22 October 2020: Clinical Research
Serum Levels of Gamma-Glutamyltransferase During Stable and Acute Exacerbations of Chronic Obstructive Pulmonary Disease
Desheng Sun12ABCEG*, Hongyan Liu3DF, Yao Ouyang1B, Xiansheng Liu2G, Yongjian Xu2CDOI: 10.12659/MSM.927771
Med Sci Monit 2020; 26:e927771
Abstract
BACKGROUND: One of the most important factors in the pathogenesis of COPD (chronic obstructive pulmonary disease) is oxidative stress. GGT (gamma-glutamyltransferase) has been regarded as a novel marker of oxidative stress over the last few years. This study aimed to compare the serum levels of GGT during stable and acute exacerbations of COPD at a single center.
MATERIAL AND METHODS: The research included 117 patients with AECOPD (acute exacerbation of chronic obstructive pulmonary disease), 107 patients with stable COPD, and 112 control subjects. Serum GGT, spirometry function, and other clinical parameters (anthropometric and biochemical measurements) were evaluated and compared among the subjects.
RESULTS: Serum GGT was elevated in patients with stable COPD in comparison to the control subjects. Its level was inversely related to lung function. It was also significantly higher in AECOPD patients compared to stable COPD patients. We also found that a GGT level of 21.2 IU/L displays a reliable diagnostic prediction of COPD and that a GGT level of 26.5 IU/L can be applied to predict the exacerbation of COPD.
CONCLUSIONS: Our research demonstrates that serum GGT level is inversely associated with pulmonary function and may serve as a biomarker during the progression of COPD. The monitoring of GGT values can be applied to evaluating COPD and its exacerbation risk.
Keywords: gamma-Glutamyltransferase, Pulmonary Disease, Chronic Obstructive, Respiratory Function Tests, Case-Control Studies, Disease Progression, Lung, Severity of Illness Index
Background
Chronic obstructive pulmonary disease (COPD) is the fourth leading cause of death worldwide and the only chronic disease that has continuously showed an increase in mortality [1–3]. COPD patients who frequently experience exacerbations have adverse impacts on their life quality, spirometry function, and prognosis [4]. Moreover, exacerbation also increases social and financial costs [5,6]. Consequently, effective and timely prevention of COPD exacerbation is of great significance for patients and medical workers. Acute exacerbation of COPD is defined as an event in the course of the disease, characterized by a change in the patient’s baseline dyspnea, cough, and/or sputum production, that is beyond normal day-to-day variations, and may warrant a change in regular medication [7,8]. However, there is still a lack of effective biomarkers for COPD and its acute exacerbation.
It is known that the most important factors in the pathogenesis of COPD are inflammation and oxidative stress [9]. In COPD, inflammation occurs in the airways, blood vessels, and lung parenchyma and has generalized effects. Oxidative stress has been determined to be involved in many processes associated with chronic inflammation. It produces direct damaging effects to the lungs and activates the molecular mechanisms that aggravate pulmonary inflammation [9].
Serum gamma-glutamyltransferase (GGT) is present in high concentrations in the biliary tree and is traditionally used in clinical practice as a biomarker for liver disease [10]. Since GGT is found in several other organs, including the lungs, it is unlikely to be a specific marker solely for biliary or liver disease [11]. Over the last few years GGT has become regarded as a novel biomarker for oxidative stress, and many studies have shown that GGT values within set reference ranges are predictive of several diseases, including chronic kidney disease, cardiovascular disease, type 2 diabetes, and cancer [12]. Nowadays, serum GGT levels are being applied as a marker for oxidative stress [13]. There are several previous studies on the relationship between GGT and COPD [14–18]. However, their results are not consistent. Bozkus et al. demonstrated that serum GGT may be helpful in grading the severity of COPD [15], while some scientists found serum GGT were similar in patients with different severity of disease [17]. Ermis et al. found significantly higher the GGT activity in COPD patients compared to healthy controls [14], while other scientists found no difference [16]. In addition, they did not compare healthy controls, stable COPD, and AECOPD at the same time, nor did they analyze their relationship with lung function.
Therefore, the present study aimed to compare the serum levels of GGT during stable and acute exacerbations of COPD at a single center. We sought to examine serum GGT activity and to elucidate its predictive value in the development of COPD. We also aimed to investigate its relationship to pulmonary function.
Material and Methods
STUDY POPULATION:
This study was based on data from 3675 individuals who were hospitalized or examined in our institution with reproducible or acceptable spirometry results between December 1, 2016 and January 31, 2019. All subjects in this research were 40 years or older and either COPD patients or control subjects whose demographic (age, sex) and clinical (COPD status, smoking status, comorbidities) data were recorded. Blood samples were drawn for diagnostics and screening, including GGT activity, hepatic function, and renal function, at the time of admission or medical examination. Participants with pulmonary diseases other than COPD, a history of asthma, or pulmonary tuberculosis, which could also bring about airflow limitations, were excluded from the study. Additionally, people with abnormal liver or biliary tract function; neoplastic pathologies; gastrointestinal, renal, and endocrine diseases; or regular alcohol consumption were eliminated from the study. Thus, a final total 336 participants were involved in the final analyses. (Details of the exclusions are presented in Figure 1)
The diagnostic criteria for COPD were based on the Global Initiative for Chronic Obstructive Pulmonary Disease [7,19]. The COPD population comprised 107 patients in the stable phase (without the need for hospitalization or therapy modification for at least 3 months) and 117 patients with acute exacerbation of chronic obstructive pulmonary disease (AECOPD). Acute exacerbation was defined as a quick deterioration of a patient’s state with the appearance of the following clinical manifestations: aggravated breathlessness, stickier and thicker sputum, or needing therapy modification. The control group was the population without respiratory diseases. Similar to the exclusion criteria mentioned above, this group also excluded liver or biliary tract dysfunction, tumor lesions, gastrointestinal, renal and endocrine diseases, and people who often drank alcohol.
The study was implemented based on the principles of the Helsinki Declaration. Due to the retrospective nature of the study, the requirement for informed written consent was waived. This research was approved by the Institutional Ethics Review Board of the Affiliated Hospital of Zunyi Medical University. All personal information has already been anonymized and de-identified prior to analysis to protect patient privacy.
ANTHROPOMETRIC AND BIOCHEMICAL MEASUREMENTS:
Epidemiological (age, sex, comorbidities, and smoking status) data were collected, and the weights and heights of the subjects were measured. Body mass index (BMI) was calculated as the ratio of body weight (kg) to height squared (m2). Blood specimens were obtained, processed, and delivered to the Medical Examination Department, and analyzed within 12 h.
The serum levels of GGT, total bilirubin, alanine aminotransferase (ALT), aspartate transaminase (AST), blood urea nitrogen (BUN), and creatinine were measured by a Hitachi 7020 automatic biochemistry analyzer (Hitachi, Japan).
Using a commercially available enzyme-linked immunosorbent assay (ELISA) kit (Gudo Biotech, Shanghai, China), GGT protein level was determined in serum. The main process of the ELISA method was as follows: Allow all reagents to reach room temperature (20–25°C) for 15–30 min; Remove the enzyme plate and add 50 μL standard solution into the blank micropore according to the order of the standard; add a 50 μL sample into the blank micropore and add 50 μL distilled water to the blank control; add 10 μL biotin into the sample hole; add 100 μL enzyme-labeling solution into each pore; seal the enzyme label plate with sealing glue and incubate at 37°C 1 h; fully clean the enzyme label plate 3–5 times to keep sufficient water pressure in each hole; dry the enzyme plate thoroughly with absorbent paper after washing; add 50 μL of chromogenic reagent A and B to each hole; react in the dark for 15 min at 20–25°C; and add 50 μL termination solution to each hole to terminate the reaction. Then, the absorbance data were collected and recorded by enzyme-linked immunosorbent assay. Curve-fitting statistical software was used to plot a 4-parameter logistic curve ft to the standards, and the results were then calculated for the test samples. The normal range for serum GGT levels for our laboratory is 10–60 IU/L.
SPIROMETRY DATA:
Spirometry testing was performed using dry rolling seal lung volume meters (SensorMedics, USA) by professional staff with patients in a seated position. A bronchodilator was not given before the spirometry test. The forced expiratory volume in one second (FEV1) and the forced vital capacity (FVC) were measured according to the criteria presented by the American Thoracic Society (ATS) [20]. All participants had at least 3 acceptable and reproducible respiratory curves with a difference of less than 150 ml between the 2 highest FEV1 and FVC results being required for inclusion in the analysis. The percent predicted values of the following predictive equations, as described by Jia et al. [21], were determined to calculate lung function:
Participants with pre-bronchodilator FEV1/FVC (forced expiratory volume in one second/forced vital capacity ratio) <0.7 were required to undergo post-bronchodilator spirometry testing. Participants with post-bronchodilator FEV1/FVC <0.7 were categorized as having COPD.
STATISTICAL ANALYSES:
Statistical analyses were performed with SPSS software (version 19.0) and GraphPad Prism (version 6.0). All data were tested for normal distribution using the Shapiro-Wilk test and for homogeneity of variances using Levene’s test. If the data were normally distributed and showed similar variances, a one-way analysis of variance (ANOVA) was performed to compare means among the 3 groups. When the ANOVA results showed significant differences, multiple comparisons of means were used to perform a Tukey HSD post hoc test. If the data did not show similar variances, a non-parametric Kruskal-Wallis analysis for comparing the median was performed and a Mann-Whitney analysis for multiple comparisons was also used if the Kruskal-Wallis analysis showed significant differences. Our missing data analysis procedures used missing at random (MAR) assumptions. We used the the MICE (multivariate imputation by chained equations) method of multiple multivariate imputation.
Correlations between GGT activity and the other parameters were measured with Pearson or Spearman correlation analysis, as appropriate. A value of
Receiver-operating characteristic (ROC) analysis was performed to calculate the cut-off values of serum GGT levels to predict COPD and its exacerbation with optimal specificity and sensitivity.
Results
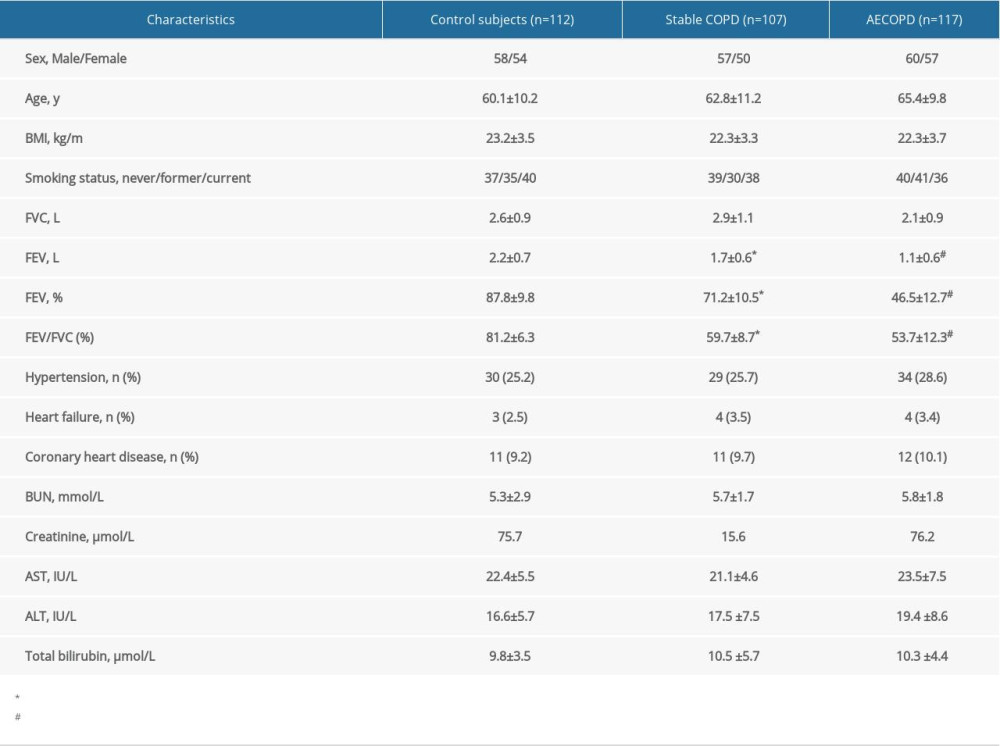
As is depicted in Table 1, there was no significant difference among the 3 groups on the basis of age, sex, comorbidities (hypertension, coronary heart diseases, and heart failure), BMI, ALT, AST, total bilirubin, BUN, serum creatinine, and overall smoking status (all
All spirometric results, except the FVC, were better in patients with stable COPD when compared to those with AECOPD, but were worse than those in the control participants (Table 1).
A statistically significant increase of serum GGT activity was found in stable COPD patients when compared to healthy controls (24.0±7.2
There were significant negative correlations between GGT levels and forced expiratory volume in one second as the percentage of the predicted value (FEV1%) (
GGT activity was further tested using an ROC curve for diagnostic specificity and sensitivity (Figure 4). Analysis showed that a cut-off value for serum GGT activity of 21.2 IU/L exhibited good diagnostic accuracy for the evaluation of oxidative stress (AUC=0.789; 95% CI, 0.681–0.823;
Discussion
A few studies have investigated or discussed the relationship between GGT and COPD [14–18], but none of them compared healthy controls, stable COPD, and AECOPD at the same time, and some of their results are contradictory. One research team found significant ly higher GGT activity in COPD patients compared to healthy controls [14], while another group found no difference [16]. In addition, none of the research groups mentioned above analyzed the relationship between GGT and lung function. We compared serum GGT levels among healthy individuals, stable COPD patients, and AECOPD patients, and studied the associations with pulmonary function.
We evaluated the possibility of applying serum GGT as a biomarker in monitoring the level of oxidative stress in COPD patients. We found higher GGT values in the sera of individuals with clinically stable COPD compared to control subjects. Furthermore, serum GGT activity was remarkably higher in subjects with AECOPD compared with patients in the stable phase. This is consistent with previously published data for C-reactive protein (CRP) [22–24], but in contrast with results for GGT obtained from research with limited samples (29 COPD patients) [16]. Systemic inflammation has been proven to be critical in the pathogenesis of COPD, although the precise mechanism remains unclear. There have been many studies proving the positive correlation between the degree of airflow limitation and increased activity of inflammatory cytokines such as CRP [25,26]. It has also been proven that an increase in GGT activity in inflammatory reactions increases antioxidant defense [27].
The present study demonstrated that a GGT level of 21.2 IU/L provides a reliable diagnostic prediction of COPD, and a GGT level of 26.5 IU/L acts as an indicator for determining the exacerbation of COPD in clinical practice.
An abnormal increase of the GGT value is commonly considered to be a biomarker of liver damage resulting from other causes, including hepatitis virus, parasites, and bile duct diseases. All these associated diseases had been excluded before enrollment. Other factors that can affect serum GGT levels, such as diets high in fruit and vegetables, drugs, and alcohol intake were reported [28,29]. People who often drank alcohol were excluded from the study, and there was no significant difference in smoking status among the 3 groups. However, specific dietary factors were not compared in detail in this study, which might have some impact on the results.
Up to now, the mechanisms underlying the association of GGT, traditionally viewed as a hepatic enzyme, with lung function and COPD, have been unclear. A possible explanation is that GGT is associated with respiratory function as a marker of oxidative stress [27,30]. GGT activity is activated by the burden of oxidative stress to maintain glutathione (an essential intracellular antioxidant) [31]. One study demonstrated that the values of antioxidant vitamins were inversely related to GGT activity [32] and that GGT activity positively indicated F2-isoprostanes, a generally accepted marker of oxidative stress. An elevated oxidant burden has been regarded as a vital part of the pathophysiology of COPD [33]. Many scientists have reported that excessive oxidative stress can cause transcriptional regulation of inflammatory genes, hyperplasia of mucous glands, and irreversible damage of the antioxidant system in airway epithelial cells [34]. Studies have demonstrated that worsened pulmonary function is related to antioxidant reduction, which is consistent with oxidative stress playing an important role in COPD development [27]. Consequently, our research may have indicated the link between oxidative stress and COPD.
Several studies showed an association between COPD and low BMI [35–38]. However, there was no significant correlation between them in our study. BMI depends on weight and the square of height. Since mass increases to the third power of linear dimensions, taller people with the same body shape have a higher BMI [39]. BMI ignores variations in physical characteristics, and also fails to consider loss of height through aging. Due to these shortcomings, some scientists say that BMI is an easy but inaccurate measure, which should be revised [40].
Our study has several strengths and limitations. The lack of baseline difference in liver function (except for GGT) or complications within the 3 groups suggests that the elevated GGT values are the result of the COPD itself and its exacerbation. Considering variations due to racial differences, we selected the predictive equations most suitable to the population of south-west China. Moreover, technical errors in the spirometry test were minimized by applying a standardized guideline for evaluation.
The most important limitation of our research was the relatively small sample size, which limits forming strong conclusions, and a larger epidemiological study is needed. In addition, the serum levels of GGT of 21.2 IU/L for COPD and of 26.5 IU/L were very close to each other and might not be useful in clinical practice. Finally, the common causes of acute exacerbation of COPD, such as infection, were not investigated in this study, and this needs to be addressed in subsequent research.
Conclusions
We found that in individuals aged 40 years or older, representative of the south-western Chinese population, increased serum GGT values are related to decreased lung function and increased prevalence of COPD. Therefore, serum GGT may be a potential marker to indicate the risk of COPD and a useful parameter to evaluate exacerbation. Confirmation of the underlying mechanism needs further clarification through future studies.
Figures
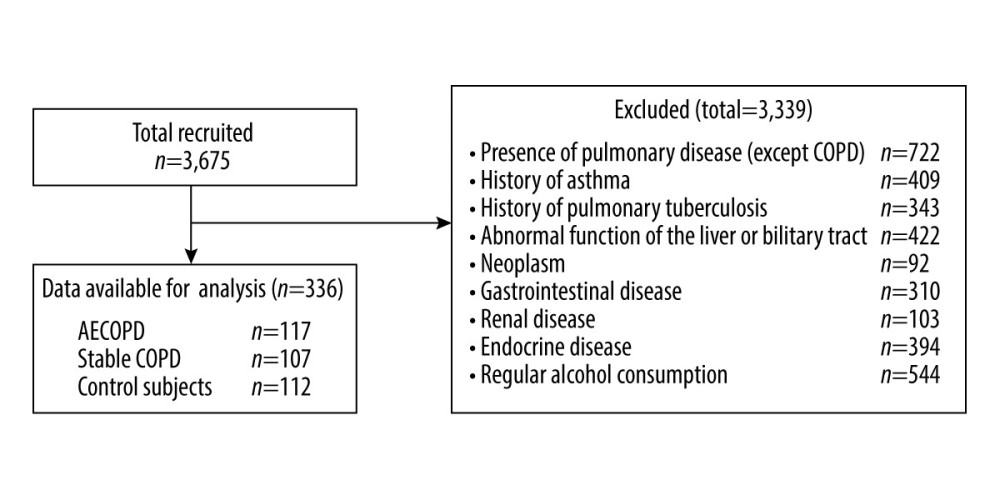 Figure 1. Participant recruitment and follow-up flow chart.
Figure 1. Participant recruitment and follow-up flow chart. 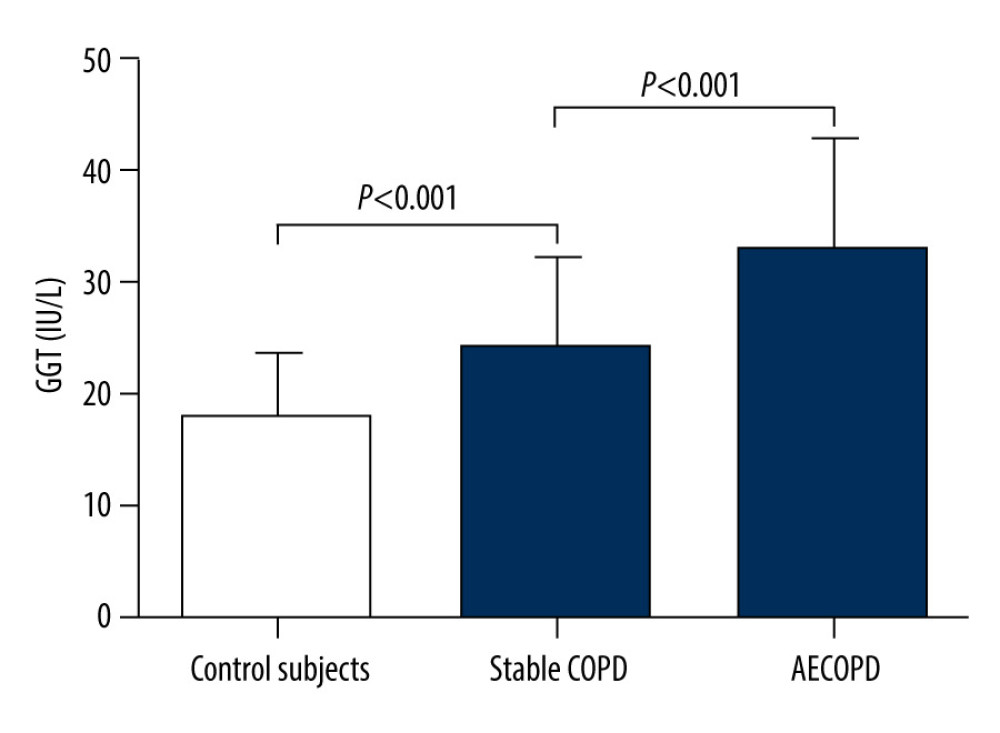 Figure 2. Serum gamma-glutamyltransferase (GGT) levels in the 3 groups.
Figure 2. Serum gamma-glutamyltransferase (GGT) levels in the 3 groups. 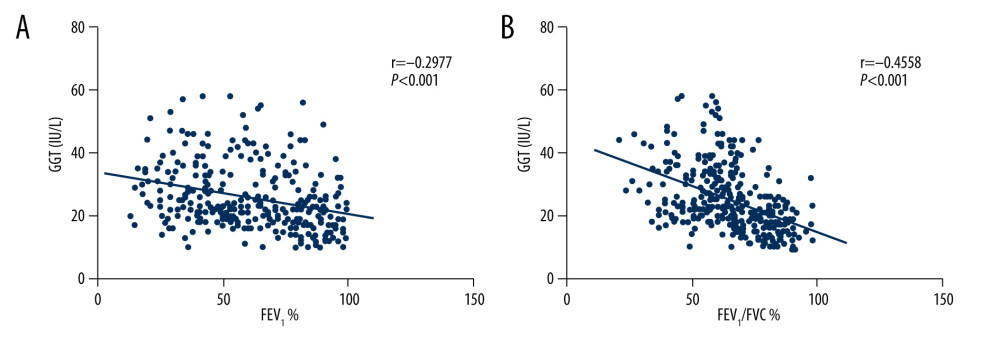 Figure 3. Correlations between GGT and forced expiratory volume in one second as the percentage of the predicted value (FEV1%) (A), GGT and forced expiratory volume in one second/forced vital capacity ratio (FEV1/FVC) (B).
Figure 3. Correlations between GGT and forced expiratory volume in one second as the percentage of the predicted value (FEV1%) (A), GGT and forced expiratory volume in one second/forced vital capacity ratio (FEV1/FVC) (B). 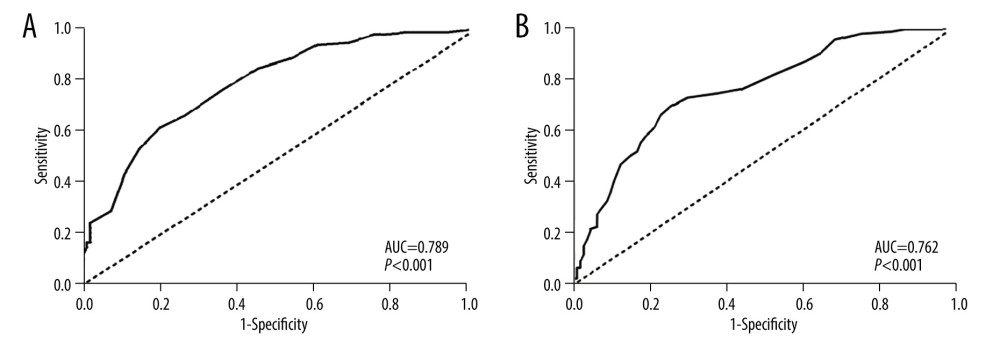 Figure 4. Receiver-operating characteristic (ROC) curves for GGT activity to evaluate COPD (A) and its acute exacerbation (B). AUC – area under the curve.
Figure 4. Receiver-operating characteristic (ROC) curves for GGT activity to evaluate COPD (A) and its acute exacerbation (B). AUC – area under the curve. References
1. Fu Z, Jiang HW, Xu ZY, Objective secondhand smoke exposure in chronic obstructive pulmonary disease patients without active smoking: The US National Health and Nutrition Examination Survey (NHANES) 2007–2012: Ann Transl Med, 2020; 8; 445
2. Sun D, Ouyang Y, Gu Y, Liu X, Cigarette smoke-induced chronic obstructive pulmonary disease is attenuated by CCL20-blocker: A rat model: Croat Med J, 2016; 57; 363-70
3. Kim V, Criner GJ, Chronic bronchitis and chronic obstructive pulmonary disease: Am J Respir Crit Care Med, 2013; 187; 228-37
4. Zhao YF, Jiang YP, Zhou LF, Wu XL, The value of assessment tests in patients with acute exacerbation of chronic obstructive pulmonary disease: Am J Med Sci, 2014; 347; 393-99
5. Yang B, Choi H, Lim JH, The disease burden of bronchiectasis in comparison with chronic obstructive pulmonary disease: A national database study in Korea: Ann Transl Med, 2019; 7; 770
6. Bandurska E, Damps-Konstanska I, Popowski P, Cost-effectiveness analysis of integrated care in management of advanced chronic obstructive pulmonary disease (COPD): Med Sci Monit, 2019; 25; 2879-85
7. Barnes PJ, Chronic obstructive pulmonary disease: N Engl J Med, 2000; 343; 269-80
8. Gold PM, The 2007 GOLD Guidelines: A comprehensive care framework: Respir Care, 2009; 54; 1040-49
9. Dhakal N, Lamsal M, Baral N, Oxidative stress and nutritional status in chronic obstructive pulmonary disease: J Clin Diagn Res, 2015; 9; BC01-04
10. Xiong Y, Ye Y, Li P, Serum NOX2 as a new biomarker candidate for HBV-related disorders: Am J Transl Res, 2018; 10; 2350-61
11. Holme J, Dawkins PA, Stockley EK, Studies of gamma-glutamyl transferase in alpha-1 antitrypsin deficiency: COPD, 2010; 7; 126-32
12. Targher G, Elevated serum gamma-glutamyltransferase activity is associated with increased risk of mortality, incident type 2 diabetes, cardiovascular events, chronic kidney disease and cancer – a narrative review: Clin Chem Lab Med, 2010; 48; 147-57
13. Gohel MG, Chacko AN, Serum GGT activity and hsCRP level in patients with type 2 diabetes mellitus with good and poor glycemic control: An evidence linking oxidative stress, inflammation and glycemic control: J Diabetes Metab Disord, 2013; 12; 56
14. Biljak VR, Rumora L, Cepelak I, Gamma-glutamyltransferase and C-reactive protein in stable chronic obstructive pulmonary disease: Coll Antropol, 2013; 37; 221-27
15. Bozkus F, Dikmen N, Sahin H, Samur A, Serum gamma-glutamyl transferase activity as a potential novel cardiovascular biomarker in COPD: Resp Care, 2016; 61; 1465-71
16. Cepelak I, Dodig S, Romic D, Enzyme catalytic activities in chronic obstructive pulmonary disease: Arch Med Res, 2006; 37; 624-29
17. Ermis H, Celik MR, Gulbas G, Relationship between serum gamma-glutamyltransferase levels and acute exacerbation of chronic obstructive pulmonary disease: Pol Arch Med Wewn, 2013; 123; 85-90
18. Zinellu E, Zinellu A, Fois AG, Circulating biomarkers of oxidative stress in chronic obstructive pulmonary disease: a systematic review: Resp Res, 2016; 17; 150
19. Vestbo J, Hurd SS, Agusti AG, Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary: Am J Respir Crit Care Med, 2013; 187; 347-65
20. Redlich CA, Tarlo SM, Hankinson JL, Official American Thoracic Society technical standards: Spirometry in the occupational setting: Am J Respir Crit Care Med, 2014; 189; 983-93
21. Jia J, Mao B, Guo X, Wang J, Normal values of lung function in the population of South-West China: Nationwide normal values of lung function, 1990; 38-48, PUMC& Beiiing Medical University Publication
22. Bathoorn E, Liesker JJ, Postma DS, Change in inflammation in out-patient COPD patients from stable phase to a subsequent exacerbation: Int J Chron Obstruct Pulmon Dis, 2009; 4; 101-9
23. Weis N, Almdal T, C-reactive protein – can it be used as a marker of infection in patients with exacerbation of chronic obstructive pulmonary disease?: Eur J Intern Med, 2006; 17; 88-91
24. Hurst JR, Donaldson GC, Perera WR, Use of plasma biomarkers at exacerbation of chronic obstructive pulmonary disease: Am J Respir Crit Care Med, 2006; 174; 867-74
25. Cordoba-Lanus E, Baz-Davila R, Espinoza-Jimenez A, IL-8 gene variants are associated with lung function decline and multidimensional BODE index in COPD patients but not with disease susceptibility: A validation study: COPD, 2015; 12; 55-61
26. Mannino DM, Ford ES, Redd SC, Obstructive and restrictive lung disease and markers of inflammation: Data from the Third National Health and Nutrition Examination: Am J Med, 2003; 114; 758-62
27. Mistry D, Stockley RA, Gamma-glutamyl transferase: the silent partner?: COPD, 2010; 7; 285-90
28. Lee DH, Steffen LM, Jacobs DR, Association between serum gamma-glutamyltransferase and dietary factors: The Coronary Artery Risk Development in Young Adults (CARDIA) Study: Am J Clin Nutr, 2004; 79; 600-5
29. Park EY, Lim MK, Oh JK, Independent and supra-additive effects of alcohol consumption, cigarette smoking, and metabolic syndrome on the elevation of serum liver enzyme levels: PLoS One, 2013; 8; e63439
30. Jansen E, Beekhof P, Viezeliene D, Long-term stability of cancer biomarkers in human serum: biomarkers of oxidative stress and redox status, homocysteine, CRP and the enzymes ALT and GGT: Biomark Med, 2015; 9; 425-32
31. Zhang H, Forman HJ, Choi J, Gamma-glutamyl transpeptidase in glutathione biosynthesis: Methods Enzymol, 2005; 401; 468-83
32. Kumar AK, Vijayalakshmi K: Appl Biochem Biotechnol, 2015; 175; 410-20
33. Lin JL, Thomas PS, Current perspectives of oxidative stress and its measurement in chronic obstructive pulmonary disease: COPD, 2010; 7; 291-306
34. Tomita K, Barnes PJ, Adcock IM, The effect of oxidative stress on histone acetylation and IL-8 release: Biochem Biophys Res Commun, 2003; 301; 572-77
35. Zhou Y, Wang D, Liu S, The association between BMI and COPD: The results of two population-based studies in Guangzhou, China: COPD, 2013; 10; 567-72
36. Yang L, Zhou M, Smith M, Body mass index and chronic obstructive pulmonary disease-related mortality: A nationally representative prospective study of 220,000 men in China: Int J Epidemiol, 2010; 39; 1027-36
37. Celli BR, Cote CG, Marin JM, The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease: N Engl J Med, 2004; 350; 1005-12
38. Harik-Khan RI, Fleg JL, Wise RA, Body mass index and the risk of COPD: Chest, 2002; 121; 370-76
39. Garrido-Miguel M, Cavero-Redondo I, Alvarez-Bueno C, Prevalence and trends of thinness, overweight and obesity among children and adolescents aged 3–18 years across Europe: A protocol for a systematic review and meta-analysis: BMJ Open, 2017; 7; e018241
40. Dhurandhar EJ, The downfalls of BMI-focused policies: Int J Obes (Lond), 2016; 40; 729-30
Figures
 Figure 1. Participant recruitment and follow-up flow chart.
Figure 1. Participant recruitment and follow-up flow chart. Figure 2. Serum gamma-glutamyltransferase (GGT) levels in the 3 groups.
Figure 2. Serum gamma-glutamyltransferase (GGT) levels in the 3 groups. Figure 3. Correlations between GGT and forced expiratory volume in one second as the percentage of the predicted value (FEV1%) (A), GGT and forced expiratory volume in one second/forced vital capacity ratio (FEV1/FVC) (B).
Figure 3. Correlations between GGT and forced expiratory volume in one second as the percentage of the predicted value (FEV1%) (A), GGT and forced expiratory volume in one second/forced vital capacity ratio (FEV1/FVC) (B). Figure 4. Receiver-operating characteristic (ROC) curves for GGT activity to evaluate COPD (A) and its acute exacerbation (B). AUC – area under the curve.
Figure 4. Receiver-operating characteristic (ROC) curves for GGT activity to evaluate COPD (A) and its acute exacerbation (B). AUC – area under the curve. Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952