13 October 2020: Animal Study
Interaction of Orexin A and Vasopressin in the Brain Plays a Role in Blood Pressure Regulation in WKY and SHR Rats
Stanisław Kowalewski1ABDEF, Katarzyna Czarzasta1ADEF*, Liana Puchalska1CDE, Ewa Szczepańska-Sadowska1ADE, Agnieszka Wsol1BDE, Agnieszka Cudnoch-Jędrzejewska1EFGDOI: 10.12659/MSM.926825
Med Sci Monit 2020; 26:e926825
Abstract
BACKGROUND: Orexin A (OXA) and vasopressin (AVP) exert a central hypertensive effect due to an increase in sympathetic nerve activity. To date, little is known about the interaction of these 2 neuropeptides in the central regulation of blood pressure. The present study compared the consequences of infusion into the left cerebral ventricle (ICV) of OXA on mean arterial blood pressure (MABP) in normotensive (WKY) and spontaneously hypertensive (SHR) rats, and explored whether the central pressor action of OXA in these 2 strains depends on activation of brain AVP V1a receptors (V1aR).
MATERIAL AND METHODS: Ten groups of experiments were performed on 12-week-old WKY and SHR rats implanted with ICV cannulas for infusion of OXA (3 nmol) and V1aR antagonist (V1aRANT, 500 ng), administered separately and together. Levels of V1aR and OXR in the medulla oblongata of WKY and SHR rats were compared in separate series.
RESULTS: We found that: 1) OXA significantly increased MABP only in WKY rats, 2) V1aRANT prevented an increase in MABP induced by OXA in WKY rats and decreased MABP in SHR rats, 3) OXA abolished the hypotensive action of V1aRANT in SHR rats, and 4) SHR rats had significantly higher levels of OX1R and V1aR proteins and OX1R mRNA in the brain medulla.
CONCLUSIONS: The present study shows that OXA and AVP can interact in the brain to affect blood pressure regulation, and that this interaction differs in normotension and hypertension.
Keywords: arterial pressure, orexin receptors, Rats, Inbred SHR, Rats, Inbred WKY, Receptors, Vasopressin, Blood Pressure, Brain, orexins, Species Specificity, Sympathetic Nervous System, Vasopressins
Background
A growing number of studies provide evidence that orexin (OX) and vasopressin (AVP) exert significant central pressor effects and that their pro-hypertensive actions are altered in various types of hypertension [1–3]. Orexins are synthesized in the brain in 2 isoforms, orexin A (OXA, hypocretin 1) and orexin B (OXB, hypocretin 2), both having the same polypeptide precursor (prepro-orexin) [4]. Two types of orexin receptors (orexin-1 receptor [OX1R] and orexin-2 receptor [OX2R]) are present in several
With regard to the role of AVP in hypertension and its interaction with orexin, it has been shown that SHR rats have higher levels of systemic vasopressin [1]. In addition, there is evidence that ICV injection of OXA increases the AVP mRNA level in rats [6]. However, there is still no information on whether the brain vasopressinergic system, in particular the AVP V1a receptors (V1aR), which are the main receptors mediating the central pressor effects of vasopressin [7], interacts with the brain OXA in the regulation of blood pressure, and whether this interaction is altered in spontaneous hypertension. Therefore, the main goal of this study was to compare the outcome of centrally administered orexin A (OXA) on arterial blood pressure in WKY and SHR rats, and to determine whether putative differences in the responsiveness of WKY and SHR rats to pressor action of orexin depend on differences in stimulation of brain V1aR in these strains. In addition, we assessed the expression of OX1R and V1aR in the brain medulla of WKY and SHR rats to assess whether differences in the regulation of MABP by OXA and AVP in these strains results from differences in OX1R and V1aR expressions.
Material and Methods
RATS:
The experiment was carried out on 25 normotensive (WKY/Clzd) and 29 spontaneously hypertensive (SHR/Clzd) 12-week-old male rats. Before the experiment, the rats were adapted to separate cages in the following laboratory conditions: 12 h light-dark cycle, temperature 21–23°C; humidity ±60%;), and provided with standard diet and water
EXPERIMENTAL DESIGN:
The study was separated into 2 stages. Stage I consisted of experiments (ICV infusions and cardiovascular measurements) performed on 5 groups of WKY rats and 5 groups of SHR rats. Stage II consisted of experiments performed on separate groups of animals and was designed to evaluate OX1R and V1aR expression in the brain of WKY and SHR rats.
EXPERIMENTAL GROUPS OF STAGE I:
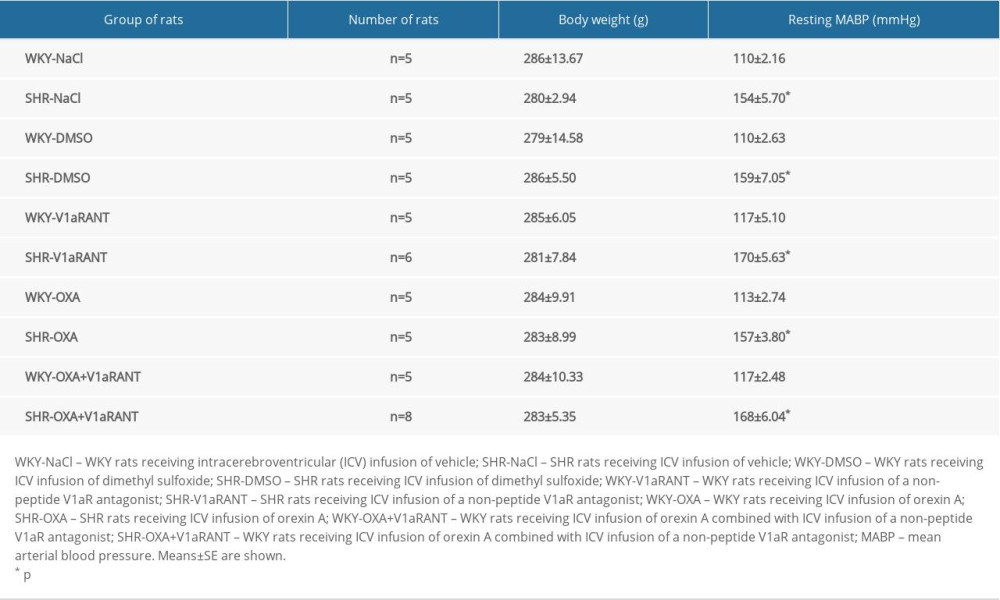
The course of experiments performed in stage I of the study is shown in Figure 1. The rats were classified into 10 groups: 1) WKY rats (n=5) receiving intracerebroventricular (ICV) infusion of vehicle (saline – 0.9% NaCl; 10 μl/15 min; Figure 1A; WKY-NaCl); 2) SHR rats (n=5) receiving the same ICV infusion of saline as in group 1 (Figure 1A; SHR-NaCl); 3) WKY rats (n=5) receiving ICV infusion of OXA (Orexin A, Human, Rat, Mouse; Phoenix Pharmaceuticals, Inc. 003–30; 3 nmol/10 μl 0.9% NaCl/15 min; Figure 1A; WKY-OXA); 4) SHR rats (n=5) receiving the same ICV infusion of OXA as in group 3 (Figure 1A; SHR-OXA); 5) WKY rats (n=5) receiving ICV infusion of DMSO (dimethyl sulfoxide; Sigma-Aldrich D8418; 10 μl 10% DMSO in 0.9% NaCl/15 min; Figure 1B; WKY-DMSO); 6) SHR rats (n=5) receiving the same ICV infusion of DMSO as in group 5 (Figure 1B; SHR-DMSO); 7) WKY rats (n=5) receiving ICV infusion of a non-peptide V1aR antagonist (V1aRANT; SR49059; Sanofi Avensis; Sigma-Aldrich S5701; 500 ng/10 μl) in DMSO (Figure 1B; WKY-V1aRANT); 8) SHR rats (n=6) receiving the same ICV infusion of V1aRANT in DMSO as in group 7 (Figure 1B; SHR-V1aRANT); 9) WKY rats (n=5) receiving ICV infusion of OXA (3 nmol/10 μl 0.9% NaCl/15 min) combined with ICV infusion of V1aRANT (500 ng/10 μl of 10% DMSO/15 min), which was introduced 20 min after the start of OXA infusion (Figure 1C; WKY–OXA+V1aRANT); 10) SHR rats (n=8) receiving the same ICV infusion of OXA combined with ICV infusion of V1aRANT as in group 9.
The effective doses of chemical compounds were established in preliminary experiments. In experiments with ICV infusions of SR49059 (groups 5–10), DMSO was used as a vehicle because this compound appears to be a good vehicle for nonlinear peptide antagonists [8,9]. In preliminary experiments, we did not find significant differences between resting MABP fluctuations in rats administered an ICV infusion of either 0.9% NaCl or 10% DMSO in 0.9% NaCl (WKY-NaCl, n=5, MABP=110±2.16 mm Hg vs. WKY-DMSO+NaCl, n=5, MABP=110±2.63; SHR-NaCl, n=5, MABP=154±5.70 mmHg vs. SHR-DMSO+NaCl, n=5, MABP=159±7.05 mmHg).
IMPLANTATION OF THE BRAIN CANNULA: Twelve-week-old rats were anaesthetized (Ketamine/Xylazine: 75 mg/1000 g body wt i.p./7 mg/1000 g body wt i.p., respectively). The implantations of the left cerebral ventricle cannula were carried out in accordance with the method described in our previous studies [10–12]. Following surgery, the animals were housed in individual cages and were administered a painkiller (Paracetamol 3 mg/1000 g body wt., orally for 2 days) and an antibiotic (Baytril 2.5% 5 mg/1000 g body wt., s.c. injection for 2 days).
IMPLANTATION OF THE AORTIC CATHETER: One week after ICV cannula implantation, the rats were anesthetized with Ketamine/Xylazine, as described above, and an arterial catheter was introduced into the abdominal aorta via the femoral artery to measure blood pressure, as was described earlier [10–12]. After surgery, the rats received an analgesic and an antibiotic, as described above.
ARTERIAL BLOOD PRESSURE MEASUREMENTS:
Systolic, diastolic, and pulse pressure were monitored using a recording system (BIOPAC MP100). MABP (mmHg) was specified as the space below the arterial pressure curve divided by the heart cycle period. During the experiment, the rats stayed in their home cages and at least 10 min were allowed for adaptation to the ICV cannula insertion and stabilization of arterial blood pressure. Averaged results from the 10-min resting period preceding ICV infusions were similar to averaged results from 5-min central administration.
ANIMALS AND TISSUE DISSECTION: Experiments of stage II were performed on 12-week-old WKY (n=7) and SHR (n=7) male rats. The rats were anesthetized with ketamine, as described in part I, and sacrificed by decapitation. The brain and the brain medulla fragments were harvested according to a previously described procedure [13]. The frontal cuts to isolate the brain medulla were made at −11.0 mm and −15.0 mm caudally from the bregma.
REAL-TIME PCR ANALYSIS OF THE OXR1 AND V1AR: Fragments of the medulla oblongata homogenized and the RNA were obtained as described earlier [14]. RT-PCR analysis was performed with the TaqMan® RNA-to-Ct™ 1-Step Kit and the starter for the following gene: rat OXR1 (Hcrtr1, Rn00565032_m1; Life Technologies); rat V1aR (Avpr1a, Rn00583910_m1; Life Technologies) tagged with a FAM pigment, a starter for the rat GADPH (Rn01775763_g1; Life Technologies) tagged with a VIC pigment. The RT-PCR analysis was performed as described previously [12,14], using a ViiA™ 7 Real-Time PCR System thermocycler (Life Technologies). The comparative gene expressions are shown as ΔCt in arbitrary units.
WESTERN BLOT ANALYSIS OF THE OX1R AND V1AR PROTEINS LEVELS:
Fragments of the medulla oblongata were homogenized and western blot analysis was performed according to the protocol described earlier [14]. We used the following antibodies: a primary goat polyclonal antibody against OX1R (ab224368; Abcam), a primary rabbit polyclonal antibody against V1aR (sc-30025; Santa Cruz Biotechnology), a primary rabbit polyclonal antibody anti-β Actin (ab8227; Abcam), and a secondary antibody: mouse anti-rabbit conjugated to Horseradish Peroxidase (HRP) (sc-2357; Santa Cruz Biotechnology). The particular bands were shown using the colorimetric technique using the Amplified Opti-4CN Substrate Kit (Bio-Rad), and measured by densitometry using the ChemiDoc™ MP Imaging System (Bio-Rad). OX1R and V1aR protein levels were standardized by β-actin and described as a comparative relationship.
STATISTICAL ANALYSIS:
Statistical analysis was carried out using Statistica 13.3 software. The significance of differences between the tested variables was calculated using one-way ANOVA with the post hoc Tukey test for normal distributions and ANOVA with the rank sign Kruskal-Wallis test with Dunn’s post hoc test for non-parametric data. The differences were regarded as significant if p was <0.05. Results are expressed as means±standard errors (SE).
Results
CHARACTERISTICS OF RATS:
Table 1 shows that the body weight was similar in all of the experimental groups of rats. The resting MABP values were significantly higher in SHR rats in comparison with WKY rats (Table 1).
IMPACT OF CENTRAL INFUSION OF OXA ON MEAN ARTERIAL BLOOD PRESSURE IN WKY AND SHR RATS:
The data presented in Figure 2A show that ICV infusion of the same doses of OXA elicited growth in MABP in WKY rats but it did not elicit changes in MABP in SHR rats. ANOVA for groups 1–4 demonstrated that the differences in MABP responses between WKY and SHR rats receiving either saline or OXA started to be significant at 30 min of OXA infusion and persisted until the end of the infusion {ANOVA at 30 min: [F(3,16)=6.938, p<0.01], at 40 min: [F(3,16)=8.819, p<0.01], and at 50 min: [F(3,16)=14.132, p<0.001]}. As shown in Figure 2A, there were significant differences at the same time points between ΔMABP in the WKY rats receiving ICV infusions of saline and OXA (p<0.001) and between the WKY and SHR rats receiving ICV infusions of OXA (p<0.001). ICV infusion of saline alone did not produce significant changes in ΔMABP in the WKY rats or in the SHR rats.
IMPACT OF CENTRAL INFUSION OF V1ARANT ON MEAN ARTERIAL BLOOD PRESSURE IN WKY AND SHR RATS:
ANOVA showed significant differences between ΔMABP in WKY and SHR rats receiving ICV infusions of DMSO and V1aRANT [F(3,17)=18.389, p<0.001]. A comparison of blood pressure changes in groups 5–8 revealed that in SHR rats, ICV infusion of V1aRANT significantly reduced MABP below baseline, while in WKY rats, infusion of the same dose of V1aRANT was not effective. Changes in MABP in SHR rats receiving ICV infusion of V1aRANT differed significantly from changes that took place during ICV infusion of V1aRANT in WKY rats (p<0.001) (Figure 2B) and from changes in MABP induced by ICV infusion of the vehicle in WKY and SHR rats (Figure 2B).
EFFECT OF COMBINED ICV INFUSIONS OF OXA AND V1ARANT ON MABP IN WKY AND SHR RATS:
An analysis of ΔMABP in groups 7–10 (Figure 2C) showed that, both in WKY rats and in SHR rats, changes in MABP that took place during the combined infusion of OXA and V1aRANT significantly differ from those induced by separate infusion of these compounds {ANOVA: [F(3,20)=3.657, p<0.05]}. No significant differences in ΔMABP were found between the WKY rats receiving ICV infusion of OXA together with V1aRANT (Figure 2C). However, the post hoc analysis revealed that infusion of V1aRANT during the time corresponding to the maximum central pressor effect of OXA showed a decreasing trend in the central pressor action of OXA in WKY rats (Figure 2A, 2C). Moreover, ICV infusion of OXA abolished the hypotensive effect of V1aRANT in SHR rats (Figure 2C, p<0.05).
EXPRESSION OF OX1R AND V1AR IN THE BRAIN MEDULLA OF WKY AND SHR RATS:
Expression of OX1R mRNA was significantly higher [F(1,12)=13.151, p<0.01] in the medulla oblongata of SHR rats than in the brain medulla of WKY rats (Figure 3A). There was also a higher OX1R protein level in the brain medulla of SHR rats than in the medulla of WKY rats [F(1,12)=5.670, p<0.05] (Figure 3B). Expression of V1aR mRNA in the brain medulla of WKY and SHR rats was similar (Figure 3C), while the V1aR protein level was significantly higher [F(1,12)=7.150, p<0.05] in the brain medulla of SHR rats than in the medulla of WKY rats (Figure 3D).
Discussion
The most important new information emerging from the present study is that OXA infusion into the periventricular system causes a different pressure response in spontaneously hypertensive (SHR) rats compared with WKY normotensive rats. We also provide evidence that centrally acting OXA interacts with vasopressin in the regulation of blood pressure in WKY and SHR rats, but the functional significance of this interaction in these 2 strains of rats differs. The finding that ICV infusion of OXA or its topical administration into the pressor regions of the brain elevates blood pressure agrees with previous findings on normotensive Wistar and Sprague-Dawley rats [3,15,16]. In the present study, ICV infusion of OXA caused a significant and long-lasting increase in MABP only in normotensive WKY rats, whereas SHR rats treated with the same dose of OXA and exposed to the same experimental procedures did not respond with significant changes in MABP. In previous studies, the sensitivity of WKY and SHR rats to centrally acting orexins was not compared; however, it has been found that blockade of orexin receptors by the dual orexin receptor antagonist almorexant, administered orally, significantly decreases blood pressure and CSF norepinephrine levels in SHR rats, whereas it is not effective in WKY rats. These findings suggested enhanced activation of orexin receptors in SHR by endogenous orexins [5]. This assumption is supported by our finding in the present study showing higher expressions of OX1R mRNA and protein in the brain medulla of SHR in comparison with WKY rats, and by the experiments of Lee et al. [17] demonstrating higher expression of hypothalamic orexin A-immunoreactive (OXA-IR) cells in SHR rats than in WKY rats. Therefore, the relative insensitivity of SHR rats to the pressor action of OXA cannot be explained by the downregulation of OX1R, but it probably results from the maximum activation of the brain orexigenic neurons by endogenous OXA. In addition, it has also been shown that the orexinergic system affects the regulation of blood pressure due to increased activity of the sympathetic nervous system [3,4,18,19]. Increased sympathetic activity has been shown to be the main mechanism underlying the development and maintenance of hypertension in SHR rats [4,20]. Based on the above data, it can be assumed that the sympathetic nervous system activity of SHR rats is at a maximum and cannot be further stimulated by exogenic OXA. Therefore, in this study, no MABP changes were observed in SHR rats after ICV infusion with OXA. Lee et al. [17] showed that application of larger (50 pmol) doses of orexin-2R agonist directly to the RVLM elicits higher pressor responses in 16-week-old SHR rats than in WKY rats. Therefore, it is possible that the responsiveness to the pressor action of OXA may depend on the dose of this peptide and on the age of the tested animals.
Vasopressin is one of the factors that can determine pressor sensitivity to centrally acting OXA. It is known that AVP, acting via V1aR in the brain and periphery, exerts a key influence in the control of blood pressure, and that its release and pressor action are enhanced in SHR rats [1]. There is also evidence that OXA stimulates AVP synthesis in rats, as shown by elevation of the hypothalamic AVP mRNA level after its ICV administration [6]. The present study showed that blockade of the central V1aR significantly reduced the resting MABP in SHR rats but it was not effective in WKY rats. We also found that SHR rats have a higher level of protein V1aR in the brain medulla than WKY rats. These findings support the hypothesis that the central V1aR are more intensively engaged in MABP regulation in SHR rats than in WKY rats.
The results presented in Figure 2 show remarkable interactions between OXA and AVP in the central regulation of MABP in WKY and SHR rats. It should be noted that in the experiments in which the interaction was analyzed, the ICV infusion of V1aRANT was introduced 20 min after the start of OXA administration, immediately preceding the significant elevation of MABP after ICV infusion of OXA alone in WKY rats. The present study shows that ICV administration of OXA in WKY rats does not elevate MABP if it is associated with ICV administration of V1aRANT, which suggests that the pressor effect of OXA is mediated by the stimulation of V1aR (Figure 2A, 2C). In SHR rats, the administration of OXA abolished the hypotensive effect of ICV infusion of V1aRANT administered alone (Figure 2C), which shows that OXA and AVP interact in blood pressure regulation and that the increased delivery of OXA may compensate for the reduced stimulation of V1aR.
At present, the mechanism underlying the interaction between orexin and vasopressin has not been established and it is not known whether the interaction occurs directly between OXA and AVP or whether it is mediated by some other compounds. In this context, the role of angiotensin II and opioid peptides should be considered, as angiotensin II is a potent stimulator of vasopressin secretion [7] and indirect evidence shows that stimulation of AT1 receptors may be necessary for the appropriate prepro-orexin expression in the rat brain [21]. The opioid peptide dynorphin is co-released with orexin and reduces the postsynaptic effects of orexin and counteracts its cellular and behavioral effects [22]. Dynorphin levels in the hypothalamus, periaqueductal gray matter, and hippocampus differ in SHR and WKY rats [23] and ICV administration of dynorphin causes a significant decrease in blood pressure and in the secretion of vasopressin [24,25]. Therefore, it is possible that the interaction of orexin and vasopressin are modulated by dynorphin A, and it is possible that this modulation differs between WKY and SHR rats.
Conclusions
The present study provides evidence for significant intra-brain interaction of orexin and vasopressin in blood pressure regulation in WKY and SHR rats. Under resting conditions, the interaction between the endogenous vasopressinergic and orexinergic systems plays an essential role only in SHR rats, whereas it becomes significant in WKY rats during activation. Although the above observations are primarily of a basic research nature, they may also have a significant clinical implication, especially for patients with resistant hypertension. It has not yet been explained why some hypertensive patients do not respond to the recommended treatment. The present results suggest that the orexinergic system and its interactions with other neuropeptides, including vasopressin, may be involved in the mechanism of drug-resistance hypertension, because ICV OXA infusion did not cause changes in MABP, but abolished the hypotensive effect of V1aRANT in SHR rats.
Figures
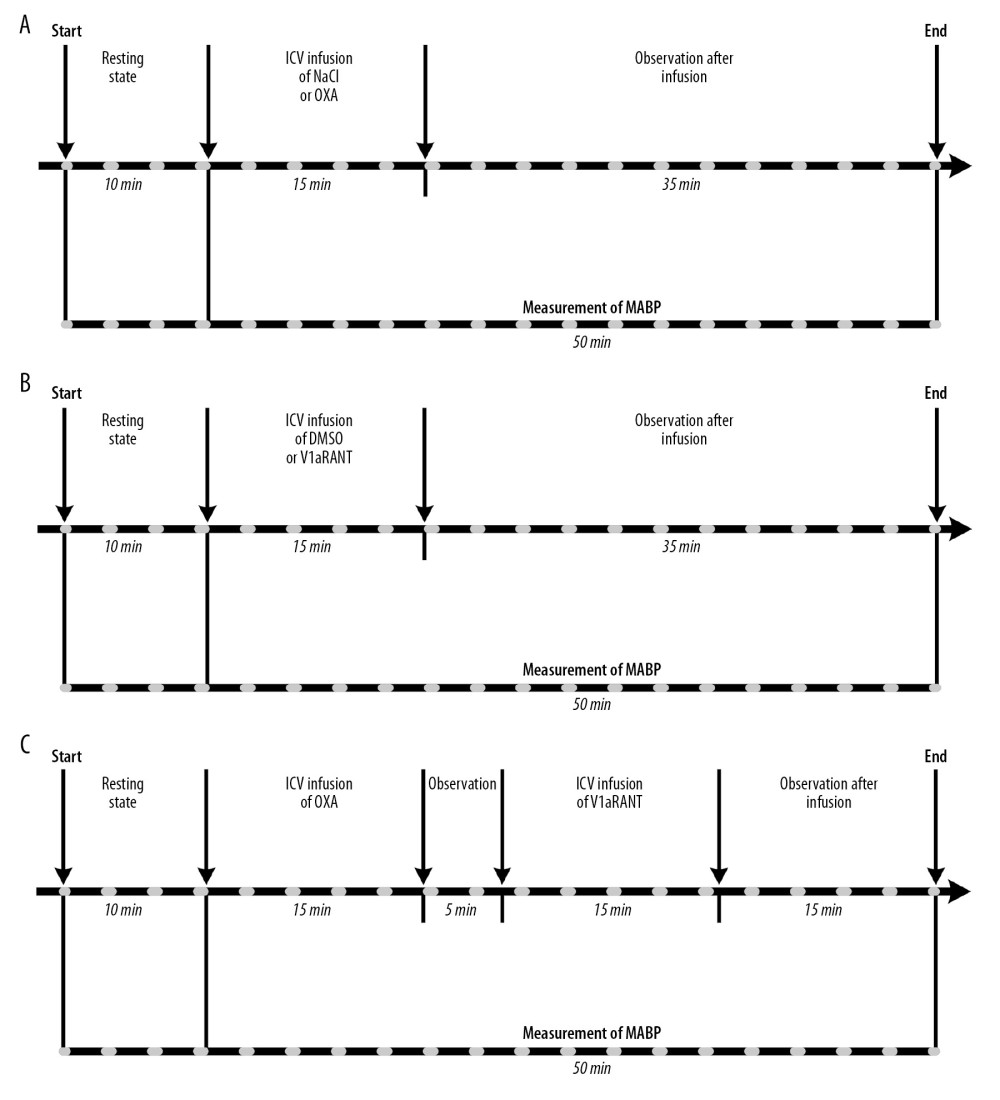 Figure 1. Design of experiments with intracerebroventricular (ICV) infusions of orexin A in saline (NaCl) or NaCl alone (A), V1aR antagonist (V1aRANT) in dimethyl sulfoxide (DMSO)or DMSO alone (B), and OXA in DMSO followed by V1aRANT in DMSO (C). MABP – mean arterial blood pressure.
Figure 1. Design of experiments with intracerebroventricular (ICV) infusions of orexin A in saline (NaCl) or NaCl alone (A), V1aR antagonist (V1aRANT) in dimethyl sulfoxide (DMSO)or DMSO alone (B), and OXA in DMSO followed by V1aRANT in DMSO (C). MABP – mean arterial blood pressure. 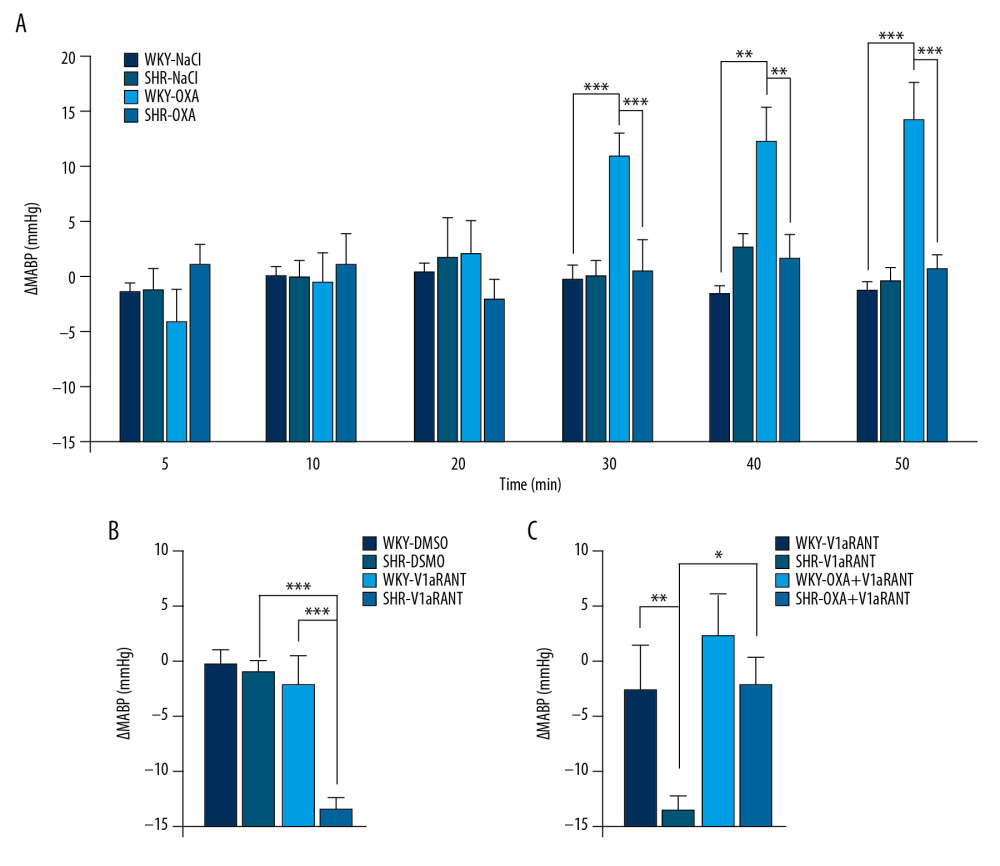 Figure 2. Changes in mean arterial blood pressure (ΔMABP) during ICV infusion of orexin A in saline (NaCl) or NaCl alone (A), V1aR antagonist (V1aRANT) in dimethyl sulfoxide (DMSO)or DMSO alone (B), and OXA in NaCl followed by V1aRANT in DMSO (C). SHR – spontaneously hypertensive rats; WKY – Wistar Kyoto rats. Means±SE are shown. ** p<0.01; *** p<0.001.
Figure 2. Changes in mean arterial blood pressure (ΔMABP) during ICV infusion of orexin A in saline (NaCl) or NaCl alone (A), V1aR antagonist (V1aRANT) in dimethyl sulfoxide (DMSO)or DMSO alone (B), and OXA in NaCl followed by V1aRANT in DMSO (C). SHR – spontaneously hypertensive rats; WKY – Wistar Kyoto rats. Means±SE are shown. ** p<0.01; *** p<0.001. 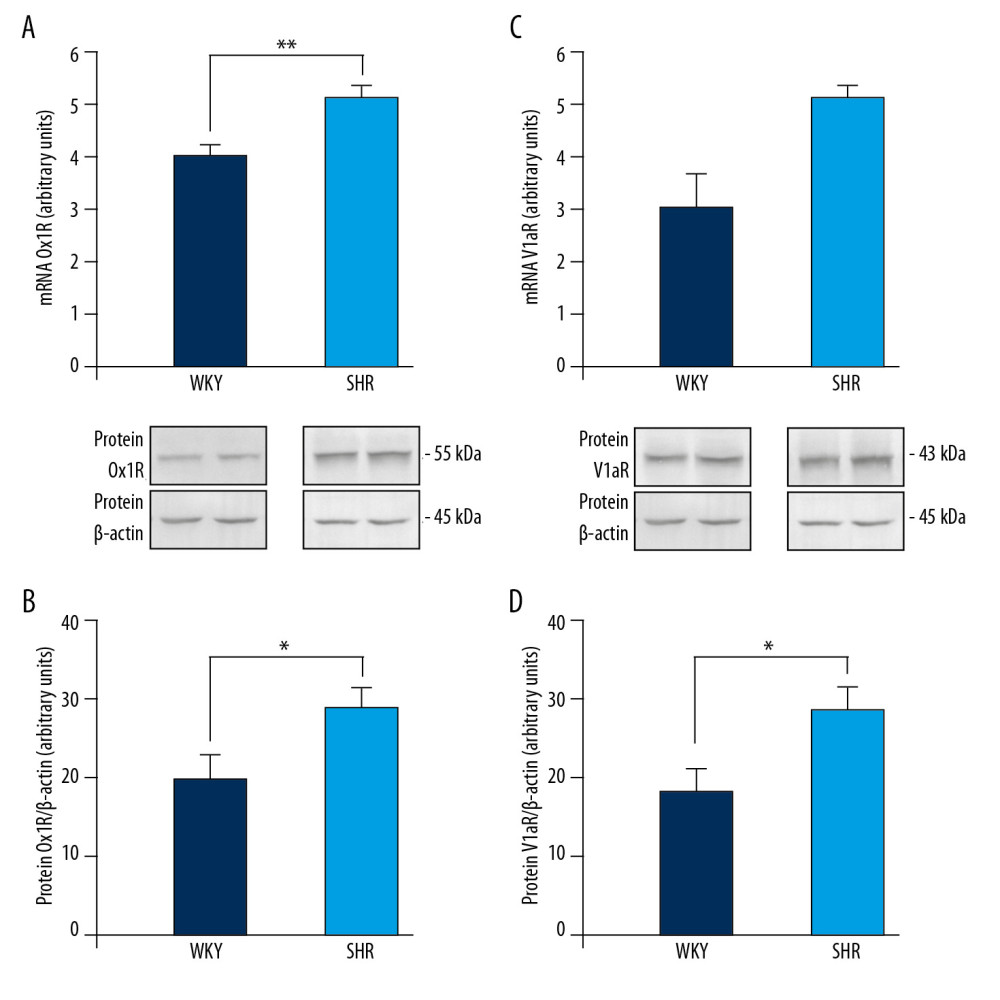 Figure 3. Expression of orexin-1 receptor (OX1R) mRNA (A) and protein (B), and vasopressin V1a receptor (V1aR) mRNA (C) and protein (D) in the brain medulla of spontaneously hypertensive (SHR) and Wistar Kyoto (WKY) rats. Means±SE are shown. * p<0.05, ** p<0.01.
Figure 3. Expression of orexin-1 receptor (OX1R) mRNA (A) and protein (B), and vasopressin V1a receptor (V1aR) mRNA (C) and protein (D) in the brain medulla of spontaneously hypertensive (SHR) and Wistar Kyoto (WKY) rats. Means±SE are shown. * p<0.05, ** p<0.01. References
1. Burrell LM, Risvanis J, Dean RG, Age-dependent regulation of renal vasopressin V(1A) and V2 receptors in rats with genetic hypertension: Implications for the treatment of hypertension: J Am Soc Hypertens, 2013; 7; 3-13
2. Lee YH, Dai YW, Huang SC, Blockade of central orexin 2 receptors reduces arterial pressure in spontaneously hypertensive rats: Exp Physiol, 2013; 98; 1145-55
3. Rani M, Kumar R, Krishan P, Implicating the potential role of orexin in hypertension: Naunyn Schmiedebergs Arch Pharmacol, 2017; 390; 667-76
4. Huber MJ, Chen QH, Shan Z, The orexin system and hypertension: Cell Mol Neurobiol, 2018; 38; 385-91
5. Li A, Hindmarch CC, Nattie EE, Paton JF, Antagonism of orexin receptors significantly lowers blood pressure in spontaneously hypertensive rats: J Physiol, 2013; 591; 4237-48
6. Al-Barazanji KA, Wilson S, Baker J, Central orexin-A activates hypothalamic-pituitary-adrenal axis and stimulates hypothalamic corticotropin releasing factor and arginine vasopressin neurones in conscious rats: J Neuroendocrinol, 2001; 13; 421-24
7. Szczepanska-Sadowska E, Zera T, Sosnowski P, Vasopressin and related peptides; Potential value in diagnosis, prognosis and treatment of clinical disorder: Curr Drug Metab, 2017; 18; 306-45
8. Kleindienst A, Dunbar JG, Glisson R, Effect of dimethyl sulfoxide on blood-brain barrier integrity following middle cerebral artery occlusion in the rat: Acta Neurochir Suppl, 2006; 96; 258-62
9. Wsol A, Szczepanska-Sadowska E, Kowalewski S, Oxytocin differently regulates pressor responses to stress in WKY and SHR rats: The role of central oxytocin and V1a receptors: Stress, 2014; 17; 117-25
10. Cudnoch-Jedrzejewska A, Szczepanska-Sadowska E, Dobruch J, Brain vasopressin V(1) receptors contribute to enhanced cardiovascular responses to acute stress in chronically stressed rats and rats with myocardial infarcton: Am J Physiol Regul Integr Comp Physiol, 2010; 298; R672-80
11. Cudnoch-Jedrzejewska A, Gomolka R, Szczepanska-Sadowska E, High-fat diet and chronic stress reduce central pressor and tachycardic effects of apelin in Sprague-Dawley rats: Clin Exp Pharmacol Physiol, 2015; 42; 52-62
12. Czarzasta K, Cudnoch-Jedrzejewska A, Szczepanska-Sadowska E, The role of apelin in central cardiovascular regulation in rats with post-infarct heart failure maintained on a normal fat or high fat diet: Clin Exp Pharmacol Physiol, 2016; 43; 983-94
13. Milik E, Szczepanska-Sadowska E, Dobruch J, Altered expression of V1a receptors mRNA in the brain and kidney after myocardial infarction and chronic stress: Neuropeptides, 2014; 48; 257-66
14. Czarzasta K, Wojno O, Zera T, The influence of post-infarct heart failure and high fat diet on the expression of apelin APJ and vasopressin V1a and V1b receptors: Neuropeptides, 2019; 78; 101975
15. Samson WK, Bagley SL, Ferguson AV, White MM, Hypocretin/orexin type 1 receptor in brain: Role in cardiovascular control and the neuroendocrine response to immobilization stress: Am J Physiol Regul Integr Comp Physiol, 2007; 292; R382-87
16. Smith PM, Samson WK, Ferguson AV, Cardiovascular actions of orexin-A in the rat subfornical organ: J Neuroendocrinol, 2007; 19; 7-13
17. Lee YH, Tsai MC, Li TL, Spontaneously hypertensive rats have more orexin neurons in the hypothalamus and enhanced orexinergic input and orexin 2 receptor-associated nitric oxide signalling in the rostral ventrolateral medulla: Exp Physiol, 2015; 100; 993-1007
18. Carrive P, Orexin, orexin receptor antagonists and central cardiovascular control: Front Neurosci, 2013; 7; 257
19. Li A, Nattie E, Orexin, cardio-respiratory function, and hypertension: Front Neurosci, 2014; 8; 22
20. Judy WV, Watanabe AM, Henry DP, Sympathetic nerve activity: Role in regulation of blood pressure in the spontaneously hypertensive rat: Circ Res, 1976; 38; 21-29
21. Müller-Fielitz H, Lau M, Geißler C, Preventing leptin resistance by blocking angiotensin II AT1 receptors in diet-induced obese rats: Br J Pharmacol, 2015; 172; 857-68
22. Matzeu A, Kallupi M, George O, Dynorphin counteracts orexin in the paraventricular nucleus of the thalamus: Cellular and behavioral evidence: Neuropsychopharmacology, 2018; 43; 1010-20
23. Tan-No K, Terenius L, Silberring J, Nylander I, Levels of dynorphin peptides in the central nervous system and pituitary gland of the spontaneously hypertensive rat: Neurochem Int, 1997; 31; 27-32
24. Haaf JA, Maigret C, Andringa-Bakker EA: Acta Endocrinol (Copenh), 1987; 114; 96-101
25. Wells T, Forsling ML, Kappa-opioid modulation of vasopressin secretion in conscious rats: J Endocrinol, 1991; 129; 411-16
Figures
 Figure 1. Design of experiments with intracerebroventricular (ICV) infusions of orexin A in saline (NaCl) or NaCl alone (A), V1aR antagonist (V1aRANT) in dimethyl sulfoxide (DMSO)or DMSO alone (B), and OXA in DMSO followed by V1aRANT in DMSO (C). MABP – mean arterial blood pressure.
Figure 1. Design of experiments with intracerebroventricular (ICV) infusions of orexin A in saline (NaCl) or NaCl alone (A), V1aR antagonist (V1aRANT) in dimethyl sulfoxide (DMSO)or DMSO alone (B), and OXA in DMSO followed by V1aRANT in DMSO (C). MABP – mean arterial blood pressure. Figure 2. Changes in mean arterial blood pressure (ΔMABP) during ICV infusion of orexin A in saline (NaCl) or NaCl alone (A), V1aR antagonist (V1aRANT) in dimethyl sulfoxide (DMSO)or DMSO alone (B), and OXA in NaCl followed by V1aRANT in DMSO (C). SHR – spontaneously hypertensive rats; WKY – Wistar Kyoto rats. Means±SE are shown. ** p<0.01; *** p<0.001.
Figure 2. Changes in mean arterial blood pressure (ΔMABP) during ICV infusion of orexin A in saline (NaCl) or NaCl alone (A), V1aR antagonist (V1aRANT) in dimethyl sulfoxide (DMSO)or DMSO alone (B), and OXA in NaCl followed by V1aRANT in DMSO (C). SHR – spontaneously hypertensive rats; WKY – Wistar Kyoto rats. Means±SE are shown. ** p<0.01; *** p<0.001. Figure 3. Expression of orexin-1 receptor (OX1R) mRNA (A) and protein (B), and vasopressin V1a receptor (V1aR) mRNA (C) and protein (D) in the brain medulla of spontaneously hypertensive (SHR) and Wistar Kyoto (WKY) rats. Means±SE are shown. * p<0.05, ** p<0.01.
Figure 3. Expression of orexin-1 receptor (OX1R) mRNA (A) and protein (B), and vasopressin V1a receptor (V1aR) mRNA (C) and protein (D) in the brain medulla of spontaneously hypertensive (SHR) and Wistar Kyoto (WKY) rats. Means±SE are shown. * p<0.05, ** p<0.01. In Press
06 Mar 2024 : Clinical Research
Comparison of Outcomes between Single-Level and Double-Level Corpectomy in Thoracolumbar Reconstruction: A ...Med Sci Monit In Press; DOI: 10.12659/MSM.943797
21 Mar 2024 : Meta-Analysis
Economic Evaluation of COVID-19 Screening Tests and Surveillance Strategies in Low-Income, Middle-Income, a...Med Sci Monit In Press; DOI: 10.12659/MSM.943863
10 Apr 2024 : Clinical Research
Predicting Acute Cardiovascular Complications in COVID-19: Insights from a Specialized Cardiac Referral Dep...Med Sci Monit In Press; DOI: 10.12659/MSM.942612
06 Mar 2024 : Clinical Research
Enhanced Surgical Outcomes of Popliteal Cyst Excision: A Retrospective Study Comparing Arthroscopic Debride...Med Sci Monit In Press; DOI: 10.12659/MSM.941102
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952