22 November 2020: Clinical Research
Surgery After Ultrasound-Guided Radiofrequency Ablation for Papillary Thyroid Carcinoma in 21 Patients: A Retrospective Study from a Single Center in China
Wei Sun1ABCDEF, Hao Zhang1ABCDG*, Liang He2DE, Ting Zhang1BE, Zhihong Wang1BCD, Wenwu Dong1CDE, Yingling Jiang1EDOI: 10.12659/MSM.928391
Med Sci Monit 2020; 26:e928391
Abstract
BACKGROUND: Radiofrequency ablation (RFA) is used to treat various cancers, but its use in thyroid cancer remains controversial. The aim of this study was to investigate surgical findings after RFA for papillary thyroid cancer (PTC).
MATERIAL AND METHODS: The study included 21 patients (average age 44.9±13.3 years) who had biopsy-confirmed thyroid cancer treated with RFA in multiple hospitals. Surgery was done in the First Hospital of China Medical University.
RESULTS: The 21 patients had a total of 32 thyroid nodules that were treated with RFA. Twenty-eight nodules were malignant, and 4 nodules were benign. Before RFA, 17 of the malignant nodules were >1 cm and 11 were ≤1 cm. Among the 28 malignant nodules, post-ablation lesions adhered to or invaded the structures surrounding the thyroid in 17 (60.7%), 19 (67.9%), and 22 (78.6%) nodules evaluated with ultrasound, contrast-enhanced computed tomography, and intraoperatively, respectively. Based on pathology results, 7 (33.3%) of the 21 patients had bilateral cancer. Ten (47.6%) of the 21 patients had central lymph node metastasis and 2 (9.5%) had lateral lymph node metastasis. For 5 (15.6%) of the 32 nodules, the fine-needle aspiration results were not consistent with the postoperative pathological results. Five (23.8%) of the 21 patients with lymph node metastasis had clinically negative (CN0) lesions.
CONCLUSIONS: RFA for PTC primary lesions may be incomplete and leave residual lymph node metastasis, even in lesions ≤1 cm. RFA should be recommended with caution in the treatment of operable patients with primary PTC.
Keywords: Catheter Ablation, Thyroid Nodule, Contrast Media, Postoperative Care, Preoperative Care, radiofrequency ablation, Thyroid Cancer, Papillary, Tomography, X-Ray Computed, Ultrasonography
Background
Thyroid cancer is a common malignancy, and its incidence has dramatically increased over the past several decades [1]. With the exception of anaplastic thyroid cancer, surgery is the main treatment for primary thyroid cancer, followed by radioactive iodine therapy and/or thyroid hormone therapy [2]. Thermal ablation – including radiofrequency ablation (RFA), laser ablation, and microwave ablation – is a nonsurgical, minimally invasive technique that has been widely used to treat hepatocellular cancer, renal cancer, and prostate cancer [3–5]. Due to the lack of long-term follow-up and controlled trials, the application scope and the indications, effectiveness, and safety of thermal ablation in the management of thyroid cancer remains controversial. Both the 2015 Italian opinion statement on RFA for thyroid nodules [6] and the 2017 Korean Thyroid Radiofrequency Ablation Guideline [7] recommended the use of thermal ablation for benign thyroid nodules and recurrent thyroid cancers at the thyroidectomy bed or for cervical lymph nodes in patients at high surgical risk or in those who refuse surgery. The 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer indicated that thermal ablation could be useful to treat high-risk patients who refuse additional surgery or patients with individual distant metastatic lesions [8].
Recently, RFA has been reported to be an effective and safe treatment for papillary thyroid microcarcinoma (PTMC). A recent meta-analysis of 11 eligible studies including 715 PTMC patients indicated that the complete disappearance and recurrence of PTMC were 57.6% and 0.4%, respectively, after thermal ablation. The pooled estimates of mean volume reduction and its rate were 73.5 mm3 and 98.1%, respectively. Overall and major complications occurred in 3.2% and 0.7% of cases, respectively [9]. However, other studies have reported that using thermal ablation to treat PTC or PTMC could lead to residual primary lesions and lymph node metastases [10–12].
In this study, we retrospectively analyzed data from 21 patients with PTC or PTMC who required surgery for post-ablation lesions in our hospital following an initial diagnosis and treatment with RFA in other hospitals. The aim of this study was to describe and investigate surgical characteristics and outcomes after RFA.
Material and Methods
STUDY DESIGN AND PATIENTS:
A total of 21 patients underwent thyroid surgery in the First Hospital of China Medical University after RFA treatment between November 2014 and January 2019.
We retrospectively analyzed data from 21 patients who required further management of post-ablation lesions in our hospital after primary PTC was diagnosed with fine-needle aspiration (FNA) and treated with RFA in other hospitals. Patients were eligible for surgery if ultrasound or contrast-enhanced computed tomography (CT) suggested malignancy of the post-ablation lesions, or if clinical evidence of cervical lymph node metastasis was present after RFA. Additionally, patients who requested further surgical treatment were considered. We reviewed the patient medical records for clinical, radiological, surgical, and pathological data. We extracted data from the records on age, sex, FNA results, RFA position, nodule size and ultrasound findings before and after RFA, contrast-enhanced CT imaging before surgery, extent of thyroid surgery, postoperative pathology outcomes, and the number of days between RFA and surgery. The ultrasound results were recorded according to the risk stratification of the Thyroid Imaging Reporting and Data System (TI-RADS) [13,14]. The patients were categorized and described according to the size of tumor lesions (i.e., ≤1 cm or >1 cm). The malignancy percentage was classified into the following TI-RADS categories at our hospital: category 1 (normal thyroid gland), category 2 (0% risk of malignancy), category 3 (<5% risk of malignancy), category 4a (5–10% risk of malignancy), category 4b (11–50% risk of malignancy), category 4c (51–85% risk of malignancy), category 5 (>86% risk of malignancy), and category 6 (biopsy-proven malignancy).
Our study was approved by the Ethics Committee of the First Affiliated Hospital of China Medical University, Shenyang, China. Written informed consent was obtained from all study participants.
SURGERY:
The extent of surgery was based on the FNA results prior to RFA, the characteristics of ultrasound or contrast-enhanced CT before surgery, and the pathological findings during the operation. For lesions associated with thyroid cancer, lobectomy plus isthmectomy and total thyroidectomy were performed. For lymph node dissection, routine ipsilateral or bilateral central lymph node dissections were performed. A modified lateral lymph node dissection (II–Vb levels) was performed in patients with clinically evident lateral neck lymph node metastasis.
STATISTICAL ANALYSIS:
SPSS 13.0 software (SPSS, USA) was used for statistical analyses. Continuous variables are expressed as mean±standard deviation. The 2 groups were compared by
Results
BASELINE CHARACTERISTICS AND CLINICAL FEATURES:
A total of 21 patients (15 women and 6 men; average age 44.9±13.3 years) were included in our study (Table 1). All FNA biopsies were done before RFA treatment, and all patients had 1 or more malignant lesions. Prior to their arrival at our hospital, 5 patients underwent RFA on the right side of the thyroid, 5 patients underwent RFA on the left side of the thyroid, and 11 patients underwent bilateral thyroid RFA. In our hospital, 14 patients received total thyroidectomy and 7 patients received lobectomy plus isthmectomy. Unilateral central lymph node dissection (CLND) was performed in 13 patients and bilateral CLND was done in 8 patients. Two patients underwent modified lateral neck dissection with clinically evident lateral neck lymph node metastasis (II–Vb levels). The median interval between RFA and surgery was 30 days (range 6–368 days).
CHANGES IN NODAL SIZE AND TI-RADS GRADE BEFORE AND AFTER RFA ON ULTRASOUND:
A total of 32 thyroid nodules underwent RFA. According to the final pathology report, 28 nodules were malignant and 4 were benign. The 28 malignant nodules were grouped based on whether their diameter was smaller or larger than 1 cm on ultrasound before RFA. The tumor size of 17 of these nodules were >1 cm, while 11 nodules were ≤1 cm (Table 1). In a comparison of the tumor size of the nodules before and after RFA, we found no significant change in the diameter of nodules >1 cm before (2.4±0.8 cm) and after ablation (2.4±0.9 cm). However, for nodules ≤1 cm, the tumor diameter significantly increased after ablation (1.4±0.9 cm) compared with its size before ablation (0.7±0.2 cm) (P<0.05) (Figure 1A–1C). In addition, the TI-RADS grade was analyzed before and after RFA. The proportion of patients with a TI-RADS grade above 4b was 28.1% before RFA and 56.3% after RFA (P=0.023) (Table 1).
PREOPERATIVE ULTRASOUND, CONTRAST-ENHANCED CT EVALUATION, AND INTRAOPERATIVE FINDINGS:
Preoperative ultrasound evaluation indicated that 20 patients had at least 1 nodule with a TI-RADS grade above 4a and 1 patient had nodules with a TI-RADS grade of 3. Among the 28 malignant nodules, post-ablation nodules were found to have adhered to or invaded the structures surrounding the thyroid in 17 (60.7%) nodules evaluated with ultrasound and in 19 (67.9%) nodules evaluated with contrast-enhanced CT. During surgery, we found that the strap muscles were markedly swollen in 22 (78.6%) of 28 nodules, which made separating them from the post-ablation thyroid lesions difficult (Table 2). After surgery, all patients recovered well without surgical complications. However, 2 patients (numbers 5 and 18) had hoarseness and unilateral vocal cord fixation associated with RFA. During surgery, we found that the thyroid lesions had adhered to or invaded the recurrent laryngeal nerve in these 2 patients (Figure 2A, 2B). Moreover, when malignant nodules were grouped according to ultrasound size before RFA, we found that the incidence of adherence to or invasion of the structures surrounding the thyroid was significantly higher in patients with nodules >1 cm compared with those with nodules ≤1 cm, regardless of preoperative ultrasound, CT, or intraoperative exploration (Table 2).
POSTOPERATIVE PATHOLOGY:
Among the 21 patients with residual PTC lesions (Table 1), cancer was bilateral in 7 (33.3%) (Figure 3A, 3B). Central lymph node metastasis was found in 10 (47.6%) of the 21 patients, and lateral lymph node metastasis was found in 2 (9.5%) patients (Figure 3C). Further, we found that 23.8% (5/21) patients had no lymph node metastasis before or during surgery (CN0), yet they were among the 10 patients with lymph node metastasis finally confirmed by postoperative pathology (Figure 3D). In 15.6% (5/32) patients, the FNA results were not consistent with the postoperative pathological results (Figure 3D). All of the nodules in these patients were ≤1 cm according to ultrasound before RFA, and the FNA results indicated follicular epithelial cell cancer; however, the pathology results showed that a PTC lesion was present (patients 4, 7, 14, 19, 21) (Supplementary Table 1).
Discussion
In this study, we retrospectively analyzed data from 21 patients with primary PTC who required further operation of post-ablation lesions in our hospital after they received an initial diagnosis and treatment with RFA in other hospitals. The results indicated that RFA for thyroid cancer primary lesions can be incomplete and leave residual lymph node metastasis even in primary lesions ≤1 cm. The use of RFA for treating operable PTC is still controversial.
In our study, nodules larger than 1 cm accounted for 60.7% of the 28 malignant nodules, indicating that such nodules are more likely to have residual lesions remaining after RFA. The result may be due to the size of the thyroid and the need to protect surrounding tissue, which make complete ablation of nodules >1 cm difficult [10]. This finding was similar to that of Ma et al. [11], who studied a total of 19 malignant residual nodules after RFA and found that 11 (57.9%) nodules were >1 cm and 8 (42.1%) nodules were ≤1 cm. We also found that residual cancer tissue still existed in 11 nodules that were ≤1 cm. A few studies have reported that RFA is effective in the treatment of PTC or PTMC [15,16]. For example, Lim et al. [17] reported using RFA to treat 152 PTMCs from 133 patients. Complete disappearance was found in 91.4% (139/152) of ablated tumors. Among the 13 tumors without complete disappearance, no tumor displayed any regrowth of the residual ablated lesion during the follow-up period [17]. From the results of the current study, clinicians should consider the possibility of malignancy remaining after RFA. Because not all PTMC is indolent and the biological aggressiveness may differ, various levels of thyroid cancer may lead to different prognoses [18]. In addition, we found that for the 11 nodules that were ≤1 cm, the tumor diameter was significantly increased after ablation and the TI-RADS grade was more likely to indicate a malignant character after RFA.
Through ultrasound, CT, and intraoperative evaluation, we found that RFA could lead to adhesion or invasion between the thyroid and surrounding tissues (strap muscles, recurrent laryngeal nerve). Heat during RFA can cause the strap muscles to become swollen, and subsequent adhesion or invasion can increase surgical difficulty, influence the choice of surgical methods, and complicate T-staging by surgeons [10,19]. Moreover, we found that the incidence of adherence to or invasion of the structures surrounding the thyroid after ablation in patients with nodules larger than 1 cm was significantly higher than in patients with nodules smaller than 1 cm, regardless of preoperative ultrasound, CT, or intraoperative exploration. Therefore, in patients needing another operation after RFA, surgery may be more difficult when tumors have a diameter >1 cm, with an associated higher risk of complications and greater difficulty in determining the TNM stage [19].
Evaluation technology before RFA is another important factor limiting the use of RFA for thyroid cancer. By comparing the postoperative pathology with the preoperative FNA and imaging data, we found that preoperative findings for 15.6% (5/32) of the nodules were not consistent with the postoperative results; all 5 of these nodules were <1 cm. The discrepant results may be due to the greater difficulty in obtaining samples of pathological tissues by FNA when nodules are <1 cm [20]. Nachiappan et al. [21] also reported that the possibility of obtaining inadequate target cells from the sample material could be as high as 33.6% with FNA [21]. More accurate pathological results before RFA would lead to different follow-up intensities and treatment strategies. Another problem is that cervical lymph node metastasis is very common in PTC, but the sensitivity of detection of lymph node metastasis by ultrasound and CT is relatively low [22]. Cervical lymph node metastasis is observed in 20–90% of CN0 patients [23,24]. We found that 23.8% (5/21) of patients with lymph node metastasis had a staging of CN0. At present, metastasis cannot be identified through any preoperative evaluation and lymph nodes containing metastases cannot be removed through RFA. Two randomized controlled trials of surgery and RFA showed patients with CN0 staging in both groups, with lymph node metastasis rates in the operation group and RFA group being 20.0% and 28.6%, respectively [25,26]. Therefore, it can be speculated that residual lymph node metastases will be present following RFA treatment.
The main limitation of this retrospective study was selection bias. Since all of the nodules were pathologically proven to be residual, we do not know how many patients underwent RFA in other hospitals and how many had residual lesions. In addition, the pre-RFA ultrasounds were obtained in other hospitals and initially interpreted by other physicians. The images were reassessed by 2 experienced doctors in our hospital based on the image features and the ultrasound descriptions, and this may have led to some bias in the TI-RADS grade. Further studies are needed to investigate the factors influencing RFA in thyroid cancer.
Conclusions
In conclusion, our retrospective study demonstrated that the use of RFA to treat primary thyroid cancer lesions might leave residual lymph node metastases even when the primary lesions are ≤1 cm. Lesions >1 cm are more likely to adhere to or invade the structures surrounding the thyroid and to need surgery following RFA. RFA should be recommended with caution for treating patients with primary thyroid cancer that is operable.
Figures
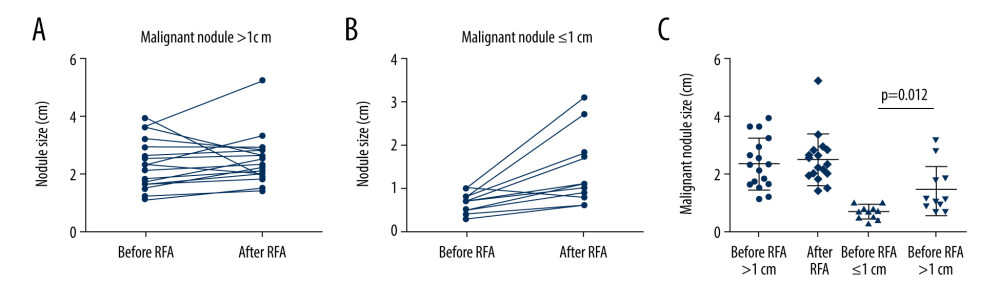 Figure 1. Changes in nodal size before and after radiofrequency ablation (RFA) on ultrasound. (A) The change tendency of size >1 cm malignant nodules before and after RFA. (B) The change tendency of size ≤1 cm malignant nodules before and after RFA. (C) Scatter plot of sizes of malignant nodules >1 cm or ≤1 cm.
Figure 1. Changes in nodal size before and after radiofrequency ablation (RFA) on ultrasound. (A) The change tendency of size >1 cm malignant nodules before and after RFA. (B) The change tendency of size ≤1 cm malignant nodules before and after RFA. (C) Scatter plot of sizes of malignant nodules >1 cm or ≤1 cm. 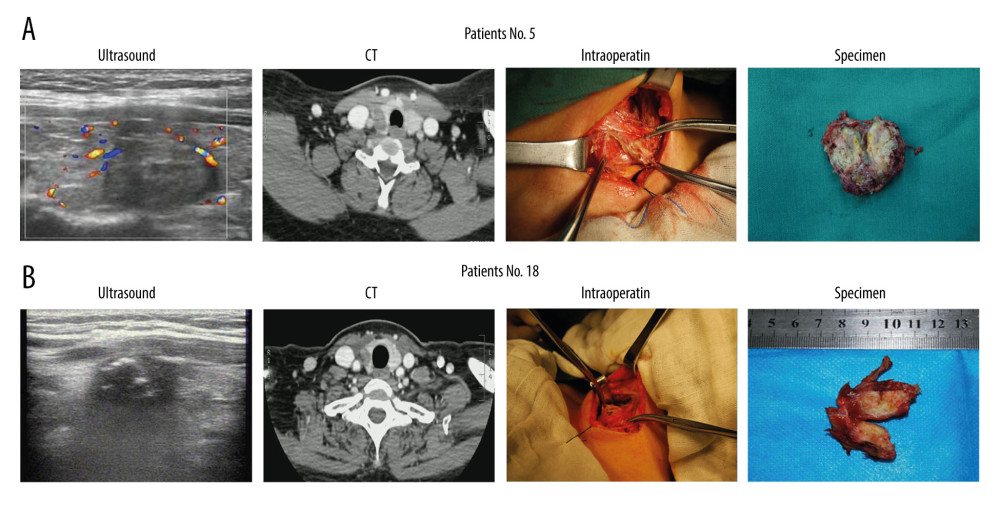 Figure 2. Preoperative ultrasound, contrast-enhanced computed tomography (CT) evaluation and intraoperative findings. (A, B) Ultrasound, CT, intraoperative, and nodule specimens of 2 patients (numbers 5 and 18) in whom the thyroid lesion simultaneously adhered to or invaded the strap muscles and recurrent laryngeal nerve.
Figure 2. Preoperative ultrasound, contrast-enhanced computed tomography (CT) evaluation and intraoperative findings. (A, B) Ultrasound, CT, intraoperative, and nodule specimens of 2 patients (numbers 5 and 18) in whom the thyroid lesion simultaneously adhered to or invaded the strap muscles and recurrent laryngeal nerve. 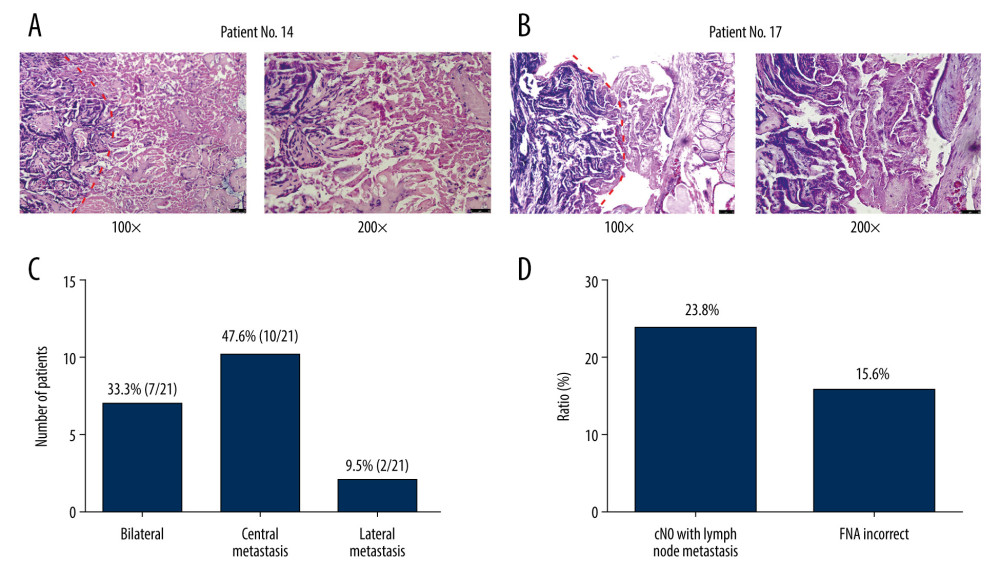 Figure 3. Postoperative pathology. (A, B) Pathological section showed necrotic papillary thyroid microcarcinoma (PTMC) tissue on the right side after radiofrequency ablation (RFA), but residual papillary thyroid carcinoma (PTC) tissue was still seen on the left side. Patient 14 (A) and patient 17 (B). (C) Incidence of bilateral cancer, central lymph node metastasis, and lateral lymph node metastasis in 21 patients. (D) Incidence of CN0 PTC and incorrect fine-needle aspiration results in 21 patients.
Figure 3. Postoperative pathology. (A, B) Pathological section showed necrotic papillary thyroid microcarcinoma (PTMC) tissue on the right side after radiofrequency ablation (RFA), but residual papillary thyroid carcinoma (PTC) tissue was still seen on the left side. Patient 14 (A) and patient 17 (B). (C) Incidence of bilateral cancer, central lymph node metastasis, and lateral lymph node metastasis in 21 patients. (D) Incidence of CN0 PTC and incorrect fine-needle aspiration results in 21 patients. Tables
Table 1. Baseline characteristics and clinical features of 21 patients with residual papillary thyroid carcinoma.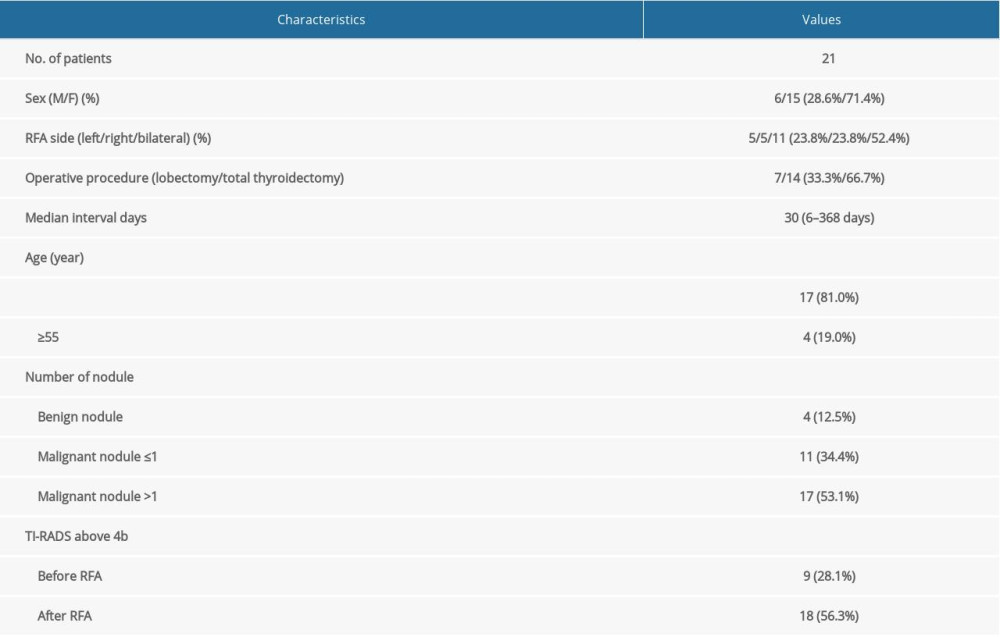 Table 2. The incidence of post-ablation lesions that adhered to or invaded the structures surrounding the thyroid.
Table 2. The incidence of post-ablation lesions that adhered to or invaded the structures surrounding the thyroid.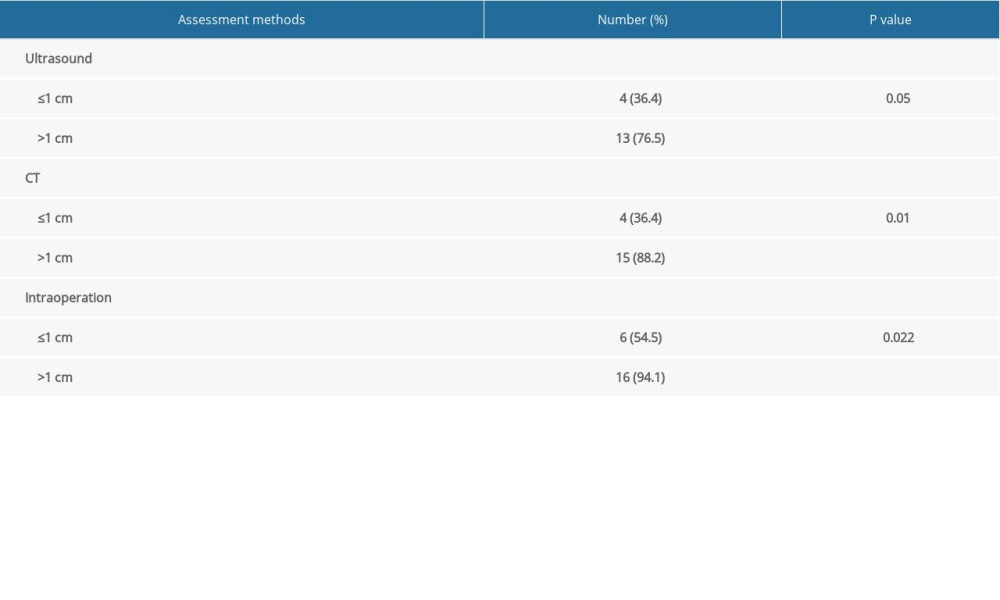 Supplementary Table 1. Characteristics and clinical features of 21 residual patients one by one.
Supplementary Table 1. Characteristics and clinical features of 21 residual patients one by one.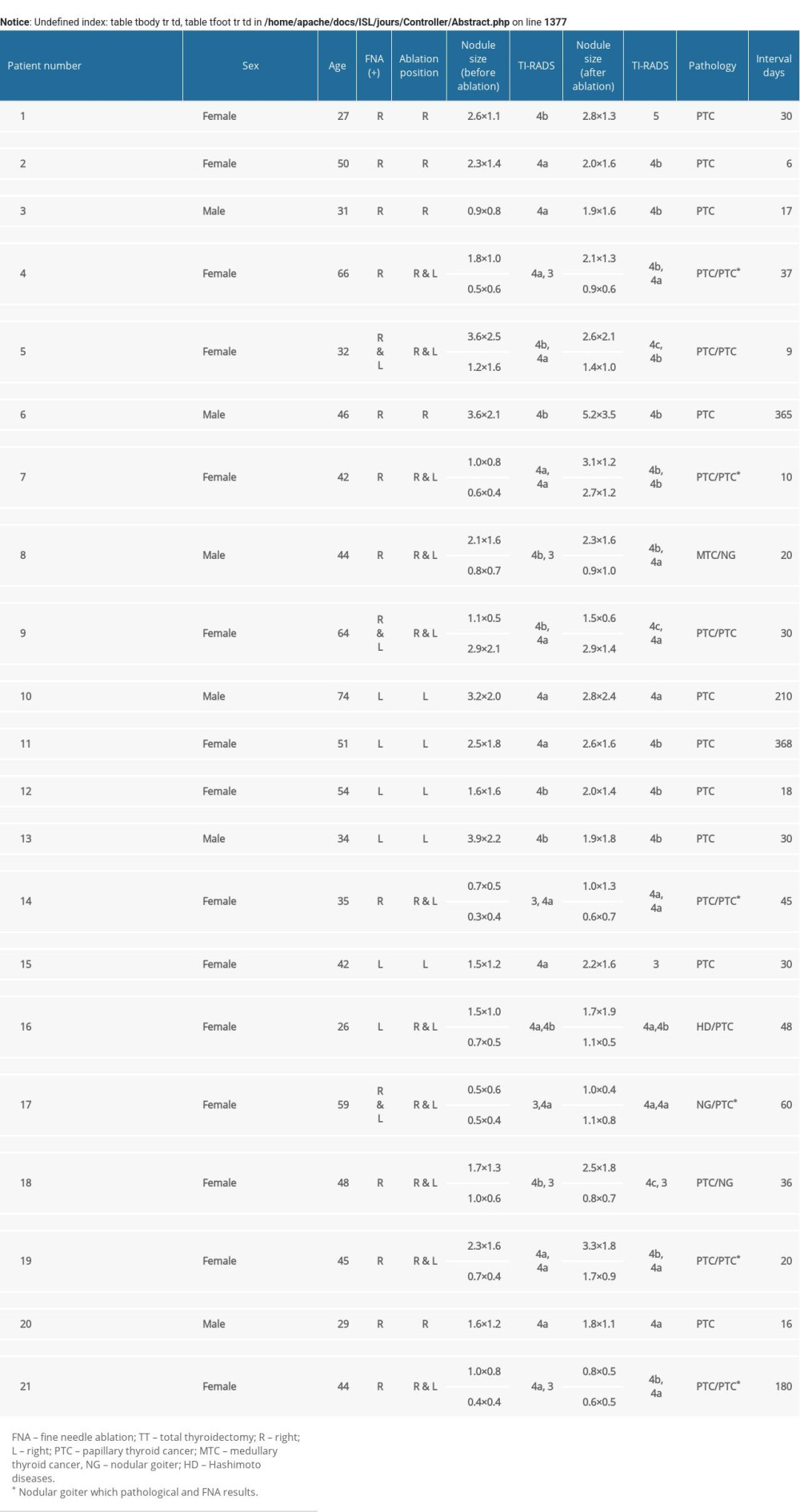
References
1. Lim H, Devesa SS, Sosa JA, Trends in thyroid cancer incidence and mortality in the United States, 1974–2013: JAMA, 2017; 317(13); 1338-48
2. Sun W, Lan X, Zhang H, Neat1_2 functions as a competing endogenous RNA to regulate ATAD2 expression by sponging microRNA-106b-5p in papillary thyroid cancer: Cell Death Dis, 2018; 9(3); 380
3. Yu J, Liang P, Yu XL, US-guided percutaneous microwave ablation versus open radical nephrectomy for small renal cell carcinoma: Intermediate-term results: Radiology, 2014; 270(3); 880-87
4. Qu P, Yu X, Liang P, Contrast-enhanced ultrasound in the characterization of hepatocellular carcinomas treated by ablation: Comparison with contrast-enhanced magnetic resonance imaging: Ultrasound Med Biol, 2013; 39(9); 1571-79
5. Werntz RP, Eggener SE, Novel focal therapy treatment options for prostate cancer: Curr Opin Urol, 2018; 28(2); 178-83
6. Garberoglio R, Aliberti C, Appetecchia M, Radiofrequency ablation for thyroid nodules: which indications? The First Italian Opinion Statement: J Ultrasound, 2015; 18(4); 423-30
7. Kim JH, Baek JH, Lim HK, 2017 Thyroid radiofrequency ablation guideline: Korean Society of Thyroid Radiology: Korea J Radiol, 2018; 19(4); 632-55
8. Haugen BR, Alexander EK, Bible KC, 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American Thyroid Association Guidelines Task Force on thyroid nodules and differentiated thyroid cancer: Thyroid, 2016; 26(1); 1-133
9. Choi Y, Jung SL, Efficacy and safety of thermal ablation techniques for the treatment of primary papillary thyroid microcarcinoma: A systematic review and meta-analysis: Thyroid, 2020; 30(5); 720-31
10. Kim HY, Ryu WS, Woo SU, Primary papillary thyroid carcinoma previously treated incompletely with radiofrequency ablation: J Cancer Res Ther, 2010; 6(3); 310-12
11. Ma B, Wei W, Xu W, Surgical confirmation of incomplete treatment for primary papillary thyroid carcinoma by percutaneous thermal ablation: A retrospective case review and literature review: Thyroid, 2018; 28(9); 1134-42
12. Valcavi R, Piana S, Bortolan GS, Ultrasound-guided percutaneous laser ablation of papillary thyroid microcarcinoma: A feasibility study on three cases with pathological and immunohistochemical evaluation: Thyroid, 2013; 23(12); 1578-82
13. Shin JH, Baek JH, Chung J, Ultrasonography diagnosis and imaging-based management of thyroid nodules: Revised Korean society of thyroid radiology consensus statement and recommendations: Korea J Radiol, 2016; 17(3); 370-95
14. Kwak JY, Han KH, Yoon JH, Thyroid imaging reporting and data system for US features of nodules: A step in establishing better stratification of cancer risk: Radiology, 2011; 260(3); 892-99
15. Wu R, Luo Y, Tang J, Ultrasound-guided radiofrequency ablation for papillary thyroid microcarcinoma: A retrospective analysis of 198 patients: Int J Hyperthermia, 2020; 37(1); 168-74
16. Zhang M, Luo Y, Zhang Y, Tang J, Efficacy and safety of ultrasound-guided radiofrequency ablation for treating low-risk papillary thyroid microcarcinoma: A prospective study: Thyroid, 2016; 26(11); 1581-87
17. Lim HK, Cho SJ, Baek JH, US-guided radiofrequency ablation for low-risk papillary thyroid microcarcinoma: Efficacy and safety in a large population: Korean J Radiol, 2019; 20(12); 1653-61
18. Montero-Conde C, Martín-Campos JM, Lerma E, Molecular profiling related to poor prognosis in thyroid carcinoma. Combining gene expression data and biological information: Oncogene, 2008; 27(11); 1554-61
19. Dong W, Zhang H, Zhang P, Re-operation for papillary thyroid carcinoma after radiofrequency ablation therapy: A clinical analysis of 5 cases: Chin J Prac Surg, 2015; 35; 653-55
20. Dong Y, Mao M, Zhan W, Size and ultrasound features affecting results of ultrasound-guided fine-needle aspiration of thyroid nodules: J Ultrasound Med, 2018; 37(6); 1367-77
21. Nachiappan AC, Metwalli ZA, Hailey BS, The thyroid: review of imaging features and biopsy techniques with radiologic-pathologic correlation: Radiographics, 2014; 34(2); 276-93
22. Kim E, Park JS, Son KR, 2008 Preoperative diagnosis of cervical metastatic lymph nodes in papillary thyroid carcinoma: Comparison of ultrasound, computed tomography, and combined ultrasound with computed tomography: Thyroid, 2008; 18(4); 411-18
23. Grebe SK, Hay ID, Thyroid cancer nodal metastases: Biologic significance and therapeutic considerations: Surg Oncol Clin N Am, 1996; 5(1); 43-63
24. Kouvaraki MA, Shapiro SE, Fornage BD, Role of preoperative ultrasonography in the surgical management of patients with thyroid cancer: Surgery, 2003; 134(6); 946-54
25. Chen H, Zhang C, Huang P, Microwave ablation and surgical resection of papillary thyroid microcarcinoma: Comparative analysis of clinical efficacy, safety and economy: Chin J Med Ultrasound, 2018; 15(4); 275-80
26. Cao W, Wang Y, Zhou N, Study on ultrasound-guided microwave ablation and endoscopic surgery of papillary thyroid carcinoma: Chin Comput Med Imaging, 2018; 24(3); 254-57
Figures
 Figure 1. Changes in nodal size before and after radiofrequency ablation (RFA) on ultrasound. (A) The change tendency of size >1 cm malignant nodules before and after RFA. (B) The change tendency of size ≤1 cm malignant nodules before and after RFA. (C) Scatter plot of sizes of malignant nodules >1 cm or ≤1 cm.
Figure 1. Changes in nodal size before and after radiofrequency ablation (RFA) on ultrasound. (A) The change tendency of size >1 cm malignant nodules before and after RFA. (B) The change tendency of size ≤1 cm malignant nodules before and after RFA. (C) Scatter plot of sizes of malignant nodules >1 cm or ≤1 cm. Figure 2. Preoperative ultrasound, contrast-enhanced computed tomography (CT) evaluation and intraoperative findings. (A, B) Ultrasound, CT, intraoperative, and nodule specimens of 2 patients (numbers 5 and 18) in whom the thyroid lesion simultaneously adhered to or invaded the strap muscles and recurrent laryngeal nerve.
Figure 2. Preoperative ultrasound, contrast-enhanced computed tomography (CT) evaluation and intraoperative findings. (A, B) Ultrasound, CT, intraoperative, and nodule specimens of 2 patients (numbers 5 and 18) in whom the thyroid lesion simultaneously adhered to or invaded the strap muscles and recurrent laryngeal nerve. Figure 3. Postoperative pathology. (A, B) Pathological section showed necrotic papillary thyroid microcarcinoma (PTMC) tissue on the right side after radiofrequency ablation (RFA), but residual papillary thyroid carcinoma (PTC) tissue was still seen on the left side. Patient 14 (A) and patient 17 (B). (C) Incidence of bilateral cancer, central lymph node metastasis, and lateral lymph node metastasis in 21 patients. (D) Incidence of CN0 PTC and incorrect fine-needle aspiration results in 21 patients.
Figure 3. Postoperative pathology. (A, B) Pathological section showed necrotic papillary thyroid microcarcinoma (PTMC) tissue on the right side after radiofrequency ablation (RFA), but residual papillary thyroid carcinoma (PTC) tissue was still seen on the left side. Patient 14 (A) and patient 17 (B). (C) Incidence of bilateral cancer, central lymph node metastasis, and lateral lymph node metastasis in 21 patients. (D) Incidence of CN0 PTC and incorrect fine-needle aspiration results in 21 patients. Tables
 Table 1. Baseline characteristics and clinical features of 21 patients with residual papillary thyroid carcinoma.
Table 1. Baseline characteristics and clinical features of 21 patients with residual papillary thyroid carcinoma. Table 2. The incidence of post-ablation lesions that adhered to or invaded the structures surrounding the thyroid.
Table 2. The incidence of post-ablation lesions that adhered to or invaded the structures surrounding the thyroid. Table 1. Baseline characteristics and clinical features of 21 patients with residual papillary thyroid carcinoma.
Table 1. Baseline characteristics and clinical features of 21 patients with residual papillary thyroid carcinoma. Table 2. The incidence of post-ablation lesions that adhered to or invaded the structures surrounding the thyroid.
Table 2. The incidence of post-ablation lesions that adhered to or invaded the structures surrounding the thyroid. Supplementary Table 1. Characteristics and clinical features of 21 residual patients one by one.
Supplementary Table 1. Characteristics and clinical features of 21 residual patients one by one. In Press
06 Mar 2024 : Clinical Research
Comparison of Outcomes between Single-Level and Double-Level Corpectomy in Thoracolumbar Reconstruction: A ...Med Sci Monit In Press; DOI: 10.12659/MSM.943797
21 Mar 2024 : Meta-Analysis
Economic Evaluation of COVID-19 Screening Tests and Surveillance Strategies in Low-Income, Middle-Income, a...Med Sci Monit In Press; DOI: 10.12659/MSM.943863
10 Apr 2024 : Clinical Research
Predicting Acute Cardiovascular Complications in COVID-19: Insights from a Specialized Cardiac Referral Dep...Med Sci Monit In Press; DOI: 10.12659/MSM.942612
06 Mar 2024 : Clinical Research
Enhanced Surgical Outcomes of Popliteal Cyst Excision: A Retrospective Study Comparing Arthroscopic Debride...Med Sci Monit In Press; DOI: 10.12659/MSM.941102
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








