22 February 2021: Clinical Research
Fractional Exhaled Nitric Oxide (FeNO) Integrating Airway Hyperresponsiveness (AHR) Examination Promotes Etiologic Diagnosis and Treatment for Children with Chronic Cough
Haiyan Zhu1BCDEF, Chuangli Hao2BCDE, Xingmei Yu2BCDF, Rongrong Zhang1BCD, Wendi Zhou1BCF, Xingzhen Sun1BCF, Yufang Yuan1BCF, Zhaofang Tian1ABDEG*DOI: 10.12659/MSM.928502
Med Sci Monit 2021; 27:e928502
Abstract
BACKGROUND: Chronic cough is the main reason why parents seek medical treatment for their children. This study aimed to evaluate changes in airway function and inflammation levels and associated values in diagnosing and treating chronic cough.
MATERIAL AND METHODS: This study involved 118 children with chronic cough, including 45 cough-variant asthma (CVA) patients, 53 upper-airway cough syndrome (UACS) patients, and 20 post-infection cough (PIC) patients. Chronic cough was diagnosed as described by guidelines of the American College of Chest Physicians for evaluating chronic cough. Pulmonary ventilation function and airway hyperresponsiveness (AHR) were evaluated. Fractional exhaled nitric oxide (FeNO) levels and eosinophilic airway inflammation were measured. Eosinophil (EOS) count in sputum was also examined. CVA patients were treated with inhaled glucocorticoids, which have anti-inflammatory effects.
RESULTS: FeNO and sputum EOS levels were higher in CVA patients compared with UACS and PIC patients (P<0.05). CVA patients demonstrated significantly higher small airway indexes, including 25% forced expiratory flow (FEF), 50% FEF, and 75% FEF, compared with UACS and PIC patients (P<0.05). FeNO level was positively correlated with EOS in sputum (r=0.468, P=0.0001) and cough symptom scores (r=0.402, P<0.05). FeNO, EOS, and cough symptoms were significantly improved in CVA patients after glucocorticoid treatment. AHR was improved in all chronic cough patients after treatment. Cough-relief CVA patients demonstrated significantly higher FeNO levels compared with those without cough relief (P<0.05).
CONCLUSIONS: FeNO integrating pulmonary function and AHR examination can improve etiologic diagnosis and treatment for chronic cough in children.
Keywords: airway remodeling, Cough, Diagnosis, Nitric Oxide, Pediatrics, Asthma, Breath Tests, Child, Chronic Disease, Diagnostic Tests, Routine, Eosinophils, Exhalation, Lung, ROC Curve, Respiratory Hypersensitivity, Sputum
Background
Chronic cough is the main reason why parents seek medical treatment for their children [1,2]. The Children’s Chronic Cough Guideline defines cough for more than 4 weeks as chronic cough, and its etiology is complex and diverse [3]. Chronic cough is closely related to the adverse effects of inappropriate drug administration, multiple physician visits, and impaired life quality [4,5]. Cough-variant asthma (CVA) accounts for the highest proportion of chronic cough among school-age children, but its diagnosis is difficult [6]. Especially for nonspecific chronic cough, which lacks the associated clinical symptoms, diagnosis and therapy are relatively difficult [7].
Pulmonary function is commonly used to reflect diagnosis and treatment for children’s chronic cough, and the airway reversibility test is of diagnostic value for etiologic diagnosis [8]. There is an appropriate correlation between level of fractional exhaled nitric oxide (FeNO) and eosinophilic inflammation of the airway in bronchial asthma patients [9,10]. The Global Initiative for Asthma Guidelines [11] and China’s Guidelines for the Prevention and Treatment of Children’s Bronchial Asthma [8] also list FeNO as a noninvasive biomarker for inflammation of the asthmatic airway together with sputum inflammatory cell detection. The consensus of the American Thoracic Society and the European Respiratory Society (ATS/ERS) [12] also confirmed the potential value of FeNO as a biomarker for airway inflammation in reflecting eosinophilic airway inflammation and predicting the response of glucocorticoid therapy.
This study focused on changes in airway function and inflammation levels in school-age children with chronic cough and evaluated their values for clinical diagnosis and treatment. This study provides a basis for early diagnosis and follow-up treatment of chronic cough.
Material and Methods
PATIENTS:
The present study involved 118 children who had a cough for more than 4 weeks from April 2012 to December 2014 and were seen in the Respiratory Outpatient Department of Children’s Hospital Affiliated to Suzhou University. The involved patients were divided into CVA, upper-airway cough syndrome (UACS), and post-infection cough (PIC) groups according to the criteria of the Guidelines for Diagnosis and Treatment of Chronic Cough in Chinese Children (revised in 2013) [3]. All of the above patients were consecutively and randomly selected.
Inclusive criteria were: (1) chronic cough lasting more than 4 weeks, with cough as the main symptom or the only symptom; (2) X-ray chest examination showing no obvious pathological changes; (3) no use of glucocorticoids, antihistamines, bronchodilators, or other drugs that could affect the examination in the past 2 weeks; (4) patients who could complete the relevant examinations and follow-up treatments, and with clear etiologic diagnosis; (5) no previous serious diseases.
DIAGNOSTIC CRITERIA:
Diagnosis of chronic cough refers to Guidelines for the Diagnosis and Treatment of Chronic Cough in Children (Trial) [3] and Guidelines for Evaluating Chronic Cough in Pediatrics of the American College of Chest Physicians (ACCP) [13]. The diagnostic items were listed as follows: (1) detailed inquiry of medical history and comprehensive physical examination; (2) chest X-ray examination; (3) auxiliary examination including pulmonary ventilation function, FeNO, induced sputum cytology, nasal sinus computed tomography examination, skin allergen test, blood routine tests, mycoplasma antibody, tuberculosis antibody; (4) diagnosis based on examination results and treatment response; (5) about 4 weeks after treatment and 48 h after drug withdrawal, examination of lung function, FeNO, and induced sputum.
EVALUATION OF PULMONARY VENTILATION FUNCTION:
In the present research, the pulmonary ventilation function was measured using an impulse oscillometry pulmonary function instrument (Mode: MasterScreen, Jaeger Company, Hoechberg, Germany). In brief, the temperature, pressure, humidity, gas, and volume were corrected and recorded routinely before the daily measurements. The conventional ventilation measurements were carried out according to the recommended standards of the ERS [14].
AIRWAY HYPERRESPONSIVENESS (AHR) EVALUATION:
AHR evaluation was conducted according to a previous study [15]. Bronchial provocation tests were conducted when baseline post-salbutamol forced expiratory volume in 1 s (FEV1) was more than 70% predicted. For every experiment, a nose clip was worn first and then an aerosol was inhaled through the mouth by tidal breathing for 120 s. Phosphate-buffered saline (Beyotime Biotech, Shanghai, China) was first inhaled, followed by 2-min intervals of 2-fold increased concentrations (from 0.01 mg to 1.28 mg) of histamine acid phosphate (Sigma-Aldrich, St. Louis, MO, USA). The response of the treatment was measured using FEV1 at the beginning for the above experiment and at 2 min after each inhalation. Finally, inhalation was continued until the FEV1 was reduced by 20% or the highest cumulative dosage (2.4 mg) was administrated. Findings were represented as the required concentration for the etiology with a decrease of 20% in FEV1 (PD20-FEV1). According to the PD20-FEV1 [15], the severity of AHR was divided into 4 grades, including severe (<0.03 mg), moderate (0.03–0.24 mg), mild (0.25–0.98 mg), and extremely mild (0.99–2.2 mg).
MEASUREMENT OF FENO:
FeNO levels were measured using an electrochemical eNO determination system (Niox Mino eNO analyzer, Aerocrine, Solna, Sweden). The detection method for measuring FeNO was carried out according to the ATS/ERS operation standard and product specification [16]. Here, the main detection index was FeNO, represented by the following formula: 1 part per billion (ppb)=1×10−9 mol/L). Briefly, the method involved asking the child to exhale as much as possible, holding the detector filter tightly in his mouth, and the child inhaling deeply and exhaling evenly with a steady air flow. The expiratory velocity was set to 50 ms and the expiratory time was set as 10 s. Values were recorded.
CLASSIFICATION AND COUNTING OF INDUCED SPUTUM CELLS:
The induced sputum cells were counted according to the method introduced in previous literature [17]. In brief, the sputum was induced by atomization of 3% hypertonic saline solution (Beyotime Biotech). Then, the sputum was treated with 0.1% dithiothreitol (Sigma-Aldrich) and the cells were classified and counted after 37°C water bath treatment, filtration, centrifugal precipitation, sediment smear, and hematoxylin and eosin staining. An eosinophil (EOS) count of sputum >3% was used as a positive standard.
THERAPEUTIC STRATEGY:
In this study, chronic cough was diagnosed and treated according to the Guidelines of Diagnosis and Treatment for Pediatric Chronic Cough (2008) [18,19] recommended by the Asthma Group of the Society of Respiratory Diseases of the Chinese Medical Association. Children with CVA were treated with inhaled corticosteroid (salmeterol fluticasone powder) at a dosage of 50 μg of salmeterol and 100 μg of fluticasone twice a day. One month after inhalation treatment, the patients were followed up regularly for at least 8 weeks. Children in the PIC group, especially patients with severe symptoms, were given montelukast sodium orally at a dosage of 5 mg once a day until the symptoms were relieved. For children in the UACS group, depending on the different diseases of the upper airway that cause chronic cough, different therapeutic programs were adopted: (1) for allergic rhinitis, the children were administered antihistamine drugs and a glucocorticoid (nasal spray). (2) For sinusitis, the children were orally given antibiotics, including amoxicillin+clavulanate potassium or amoxicillin+azithromycin for at least 2 weeks. (3) For proliferative body hypertrophy, children were given glucocorticoid nasal spray combining administration with montelukast sodium orally for 1–3 months.
All of the patients with CVA, UACS, and PIC received the above strategies. Four weeks after treatment, FeNO was re-examined and induced sputum cell count and bronchial provocation tests were performed to evaluate the relief of cough symptoms and the score of cough symptoms. Children with CVA were treated with inhaled glucocorticoids for anti-inflammation and followed up 4 weeks later.
STATISTICAL ANALYSIS:
Data were analyzed with professional SPSS software (version 17.0, SPSS, Inc., Chicago, IL, USA). Quantitative data were assigned as mean±SD and analyzed using Tukey’s post hoc test with validated analysis of variance among groups. The categorical data were represented as quartile interval of median and analyzed using the chi-square test. The correlation between FeNO and sputum EOS ratio was analyzed using linear regression correlation analysis. A
Results
GENERAL INFORMATION:
In this study, there were 68 boys and 50 girls (1.36: 1). Mean age of the patients was 9.3±1.1 years, and cough course ranged from 1 month to 76 months. Among 118 cases of chronic cough, there were 45 cases of CVA, 53 cases of UACS, and 20 cases of PIC. Statistical analysis showed that no remarkable differences were discovered for the age, height, or weight among all of the etiologic groups (
HIGHER FENO AND SPUTUM EOS LEVELS WERE EXHIBITED IN CVA PATIENTS COMPARED WITH UACS AND PIC PATIENTS:
Levels of FeNO in the CVA group were remarkably higher compared with those of the UACS and PIC groups (Table 1, all P<0.05). However, no significant differences were seen between the UACS and the PIC groups (P>0.05). EOS levels in induced sputum in the CVA group were obviously higher compared with those in the UACS and the PIC groups (P<0.05). There were also no remarkable differences for cough symptom scores among all 3 groups (P>0.05).
CVA PATIENTS DEMONSTRATED HIGHER SMALL AIRWAY INDEXES COMPARED WITH UACS AND PIC PATIENTS:
In this study, all 118 children with chronic cough completed routine pulmonary ventilation function tests. For patients with CVA, PIC, and UACS, no obvious differences were illustrated for the baseline pulmonary function parameters (FEV1% predicted and FEV 1/forced vital capacity [FVC]) for all 2-2 comparisons (Table 2, P>0.05). However, the 25% forced expiratory flow (FEF 25%), 50% FEF (FEF 50%), and 75% FEF (FEF 75%) values in the CVA group were remarkably lower compared with those of the UACS and the PIC groups (Table 2, P<0.05); no obvious differences were found between the UACS and the PIC groups (P>0.05).
FENO LEVELS POSITIVELY CORRELATED WITH EOS IN SPUTUM AND COUGH SYMPTOM SCORES:
The liner regression correlation analysis showed that FeNO in children with chronic cough before treatment was significantly correlated with EOS proportion in the induced sputum (Figure 1A, r=0.468, P=0.0001). There was also a significant correlation between FeNO and total cough symptom scores in children with chronic cough before treatment (Figure 1B, r=0.402, P=0.001). However, no significant correlation was discovered between the FeNO levels and FEV1 value (r=0.14, P=0.257) or FEV1/FVC (r=0.23, P=0.185). Additionally, FeNO was negatively correlated with small airway indicators, including maximal mid-expiratory flow (MMEF) and mid-expiratory flow at 50% and 25% (MEF50, MEF25) in children with chronic cough before treatment (r=−0.276, −0.257, −0.334, respectively; P=0.011, 0.018, 0.002, respectively).
FENO, EOS, AND COUGH SYMPTOMS WERE SIGNIFICANTLY IMPROVED IN CVA PATIENTS AFTER TREATMENT:
For the CVA patients, the FeNO levels, EOS in induced sputum, and cough symptom scores were significantly decreased after treatment compared with those before treatment (Table 3, all P=0.001). For both the UACS group and the PIC group, cough symptom scores were significantly decreased after treatment compared with before treatment (Table 3, all P<0.001); however, there were no significant differences for FeNO levels and EOS in sputum between the before- and after-treatment groups (P>0.05).
AHR WAS IMPROVED IN ALL CHRONIC COUGH PATIENTS AFTER TREATMENT:
According to the AHR test, a bronchodilation test was conducted in 2 patients of the CVA group because of FEV1 <70%. The positive rate of AHR in the CVA group was significantly higher compared with that in the PIC group and the UACS group (Table 3, both P<0.05). At 4 weeks after treatment, all patients in the CVA group remained positive; however, in most cases the AHR in the PIC group and in the UACS group became negative.
For the severity of AHR at the time of initial diagnosis, 0, 13, 29, and 3 children were severe, moderate, mild, and extremely mild, respectively, in the CVA group (45 children with AHR), compared with 0, 0, 11, and 6 in the UACS group (17 children with AHR) and 0, 0, 8, and 6 in the PIC group (14 children with AHR). However, at 4 weeks after treatment, 0, 4, 39, and 2 children were severe, moderate, mild, and extremely mild, respectively, in the CVA group (45 children with AHR), compared with 0, 0, 2, and 4 in the UACS group (6 children with AHR) and 0, 0, 1, and 1 in the PIC group (2 children with AHR). The PD20-FEV1 value in the CVA group was significantly lower compared with those in the UACS group and the PIC group both at time of initial diagnosis and 4 weeks after treatment (follow-up) (Table 3, all P<0.01). However, no remarkable difference was found between the UACS and PIC groups (P=0.081). For the intragroup comparison, there were significant differences between initial diagnosis and 4 weeks after treatment (all P<0.05).
COUGH-RELIEF CVA PATIENTS DEMONSTRATED HIGHER FENO LEVELS:
In this study, judgment of cough relief was based on parents’ records and cough diaries. The improvement of cough for more than 3 days or by more than 75% was considered a non-cough state [20]. Among 45 cases of CVA treated with anti-inflammatory therapy, there were 39 cases relieved and 6 cases without effective relief. The baseline FeNO value of CVA children in the cough-relief group was 40±12 ppb, which was significantly higher compared with that in CVA children without relief (23±10 ppb; Figure 2, P=0.005).
Discussion
In this study, according to the diagnosis of chronic cough in school-age children, the first 3 causes for chronic cough were UACS (44.9%), CVA (38.2%), and PIC (16.9%). However, a multicenter study on etiologic components of chronic cough in Chinese children in 2012 demonstrated that the top 3 causes for chronic cough were CVA (41.95%), UACS (24.71%), and PIC (21.73%) [21]. Asiloy et al [22] reported that the top 4 causes for chronic cough in children are asthma and asthma-like diseases (25%), chronic persistent bacterial bronchitis (PBB, 23%), UACS (20%), and PBB combined with asthma (12%). According to the above description, the distribution of etiology in this study was equal to that of the multicenter study of Chinese children, but the proportions were different. At the same time, there are significant distinctions for the proportion of etiologic factors between our data and the foreign literature.
In the ACCP guidelines, Chang and Glomb [13] pointed out that the differences in etiologic diagnosis mainly depend on hospitals, diagnostic criteria, research subjects, follow-up rate, and length of cough course, and also depend on the physical examination of children, the means of auxiliary examination, and the content of investigation. We compared other subjects with children of all ages, and the diagnostic basis was different. These differences may be caused by national and regional environments.
CVA is thought to have the same pathogenesis as typical asthma, and both have AHR and allergic inflammation with EOS infiltration. Induced sputum EOS and FeNO are the most important markers of airway inflammation [23] and are closely related to AHR [24]. In this study, induced sputum test results showed that CVA airways had significant EOS inflammation and the highest level of FeNO.
AHR is an important reference index in diagnosing and differentially diagnosing chronic cough [25]. Although there is a certain proportion of AHR in UACS and PIC [26], lymphocytes and neutrophils are the main causes of airway inflammation in UACS and PIC [27]. Our results showed that UACS and PIC airways had non-EOS-induced inflammation and the level of FeNO was not high, which was significantly different from CVA inflammation types. At the same time, UACS and PIC also have a certain proportion of AHR (AHR positive), so AHR can be combined with induced sputum examination for diagnosis of non-EOS inflammation and low level of FeNO, basically excluding CVA.
FeNO can not only reflect airway inflammation sensitively, but can also be sensitive to glucocorticoid therapy [28]. Glucocorticoids can inhibit the effect of inducible NO synthase, so there will be a decrease in FeNO level after hormone treatment. FeNO levels can be significantly reduced 2–3 days after inhaled corticosteroid (ICS) treatment, and the maximum effect can be achieved at 2–4 weeks, which could predict a high level of FeNO [29]. Therefore, FeNO is considered a good tool for assessing patients’ ICS response [30]. In this study, after inhalation of glucocorticoid and anti-inflammatory treatment, the level of FeNO and EOS count in induced sputum of CVA patients decreased significantly, accompanied by a certain degree of reduction of AHR, which is consistent with the literature [31,32]. Inhalation of low-dose glucocorticoids can improve AHR in CVA patients in a short time, mainly by reducing airway EOS inflammation. FeNO has an obvious correlation with EOS in sputum [33]; therefore, FeNO can replace the tedious EOS counting examination in induced sputum to some extent when it is impossible to carry out induced sputum examination. In addition, the FeNO level of some children who meet the diagnostic criteria of CVA is normal, suggesting that normal FeNO cannot be excluded from the diagnosis of CVA [34]. Therefore, FeNO can supplement the insufficiency of pulmonary function, but cannot completely replace the pulmonary function examination.
The former study [35] of our team demonstrated that there was no significant correlation between cough symptom score and the size of PD20; however, the present study illustrated that a good correlation existed between levels of FeNO and cough symptom scores, suggesting that FeNO is a laboratory indicator closely related to the evaluation of chronic cough symptoms. The former document [36] has shown that small airway dysfunction is a characteristic of CVA, and it is mainly a minor airway dysfunction. Feng-Jia et al [37] also showed that the small airway function of CVA patients was significantly lower than that of non-CVA patients. This study reached a consistent conclusion, indicating the small airway dysfunction of CVA. A previous study [38] also reported that FeNO was correlated with small airway function. In this study, MMEF, MEF50, MEF25, and other small airway function indicators have a good correlation with levels of FeNO, suggesting that small airway dysfunction may be caused by airway inflammation. Therefore, clinical work should focus on the improvement of small airway function.
Conclusions
The etiology of chronic cough in children is complex. One important etiology is CVA, characterized by eosinophilic airway infiltration and inflammation and AHR, following associated high levels of FeNO and small airway dysfunction. There was a certain proportion of AHR in UACS and PIC; however, the severity of AHR was lower than in CVA, and had no EOS airway inflammation. After anti-inflammatory treatment with glucocorticoids, levels of FeNO decreased with the improvement of pulmonary function in children with CVA. Therefore, FeNO combined with pulmonary function and AHR examination can improve the diagnosis and treatment of children with chronic cough.
Figures
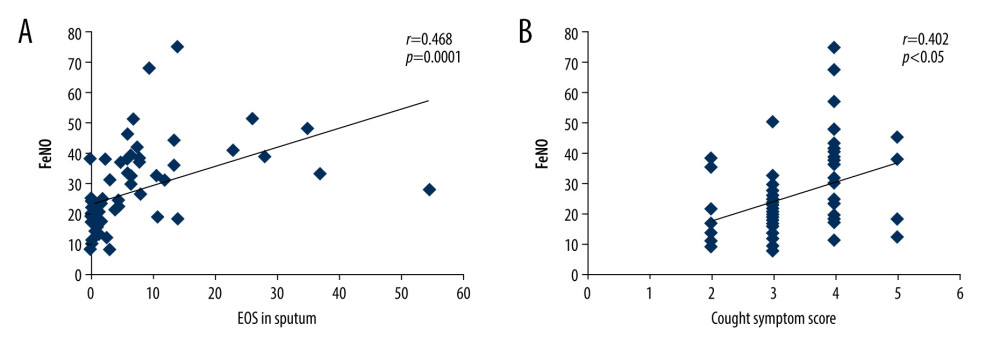 Figure 1. Linear correlation analysis for the correlation between fractional exhaled nitric oxide (FeNO) levels and eosinophils (EOS) in sputum (A) or cough symptom scores (B).
Figure 1. Linear correlation analysis for the correlation between fractional exhaled nitric oxide (FeNO) levels and eosinophils (EOS) in sputum (A) or cough symptom scores (B). ![Comparison of baseline fractional exhaled nitric oxide (FeNO) (parts per billion, [ppb]) between children with cough relief and those without cough relief at 4 weeks after the anti-inflammatory treatment. * P<0.05 vs no-relief group.](https://jours.isi-science.com/imageXml.php?i=medscimonit-27-e928502-g002.jpg&idArt=928502&w=1000) Figure 2. Comparison of baseline fractional exhaled nitric oxide (FeNO) (parts per billion, [ppb]) between children with cough relief and those without cough relief at 4 weeks after the anti-inflammatory treatment. * P<0.05 vs no-relief group.
Figure 2. Comparison of baseline fractional exhaled nitric oxide (FeNO) (parts per billion, [ppb]) between children with cough relief and those without cough relief at 4 weeks after the anti-inflammatory treatment. * P<0.05 vs no-relief group. Tables
Table 1. Comparisons for initial diagnosis of fractional exhaled nitric oxide (FeNO) and other parameters in children with chronic cough for different etiologies (χ±s).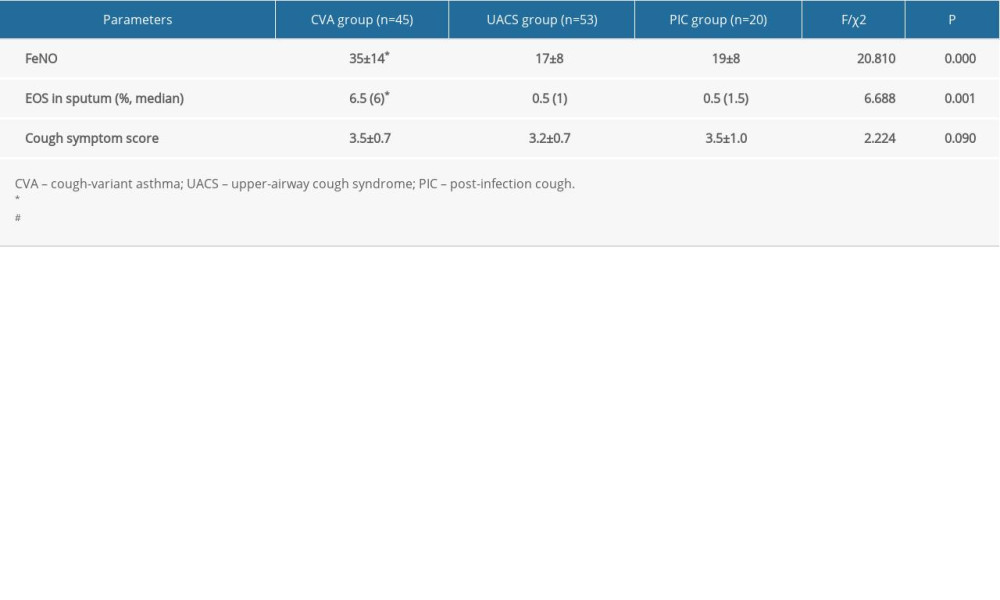 Table 2. Comparative analysis of pulmonary function indexes in children with chronic cough for the different etiologies (χ±s).
Table 2. Comparative analysis of pulmonary function indexes in children with chronic cough for the different etiologies (χ±s).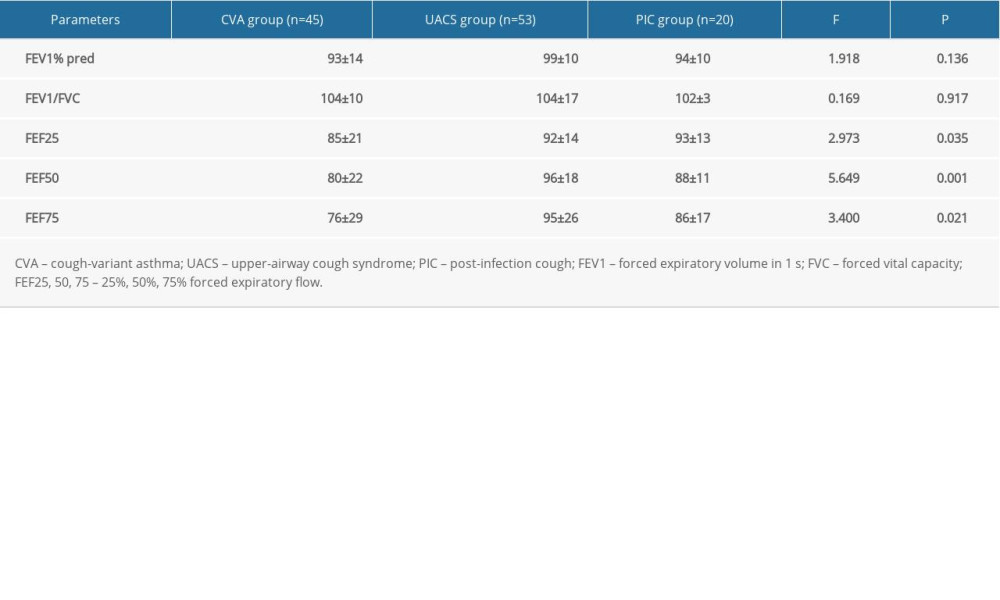 Table 3. Comparison of the levels of FeNO and other parameters before and after treatment for different etiologies of chronic cough (χ±s).
Table 3. Comparison of the levels of FeNO and other parameters before and after treatment for different etiologies of chronic cough (χ±s).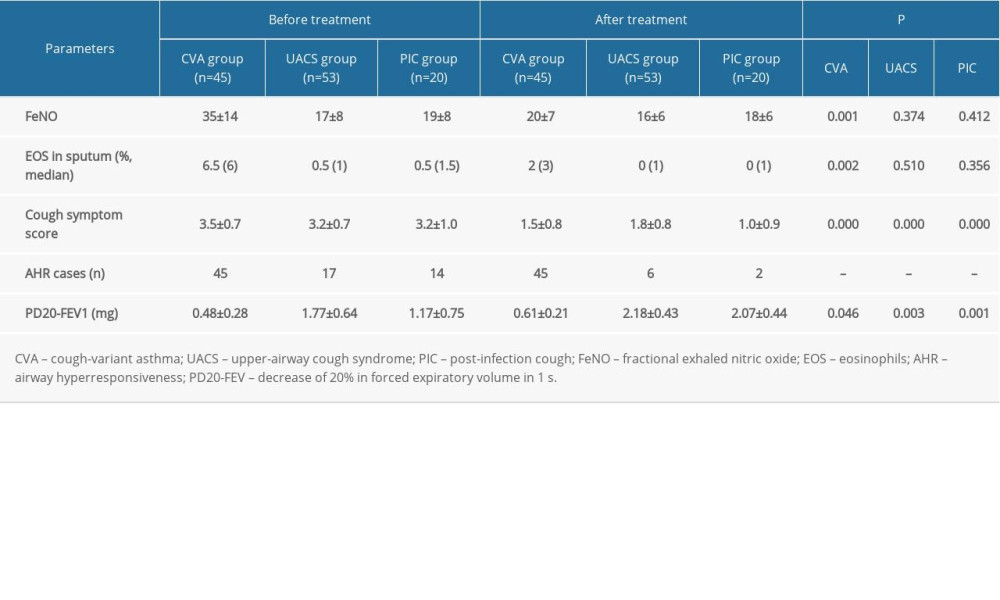
References
1. Chang AB, Oppenheimer JJ, Weinberger M, Etiologies of chronic cough in pediatric cohorts: CHEST guideline and expert panel report: Chest, 2017; 152; 607-17
2. Lei M, Wang K, Li J, Phylogenetic and epidemiological analysis of measles viruses in Shenzhen, China from January 2015 to July 2019: Med Sci Monit, 2019; 25; 9245-54
3. Guideline for diagnosis and treatment of chronic cough in Chinese children: Zhonghua Er Ke Za Zhi, 2014; 52; 184-88 [in Chinese]
4. Chang AB, Robertson CF, van Asperen PP, A multi-centre study on chronic cough in children: Burden and etiologies based on a standardized management pathway: Chest, 2012; 142; 943-50
5. Marchant JM, Newcombe PA, Juniper EF, What is the burden of chronic cough for families?: Chest, 2008; 134(2); 303-9
6. Niimi ACough variant asthma: Nihon Rinsho, 2016; 74; 1693-97 [in Japanese]
7. Chen X, Peng WS, Wang L, Etiology analysis of nonspecific chronic cough in children of 5 years and younger: Medicine (Baltimore), 2019; 98; e13910
8. Guideline for the diagnosis and optimal management of asthma in children: Zhonghua Er Ke Za Zhi, 2016; 54; 167-81 [in Chinese]
9. Zhu H, Zhang R, Hao C, Fractional exhaled nitric oxide (FeNO) combined with pulmonary function parameters shows increased sensitivity and specificity for the diagnosis of cough variant asthma in children: Med Sci Monit, 2019; 25; 3832-38
10. Chung KF, Wenzel SE, Brozek JL, International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma: Eur Respir J, 2014; 43; 343-73
11. Lin J, Zhou X, Wang C, Symbicort maintenance and reliever therapy (SMART) and the evolution of asthma management within the GINA guidelines: Expert Rev Respir Med, 2018; 12; 191-202
12. Brusasco V, Crapo R, Viegi G, Coming together: The ATS/ERS consensus on clinical pulmonary function testing: Rev Mal Respir, 2007; 24(2); S11-14
13. Chang AB, Glomb WB, Guidelines for evaluating chronic cough in pediatrics: ACCP evidence-based clinical practice guidelines: Chest, 2006; 129; 260S-83S
14. Miller MR, Hankinson J, Brusasco V, Standardisation of spirometry: Eur Respir J, 2005; 26; 319-38
15. Crapo RO, Casaburi R, Coates AL, Guidelines for methacholine and exercise challenge testing – 1999. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors: Am J Respir Crit Care Med, 2000; 161; 309-29
16. American Thoracic Society, European Respiratory Society, ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005: Am J Respir Crit Care Med, 2005; 171; 912-30
17. Gibson PG, Wlodarczyk JW, Hensley MJ, Epidemiological association of airway inflammation with asthma symptoms and airway hyperresponsiveness in childhood: Am J Respir Crit Care Med, 1998; 158; 36-41
18. Guideline for diagnosis and treatment of chronic cough in pediatrics: Zhonghua Eer Ke Za Zhi, 2008; 46; 104-7 [in Chinese]
19. Asthma group of Chinese Society of Respiratory Diseases, Guidelines for diagnosis and treatment of cough (2009): Chin J Tuberculosis Res Dis, 2016; 32; 407-13 [in Chinese]
20. Marchant JM, Masters IB, Taylor SM, Evaluation and outcome of young children with chronic cough: Chest, 2006; 129; 1132-41
21. Prospective multicenter clinical study on the causes constituents ratio of chronic cough in Chinese children: Zhonghua Er Ke Za Zhi, 2012; 50; 83-92 [in Chinese]
22. Asiloy S, Bayram E, Agin H, Evaluation of chronic cough in children: Chest, 2008; 134; 1122-28
23. Bateman ED, Hurd SS, Barnes PJ, Global strategy for asthma management and prevention: GINA executive summary: Eur Respir J, 2008; 31; 143-78
24. Jo EJ, Kim MY, Lee SE, Eosinophilic airway inflammation and airway hyperresponsiveness according to aeroallergen sensitization pattern In patients with lower airway symptoms: Allergy Asthma Immunol Res, 2014; 6; 39-46
25. Zhang YM, Lin JTThe values of fractional exhaled nitric oxide in the diagnosis and treatment of chronic cough: Zhonghua Jie He He Hu Xi Za Zhi, 2011; 34; 504-8 [in Chinese]
26. Girillo I, Medusei G, Signori A, Methacholine bronchial challenge effects on nasal symptoms and function in patients with allergic rhinitis: Eur Ann Allergy Clin Immunol, 2013; 45; 123-29
27. Braman SS, Postinfectious cough ACCP evidence-based clinical practice guidelines: Chest, 2006; 129; 138-46
28. Shimoda T, Obase Y, Kishikawa R, Influence of cigarette smoking on airway inflammation and inhaled corticosteroid treatment in patients with asthma: Allergy Asthma Proc, 2016; 37; 50-58
29. Kovesi T, Kulka R, Dales R, Exhaled nitric oxide concentration is affected by age, height, and race in healthy 9- to 12-year-old children: Chest, 2008; 133; 169-75
30. Lamon T, Didier A, Brouquieres D, Exhaled nitric oxide in chronic cough: A good tool in a multi-step approach: Respir Med Res, 2019; 76; 4-9
31. Baraket M, Oliver BG, Burgess JK, Is low dose inhaled corticosteroid therapy as effective for inflammation and remodeling in asthma? A randomized, parallel group study: Respir Res, 2012; 13; 11
32. Liu M, Liu K, Zhu N, Inflammatory mediators in induced sputum and airway hyperresponsiveness in cough variant asthma during long-term inhaled corticosteroid treatment: Mediators Inflamm, 2012; 2012 403868
33. Yin SS, Liu H, Gao X, Elevated fractional exhaled nitric oxide (FeNO) is a clinical indicator of uncontrolled asthma in children receiving inhaled corticosteroids: Int J Clin Pharmacol Ther, 2017; 55; 66-77
34. Petsky HL, Cates CJ, Li AM, Tailored interventions based on exhaled nitric oxide versus clinical symptoms for asthma in children and adult: Cochrane Database Syst Rev, 2008; 2; CD0016340
35. Yu X, Zhu H, Yang XStudy on dynamic changes of airway hyperresponsiveness in children with cough variant asthma: Zhonghua Yi Xue Za Zhi, 2014; 94; 1215-18 [in Chinese]
36. Liu L, Liu W, Liu C, Study on small airway function in asthmatics with fractional exhaled nitric oxide and impulse oscillometry: Clin Respir J, 2018; 12; 483-90
37. Feng-Jia C, Xin-Yan H, Geng-Peng L, Validity of fractional exhaled nitric oxide and small airway function indices in diagnosis of cough-variant asthma: J Asthma, 2018; 55; 750-55
38. Fang S, Chen SY, He XEvaluating the efficacy of fractional exhaled nitric oxide and impulse oscillometry in screening out cough variant asthma from patients with subacute cough: Zhonghua Yi Xue Za Zhi, 2017; 97; 2338-43 [in Chinese]
Figures
 Figure 1. Linear correlation analysis for the correlation between fractional exhaled nitric oxide (FeNO) levels and eosinophils (EOS) in sputum (A) or cough symptom scores (B).
Figure 1. Linear correlation analysis for the correlation between fractional exhaled nitric oxide (FeNO) levels and eosinophils (EOS) in sputum (A) or cough symptom scores (B).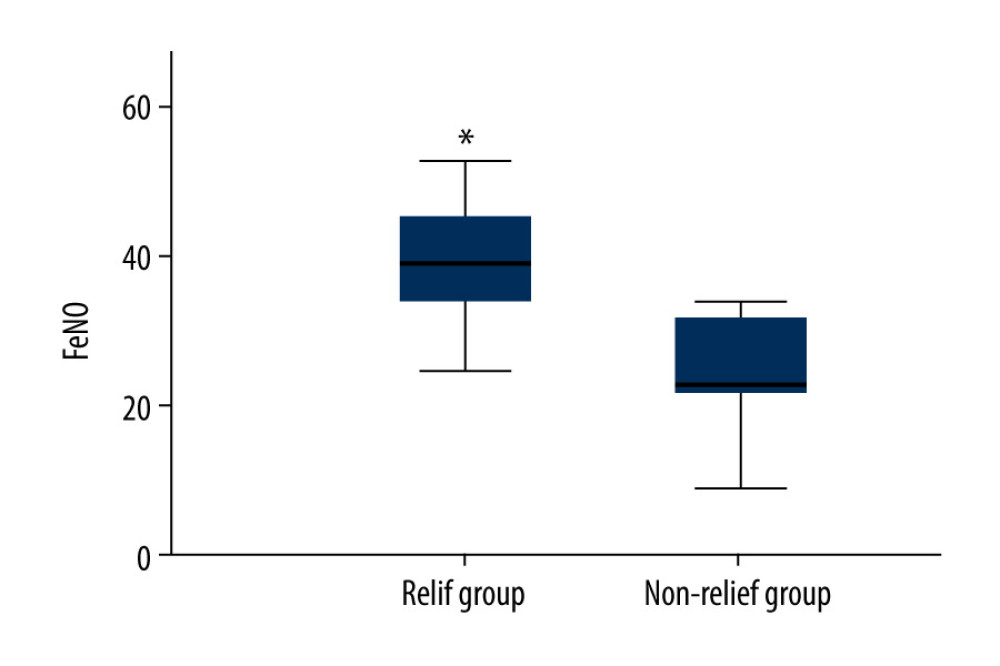 Figure 2. Comparison of baseline fractional exhaled nitric oxide (FeNO) (parts per billion, [ppb]) between children with cough relief and those without cough relief at 4 weeks after the anti-inflammatory treatment. * P<0.05 vs no-relief group.
Figure 2. Comparison of baseline fractional exhaled nitric oxide (FeNO) (parts per billion, [ppb]) between children with cough relief and those without cough relief at 4 weeks after the anti-inflammatory treatment. * P<0.05 vs no-relief group. Tables
 Table 1. Comparisons for initial diagnosis of fractional exhaled nitric oxide (FeNO) and other parameters in children with chronic cough for different etiologies (χ±s).
Table 1. Comparisons for initial diagnosis of fractional exhaled nitric oxide (FeNO) and other parameters in children with chronic cough for different etiologies (χ±s). Table 2. Comparative analysis of pulmonary function indexes in children with chronic cough for the different etiologies (χ±s).
Table 2. Comparative analysis of pulmonary function indexes in children with chronic cough for the different etiologies (χ±s). Table 3. Comparison of the levels of FeNO and other parameters before and after treatment for different etiologies of chronic cough (χ±s).
Table 3. Comparison of the levels of FeNO and other parameters before and after treatment for different etiologies of chronic cough (χ±s). Table 1. Comparisons for initial diagnosis of fractional exhaled nitric oxide (FeNO) and other parameters in children with chronic cough for different etiologies (χ±s).
Table 1. Comparisons for initial diagnosis of fractional exhaled nitric oxide (FeNO) and other parameters in children with chronic cough for different etiologies (χ±s). Table 2. Comparative analysis of pulmonary function indexes in children with chronic cough for the different etiologies (χ±s).
Table 2. Comparative analysis of pulmonary function indexes in children with chronic cough for the different etiologies (χ±s). Table 3. Comparison of the levels of FeNO and other parameters before and after treatment for different etiologies of chronic cough (χ±s).
Table 3. Comparison of the levels of FeNO and other parameters before and after treatment for different etiologies of chronic cough (χ±s). In Press
06 Mar 2024 : Clinical Research
Comparison of Outcomes between Single-Level and Double-Level Corpectomy in Thoracolumbar Reconstruction: A ...Med Sci Monit In Press; DOI: 10.12659/MSM.943797
21 Mar 2024 : Meta-Analysis
Economic Evaluation of COVID-19 Screening Tests and Surveillance Strategies in Low-Income, Middle-Income, a...Med Sci Monit In Press; DOI: 10.12659/MSM.943863
10 Apr 2024 : Clinical Research
Predicting Acute Cardiovascular Complications in COVID-19: Insights from a Specialized Cardiac Referral Dep...Med Sci Monit In Press; DOI: 10.12659/MSM.942612
06 Mar 2024 : Clinical Research
Enhanced Surgical Outcomes of Popliteal Cyst Excision: A Retrospective Study Comparing Arthroscopic Debride...Med Sci Monit In Press; DOI: 10.12659/MSM.941102
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








