31 October 2020: Animal Study
Effects of Drynaria Total Flavonoid on the Microstructure of the Mandible in Ovariectomized Rats
Hui Zeng1ABCDEFG*, Xubing Zhao2AB, Lin Wang1ABEF, Chengfang Tang1DF, Zixia Li1ABF, Na Xie1AB, Feng Wang1ABFDOI: 10.12659/MSM.926171
Med Sci Monit 2020; 26:e926171
Abstract
BACKGROUND: The aim of this study is to investigate the effects of Drynaria total flavonoids (DTF) on mandible microarchitecture, serum estrogen (E2), osteoprotegerin (OPG), and receptor activator of nuclear factor kappa-B ligand (RANKL) levels in an ovariectomy-induced osteoporosis rat model.
MATERIAL AND METHODS: Thirty female Sprague-Dawley rats were divided into 5 groups (n=6 per group): sham surgery, ovariectomy (OVX), and low-dose, middle-dose, and high-dose DTF. Mandibular osteoporosis was induced by ovariectomy; an equal amount of ovary-sized fat tissue was removed from the sham group. The DTF-treated groups were given DTF gavage at different doses for 12 weeks; the sham and OVX groups were given saline. After the treatment phase, the effects of DTF on the microarchitecture of the mandible were evaluated by measuring bone density, maximum load, morphometric parameters, and histopathological alterations. Serum E2, OPG, and RANKL levels were measured.
RESULTS: The OVX group showed obvious osteoporosis in the mandible and decreased serum E2 levels and OPG/RANKL ratio. The low-dose group did not show significant improvement in mandibular microstructure. The middle-dose group showed significantly ameliorated osteoporosis. The high-dose group had further improvement in bone microstructures and increase of OPG/RANKL over the middle-dose group. Furthermore, ovariectomy significantly decreased serum E2, but DTF treatment failed to restore serum E2 levels.
CONCLUSIONS: Ovariectomy can cause significant bone loss in the rat mandible and a decrease in serum E2 and OPG/RANKL. DTF significantly improved the mandibular microstructure and restored OPG/RANKL balance, but it did not restore the decreased serum E2 concentration following ovariectomy.
Keywords: Mandible, Osteoporosis, Osteoprotegerin, Polypodiaceae, RANK Ligand, Bone Density, Estrogens, Ovariectomy, Plant Extracts
Background
Osteoporosis is a systemic metabolic bone disease characterized by low bone mass and the degeneration of bone tissue microstructure. The main features of osteoporosis include reduced bone mineral density and bone microstructure destruction, which result in bone brittleness and an increased risk of fracture [1]. Recently, due to an aging population, the incidence of osteoporosis has increased yearly. According to epidemiological studies in China, the prevalence of osteoporosis is 21% in people aged 50 to 60 years, 58% in people aged 60 to 70 years, and 100% in people aged 70 to 80 years [2]. Osteoporosis occurs in more than 25% of women over the age of 50 [3]. Postmenopausal women have a much higher incidence of osteoporosis and pathological fracture than do men because of the dramatic decrease in estrogen levels during this time [4]. Furthermore, previous studies have shown that osteoporosis in postmenopausal women can significantly affect the mandible, and mandibular bone loss is a local manifestation of osteoporosis in the oral maxillofacial region [5]. Bone loss in the jaws can aggravate the bone resorption of periodontitis and increase the rate of tooth loss, which affects the early bone integration of dental implants and is one of the risk factors for the success of dental implants [6,7]. In recent years, the effects of systemic anti-osteoporosis reagents on the structure and bone mass of the jawbones have become a hot topic of research [8,9].
The primary regimen for preventing and treating postmenopausal osteoporosis, including jaw osteoporosis, is hormone replacement therapy (HRT), but it may cause an increased risk of cancer and other adverse effects [10]. Bisphosphonates are another type of anti-osteoporosis reagent for the prevention of bone loss and reduction of fracture risk; their adverse effects involve mainly esophageal complications and osteonecrosis of the jaw [11]. Calcitonin is a 32-amino acid peptide that binds to osteoclasts and inhibits bone resorption. The main concerns about using calcitonin are its relatively low effectiveness and association with an increased rate for certain types of cancers [12]. In recent years, researchers have discovered phytoestrogens, a group of compounds derived from plants that have estrogen-like effects [13]. The chemical structure of this class of compounds is similar to that of estrogen and can exert estrogen or antihormonal effects [14]. Some commonly used traditional Chinese medicines have been reported to contain these compounds and have been widely used to treat osteoporosis [15].
Material and Methods
REAGENTS:
The DTF was developed by the Xiyuan Hospital of China Academy of Traditional Chinese Medicine and Sichuan Institute of Traditional Chinese Medicine (batch number: 961214); each gram of the extract contained 66.67 mg of crude DTF. The stock solution was diluted with saline into specific concentrations for administration to the rats.
CONSTRUCTION OF THE OVX RAT MODEL AND GROUPING:
Thirty 3-month-old female Sprague-Dawley rats (weight: 220±30g) were obtained from the Xi’an Jiaotong University School of Medicine Laboratory Animal Center. Six rats were randomly selected as the sham surgery group; the remaining rats were surgically castrated by ovariectomy. The rats in the castration group were anesthetized with an intraperitoneal injection of 2% pentobarbital sodium (40 mg/kg). To expose the ovaries, the skin was cut from the lumbar spine along the midline of the back to make a longitudinal incision of about 2–3 cm, and then the psoas muscles were cut along the scapula under the left and right sides of the ribs. T2 ligations were made on the uterine horn where the ovary is closely connected, and the uterine horn was cut off after the ligation, the ovary removed, and the skin sutured. In the sham operation group, only a piece of fat tissue similar in size to the ovary was removed during the operation. Local antibiotics were administrated to the wound postoperatively to prevent infection and to reduce animal mortality due to surgery. One week after surgery, vaginal smears were performed for 5 consecutive days. If the estrus cycle did not occur in the ovariectomized rats, it indicated that the ovary was successfully removed. Three months after surgery, the rats that underwent sham surgery were classified into the sham group; the remaining 24 animals were randomly divided into 4 groups (n=6 per group): ovariectomy control (OVX), low-dose DTF, middle-dose DTF, and high-dose DTF groups. The low-dose, middle-dose, and high-dose groups of rats were given intragastric gavage of DTF at 54 mg/kg, 108 mg/kg, and 216 mg/kg, respectively, once per day for 12 weeks. The sham and OVX groups were given an equal volume of saline for 12 weeks. All procedures were approved by the Animal Care and Use Committee of Xi’an Medical College (#XYLS2020137), Xi’an, China.
MANDIBULAR BONE DENSITY EXAMINATION:
After 12 weeks of intragastric administration of DTF, the rats were euthanized by anesthesia. The right mandible of each group of rats was thoroughly cleaned of soft tissue and fixed with 10% neutral formaldehyde solution for bone density and maximum mandible load measurement. The bone density measurement was performed using a dual-energy X-ray bone densitometer (Norland XR-46). The measurement site was the junction of the last molar of the mandible and the mandibular branch (this site is parallel to the growth direction of the molar).
MEASUREMENT OF THE MAXIMUM LOAD OF THE MANDIBLE:
Before making the maximum load measurement, the 2 ends of the mandible (joint end and incisor end) were embedded with resin. The bottom of the resin block was parallel to the ground plane. The mandibular molar area was exposed and placed in the Shimadzu automatic control universal testing machine (model AG-IC). The loading point was the second molar of the mandible, the span was 20 mm, the loading speed was 0.5 N/min, and the force was gradually increased until the bone was completely broken. During the experiment, the bone surface was sprayed with normal saline to keep it moist.
HISTOPATHOLOGICAL EXAMINATION:
The left mandible was fixed in a 10% formaldehyde solution for 24 to 48 h, rinsed with PBS, and decalcified in 10% EDTA (pH 8.0) at 4 °C for 21 days. The decalcified specimens were rinsed thoroughly with PBS, dehydrated, and then embedded. The mandibular molar region was sliced along the near-distal direction, and the anterior teeth were sliced along the buccal-tongue direction (in 5 μm thickness). The slices were stained with hematoxylin and eosin (H&E) and observed under a light microscope.
MEASUREMENT OF BONE TISSUE MORPHOMETRIC PARAMETERS:
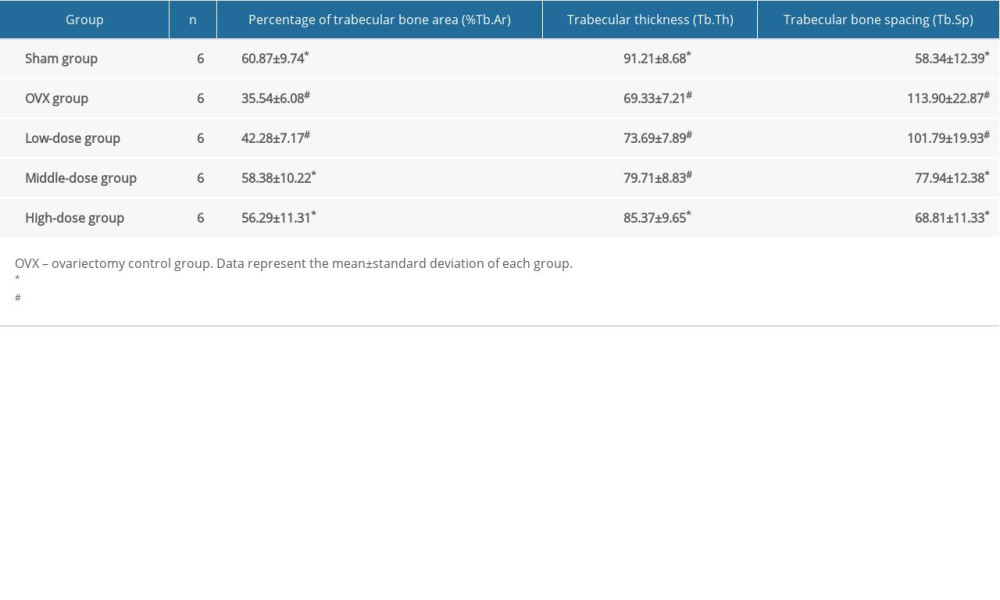
The image of the distal cancellous bone area of the root of the last mandibular molar was collected under the light microscope. To follow the principle of equidistant random sampling, 3 slices of the same specimen were selected, and 3 observation fields in each slice were selected. The images were analyzed with the Motic Med 6.0 digital medical image analysis system to measure the trabecular bone parameters. When measuring, the scale overlapped with the image, the cursor moved along the tissue structure to be measured to determine the point count, straight line or curve length, circumference, and area. Bone histometry software was then used to calculate the measured parameters: bone tissue area (T.Ar), trabecular bone area (Tb.Ar), and trabecular bone perimeter (Tb.Pm). Next, the following parameters were calculated based on the measured parameters using the corresponding formulas: trabecular bone area percentage (%Tb.Ar)=Tb.Ar/T.Ar; trabecular number thickness (Tb.Th)=(2/1.99)×(Tb.Ar/Tb.Pm); and trabecular bone spacing Tb.Sp=(T.Ar-Tb.Ar)/Tb.Pm.
MANDIBULAR CORTICAL THICKNESS MEASUREMENT:
Under the microscope, the thickness of the bone cortex of the root of the anterior teeth of the mandible was taken. The anterior, middle, and posterior points were measured. Each location was measured 3 times and the average value was calculated. Finally, the total average value of the 3 locations was calculated.
MEASUREMENT OF SERUM E2, OPG, AND RANKL LEVELS:
Rats in each group were continuously gavaged for 12 weeks. After intraperitoneal anesthesia, blood was collected from the abdominal aorta and centrifuged at 3500 rpm for 15 min. The serum was collected and stored at −70°C. Before the measurement, the samples and the kits were thawed at room temperature for 30 min. The serum E2 was detected by using a radioimmunoassay kit (Tianjin Jiuding Medical Bioengineering Co., Ltd.). The serum OPG and RANKL levels were detected by the corresponding ELISA kit (Wuhan Zhongmei Technology Co., Ltd). The measurements were carried out according to the manufactures’ instructions.
STATISTICAL ANALYSIS:
SPSS16.0 (IBM, Chicago, IL, USA) software was used to perform all statistical analyses. The means of different groups were compared with one-way ANOVA followed by least significant difference
Results
HIGH-DOSE DTF PROTECTIVE EFFECTS ON OVX-INDUCED MANDIBULAR OSTEOPOROSIS:
After the surgery and treatment, the right mandibles of the rats were compared. In the sham surgery group, the mandibles were a normal shape, with a smooth surface and no bite-like defects. However, the mandibles of the OVX group showed a rough surface and many bite-like lesions. The mandibles of the low-dose, middle-dose, and high-dose groups showed obvious improvement in appearance. The surfaces were relatively smooth and no obvious bite-like lesions were observed (Figure 1). Furthermore, the bone density of the right mandibles of the OVX group was significantly lower than that of the sham group, and the difference was statistically significant (P<0.05), indicating that the OVX-induced osteoporosis model was successfully constructed (Figure 2). After the administration of DTF for 12 weeks, the high-dose group showed a significantly higher mandible bone density than the OVX group (P<0.05), while the differences between the low-dose and middle-dose groups and the OVX group were not statistically significant (both P>0.05), suggesting that high-dose DTF treatment had protective effects on OVX-induced mandibular osteoporosis (Figure 2).
DTF WAS BENEFICIAL IN RESTORING THE MAXIMUM LOAD OF THE RIGHT MANDIBLE:
As shown in Figure 3, compared with the sham group, the maximum load of the mandible of the OVX group was significantly lower (P<0.05). We then investigated the effects of DTF on the maximum load of the mandible. The low-dose group had a higher mandible maximum load compared to that of the OVX group, although the difference was not significant (P>0.05). The middle-dose and high-dose groups had significantly higher maximum loads of the mandible compared to that of the OVX group (both P<0.05). Remarkably, the maximum load of the mandible was increased as the concentration of DTF was increased, indicating that DTF was beneficial in restoring the mandible maximum load in a dose-dependent manner. However, although the DTF-treated groups showed an improved maximum load of the mandible, this parameter in these groups was still lower than that of the sham group, suggesting that DTF did not completely restore the declined mandible maximum load following ovariectomy, and other reagents are needed to completely reverse this process (Figure 3).
DTF IMPROVED THE MORPHOMETRIC PARAMETERS OF THE MANDIBULAR TRABECULAR BONE:
Compared to in the sham group, the%Tb.Ar and Tb.Th in the OVX group were significantly lower (both P<0.05), while the Tb.Sp in the OVX group was significantly higher compared to that of the sham group (P<0.05) (Table 1). The%Tb.Ar was improved in the middle-dose and high-dose DTF groups compared to in the OVX group (both P<0.05), and the%Tb.Ar of these 2 groups was not significantly lower than that of the sham group (both P>0.05). Although the low-dose DTF group showed the trend of improved%Tb.Ar, it did not show a significantly improved%Tb.Ar compared to the OVX group (P>0.05) (Table 1). For the Tb.Th, only the high-dose group showed significant improvement in bone quality compared to the OVX group (P<0.05), and the difference in Tb.Th between the high-dose group and the sham group was not significantly different (P>0.05). The low-dose and middle-dose groups did not show significant improvement in Tb.Th (both P>0.05) (Table 1). Both the middle-dose and high-dose groups showed significantly lower Tb.Sp compared to the OVX group (both P<0.05), and the Tb.Sp of these 2 groups was not significantly different than that of the sham group (both P>0.05). The Tb.Sp of the low-dose group was not significantly improved compared to that of the OVX group (P>0.05) (Table 1). These results suggest that DTF at middle and high doses significantly improved the microarchitecture of the mandibular trabecular bone in the OVX-induced osteoporosis model.
DTF LED TO AN INCREASED MANDIBULAR CORTICAL BONE THICKNESS:
The OVX group showed significantly lower mandibular cortical bone thickness than the sham group (P<0.01). DTF at the 3 different doses significantly improved the thickness of the mandibular cortical bone (all P<0.05). Furthermore, the differences between the low-dose and high-dose groups and the sham group were significant (both P<0.05), while the difference between the middle-dose group and sham group was not significant (P>0.05) (Figure 4). These results suggest that DTF, even at a low dose, significantly improved the thickness of the mandibular cortical bone, but only high-dose DTF completely restored it.
DTF IMPROVED THE HISTOPATHOLOGICAL CHANGES IN MANDIBLE AFTER OVARY REMOVAL:
We then observed the histopathological changes in the mandibles with H&E staining. The mandibular bone trabeculae of the sham group was dense and neatly arranged, the thickness of the trabecular bone was normal, the trabecular bone spacing was small, the trabecular bones were well connected, and no abnormal bone resorption was observed (Figure 5A). In the OVX group, the mandible bones were severely absorbed, the trabecular bones were sparse, broken, and disorderly arranged, with some of them showed nibbling-like changes. Also, the trabecular bone thickness became smaller, the spacing became wider, and the marrow cavity was increased (Figure 5B). In the low-dose DTF group, the bone marrow cavity was reduced, but nibbling-like changes were still seen, and the trabecular bone structure was not significantly improved (Figure 5C). However, after treatment with middle-dose and high-dose DTF, the bone marrow cavity of the mandible was reduced, the trabecular bone was slightly thinner, the arrangement was neat, the trabecular bone space was reduced, and the thickness was normal (Figure 5D, 5E).
DTF TREATMENT INCREASED OPG AND DECREASED RANKL LEVELS:
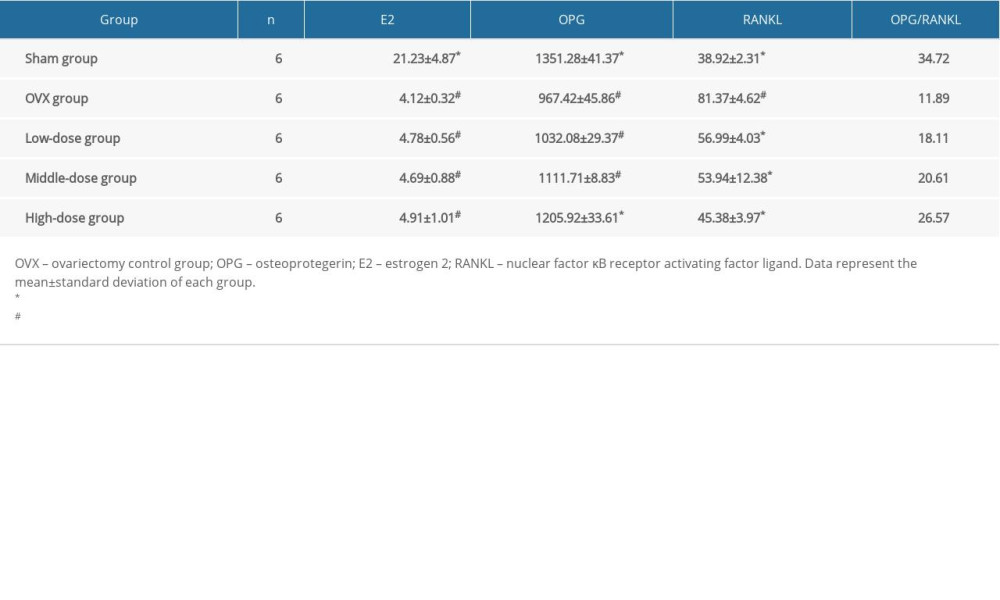
The serum E2 level in the OVX and the 3 DTF-treated groups was significantly lower than that of the sham group (all P<0.05). However, the DTF treatment at different concentrations did not increase the serum level of E2 (all P>0.05) (Table 2), suggesting that the anti-osteoporosis effect of DTF does not occur by restoring E2 production. OPG was significantly downregulated in the OVX group compared to in the sham group (P<0.05). DTF treatment led to an increased serum OPG level, but only the high-dose group showed a significant difference compared to the OVX group (P<0.05). Notably, even the high-dose group of DTF could not completely restore the serum OPG level (P<0.05, compared to the sham group) (Table 2). After the ovariectomy, the serum RANKL level was significantly upregulated in the OVX group compared to in the sham group (P<0.05). Treatment with DTF at all 3 concentrations led to a lower RANKL level compared to the OVX group (all P<0.05). The difference in the serum RANKL level between the high-dose group and the sham group was not significant, while the level in the low-dose and middle-dose groups was significantly higher than that of the sham group (both P<0.05) (Table 2). The OPL/RANKL ratio was decreased in the OVX group, but the treatment with DTF partially restored OPL/RANKL balance (Table 2). These results suggest that DTF treatment can restore the balance between OPG/RANKL after ovariectomy and therefore improve osteoporosis caused by ovary removal.
Discussion
Systemic osteoporosis is common in postmenopausal women. In some women with general osteoporosis, the mandible is also significantly affected. Therefore, many studies have investigated the relationship between systemic osteoporosis and osteoporosis of the jaw bone. In the 1960s, Groen et al. were the first to report a correlation between jaw bone loss and general osteoporosis [18]. Sindeaux et al. showed that female patients with osteoporosis have a significantly increased risk of decreased oral bone density and cortical bone thickness [19]. In recent years, studies found that the mandibular bone is the bone tissue with the fastest turnover rate, and mandibular bone loss may represent the first sign of osteoporosis [20–22]. Thus, it is accepted that mandibular osteoporosis is a local manifestation of general osteoporosis in the oral and maxillofacial regions. Jaw bone loss can increase bone resorption in periodontitis and the rate of tooth loss and affect the early osseointegration of dental implants [6,7]. Therefore, the correction of mandibular osteoporosis is of great value in the treatment of periodontitis and the success of dental implantation.
The ovariectomy rat model has been shown to represent some of the most important clinical features of osteoporosis caused by estrogen deficiency (or postmenopause) in older women [23]. Decreased estrogen levels in ovariectomized rats can lead to decreased bone density, loss of bone mass, and damage to the trabecular bone structure of the mandible. The model has been widely used as a reliable method for inducing bone loss in the mandible [23,24]. In the present study, the OVX group had typical osteoporosis-like changes, including significantly lower serum E2 levels, decreased mandibular bone density, sparse trabecular bone, and a large bone marrow cavity. Histomorphometric results showed that, compared with the sham group, the OVX group had lower Tb.Th,%Tb.Ar, and cortical bone thickness and higher Tb.Sp. Therefore, in the present study, the rat mandible osteoporosis animal model was successfully constructed by ovariectomy.
In recent years, the effect of drugs that improve systemic osteoporosis on the structure and bone mass of the jaw bone has become a hot topic of research. HRT is one of the most popular and effective regimens for preventing and treating postmenopausal osteoporosis [25]. Furthermore, HRT has been used in the treatment of mandible osteoporosis in postmenopausal women. However, although HRT can reduce bone turnover and maintain bone mass, it significantly increases the risk of breast and endometrial cancer [25]. Bisphosphonates are another type of drug widely used for the prevention and treatment of postmenopausal osteoporosis, which inhibit bone resorption with relatively few adverse effects. Oral bisphosphonates are considered the initial therapy for most postmenopausal women at high risk for fracture because they have high efficacy, favorable cost, and long-term safety data [11, 26]. Although bisphosphonates are generally safe, oral bisphosphonates are not used as initial therapy in patients with esophageal disorders. Long-term use of bisphosphonates is also associated with osteonecrosis of the jaw; although this occurs most often in cancer patients, it has been observed in postmenopausal women with osteoporosis. This raised the concern that use of bisphosphonates may decrease the survival of dental implants. Although Koka et al. showed that dental implants placed in postmenopausal women have the same survival whether or not the patients received bisphosphonate treatment [27], the outcome of using of bisphosphonates in the treatment of jaw osteoporosis in postmenopausal women is still uncertain. Calcitonin is a short peptide composed of 32 amino acids, which inhibits osteoclast activity [28]. After menopause, because of a lack of calcitonin, bone resorption is not appropriately regulated, which contributes to a decline in bone matrix formation [29]. Some studies have shown that calcitonin can stimulate the growth of bone tissue [30]. However, it is less commonly used to treat postmenopausal osteoporosis due to low efficacy and an increased risk of adverse effects [31]. Arisawa et al. reported that synthetic salmon calcitonin accelerated regeneration of bone defects in the mandibles of ovariectomized rats [32]. Notably, the role of calcitonin in jaw osteoporosis in postmenopausal women is still unclear. Some researchers thus turn to traditional herbal medicines to search for effective ingredients with fewer adverse effects. Drynaria has been widely used to treat bone diseases such as bone fracture, osteoporosis, and arthritis [33]. DTF is the sum of flavonoids contained in
Bone mass (bone density) and sclerotin (fine bone structure) are 2 important indicators of bone quality. Bone density reduction is a characteristic of bone changes in osteoporosis, and the use of dual-energy X-ray detection of bone density is the criterion standard for the diagnosis of osteoporosis [38]. In the present study, the dual-energy X-ray bone densitometer was thus used to measure the mandibular bone density of the rats. The results showed that compared with the OVX group, the bone density of mandibular bone was increased after the intervention of DTF, and the difference between the high-dose group and OVX group was statistically significant (
The OPG/RANKL/RANK signaling pathway is critical in regulating the activity of osteoclasts [42]. OPG is an inhibitory factor of osteoclastogenesis whose main function is to maintain normal mineralization of bone. RANKL induces osteoclast differentiation, enhances the viability of mature osteoclasts, and prevents osteoclast apoptosis. RANK is the only target receptor of RANKL, and OPG can compete with RANKL to bind this receptor [42]. Therefore, the balance of OPG and RANKL is important in regulating the activity of osteoclasts. An increased OPG/RANKL ratio indicates increased bone formation and reduced bone destruction, while a decreased OPG/RANKL ratio suggests decreased bone formation and increased bone reabsorption [42]. Studies have shown that postmenopausal osteoporosis patients have elevated serum RANKL levels and low OPG levels [43]. Inhibiting the expression of RANKL may become a new therapeutic strategy for osteoporosis [44]. The results of the present study showed that the serum OPG level of the OVX group was significantly lower than that of the sham group (
Because this study aims to evaluate the efficacy of DTF in treating jaw osteoporosis in ovariectomized rats, and we need to identify an optimal dose of DTF, we did not design a head-to-head comparison of DTF with other commonly used therapeutic drugs for osteoporosis, such as HRT, bisphosphonates, and calcitonin. This is a limitation of this study; the inclusion of a group treated with HRT would represent a better design. In our future studies, we will compare the efficacy of DTF with that of widely used treatments and investigate the potential of combining DTF with these therapies to further improve the outcome of jaw osteoporosis in postmenopausal women.
Conclusions
In summary, ovariectomy can induce bone loss and osteoporosis-like changes in the mandible of rats. DTF significantly improved the mandible osteoporosis in the OVX rats. It inhibited the destruction and reabsorption of the mandible, increased bone density, maximum load, and cortical bone thickness and improved the microstructure of the mandible, indicating that it has a good phytoestrogen effect. The high-dose group had a stronger effect than the medium-dose and low-dose groups. Furthermore, DTF does not improve mandible osteoporosis through regulating E2, since DTF cannot increase serum E2 concentration in ovariectomized rats. However, DTF may inhibit bone resorption and promote bone formation by regulating the OPG/RANKL ratio. The mechanism of DTF-induced bone protection and the optimal dosage and schedule of DTF treatment needs to be evaluated in future studies.
Figures
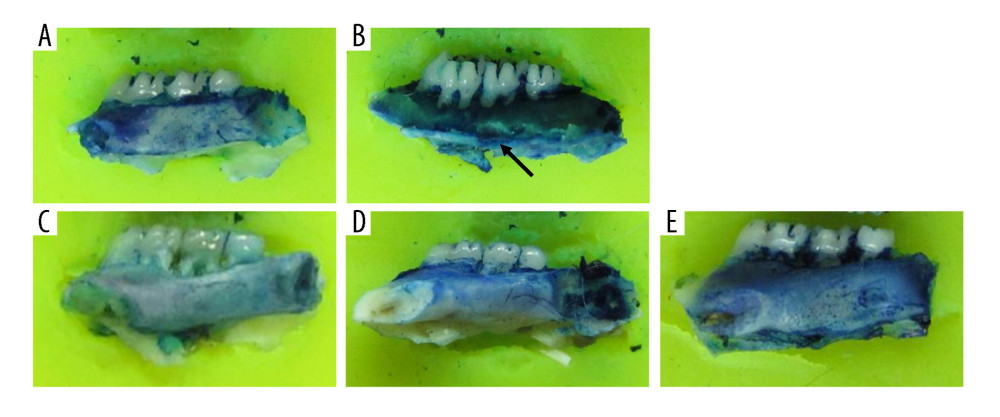 Figure 1. Drynaria total flavonoid (DTF) has protective effects on ovariectomy-induced mandibular osteoporosis. The representative images of the right mandible of the rats in each group are shown. (A) Sham group, (B) ovariectomy control (OVX) group, (C) low-dose group, (D) middle-dose group, and (E) high-dose group. The arrow indicates the bite-like changes in the OVX group.
Figure 1. Drynaria total flavonoid (DTF) has protective effects on ovariectomy-induced mandibular osteoporosis. The representative images of the right mandible of the rats in each group are shown. (A) Sham group, (B) ovariectomy control (OVX) group, (C) low-dose group, (D) middle-dose group, and (E) high-dose group. The arrow indicates the bite-like changes in the OVX group. 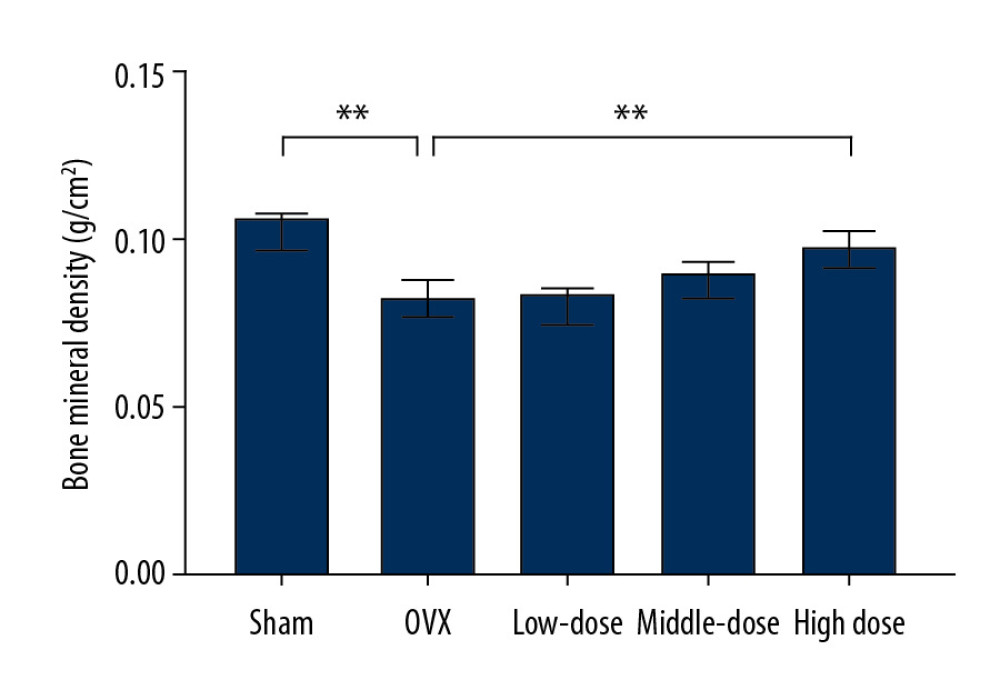 Figure 2. High-dose Drynaria total flavonoid (DTF) led to increased bone mineral density in ovariectomy-induced mandibular osteoporosis. The bone mineral density of the right mandible of each group was measured by a dual-energy X-ray bone densitometry. Data represent the mean±SE of each group. * P<0.05, ** P<0.01, both compared with the ovariectomy control (OVX) group.
Figure 2. High-dose Drynaria total flavonoid (DTF) led to increased bone mineral density in ovariectomy-induced mandibular osteoporosis. The bone mineral density of the right mandible of each group was measured by a dual-energy X-ray bone densitometry. Data represent the mean±SE of each group. * P<0.05, ** P<0.01, both compared with the ovariectomy control (OVX) group. 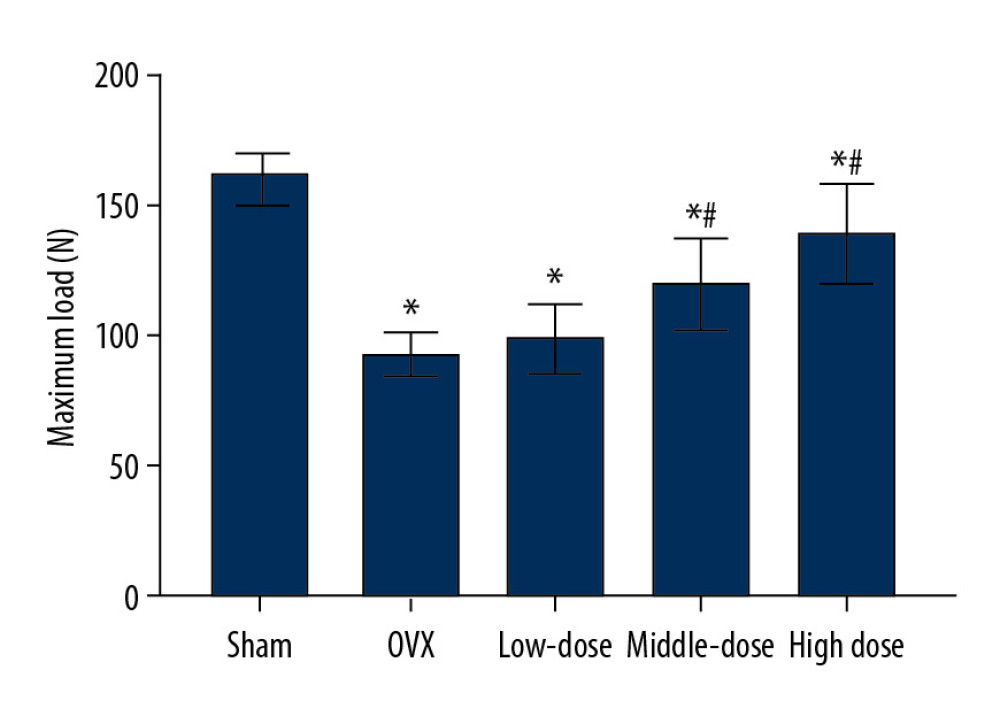 Figure 3. Drynaria total flavonoid (DTF) was beneficial in restoring the maximum load of the right mandible. The maximum load of the right mandible in each group was measured. Data represent the mean±SE of each group. * P<0.05, compared with the sham surgery group; # P<0.05, compared with the ovariectomy control (OVX) group.
Figure 3. Drynaria total flavonoid (DTF) was beneficial in restoring the maximum load of the right mandible. The maximum load of the right mandible in each group was measured. Data represent the mean±SE of each group. * P<0.05, compared with the sham surgery group; # P<0.05, compared with the ovariectomy control (OVX) group. 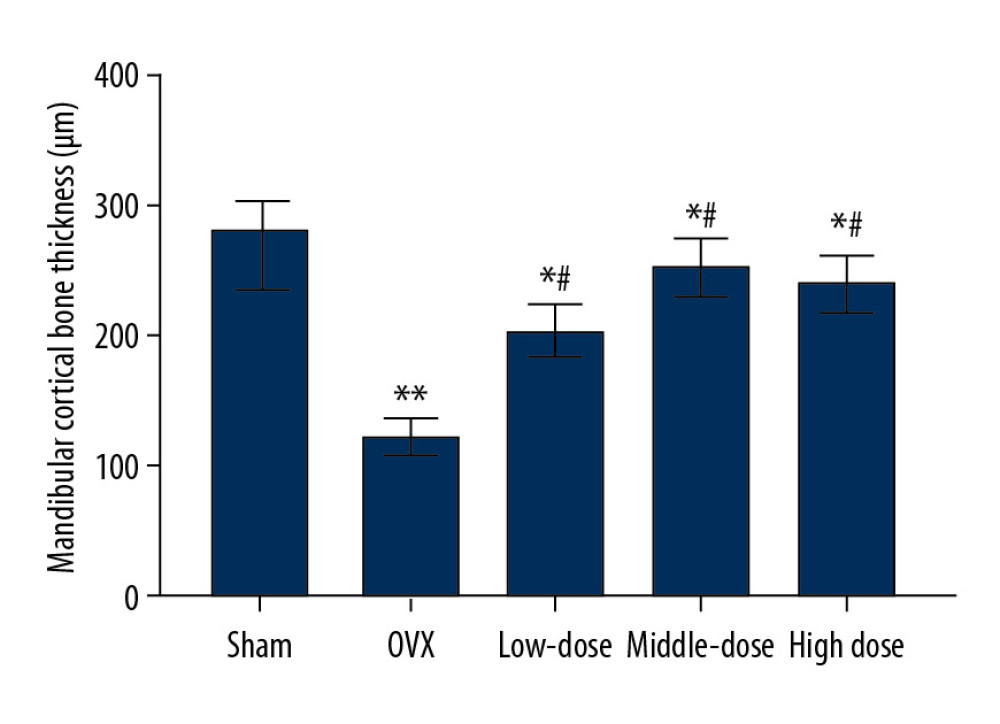 Figure 4. Drynaria total flavonoid (DTF) led to an increased mandibular cortical bone thickness. The mandibular cortical bone thickness of each group was measured. Data represent the mean±SE of each group. * P<0.05, ** P<0.01, compared with the sham surgery group; # P<0.05, compared with the ovariectomy control (OVX) group.
Figure 4. Drynaria total flavonoid (DTF) led to an increased mandibular cortical bone thickness. The mandibular cortical bone thickness of each group was measured. Data represent the mean±SE of each group. * P<0.05, ** P<0.01, compared with the sham surgery group; # P<0.05, compared with the ovariectomy control (OVX) group. 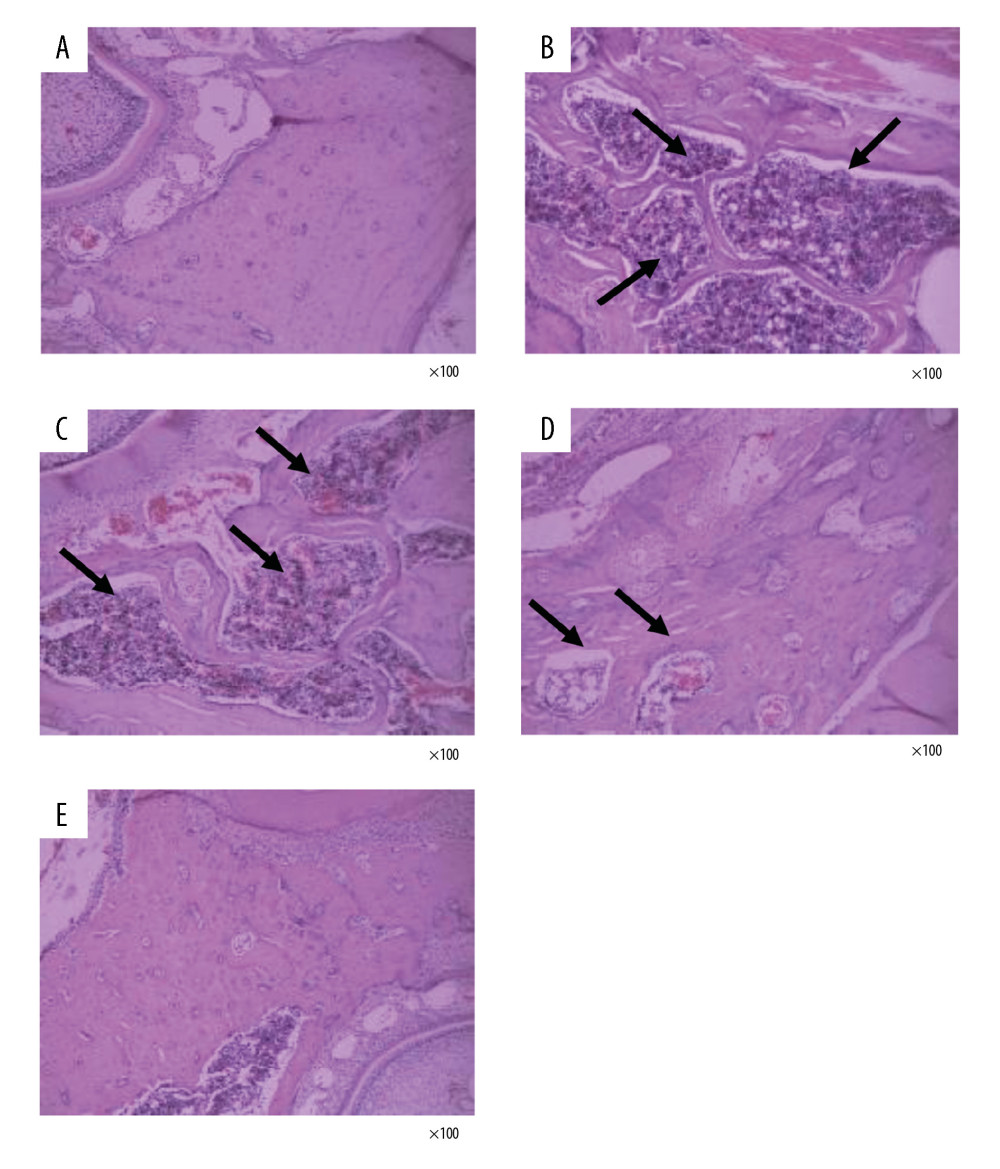 Figure 5. Drynaria total flavonoids (DTF) improved the histopathological changes in rat mandibles after ovary removal. The histopathological changes of the mandible of each group were investigated by H&E staining (×100). (A) Sham group, (B) ovariectomy control (OVX) group, (C) low-dose group, (D) middle-dose group, and (E) high-dose group. The arrows indicate the osteoporotic lesions in the mandible, including abnormal bone resorption, reduced number of trabeculae, and bite-like changes.
Figure 5. Drynaria total flavonoids (DTF) improved the histopathological changes in rat mandibles after ovary removal. The histopathological changes of the mandible of each group were investigated by H&E staining (×100). (A) Sham group, (B) ovariectomy control (OVX) group, (C) low-dose group, (D) middle-dose group, and (E) high-dose group. The arrows indicate the osteoporotic lesions in the mandible, including abnormal bone resorption, reduced number of trabeculae, and bite-like changes. References
1. Wright NC, Saag KG, Dawson-Hughes B, The impact of the new National Bone Health Alliance (NBHA) diagnostic criteria on the prevalence of osteoporosis in the United States: supplementary presentation: Osteoporosis Int, 2017; 28(11); 3283-84
2. Chen P, Li Z, Hu Y, Prevalence of osteoporosis in China: A meta-analysis and systematic review: BMC Public Health, 2016; 16(1); 1039
3. Melton LJ, How many women have osteoporosis now?: J Bone Miner Res, 1995; 10(2); 175-77
4. Bonnick SL, Osteoporosis in men and women: Clin Cornerstone, 2006; 8(1); 28-39
5. Oliveira ML, Pedrosa EF, Cruz AD, Relationship between bone mineral density and trabecular bone pattern in postmenopausal osteoporotic Brazilian women: Clin Oral Investig, 2013; 17(8); 1847-53
6. Wactawski-Wende J, Periodontal diseases and osteoporosis: Association and mechanisms: Ann Periodontol, 2001; 6(1); 197-208
7. Merheb J, Temmerman A, Rasmusson L, Influence of skeletal and local bone density on dental implant stability in patients with osteoporosis: Clin Implant Dent Res, 2016; 18(2); 253-60
8. Dervis E, Oral implications of osteoporosis: Oral Surg Oral Med Oral Pathol Oral Radiol Endod, 2005; 100(3); 349-56
9. Munakata M, Tachikawa N, Honda E, Influence of menopause on mandibular bone quantity and quality in Japanese women receiving dental implants: Arch Osteoporos, 2011; 6(1–2); 51-57
10. Tella SH, Gallagher JC, Prevention and treatment of postmenopausal osteoporosis: J Steroid Biochem Mol Biol, 2014; 142; 155-70
11. Imam B, Aziz K, Khan M, Role of bisphosphonates in postmenopausal women with osteoporosis to prevent future fractures: a literature review: Cureus, 2019; 11(8); e5328
12. Wells G, Chernoff J, Gilligan JP, Krause DS, Does salmon calcitonin cause cancer? A review and meta-analysis: Osteoporos Int, 2016; 27(1); 13-19
13. Rietjens IMCM, Louisse J, Beekmann K, The potential health effects of dietary phytoestrogens: Br J Pharmacol, 2017; 174(11); 1263-80
14. Poluzzi E, Piccinni C, Raschi E, Phytoestrogens in postmenopause: The state of the art from a chemical, pharmacological and regulatory perspective: Curr Med Chem, 2014; 21(4); 417-36
15. Zhao H, Zhao N, Zheng P, Prevention and treatment of osteoporosis using Chinese Medicinal plants: Special emphasis on mechanisms of immune modulation: J Immunol Res, 2018; 2018 6345857
16. Zhang Y, Jiang J, Shen H: Drug Des Devel Ther, 2017; 11; 1881-90
17. Song SH, Zhai YK, Li CQ: Bone Rep, 2016; 5; 262-73
18. Groen JJ, Duyvensz F, Halsted JA, Diffuse alveolar atrophy of the jaw (non-inflammatory form of paradental disease) and pre-senile osteoporosis: Gerontol Clin, 1960; 2; 68-86
19. Sindeaux R, Figueiredo PT, de Melo NS, Fractal dimension and mandibular cortical width in normal and osteoporotic men and women: Maturitas, 2014; 77(2); 142-48
20. Huja SS, Fernandez SA, Hill KJ, Li Y, Remodeling dynamics in the alveolar process in skeletally mature dogs: Anat Rec A Discov Mol Cell Evol Biol, 2006; 288(12); 1243-49
21. Jonasson G, Skoglund I, Rythen M, The rise and fall of the alveolar process: Dependency of teeth and metabolic aspects: Arch Oral Biol, 2018; 96; 195-200
22. Jonasson G, Rythén M, Alveolar bone loss in osteoporosis: A loaded and cellular affair?: Clin Cosmet Investig Dent, 2016; 8; 95-103
23. Johnston BD, Ward WE, The ovariectomized rat as a model for studying alveolar bone loss in postmenopausal women: Biomed Res Int, 2015; 2015 635023
24. Yousefzadeh N, Kashfi K, Jeddi S, Ghasemi A, Ovariectomized rat model of osteoporosis: A practical guide: Excli J, 2020; 19; 89-107
25. Gambacciani M, Levancini M, Hormone replacement therapy and the prevention of postmenopausal osteoporosis: Prz Menopauzalny, 2014; 13(4); 213-20
26. Bock O, Felsenberg D, Bisphosphonates in the management of postmenopausal osteoporosis – optimizing efficacy in clinical practice: Clin Interv Aging, 2008; 3(2); 279-97
27. Koka S, Babu NM, Norell A, Survival of dental implants in post-menopausal bisphosphonate users: J Prosthodont Res, 2010; 54(3); 108-11
28. Eastell R, Treatment of postmenopausal osteoporosis: N Engl J Med, 1998; 338(11); 736-46
29. Macintyre I, Evans IMA, Hobitz HHG, Chemistry, physiology, and therapeutic applications of calcitonin: Arthritis Rheum, 1980; 23(10); 1139-47
30. Chesnut CH, Majumdar S, Newitt DC, Effects of salmon calcitonin on trabecular microarchitecture as determined by magnetic resonance imaging: Results from the QUEST study: J Bone Miner Res, 2005; 20(9); 1548-61
31. Eastell R, Rosen CJ, Black DM, Pharmacological management of osteoporosis in postmenopausal women: An endocrine society clinical practice guideline: J Clin Endocrinol Metab, 2019; 104(5); 1595-622
32. Arisawa EAL, Brandão AAH, Almeida JD, da Rocha RF, Calcitonin in bone-guided regeneration of mandibles in ovariectomized rats: Densitometric, histologic and histomorphometric analysis: Int J Oral Max Surg, 2008; 37(1); 47-53
33. Gan D, Xu X, Chen D: Med Sci Monit, 2019; 25; 5700-16
34. Guo Y, Li PF, Shu XCInvolvement of Wnt/beta-catenin signaling in the osteogenesis of bone marrow mesenchymal stem cells induced by drynaria total flavonoids: Zhonghua Yi Xue Za Zhi, 2012; 92(32); 2288-91 [in Chinese]
35. Wang X, Zhen L, Zhang G: Phytomedicine, 2011; 18(10); 868-72
36. Chang CL, Lin CS, Lai GH, Phytochemical characteristics, free radical scavenging activities, and neuroprotection of five medicinal plant extracts: Evid Based Complement Alternat Med, 2012; 2012 984295
37. Wei X, Xu A, Shen H, Xie Y, Qianggu capsule for the treatment of primary osteoporosis: Evidence from a Chinese patent medicine: BMC Complement Altern Med, 2017; 17(1); 108
38. Garg MK, Kharb S, Dual energy X-ray absorptiometry: Pitfalls in measurement and interpretation of bone mineral density: Indian J Endocrinol Metab, 2013; 17(2); 203-10
39. Turner CH, Biomechanics of bone: determinants of skeletal fragility and bone quality: Osteoporos Int, 2002; 13(2); 97-104
40. Osterhoff G, Morgan EF, Shefelbine SJ, Bone mechanical properties and changes with osteoporosis: Injury, 2016; 47(Suppl 2); S11-20
41. Yao W, Zhang H, Jiang X: Front Pharmacol, 2018; 9; 1251
42. Boyce BF, Xing L, Functions of RANKL/RANK/OPG in bone modeling and remodeling: Arch Biochem Biophys, 2008; 473(2); 139-46
43. Jabbar S, Drury J, Fordham JN, Osteoprotegerin, RANKL and bone turnover in postmenopausal osteoporosis: J Clin Pathol, 2011; 64(4); 354-57
44. McClung MR, Inhibition of RANKL as a treatment for osteoporosis: Preclinical and early clinical studies: Curr Osteoporos Rep, 2006; 4(1); 28-33
Figures
 Figure 1. Drynaria total flavonoid (DTF) has protective effects on ovariectomy-induced mandibular osteoporosis. The representative images of the right mandible of the rats in each group are shown. (A) Sham group, (B) ovariectomy control (OVX) group, (C) low-dose group, (D) middle-dose group, and (E) high-dose group. The arrow indicates the bite-like changes in the OVX group.
Figure 1. Drynaria total flavonoid (DTF) has protective effects on ovariectomy-induced mandibular osteoporosis. The representative images of the right mandible of the rats in each group are shown. (A) Sham group, (B) ovariectomy control (OVX) group, (C) low-dose group, (D) middle-dose group, and (E) high-dose group. The arrow indicates the bite-like changes in the OVX group. Figure 2. High-dose Drynaria total flavonoid (DTF) led to increased bone mineral density in ovariectomy-induced mandibular osteoporosis. The bone mineral density of the right mandible of each group was measured by a dual-energy X-ray bone densitometry. Data represent the mean±SE of each group. * P<0.05, ** P<0.01, both compared with the ovariectomy control (OVX) group.
Figure 2. High-dose Drynaria total flavonoid (DTF) led to increased bone mineral density in ovariectomy-induced mandibular osteoporosis. The bone mineral density of the right mandible of each group was measured by a dual-energy X-ray bone densitometry. Data represent the mean±SE of each group. * P<0.05, ** P<0.01, both compared with the ovariectomy control (OVX) group. Figure 3. Drynaria total flavonoid (DTF) was beneficial in restoring the maximum load of the right mandible. The maximum load of the right mandible in each group was measured. Data represent the mean±SE of each group. * P<0.05, compared with the sham surgery group; # P<0.05, compared with the ovariectomy control (OVX) group.
Figure 3. Drynaria total flavonoid (DTF) was beneficial in restoring the maximum load of the right mandible. The maximum load of the right mandible in each group was measured. Data represent the mean±SE of each group. * P<0.05, compared with the sham surgery group; # P<0.05, compared with the ovariectomy control (OVX) group. Figure 4. Drynaria total flavonoid (DTF) led to an increased mandibular cortical bone thickness. The mandibular cortical bone thickness of each group was measured. Data represent the mean±SE of each group. * P<0.05, ** P<0.01, compared with the sham surgery group; # P<0.05, compared with the ovariectomy control (OVX) group.
Figure 4. Drynaria total flavonoid (DTF) led to an increased mandibular cortical bone thickness. The mandibular cortical bone thickness of each group was measured. Data represent the mean±SE of each group. * P<0.05, ** P<0.01, compared with the sham surgery group; # P<0.05, compared with the ovariectomy control (OVX) group. Figure 5. Drynaria total flavonoids (DTF) improved the histopathological changes in rat mandibles after ovary removal. The histopathological changes of the mandible of each group were investigated by H&E staining (×100). (A) Sham group, (B) ovariectomy control (OVX) group, (C) low-dose group, (D) middle-dose group, and (E) high-dose group. The arrows indicate the osteoporotic lesions in the mandible, including abnormal bone resorption, reduced number of trabeculae, and bite-like changes.
Figure 5. Drynaria total flavonoids (DTF) improved the histopathological changes in rat mandibles after ovary removal. The histopathological changes of the mandible of each group were investigated by H&E staining (×100). (A) Sham group, (B) ovariectomy control (OVX) group, (C) low-dose group, (D) middle-dose group, and (E) high-dose group. The arrows indicate the osteoporotic lesions in the mandible, including abnormal bone resorption, reduced number of trabeculae, and bite-like changes. In Press
05 Mar 2024 : Clinical Research
Role of Critical Shoulder Angle in Degenerative Type Rotator Cuff Tears: A Turkish Cohort StudyMed Sci Monit In Press; DOI: 10.12659/MSM.943703
06 Mar 2024 : Clinical Research
Comparison of Outcomes between Single-Level and Double-Level Corpectomy in Thoracolumbar Reconstruction: A ...Med Sci Monit In Press; DOI: 10.12659/MSM.943797
21 Mar 2024 : Meta-Analysis
Economic Evaluation of COVID-19 Screening Tests and Surveillance Strategies in Low-Income, Middle-Income, a...Med Sci Monit In Press; DOI: 10.12659/MSM.943863
10 Apr 2024 : Clinical Research
Predicting Acute Cardiovascular Complications in COVID-19: Insights from a Specialized Cardiac Referral Dep...Med Sci Monit In Press; DOI: 10.12659/MSM.942612
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952