14 November 2020: Clinical Research
Association of Carotid Plaque and Serum Lipoprotein-Associated Phospholipase A2 (LP-PLA2) with Postoperative Delirium in Geriatric Patients Undergoing Hip Replacement: A Prospective Cohort Study
Tiantian Wan1ABCDEF, Penghui Wei2ABCDE, Yong Yao3ABCDE, Hui Liu4BCE, Jianjun Li2ABCDEFG*DOI: 10.12659/MSM.927763
Med Sci Monit 2020; 26:e927763
Abstract
BACKGROUND: The aim of this study was to investigate the relationships among carotid plaque (CP), serum lipoprotein-associated phospholipase (LP-PLA2), and POD in elderly patients.
MATERIAL AND METHODS: Sixty-two elderly patients undergoing hip replacement with spinal-epidural anesthesia were divided into CP and non-CP groups based on the preoperative presence or absence of carotid atherosclerotic plaques, as assessed by ultrasound. POD was diagnosed by means of the Confusion Assessment Method (CAM). Blood samples were collected (preoperatively, postoperatively, and postoperative day 2) for the assessment of serum LP-PLA2 by enzyme-linked immunosorbent assay. The CP group was further divided into POD and no-POD subgroups based on the occurrence of POD.
RESULTS: The incidence of POD was higher in the CP group than in the non-CP group (P<0.05). While the LP-PLA2 level did not significantly differ between CP and non-CP groups preoperatively (P>0.05), it was higher in the CP group than in the non-CP group postoperatively and on postoperative day 2 (P<0.05). In the CP group, the LP-PLA2 level did not significantly differ between the subgroups preoperatively or postoperatively (P>0.05), but was significantly higher in the POD subgroup than in the no-POD subgroup on postoperative day 2 (P<0.05). Furthermore, the LP-PLA2 level on postoperative day 2 was an independent risk factor for POD (odds ratio: 1.03, 95% confidence interval: 1.00-1.07).
CONCLUSIONS: The preoperative presence of carotid plaque is closely associated with a higher incidence of POD. The potential mechanism may involve the increased expression of LP-PLA2 in the serum, which can lead to plaque destabilization and subsequent inflammatory cascades.
Keywords: 1-Alkyl-2-acetylglycerophosphocholine Esterase, carotid stenosis, Delirium, Geriatrics, Arthroplasty, Replacement, Hip, Carotid Arteries, Incidence, Logistic Models, Plaque, Atherosclerotic, Postoperative Complications, Prospective Studies
Background
Postoperative delirium (POD) is a frequent neurological complication, manifested by abrupt and transient instability in awareness, consciousness, and cognitive and perceptual abilities. POD primarily occurs in aged individuals (≥65 years old), and is related to a high risk of morbidity and mortality, long hospital stays, and greater total cost [1]. Furthermore, patients with hip fractures have a high incidence of POD (4~53%) [2].
Carotid atherosclerosis is common in elderly patients, and vulnerable plaques are more prone to microvascular changes and embolization, causing microinfarcts and silent strokes that result in cognitive decline [3]. A recent study showed that covert perioperative stroke is associated with an increased risk of perioperative delirium [4]. However, few researchers have explored the relationship between carotid plaque (CP) and POD.
Lipoprotein-associated phospholipase LP-PLA2 has proinflammatory properties and is not only associated with the formation of atherosclerotic plaques, but can also reflect the severity and stability of atherosclerotic plaques [5]. Thus, the present study aimed to explore the relationship between CP and POD, as well as the relationship between the LP-PLA2 level and POD in elderly patients, as a potential mechanism of POD induced by CP.
Material and Methods
STUDY DESIGN:
This cohort study was approved by the Ethics Committee of Qilu Hospital of Shandong University (Qingdao). Written informed consent was obtained from all participants.
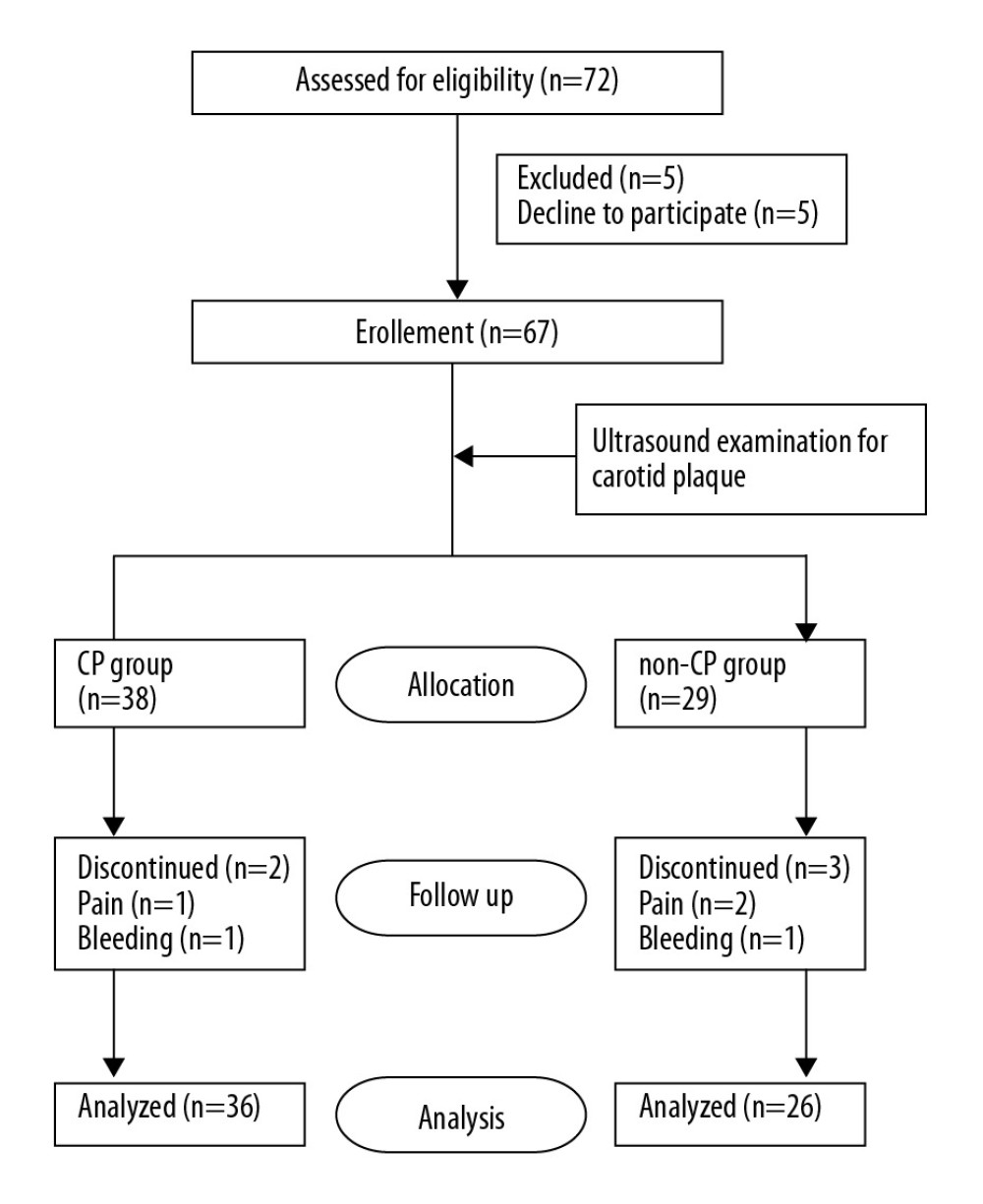
Patients (≥65 years of age) undergoing hip arthroplasty for a femoral neck fracture at Qilu Hospital of Shandong University (Qingdao) between 1 October 2016 and 30 March 2018 were recruited. The exclusion criteria were as follows: age <65 years, American Society of Anesthesiologists (ASA) physical status above III, mini-mental state examination (MMSE) [6,11] score <24, dementia due to various etiologies, history of a neurological/mental illness or an endocrine/metabolic disorder, preoperative delirium, current tranquilizer or antidepressant use, communication difficulties, severe visual or hearing impairments, illiteracy, drug or alcohol abuse, refusal to complete the study procedures, perioperative anemia (hemoglobin <90 g/L), blood loss >800 ml, and the need for an intraoperative transfusion. A total of 62 patients were included in the study (Figure 1).
High-resolution B-mode ultrasound imaging of the bilateral carotid arteries was carried out by proficient sonographers according to the same protocol using GE Vivid 7 (8–10 MHz linear-array transducer, USA). The sonographers screened all levels of the carotid trees, measuring the intima-media thickness (IMT, defined as the distance from the intima-lumen interface to the media-adventitia interface) and focal structures to define plaques. Focal structures invading into the arterial lumen were suggestive of plaque if they were sized at least ≥0.5 mm or 50% of the surrounding IMT value, or had a thickness >1.5 mm [7].
Patients were divided into CP and non-CP groups according to the preoperative presence or absence of carotid atherosclerotic plaques. The CP group was further divided into POD and no-POD subgroups based on the postoperative presence or absence of POD.
CLINICAL DATA:
Basic patient demographics and clinical data were collected, including age, sex, body mass index (BMI), ASA score, coronary artery disease, hypertension, and diabetes. The MMSE was administered by trained medical staff to test for impaired preoperative cognitive function 1 day before the surgery. Peri- and postoperative parameters (surgical time, blood loss, postoperative complications, bone cement use, drug dosage, and in-patient duration) were documented by trained medical staff.
ANESTHESIA AND SURGICAL MANAGEMENT:
For all participants, the invasive blood pressure, electrocardiogram, and pulse oxygen saturation were continuously monitored during the operative period. Spinal anesthesia was performed (L3–L4); after a successful puncture, cerebrospinal fluid reflux was observed. Subsequently, 0.5% ropivacaine 2.5–2.6 ml (AstraZeneca, UK) was injected into the subarachnoid space and the epidural catheter was placed. The anesthesia block plane was involved and was regulated at T8–S5. During the operation, 2% lidocaine (Shandong Hualu Pharmaceutical Co., Ltd., China) was added via the epidural catheter. Blood pressure and heart rate fluctuation amplitude were maintained within ±20% of the base value by using noradrenalin (Shanghai Hefeng Pharmaceutical Co., Ltd., China) and atropine (Shanghai Hefeng Pharmaceutical Co., Ltd., China). The operative time no longer than 4 h. Patient-controlled intravenous analgesia was utilized postoperatively, and the analgesic drug configuration was as follows: sufentanil (Humanwell Healthcare Group Co., Ltd., China) 2 ug/kg; dezocine (Yangtze River Pharmaceutical Group Co., Ltd., China) 10 mg; and ondansetron (Qilu Pharmaceutical Co., Ltd., China) 8 mg. Normal saline (up to 100 ml) was added, and the background infusion rate was 2 ml/h, PAC dose was 0.5 ml, and locking time was 15 min.
LABORATORY TESTS:
Venous blood was drawn preoperatively (T0), at the end of surgery (T1), and at 06: 00 on postoperative day 2 (T3). Blood samples were promptly placed into sterile EDTA test tubes and then centrifuged at 3000 g for 30 min at 4°C for the collection of serum. Serum was placed into polypropylene tubes and stored in a freezer at −80°C until further analyses. The serum expressions of Lp-PLA2 were determined by using enzyme-linked immunosorbent assay kits (R&D Systems, Wiesbaden, Germany). Preoperative biochemical analyses to determine the levels of C-reactive protein, high-density lipoprotein (HDL), low-density lipoprotein (LDL), total cholesterol (TC), triglycerides (TGs), and blood urea nitrogen (BUN) were also performed at our hospital laboratory.
DELIRIUM EVALUATION:
POD was determined on postoperative days 1, 2, and 3 (at 7: 00–9: 00 and 17: 00–19: 00) by means of the Confusion Assessment Method (CAM) diagnostic algorithm. In the CAM, the following features are evaluated: (1) acute or fluctuating onset; (2) inattention; (3) disorganized thinking; and (4) altered level of consciousness. The diagnosis of delirium by the CAM is made by the existence of features 1 and 2 and either 3 or 4. CAM evaluations were conducted by 2 trained medical staff members
STATISTICAL ANALYSIS:
Statistical analyses were performed using SPSS version 21.0 (IBM Corp., Armonk, NY). Categorical variables were compared using the chi-square test (e.g., sex, ASA, education level, coronary disease, diabetes, hypertension, stains, arrhythmia, smoking habit, bone cement, and the incidence of delirium on postoperative day 1, 2, and total) or Fisher’s exact test (e.g., the incidence of delirium on postoperative day 3) and presented as numbers and percentages. Continuous data were compared using the
Results
STUDY POPULATION:
A total of 62 geriatric patients were enrolled, including 36 in the CP group and 26 in the non-CP group. There were no statistically significant differences between the groups in the main demographics, chronic medical conditions, and peri- and postoperative parameters (Table 1) (P>0.05).
INCIDENCE OF DELIRIUM:
The incidence of delirium was significantly higher in the CP group (38.90%) than in the non-CP group (7.69%) (P<0.05). Additionally, in the CP group, POD occurred in 10 patients on postoperative day 2, which was higher than the number of patients with POD on postoperative days 1 and 3. For all patients, POD never occurred more than once (Table 1).
SERUM LP-PLA2:
There was no statistically significant difference in the preoperative LP-PLA2 level between the CP and non-CP groups. However, the LP-PLA2 level was significantly higher in the CP group than in the non-CP group postoperatively and on postoperative day 2 (Table 1) (P<0.05). Additionally, while the LP-PLA2 level did not significantly differ between the POD and no-POD subgroups preoperatively or postoperatively (P>0.05), it was significantly higher in the POD subgroup than in the no-POD subgroup on postoperative day 2 (P<0.05) (Table 2). Furthermore, the multivariate logistic analysis revealed that the Lp-PLA2 level on postoperative day 2 (OR 1.03, 95% CI 1.00–1.07) and preoperative BUN level (OR 1.80, 95% CI 1.03–3.11) were independent risk factors for POD (Table 3).
Discussion
The present study showed a significantly increased occurrence of POD in patients with CP compared to that in patients without CP. Additionally, there was a remarkable rise in LP-PLA2 postoperatively among patients with CP or emerging POD. To the best of our knowledge, the current study is the first clinical study to describe this phenomenon. It remains to be determined how CP and LP-PLA2 lead to POD.
POD, an age-related syndrome, is observed in patients with hip fractures who undergo hip surgery. In the present study, the incidence of POD was 26% among older patients undergoing hip surgery, which is similar to that in previous studies [8]. Furthermore, the incidence of POD was higher on postoperative day 2 than at other times, which is also in keeping with previous studies [8].
However, the mechanism by which POD occurs is still unclear. The National Institute for Health and Care Excellence suggested 4 major risk factors for delirium: age ≥65 years, past or present dementia and/or cognitive deficiency, present hip fracture, and severe disease (defined as a clinical condition of deterioration) [9]. In addition, Dasgupa et al. confirmed that psychotropic drug use, laboratory abnormalities, and psychopathological symptoms were associated with POD after non-cardiac surgery [11]. The present study analyzed many factors, including greater comorbidities (diabetes, hypertension, and coronary artery disease), preoperative laboratory test results (TGs, LDL, HDL, and BUN), and operative parameters. Among these, only BUN was supported as an independent risk factor for POD, which is consistent with previous studies [9]. Although the preoperative CRP level has been reported to be an independent risk factor for POD in several recent studies [11,12], this was not the case in the present study.
Carotid atherosclerosis, widely distributed in the elderly, may be connected with cognitive decline. Carotid stenosis, plaque, and IMT reflect 3 types of atherosclerosis, all of which are presumed to be connected with central nervous system functional decline [13]. Atherosclerosis stenosis may be involved in cognitive impairment via chronic cerebral hypoperfusion and embolization [14]. A study by Zhong suggested that IMT, but not plaque, is related to the occurrence of cognitive impairment during 10 years of follow-up [15]; however, recent studies have not consistently confirmed these results. Researchers found that microemboli from mechanically unstable CP could contribute to silent strokes, resulting in cognitive function decline [16,17]. Vulnerable plaques are more prone to embolization and microvascular changes, leading to microinfarcts and silent strokes. Additionally, internal carotid artery vulnerable plaques, which are strongly related to the amount of white matter hyperintensities, are associated with decreased cognitive performance [18].
Although there are numerous studies showing that CP is a risk factor for cognitive dysfunction, few studies have focused on the relationship between CP and POD. Recently, the prospective cohort study known as NeuroVISION suggested that covert stroke is involved in an increased risk of cognitive decline, and the potential development of perioperative delirium [4]. As patients with CP were more likely to develop POD than were patients without CP in the present study (38.90%
Our hypothesis is that CP, especially a vulnerable plaque, may give off some substance due to perioperative injury. This substance can invade the central nervous system, causing inflammation and dysfunction, as shown by POD. Therefore, it is critical to determine a biomarker reflecting the stability of CP. LP-PLA2 may be such a biomarker. A study by Kolodgie et al. suggested that LP-PLA2 expression is strong in the necrotic core and surrounding macrophages of vulnerable and ruptured plaques, while relatively weak staining is observed in less advanced lesions [19]. Additionally, an analysis of 167 patients undergoing carotid endarterectomy in the study by Mannheim et al. showed that the local LP-PLA2 expression is elevated in clinically unstable and raptured plaques [20]. These findings, in common with the known association of LP-PLA2 with apoptotic macrophages, imply a hypothetical role of LP-PLA2 in promoting plaque instability. Consistent with this, Sarlon-Bartoli et al. suggested that increased circulating concentrations of LP-PLA2 may be a novel maker for the prediction of CP vulnerability, as circulating LP-PLA2 is greater in individuals with unstable carotid stenosis than in patients with stable plaques [5].
The complicated mechanisms underlying the association between circulating LP-PLA2 and unstable plaques require further exploration. However, LP-PLA2 is known to promote LDL modification, boost matrix proteoglycans binding, and accelerate their assemblage and oxidation, which is a vital induction stage in endothelial activation and carotid plaque rupture [21]. Macrophages and foam cells within vulnerable plaques produce LP-PLA2, and an excess of LP-PLA2 may be expelled into the circulatory system; thus, serum LP-PLA2 could indicate the degree of unstable plaques. In the present study, we found that the LP-PLA2 levels did not differ between CP and non-CP groups preoperatively. However, the LP-PLA2 level was significantly higher in the CP group on postoperative day 2 compared to that in the non-CP group, which may indicate that CP stability was seriously damaged on postoperative day 2. Interestingly, the patients who developed POD also demonstrated a higher LP-PLA2 level on postoperative day 2 than that in patients who did not develop POD. This may explain the greater incidence of POD on postoperative day 2. Overall, the present study results are consistent with our hypothesis that CP, especially vulnerable plaques, may rupture into the circulation under a perioperative injury stimulus. Additionally, the mass of LP-PLA2 may increase following a decrease in CP stability.
After multivariate adjustments, a higher LP-PLA2 level remained a reliable marker for an increased risk of POD. Besides predicting plaque stability and being released into circulation, LP-PLA2 may affect cognitive function in other ways. A previous study demonstrated that oxidized LDL could be hydrolyzed by LP-PLA2 into 2 bioactive products: oxidized non-esterified fatty acids (oxNEFAs) and lysophosphatidylcholine (lysoPC). The majority of LP-PLA2-derived proinflammatory properties involve lysoPC [22]. Furthermore, lysoPC induces pericytes loss in the central nervous system, which has been shown to be characteristic of a damaged blood-brain barrier (BBB) [23]. Additionally, LP-PLA2 is one of several inflammatory biomarkers with a known association with the risk of dementia or impaired cognitive function [24,25]. Thus, we suggest that LP-PLA2 could be closely related to POD via 2 pathways: (1) Perioperatively, LP-PLA2 is released into the blood with a decrease in plaque stability, forming lipid microemboli that can reach the brain microcirculation, causing neurological dysfunction. (2) LP-PLA2 mediates inflammation by the hydrolysis of ox-LDL, producing lysoPC, which can impair endothelial function, resulting in increased BBB permeability, and, therefore, peripheral inflammatory factors invade the central nervous system and impair brain function.
The present study has several limitations that should be acknowledged. First, the sample size may have been insufficient to reveal a significant correlation between LP-PLA2 and POD in patients with CP, even though the trend was strong. Based on the results of the present study, we plan to further investigate these relationships in a larger sample. Second, the postoperative observation period was limited to 3 days, which is relatively short. A longer observation period may be essential for determining the relationships among delirium, LP-PLA2 level, and CP. Third, we measured the LP-PLA2 mass without analyzing its activity. LP-PLA2, as an enzyme, can be measured by either its mass or its activity; currently, a consensus on the optimal method for evaluating the Lp-PLA2 level is lacking [26]. Thus, the association between the LP-PLA2 activity and POD requires further exploration.
Conclusions
In summary, the incidence of POD in elderly patients with CP is significantly increased, and excessive postoperative LP-PLA2 levels are associated with POD. Optimizing anesthesia management for elderly patients with carotid artery plaques preoperatively and reducing the risk of plaque shedding may reduce the incidence of POD.
Tables
Table 1. Demographic and clinical characteristics and incidence of postoperative delirium and perioperative serum LP-PLA2 levels in the CP group and non-CP group.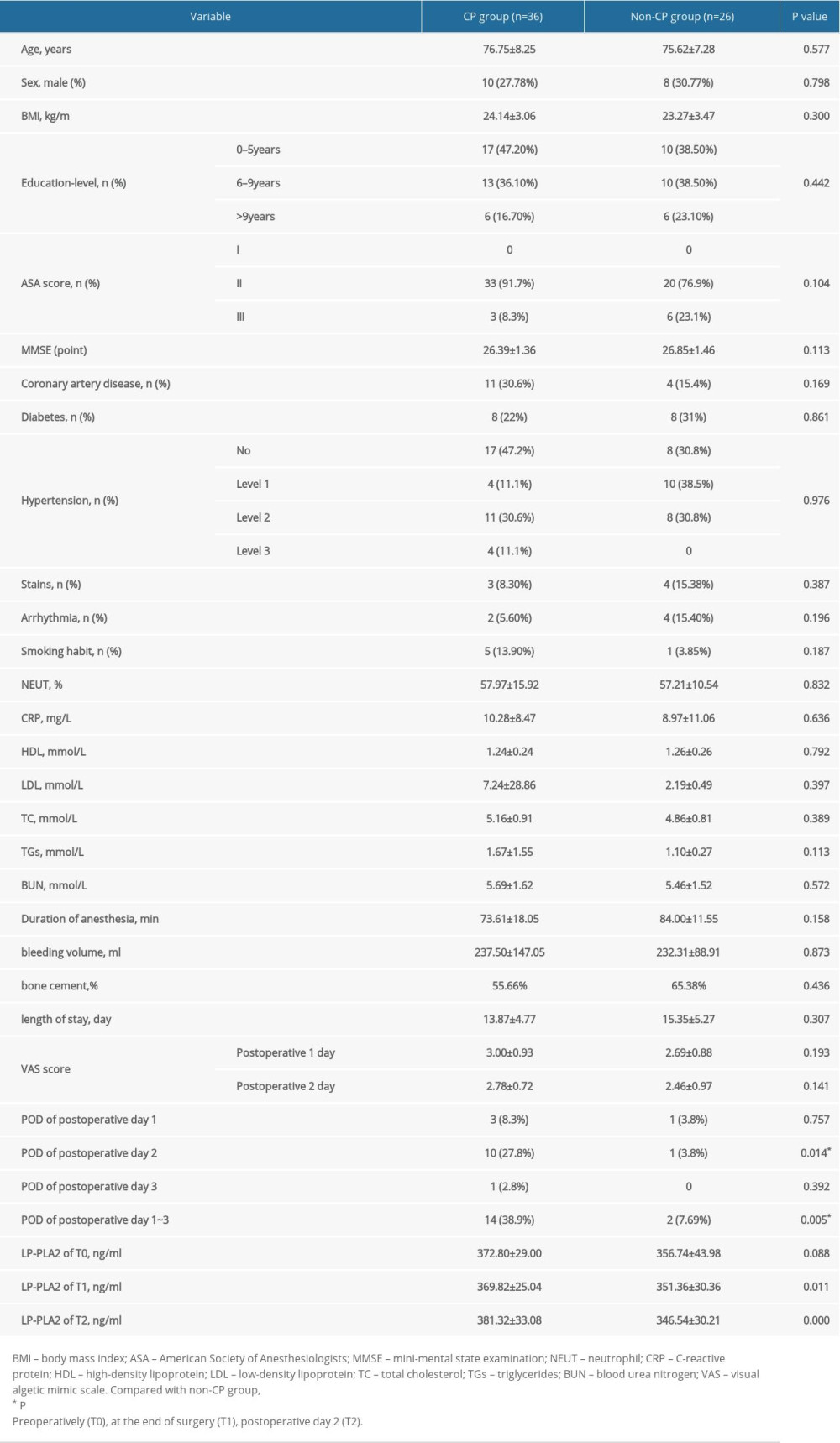 Table 2. Perioperative serum LP-PLA2 in the POD subgroup and NO-POD subgroup.
Table 2. Perioperative serum LP-PLA2 in the POD subgroup and NO-POD subgroup.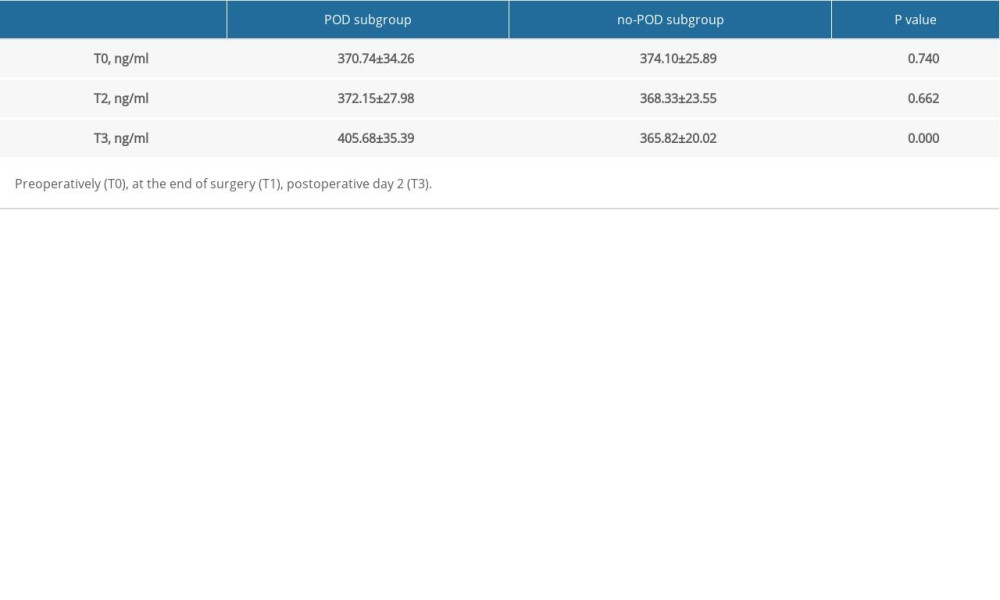 Table 3. Univariate/multiple logistic regression models for the variables associated with POD.
Table 3. Univariate/multiple logistic regression models for the variables associated with POD.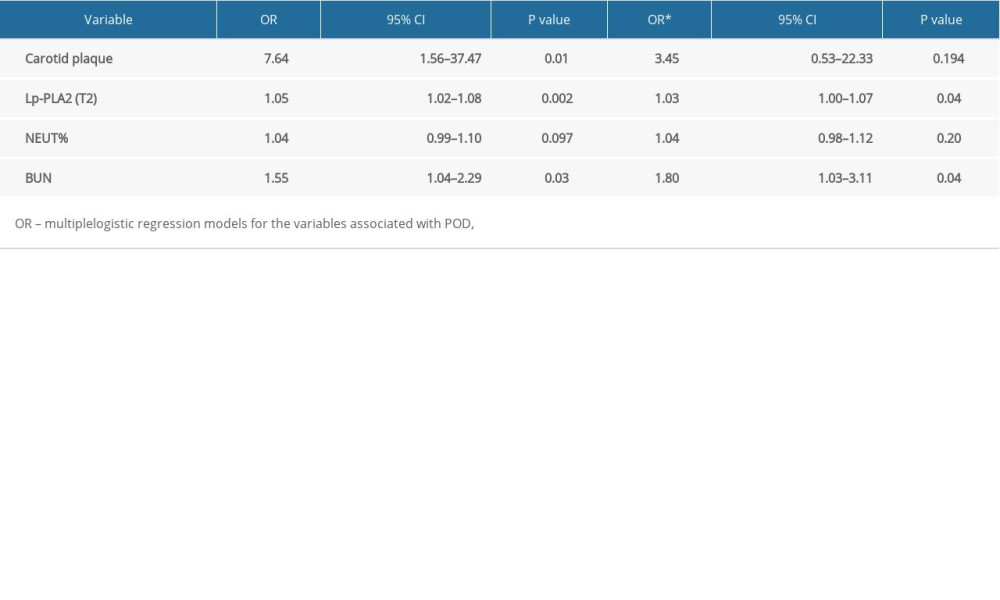
References
1. Mittal V, Muralee S, Williamson D, Review: Delirium in the elderly: A comprehensive review: Am J Alzheimer Dis Other Demen, 2011; 26(2); 97-109
2. Rizk P, Morris W, Oladeji P, Huo M, Review of postoperative delirium in geriatric patients undergoing hip surgery: Geriatr Orthop Surg Rehabil, 2016; 7(2); 100-5
3. van Veluw SJ, Shih AY, Smith EE, Detection, risk factors, and functional consequences of cerebral microinfarcts: Lancet Neurol, 2017; 16(9); 730-40
4. NeuroVISION Investigators, Perioperative covert stroke in patients undergoing non-cardiac surgery (NeuroVISION). A prospective cohort study: Lancet (London, England), 2019; 394(10203); 1022-29
5. Sarlon-Bartoli G, Boudes A, Buffat C, Circulating lipoprotein-associated phospholipase A2 in high-grade carotid stenosis: A new biomarker for predicting unstable plaque: Eur J Vasc Endovasc Surg, 2012; 43(2); 154-59
6. Papaioannou A, Fraidakis O, Michaloudis D, The impact of the type of anaesthesia on cognitive status and delirium during the first postoperative days in elderly patients: Eur J Anaesthesiol, 2005; 22(7); 492-99
7. Touboul PJ, Hennerici MG, Meairs S: Cerebrovascular diseases (Basel, Switzerland), 2012; 34(4); 290-96
8. Inouye SK, Westendorp RG, Saczynski JS, Delirium in elderly people: Lancet (London, England), 2014; 383(9920); 911-22
9. National Institute for Health and Clinical Excellence: Delirium: Diagnosis, Prevention and Management (clinical guideline 103 ) Published July 2010. www.nice.org.uk/nicemedia/live/13060/49909/49909.pdf
10. Dasgupta M, Dumbrell AC, Preoperative risk assessment for delirium after noncardiac surgery: A systematic review: J Am Geriatr Soc, 2006; 54(10); 1578-89
11. Pol RA, van Leeuwen BL, Izaks GJ, C-reactive protein predicts postoperative delirium following vascular surgery: Ann Vasc Surg, 2014; 28(8); 1923-30
12. Xiang D, Xing H, Tai H, Xie G, Preoperative C-reactive protein as a risk factor for postoperative delirium in elderly patients undergoing laparoscopic surgery for colon carcinoma: Biomed Res Int, 2017; 2017 5635640
13. Chen WH, Jin W, Lyu PY, Carotid atherosclerosis and cognitive impairment in nonstroke patients: Chin Med J (Engl), 2017; 130(19); 2375-79
14. Sztriha LK, Nemeth D, Sefcsik T, Vecsei L, Carotid stenosis and the cognitive function: J Neurol Sci, 2009; 283(1–2); 36-40
15. Zhong W, Cruickshanks KJ, Schubert CR, Carotid atherosclerosis and 10-year changes in cognitive function: Atherosclerosis, 2012; 224(2); 506-10
16. Dempsey RJ, Vemuganti R, Varghese T, Hermann BP, A review of carotid atherosclerosis and vascular cognitive decline: A new understanding of the keys to symptomology: Neurosurgery, 2010; 67(2); 484-93 discussion 493–94
17. Arntzen KA, Mathiesen EB, Subclinical carotid atherosclerosis and cognitive function: Acta Neurol Scandin Suppl, 2011(191); 18-22
18. Berman SE, Wang X, Mitchell CC, The relationship between carotid artery plaque stability and white matter ischemic injury: Neuroimage Clin, 2015; 9; 216-22
19. Kolodgie FD, Burke AP, Skorija KS, Lipoprotein-associated phospholipase A2 protein expression in the natural progression of human coronary atherosclerosis: Arterioscler Thromb Vasc Biol, 2006; 26(11); 2523-29
20. Mannheim D, Herrmann J, Versari D, Enhanced expression of Lp-PLA2 and lysophosphatidylcholine in symptomatic carotid atherosclerotic plaques: Stroke, 2008; 39(5); 1448-55
21. Mannheim D, Herrmann J, Versari D, Enhanced expression of Lp-PLA2 and lysophosphatidylcholine in symptomatic carotid atherosclerotic plaques: Stroke, 2008; 39(5); 1448-55
22. Huang F, Wang K, Shen J, Lipoprotein-associated phospholipase A2: The story continues: Med Res Rev, 2020; 40(1); 79-134
23. Muramatsu R, Kuroda M, Matoba K, Prostacyclin prevents pericyte loss and demyelination induced by lysophosphatidylcholine in the central nervous system: J Biol Chem, 2015; 290(18); 11515-25
24. Stewart RAH, Held C, Krug-Gourley S, Cardiovascular and lifestyle risk factors and cognitive function in patients with stable coronary heart disease: J Am Heart Assoc, 2019; 8(7); e010641
25. Jiang R, Chen S, Shen Y, Higher levels of lipoprotein associated phospholipase A2 is associated with increased prevalence of cognitive impairment: The APAC study: Sci Rep, 2016; 6; 33073
26. Madjid M, Ali M, Willerson JT, Lipoprotein-associated phospholipase A2 as a novel risk marker for cardiovascular disease: A systematic review of the literature: Tex Heart Inst, J, 2010; 37(1); 25-39
Tables
 Table 1. Demographic and clinical characteristics and incidence of postoperative delirium and perioperative serum LP-PLA2 levels in the CP group and non-CP group.
Table 1. Demographic and clinical characteristics and incidence of postoperative delirium and perioperative serum LP-PLA2 levels in the CP group and non-CP group. Table 2. Perioperative serum LP-PLA2 in the POD subgroup and NO-POD subgroup.
Table 2. Perioperative serum LP-PLA2 in the POD subgroup and NO-POD subgroup. Table 3. Univariate/multiple logistic regression models for the variables associated with POD.
Table 3. Univariate/multiple logistic regression models for the variables associated with POD. Table 1. Demographic and clinical characteristics and incidence of postoperative delirium and perioperative serum LP-PLA2 levels in the CP group and non-CP group.
Table 1. Demographic and clinical characteristics and incidence of postoperative delirium and perioperative serum LP-PLA2 levels in the CP group and non-CP group. Table 2. Perioperative serum LP-PLA2 in the POD subgroup and NO-POD subgroup.
Table 2. Perioperative serum LP-PLA2 in the POD subgroup and NO-POD subgroup. Table 3. Univariate/multiple logistic regression models for the variables associated with POD.
Table 3. Univariate/multiple logistic regression models for the variables associated with POD. In Press
08 Mar 2024 : Clinical Research
Evaluation of Foot Structure in Preschool Children Based on Body MassMed Sci Monit In Press; DOI: 10.12659/MSM.943765
15 Apr 2024 : Laboratory Research
The Role of Copper-Induced M2 Macrophage Polarization in Protecting Cartilage Matrix in OsteoarthritisMed Sci Monit In Press; DOI: 10.12659/MSM.943738
07 Mar 2024 : Clinical Research
Knowledge of and Attitudes Toward Clinical Trials: A Questionnaire-Based Study of 179 Male Third- and Fourt...Med Sci Monit In Press; DOI: 10.12659/MSM.943468
08 Mar 2024 : Animal Research
Modification of Experimental Model of Necrotizing Enterocolitis (NEC) in Rat Pups by Single Exposure to Hyp...Med Sci Monit In Press; DOI: 10.12659/MSM.943443
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952