phalloidin (red). Nuclear counterstain with 4′,6-diamidino-2-phenylindole (DAPI; blue). Two-dimensional (2D) culture system on day 3 (A1), silk fibroin (SF)/chitosan (Cs) (1: 1) scaffold on day 3 (B1), SF/Cs/alginate (Alg) (1: 1: 1) scaffold on day 3 (C1), 2D culture system on day 7 (A2, D1, D2), SF/Cs (1: 1) scaffold on day 7 (B2, E1, E2), SF/Cs/Alg (1: 1: 1) scaffold on day 7 (C2, F1, F2). The green arrow indicates the contractile ring around the nucleus, and the white arrow indicates the contraction ring at the location of the cell mitosis groove.
Figure 8. Fluorescence staining of actin in HCT-116 cells cultured in the extracting liquid of scaffold. F-Actin is marked with fluorescein isothiocyanate (FITC)-DY-554-phalloidin (red). Nuclear counterstain with 4′,6-diamidino-2-phenylindole (DAPI; blue). Two-dimensional (2D) culture system on day 3 (A1), silk fibroin (SF)/chitosan (Cs) (1: 1) scaffold on day 3 (B1), SF/Cs/alginate (Alg) (1: 1: 1) scaffold on day 3 (C1), 2D culture system on day 7 (A2, D1, D2), SF/Cs (1: 1) scaffold on day 7 (B2, E1, E2), SF/Cs/Alg (1: 1: 1) scaffold on day 7 (C2, F1, F2). The green arrow indicates the contractile ring around the nucleus, and the white arrow indicates the contraction ring at the location of the cell mitosis groove. Figure 9. Scanning electron microscopy images of cell morphology on the scaffolds. Silk fibroin (SF)/chitosan (Cs) (1: 1) scaffold on day 1 (A1, A2), SF/Cs (1: 1) scaffold on day 3 (C1, C2), SF/Cs (1: 1) scaffold on day 7 (E1, E2), SF/Cs/alginate (Alg) (1: 1: 1) scaffold on day 1 (B1, B2), SF/Cs/Alg (1: 1: 1) scaffold on day 3 (D1, D2), SF/Cs/Alg (1: 1: 1) scaffold on day 7 (F1, F2).
Figure 9. Scanning electron microscopy images of cell morphology on the scaffolds. Silk fibroin (SF)/chitosan (Cs) (1: 1) scaffold on day 1 (A1, A2), SF/Cs (1: 1) scaffold on day 3 (C1, C2), SF/Cs (1: 1) scaffold on day 7 (E1, E2), SF/Cs/alginate (Alg) (1: 1: 1) scaffold on day 1 (B1, B2), SF/Cs/Alg (1: 1: 1) scaffold on day 3 (D1, D2), SF/Cs/Alg (1: 1: 1) scaffold on day 7 (F1, F2). Figure 10. Images of hematoxylin and eosin staining of scaffold. Silk fibroin (SF)/chitosan (Cs) (1: 1) scaffold without cells (A1), SF/Cs/alginate (Alg) (1: 1: 1) scaffold without cells (B1), SF/Cs (1: 1) scaffold with cells on day 3 (A2), SF/Cs/Alg (1: 1: 1) scaffold with cells on day 3 (B2), SF/Cs (1: 1) scaffold with cells on day 7 (A3), SF/Cs/Alg (1: 1: 1) scaffold with cells on day 7 (B3). Spheroids formed in the SF/Cs (1: 1) scaffold are observed under oil lens of Leica DM2500 (spheroid 1). Spheroids formed in the SF/Cs/Alg (1: 1: 1) scaffold are observed under oil lens of Leica DM2500 (spheroid 2). Tumor formed subcutaneously in nude mice after inoculation with HCT-116 cells, observed under oil lens of Leica DM2500 (tumor 1, 2). Green arrows indicate the fiber band.
Figure 10. Images of hematoxylin and eosin staining of scaffold. Silk fibroin (SF)/chitosan (Cs) (1: 1) scaffold without cells (A1), SF/Cs/alginate (Alg) (1: 1: 1) scaffold without cells (B1), SF/Cs (1: 1) scaffold with cells on day 3 (A2), SF/Cs/Alg (1: 1: 1) scaffold with cells on day 3 (B2), SF/Cs (1: 1) scaffold with cells on day 7 (A3), SF/Cs/Alg (1: 1: 1) scaffold with cells on day 7 (B3). Spheroids formed in the SF/Cs (1: 1) scaffold are observed under oil lens of Leica DM2500 (spheroid 1). Spheroids formed in the SF/Cs/Alg (1: 1: 1) scaffold are observed under oil lens of Leica DM2500 (spheroid 2). Tumor formed subcutaneously in nude mice after inoculation with HCT-116 cells, observed under oil lens of Leica DM2500 (tumor 1, 2). Green arrows indicate the fiber band. Tables
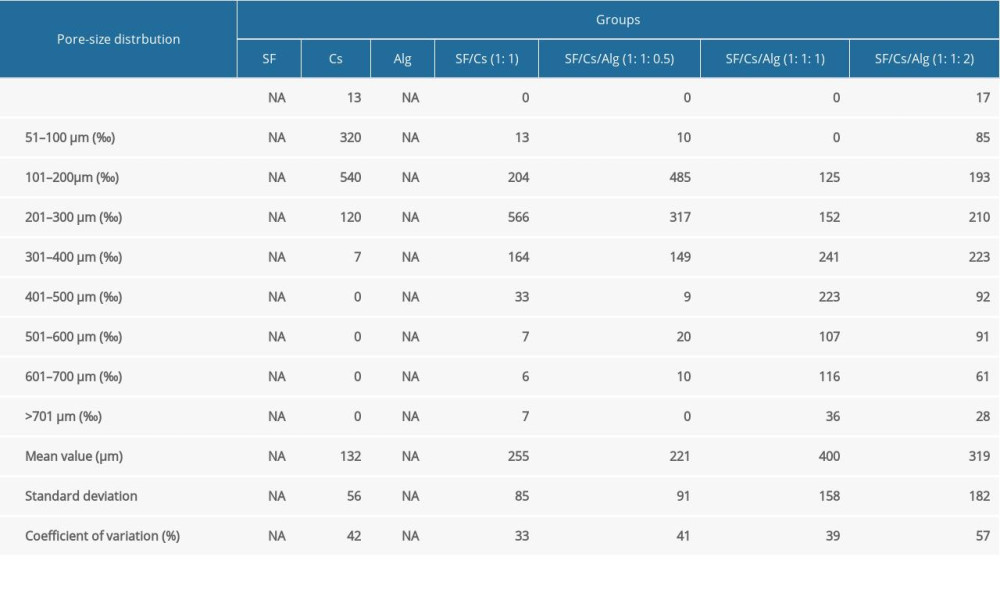 Table 1. Frequency distribution of pore size of scaffolds made from silk fibroin (SF), chitosan (Cs), alginate (Alg), SF/Cs (1: 1), SF/Cs/Alg (1: 1: 0.5), SF/Cs/Alg (1: 1: 1), and SF/Cs/Alg (1: 1: 2). Image J was used to measure the cross-sectional area of pore for each group of scaffolds and the diameter of each pore was obtained by the formula: Area=πd2/4, (d – diameter; NA – not available).
Table 1. Frequency distribution of pore size of scaffolds made from silk fibroin (SF), chitosan (Cs), alginate (Alg), SF/Cs (1: 1), SF/Cs/Alg (1: 1: 0.5), SF/Cs/Alg (1: 1: 1), and SF/Cs/Alg (1: 1: 2). Image J was used to measure the cross-sectional area of pore for each group of scaffolds and the diameter of each pore was obtained by the formula: Area=πd2/4, (d – diameter; NA – not available). Table 1. Frequency distribution of pore size of scaffolds made from silk fibroin (SF), chitosan (Cs), alginate (Alg), SF/Cs (1: 1), SF/Cs/Alg (1: 1: 0.5), SF/Cs/Alg (1: 1: 1), and SF/Cs/Alg (1: 1: 2). Image J was used to measure the cross-sectional area of pore for each group of scaffolds and the diameter of each pore was obtained by the formula: Area=πd2/4, (d – diameter; NA – not available).
Table 1. Frequency distribution of pore size of scaffolds made from silk fibroin (SF), chitosan (Cs), alginate (Alg), SF/Cs (1: 1), SF/Cs/Alg (1: 1: 0.5), SF/Cs/Alg (1: 1: 1), and SF/Cs/Alg (1: 1: 2). Image J was used to measure the cross-sectional area of pore for each group of scaffolds and the diameter of each pore was obtained by the formula: Area=πd2/4, (d – diameter; NA – not available). In Press
05 Mar 2024 : Clinical Research
Role of Critical Shoulder Angle in Degenerative Type Rotator Cuff Tears: A Turkish Cohort StudyMed Sci Monit In Press; DOI: 10.12659/MSM.943703
06 Mar 2024 : Clinical Research
Comparison of Outcomes between Single-Level and Double-Level Corpectomy in Thoracolumbar Reconstruction: A ...Med Sci Monit In Press; DOI: 10.12659/MSM.943797
21 Mar 2024 : Meta-Analysis
Economic Evaluation of COVID-19 Screening Tests and Surveillance Strategies in Low-Income, Middle-Income, a...Med Sci Monit In Press; DOI: 10.12659/MSM.943863
10 Apr 2024 : Clinical Research
Predicting Acute Cardiovascular Complications in COVID-19: Insights from a Specialized Cardiac Referral Dep...Med Sci Monit In Press; DOI: 10.12659/MSM.942612
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








