26 January 2021: Clinical Research
Predictive Value of a Thyroid-Absorbed Dose with a Shorter Effective Half-Life on Efficacy in Graves Disease Patients Receiving Iodine-131 Therapy
Feng Yu1BCEF, Ruiguo Zhang1CDF, Guizhi Zhang1B, Zhaowei Meng1B, Xiaohua Liu1B, Yajing He1B, Jian Tan1D, Renfei Wang1ADG*DOI: 10.12659/MSM.928796
Med Sci Monit 2021; 27:e928796
Abstract
BACKGROUND: Although radioiodine therapy (RIT) efficacy is thoroughly validated for Graves disease (GD), there is a lack of research on the predictive factors of RIT, especially the optimal thyroid-absorbed dose (TD) with a shorter effective half-life (Teff ≤5 days). The goal of this study was to explore the predictive value of TD in GD patients receiving RIT with a shorter Teff.
MATERIAL AND METHODS: We studied 208 GD patients receiving RIT with a shorter Teff. Plotting the receiver-operating characteristic (ROC) curve verified the accuracy of TD for predicting RIT efficacy in GD patients. In addition, we conducted univariate and multivariate analyses to investigate the influence of 14 factors, including thyroid weight, TD, 24-h radioiodine uptake rate (RAIU), the highest RAIU, thyrotrophin receptor antibody level, thyroglobulin antibody level, thyroid peroxidase antibody level, and others, on curative effects of RIT.
RESULTS: Of the 208 study participants, complete remission and the total effectiveness rates were 68.3% and 92.3%, respectively. The threshold value of TD to predict RIT efficacy was 70.2 Gy, based on ROC analysis. Univariate analysis showed that 24-h RAIU, Teff, total iodine dose, iodine dose per gram of thyroid tissue, TD, and thyrotropin receptor antibody level were significantly associated with RIT efficacy. Multivariate analysis indicated that 24-h RAIU, total iodine dose, iodine dose per gram of thyroid tissue, and TD were significant independent predictors of RIT efficacy.
CONCLUSIONS: Predicting RIT efficacy from TD with a shorter Teff was feasible in GD patients, and TD above 70.2 Gy had an especially high predictive accuracy.
Keywords: Dose-Response Relationship, Radiation, hyperthyroidism, Biomarkers, Pharmacological, Dose-Response Relationship, Drug, Graves Disease, Half-Life, Iodine, Iodine Radioisotopes, Thyroid Gland
Background
Graves disease (GD) is an autoimmune disorder caused by thyrotropin receptor antibody (TRAb) and characterized by abnormally synthesized thyroid hormone secreted in large amounts [1,2]. GD accounts for about 70% to 80% of all hyperthyroidism, with a prevalence of about 0.5% in men and 3% in women [3]. The 3 main treatment methods for GD are antithyroid drugs (ATDs), radioiodine therapy (RIT), and surgery [4]. ATDs offer a safe treatment that does not destroy the thyroid, but the course is long term and may cause drug-related adverse reactions [5]. Surgery is the most successful definitive treatment, but it carries the risk of recurrent laryngeal nerve injury or hypoparathyroidism [6,7]. RIT for hyperthyroidism was first introduced in the 1940s. Owing to its efficiency and safety, it is increasingly used as a treatment option for GD patients, primarily when thyrotoxicosis cannot be controlled by ATDs [8,9].
Several factors influence the prognosis of hyperthyroidism after RIT. Some act directly on the thyroid radioiodine uptake (RAIU), while others modify the impact of radiation on the thyroid gland, either positively or negatively. Many factors are likely interrelated in a complex fashion [10]. The effective half-life Teff is commonly known as one of the critical factors for the success of RIT in GD treatment [11]. The Teff value in most GD patients ranges from 1.6 to 7.5 days, with an average value of 5.0 days [11]. A short Teff increases the risk of RIT failure because of the rapid clearance or turnover of 131I from the thyroid gland, and failure rates can extend up to 55% [12]. Several investigators have reported factors that potentially affect RIT efficacy in GD, such as the dose of 131I administration, thyroid volume, age, RAIU, and pretreatment with ATDs [10,13–15]. However, to our knowledge, there is a lack of study on the predictive factors of RIT with a shorter Teff (Teff ≤5 days) in GD patients.
This study was a retrospective analysis of 208 GD patients treated and managed at our institution in whom RIT had a shorter Teff. This study aimed to explore the value of TD in predicting the outcome of RIT with a shorter Teff in GD patients and to discern the relevant factors affecting the prognosis of these patients.
Material and Methods
ETHICS STATEMENT:
The Ethics Committee of the Tianjin Medical University General Hospital approved this research. Written informed consent was given by all patients participating in the research. All clinical data used in this research were anonymized for analysis.
PARTICIPANTS:
Data on consecutive patients treated in our institution and referred for RIT for GD between June 2017 and February 2019 were retrospectively collected. Patients receiving RIT that had a shorter Teff (Teff ≤5 days) were enrolled for analysis. GD was diagnosed based on thyrotoxicosis, increased thyroid hormones, and decreased thyrotropin (TSH); elevated RAIU, diffuse goiter, exophthalmos, or pretibial myxedema; or positive TRAb [16]. The sample comprised 208 GD patients (161 women and 47 men, ratio 3.4: 1). The average age was 43.0±13.8 for women and 43.3±15.9 years for men. All participants had undergone unsuccessful pharmacological treatment with thyrostatic drugs.
PROCEDURES FOR RIT:
Before administration of therapeutic 131I, the subjects underwent routine qualifying examinations including evaluation of the typical clinical symptoms and signs of GD. Thyroid hormones and TSH were determined by chemiluminescence immunoassay (Abbott Laboratories, Chicago, IL, USA). Thyroglobulin antibody (TgAb) and thyroid peroxidase (TPOAb) levels were also determined by chemiluminescence immunoassay (Siemens Healthcare Diagnostics Inc, New York, NY, USA), and TRAb levels were determined by electrochemiluminescence immunoassay (Roche Diagnostics GmbH, Mannheim, Germany). Single-photon emission computerized tomography (Discovery VH; GE Healthcare, Wauwatosa, WI) was used for thyroid imaging to rule out inflammatory hyperthyroidism.
The depth (D), width (W), and length (L) of each thyroid lobe were measured by 2 radiologists using the Ultrasound System (GE Logiq 400 Pro, GE Healthcare) with a 10-MHz probe. The total thyroid volume (TV) was the sum of the products for both lobes, with volume calculated as D×W×L. The thyroid weight (TW) was calculated based on the TV: TW (g)=0.479×TV (cm3) [17].
After a low-iodine diet for about 2 weeks and an ATD discontinuation period (more than 3 days for thiamazole or more than 2 weeks for propylthiouracil) before RIT, thyroid uptake testing was done [5]. The RAIU of the thyroid was determined dynamically at 6, 24, 48, 72, and 144 h, respectively, to obtain the values of RAIU24h, RAIUmax, and Teff.
The therapeutic activity (A) of 131I for each patient was calculated using Marinelli’s formula [18]:
The TD was calculated according to the standard procedure recommended by the European Association of Nuclear Medicine for the treatment of GD [19]:
Where
ASSESSMENT OF THERAPEUTIC EFFICACY:
All 208 patients were followed for 6 months to 1 year after RIT. The following criteria were used to evaluate the therapeutic efficacy. Complete remission (CR) included euthyroidism and hypothyroidism. Euthyroidism was defined by no symptoms or signs of GD and normal serum levels of free triiodothyronine (FT3), free thyroxine (FT4), and TSH. Hypothyroidism was diagnosed if the patients showed symptoms or signs of hypothyroidism, serum levels of FT3 and FT4 were lower than normal, and the TSH level was higher than average. In partial remission (PR), the symptoms of GD were alleviated, the signs were partly resolved, and the serum FT3 and FT4 levels were significantly reduced, although not normal. No remission (NR) was defined by no significant improvement or by an increase in symptoms and signs of hyperthyroidism, with no significant decrease in serum FT3 and FT4 levels. Recurrence was identified by FT3 and FT4 levels returning to normal and then increasing again, with symptoms reappearing but not yet clinically notable [5]. We defined CR as “cured” (cured group) and persistent hyperthyroidism (including PR, NR, and recurrence) as “uncured” (uncured group).
OBSERVATION FACTORS:
The following 14 factors may be associated with curative effects of RIT with a shorter Teff in GD patients: age at diagnosis, sex, TW, disease course, disease status, RAIU24h, RAIUmax, Teff, total iodine dose, iodine dose per gram of thyroid tissue, TD, TRAb level, TgAb level, and TPOAb level.
STATISTICAL ANALYSIS:
Data were expressed as mean±standard deviation, proportions, or absolute numbers. All factors that may have affected the RIT efficacy for patients with GD were analyzed by univariate analysis, which was performed by
Results
:
All 208 patients with GD who received RIT with a shorter Teff were followed routinely for more than 6 months (Table 1). The euthyroidism and hypothyroidism rates were 35.1% (73/208) and 33.2% (69/208), respectively. The incidence of PR and NR was 24.0%, and recurrence occurred in 7.7% of patients (16/208). The overall CR rate (euthyroidism+hypothyroidism) was 68.3% (142/208), and the total effectiveness rate (euthyroidism+PR+hypothyroidism) was 92.3% (192/208).
The results shown in Figure 1 indicated that the PR and NR or recurrence rates among GD patients who received RIT with a Teff of >3 to ≤5 days were lower than the rates of the other group (χ2=4.294, P=0.038 <0.05; χ2=72.028, P<0.001, respectively). However, the CR rate for patients with a Teff of >3 to ≤5 days was higher than the other group (χ2=45.718, P<0.001). RIT was determined to be more effective for patients when Teff was >3 to ≤5 days compared with Teff ≤3 days.
:
The TD of the patients in the euthyroidism, hypothyroidism, PR, and NR or recurrence groups were 69.8±7.6 Gy, 79.0±5.0 Gy, 62.4±9.1 Gy, and 51.2±9.4 Gy, respectively. The Teff of these 4 groups were 4.0±0.5 days, 4.6±0.3 days, 3.5±0.6 days, and 2.7±0.6 days, respectively. There were significant differences in TD values among the 4 groups (F=85.64, P<0.001) and between the 2 groups (cured vs. uncured; P<0.001). The TD in the hypothyroidism group was the highest, followed by the euthyroidism group, and the lowest TD was in the NR or recurrence group (Figure 2).
:
The ROC curve was drawn to evaluate the TD accuracy in predicting the efficacy of RIT with a shorter Teff in GD treatment (Figure 3). The Youden index, defined as the value that gives the maximum correct classification, was calculated as being 63.5 Gy. This value corresponded to the established threshold value at arrow 1 in Figure 3, resulting in a sensitivity value of 88.0% and specificity value of 72.7%. At this threshold, the positive predictive value and negative predictive value were 87.4% and 73.8%, respectively (AUC: 0.863; 95% confidence interval [CI]: 0.807–0.919; P<0.001). We compared the sensitivity and specificity values one by one and finally selected the TD value with greater specificity (not the maximum) and relatively greater sensitivity. The further study demonstrated that the optimal cutoff value was 70.2 Gy, which corresponded to the established threshold value at arrow 2 in Figure 3. This value had a relatively lower sensitivity of 73.2% but a higher specificity of 81.8%, with a positive predictive value of 89.7% and a negative predictive value of 58.7%.
:
The univariate analyses of factors that may influence the efficacy of RIT with a shorter Teff (≤5 days) in GD treatment are presented in Tables 2 and 3. These analyses showed that cases with higher RAIU24h, longer Teff, higher total iodine dose, higher iodine dose per gram of thyroid tissue, higher TD, or lower TRAb level at diagnosis had a higher probability of good prognosis (P=0.030, 0.000, 0.006, 0.000, 0.000, and 0.006, respectively). However, we found no statistically significant differences in age (P=0.445), the RAIUmax (P=0.722), TW (P=0.952), sex (P=0.495), disease course (P=0.342), disease status (P=0.553), TgAb level (P=0.658), and TPOAb level at diagnosis (P=0.223).
:
Table 4 shows the results of a multivariate analysis of factors influencing the efficacy of RIT with a shorter Teff in GD treatment. Variables that were significant in the univariate analysis were entered into the binary logistic regression analysis using a stepwise method. The results revealed that RAIU24h, total iodine dose, iodine dose per gram of thyroid tissue, and TD were the independent factors predicting efficacy of RIT with a shorter Teff. Furthermore, we found patients were more likely to be cured in cases with higher RAIU24h (odds ratio [OR]: 1.073, CI: 1.024–1.124), higher total iodine dose (OR: 1.242, CI: 1.106–1.393), higher iodine dose per gram of thyroid tissue (OR: 1.422, CI: 1.220–1.658), or higher TD (OR: 1.244, CI: 1.168–1.325).
Discussion
Iodine is the primary substrate material for the synthesis of thyroid hormone by the thyroid. Consequently, the iodine isotope 131I can be actively taken up by the thyroid gland. RIT has been used for GD patients for more than 50 years [10]. It has the advantages of being noninvasive and well tolerated, and along with ATD and surgery, it has become a main therapeutic protocol for treatment of hyperthyroidism [20].
In our clinical practice, GD patients undergoing RIT with a shorter Teff (Teff ≤5 days) often have a poor prognosis and a high chance of retreatment. A short Teff reflects a high metabolic state of thyrocytes, resulting in a short residence time [10]. The TD depends on the resident time and the type of isotope, and an inadequate TD can yield poor results.
In this study, 208 patients underwent RIT with a shorter Teff (≤5 days), accounting for about 33.3% of all patients. We found that the CR rate (euthyroidism+hypothyroidism) was 68.3% (142/208), and the total effectiveness rate (euthyroidism+PR+hypothyroidism) was 92.3% (192/208). Patients were divided into 2 groups with Teff of ≤3 days and Teff of >3 to ≤5 days. A comparison of the 2 groups revealed that RIT was less effective in patients with Teff ≤3 days than in those with Teff of >3 to ≤5 days.
This study’s primary goal was to explore the predictive value of TD in GD patients receiving RIT with a shorter Teff. We found significant differences among the 4 curative effect groups, and TD was lower in patients with poor RIT efficacy. Using ROC curve analysis, we showed that TD could be used as a prognostic tool to predict the efficacy of RIT with a shorter Teff (Teff ≤5 days). For these patients, the optimal threshold value was 70.2 Gy. This value was relatively higher than the threshold value of 63.5 Gy. However, it is essential to note that the latter TD threshold is defined as the value that gives the maximum Youden index. At this threshold, the specificity value was 72.7%, which was lower than the optimal threshold’s specificity value of 81.82%. As is generally known, sensitivity reflects the ability of screening tests to identify patients, while specificity reflects the ability of screening tests to identify nonpatients [21]. In this study, finding an applicable TD cutoff value to correctly predict RIT outcome was essential. Consequently, a TD threshold value with a higher specificity may be preferred for deciding on a patient’s management. Notably, the TD threshold value of 70.2 Gy is lower than the recommended TD value of 150 Gy by the European Association of Nuclear Medicine. This different is mainly because our goal for RIT in GD is to control hyperthyroidism by rendering the patient euthyroid, rather than hypothyroid.
Many factors influence the efficacy of RIT with a shorter Teff (Teff ≤5 days) in GD patients. In this study, univariate analysis showed that RAIU24h, Teff, administration of total iodine dose, administration of iodine dose per gram of thyroid tissue, TD, and TRAb level were significantly associated with the efficacy of RIT. Furthermore, multivariate logistic regression analysis indicated that 4 variables (RAIU24h, administration of total iodine dose, administration of iodine dose per gram of thyroid tissue, and TD) were significant independent predictors of RIT efficacy.
Numerous studies have addressed the issue of the response of RIT with regard to RAIU24h. Some studies, which were mostly observational, have shown that the cure rate of RIT is inversely correlated with RAIU24h [3,12,22,23]. Several explanations may exist for this observation. First, there could be an inverse relationship between the thyroid gland’s radiosensitivity and the RAIU24h, which is supported by a theoretical model [24]. Another explanation could be that the calculated dose decreases with the increase in RAIU24h, and the iodine absorption rate varies during the treatment period. However, our study demonstrated a favorable outcome of RIT in patients with high RAIU24h. The predictive value of RAIU24h in RIT is supported by previous reports [25,26]. For example, the 6- to 24-h RAIU was positively associated with treatment failure, which indirectly suggests that a higher RAIU24h implies a higher probability of success [27]. RAIU24h is still considered an essential factor in RIT success, although these results are contradictory.
Our study found that the greater the administration of the total dose in GD patients, the higher the cure rates. We speculate that administering a large total iodine dose, especially a large iodine dose per gram of thyroid tissue, can ensure that the thyroid gland receives sufficient radiation to destroy enough hyperfunctional thyroid issues to yield a satisfactory therapeutic response.
Our study also showed that a high TD could predict a good prognosis for RIT. This result agreed with a cohort study of 700 GD patients conducted in Poland [13], which indicated the higher the TD, the higher the rate of clinical cure. As is known from radiobiology, the destructive ability of ionizing radiation is directly proportional to the dose absorbed by the tissue.
However, the present study had some limitations that deserve mention. First, patients’ radiosensitivity was not taken into account, and the individual differences were challenging to evaluate. Second, the sample size was small. Third, the follow-up period was short. Finally, we did not compare the treatment outcomes for patients based on Teff being shorter than versus longer than 5 days. Therefore, further study with a rigorous progression and longer follow-up is needed to validate our preliminary findings.
Conclusions
In conclusion, our study showed that high success rates are expected in GD patients with high RAIU24h, administration of total iodine dose, administration of iodine dose per gram of thyroid tissue, and TD (particularly TD >70.2 Gy). These finding will be clinically useful for patient management.
Figures
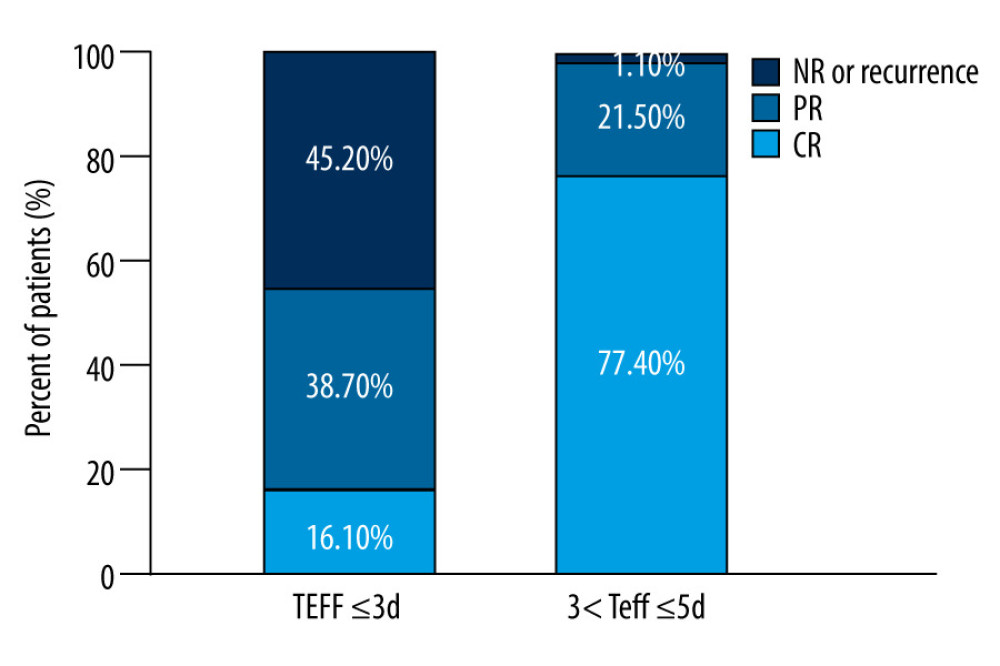 Figure 1. The distribution of efficacy for Graves disease patients between the 2 groups with effective half-life (Teff) ≤3 d and Teff of >3 to ≤5 days.
Figure 1. The distribution of efficacy for Graves disease patients between the 2 groups with effective half-life (Teff) ≤3 d and Teff of >3 to ≤5 days. 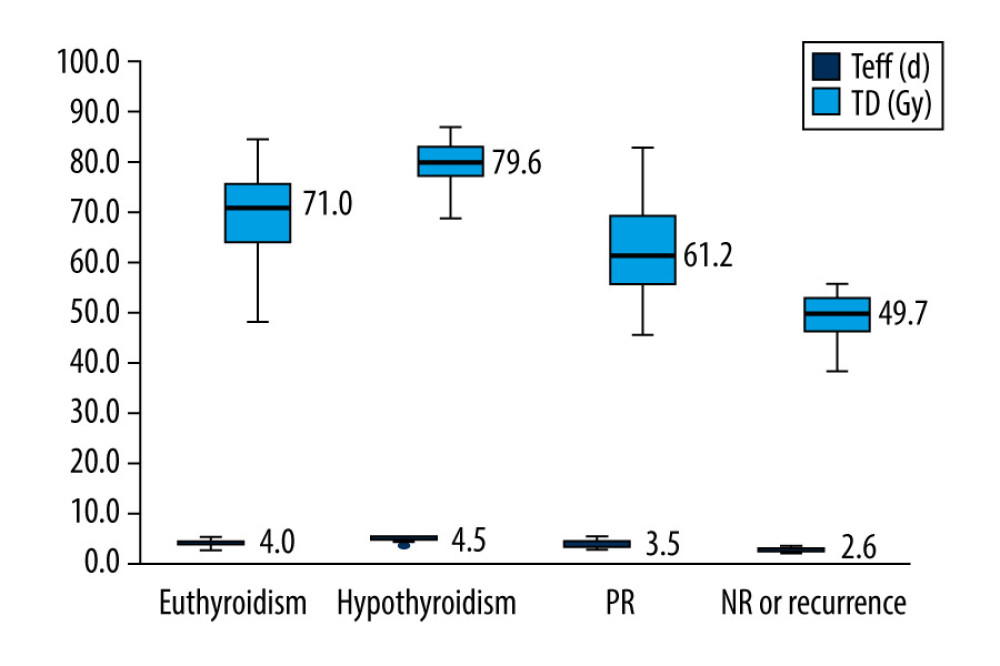 Figure 2. Box plot of thyroid-absorbed dose (TD) and effective half-life (Teff) values in the different outcomes of Graves disease patients receiving radioiodine therapy with a shorter Teff.
Figure 2. Box plot of thyroid-absorbed dose (TD) and effective half-life (Teff) values in the different outcomes of Graves disease patients receiving radioiodine therapy with a shorter Teff. 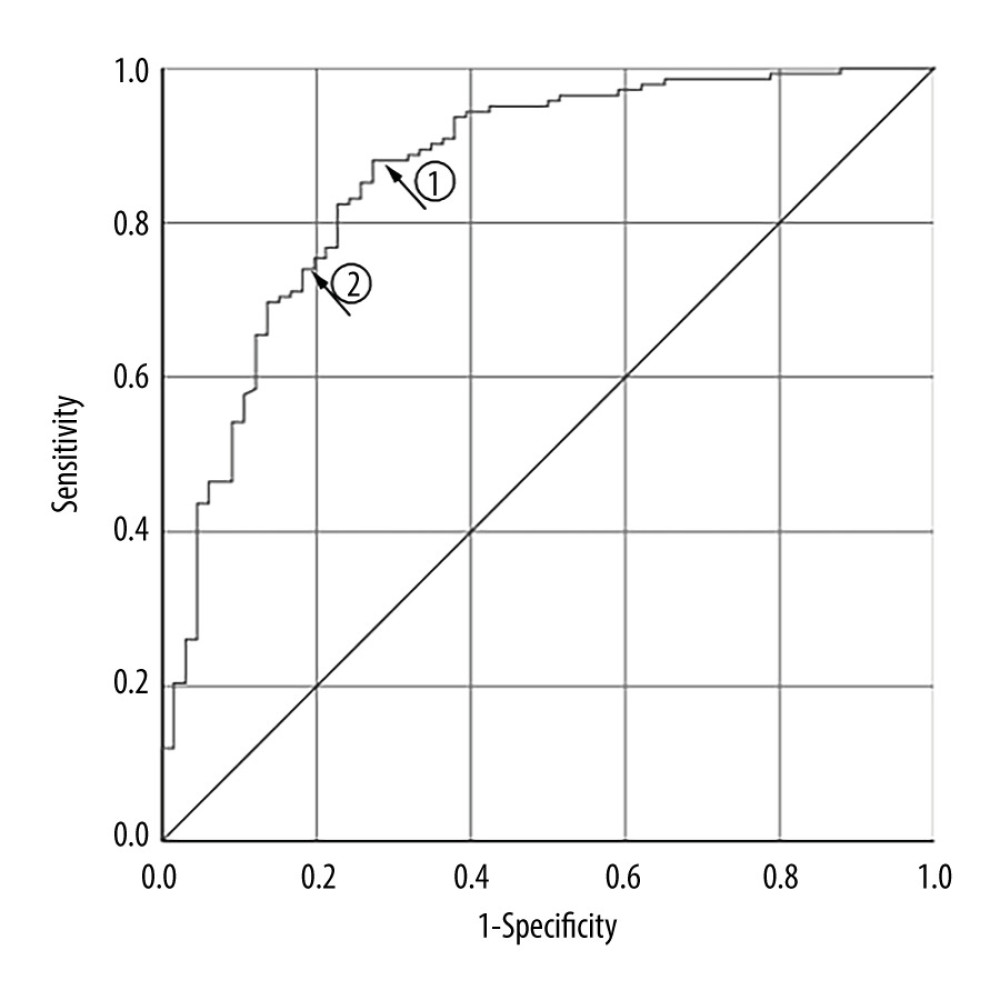 Figure 3. Receiver-operating characteristic curve for thyroid-absorbed dose (TD) in predicting radioiodine therapy (RIT) efficacy for Graves disease patients with a shorter Teff.
Figure 3. Receiver-operating characteristic curve for thyroid-absorbed dose (TD) in predicting radioiodine therapy (RIT) efficacy for Graves disease patients with a shorter Teff. References
1. Burch HB, Burman KD, Cooper DS, A 2011 survey of clinical practice patterns in the management of Graves’ disease: J Clin Endocrinol Metab, 2012; 97; 4549-58
2. Smith TJ, Hegedus L, Graves’ disease: N Engl J Med, 2016; 375; 1552-65
3. Zhao DF, Teng WP, Teng XC, Genetic study of pathogenesis of Graves’ disease: Chin Gen Pract, 2019; 8; 935-41
4. Fadeyev VV, Karseladse EA, Hyperthyroidism and other causes of thyrotoxicosis: Management guidelines of the American Thyroid Association and American Association of Clinical Endocrinologists: Thyroid, 2011; 21(6); 593-646
5. Chinese Society of Nuclear Medicine, Guidelines for Graves’ disease with hyperthyroidism treated with 131I (2013 vision): Chin J Nucl Med Mol Imaging, 2013; 33(2); 83-95
6. Genovese BM, Noureldine SI, Gleeson EM, What is the best definitive treatment for Graves’ disease? A systematic review of the existing literature: Ann Surg Oncol, 2013; 20; 660-70
7. Bartalena L, Diagnosis and management of Graves disease: A global overview: Nat Rev Endocrinol, 2013; 9; 724-34
8. Moka D, Dietlein M, Schicha H, Radioiodine therapy and thyrostatic drugs and iodine: Eur J Nucl Med Mol Imaging, 2002; 29; S486-91
9. Ross DS, Radioiodine therapy for hyperthyroidism: N Engl J Med, 2011; 364; 542-50
10. Bonnema SJ, Hegedüs L, Radioiodine therapy in benign thyroid diseases: Effects, side effects, and factors affecting therapeutic outcome: Endocr Rev, 2012; 33(6); 920-80
11. Berg GE, Michanek AM, Holmberg EC, Iodine-131 treatment of hyperthyroidism: Significance of effective half-life measurements: J Nucl Med, 1996; 37(2); 228-32
12. de Jong JA, Verkooijen HM, Valk GD, High failure rates after (131)I therapy in Graves hyperthyroidism patients with large thyroid volumes, high iodine uptake, and high iodine turnover: Clin Nucl Med, 2013; 38; 401-6
13. Szumowski P, Abdelrazek S, Kociura Sawicka A, Radioiodine therapy for Graves’ disease-retrospective analysis of efficacy factors: Endokrynol Pol, 2015; 66(2); 126-31
14. Šfiligoj D, Gaberšček S, Mekjavič PJ, Factors influencing the success of radioiodine therapy in patients with Graves’ disease: Nucl Med Commun, 2015; 36(6); 560-65
15. Liu M, Jing D, Hu J, Yin S, Predictive factors of outcomes in personalized radioactive iodine ((131)I) treatment for Graves’ disease: Am J Med Sci, 2014; 348(4); 288-93
16. Endocrinology Branch of Chinese Medical Association, Chinese guidelines for diagnosis and treatment of thyroid disease: Chin J Int Med, 2007; 46; 876-82
17. Gierach M, Gierach J, Pilecki S, The estimation of the goiter by means of ultrasonography and scintigraphy (SPECT) with using 131I: Endokrynol Pol, 2007; 58(5); 403-7
18. Tan J, Zhang GZ, Zhou YB, Practical calculation method and application of 131I therapeutic dosage for hyperthyroidism: Chin J Ctrl Ende Dis, 1995; 10; 21-22
19. Hänscheid H, Canzi C, Eschner W, EANM dosimetry committee series on standard operational procedures for pretherapeutic dosimetry II: Dosimetry prior to radioiodine therapy of benign thyroid diseases: Eur J Nucl Med Mol Imaging, 2013; 40(7); 1126-34
20. Knapska-Kucharska M, Oszukowska L, Lewiński A, Analysis of demographic and clinical factors affecting the outcome of radioiodine therapy in patients with hyperthyroidism: Arch Med Sci, 2010; 6(4); 611-16
21. Palinkas M, de Canto GL, Rodrigues LA, The real role of sensitivity, specificity and predictive values in the clinical assessment: J Clin Sleep Med, 2016; 12(2); 279-80
22. Walter MA, Christ-Crain M, Eckard B, Radioiodine therapy in hyperthyroidism: Inverse correlation of pretherapeutic iodine uptake level and post-therapeutic outcome: Eur J Clin Invest, 2004; 34(5); 365-70
23. Kristoffersen US, Hesse B, Rasmussen AK, Radioiodine therapy in hyperthyroid disease: Poorer outcome in patients with high 24 hs radioiodine uptake: Clin Physiol Funct Imaging, 2006; 26(3); 167-70
24. Di Martino F, Traino AC, Brill AB, A theoretical model for prescription of the patient-specific therapeutic activity for radioiodine therapy of Graves’ disease: Phys Med Biol, 2002; 47(9); 1493-99
25. Nordyke RA, Gilbert FI, Optimal iodine-131 dose for eliminating hyperthyroidism in Graves’ disease: J Nucl Med, 1991; 32(3); 411-16
26. Sabri O, Zimny M, Schreckenberger M, Characterization of therapy failures in radioiodine therapy of Graves’ disease without simultaneous antithyroid agents: Nuklearmedizin, 2001; 40(1); 1-6 [in German]
27. Xing YZ, Zhang K, Jin G, Predictive factors for the outcomes of Graves’ disease patients with radioactive iodine (131I) treatment: Biosci Rep, 2020; 40(1); BSR20191609
Figures
 Figure 1. The distribution of efficacy for Graves disease patients between the 2 groups with effective half-life (Teff) ≤3 d and Teff of >3 to ≤5 days.
Figure 1. The distribution of efficacy for Graves disease patients between the 2 groups with effective half-life (Teff) ≤3 d and Teff of >3 to ≤5 days. Figure 2. Box plot of thyroid-absorbed dose (TD) and effective half-life (Teff) values in the different outcomes of Graves disease patients receiving radioiodine therapy with a shorter Teff.
Figure 2. Box plot of thyroid-absorbed dose (TD) and effective half-life (Teff) values in the different outcomes of Graves disease patients receiving radioiodine therapy with a shorter Teff. Figure 3. Receiver-operating characteristic curve for thyroid-absorbed dose (TD) in predicting radioiodine therapy (RIT) efficacy for Graves disease patients with a shorter Teff.
Figure 3. Receiver-operating characteristic curve for thyroid-absorbed dose (TD) in predicting radioiodine therapy (RIT) efficacy for Graves disease patients with a shorter Teff. Tables
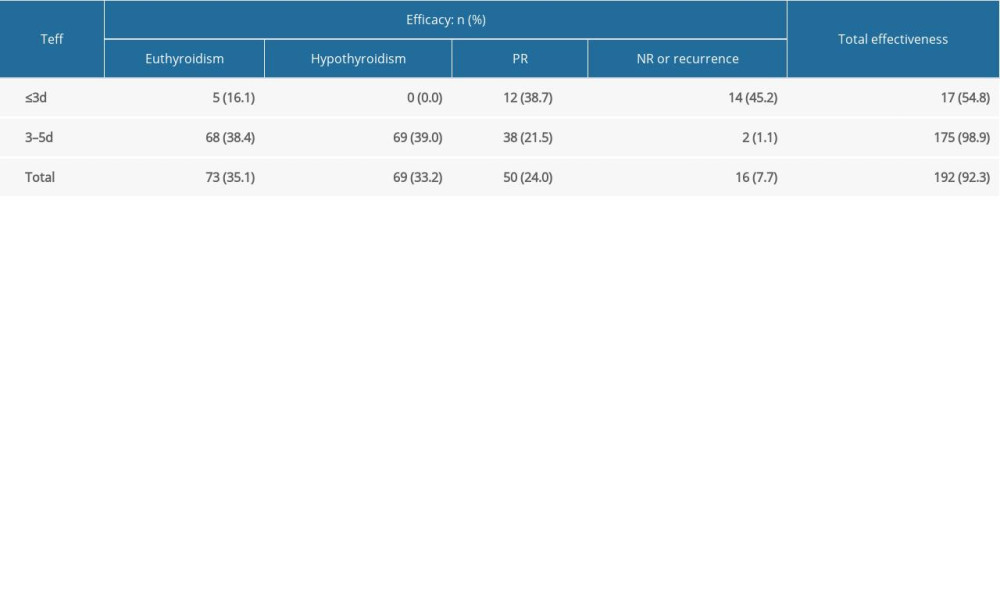 Table 1. The efficacy after a single RIT for GD patients with shorter Teff.
Table 1. The efficacy after a single RIT for GD patients with shorter Teff.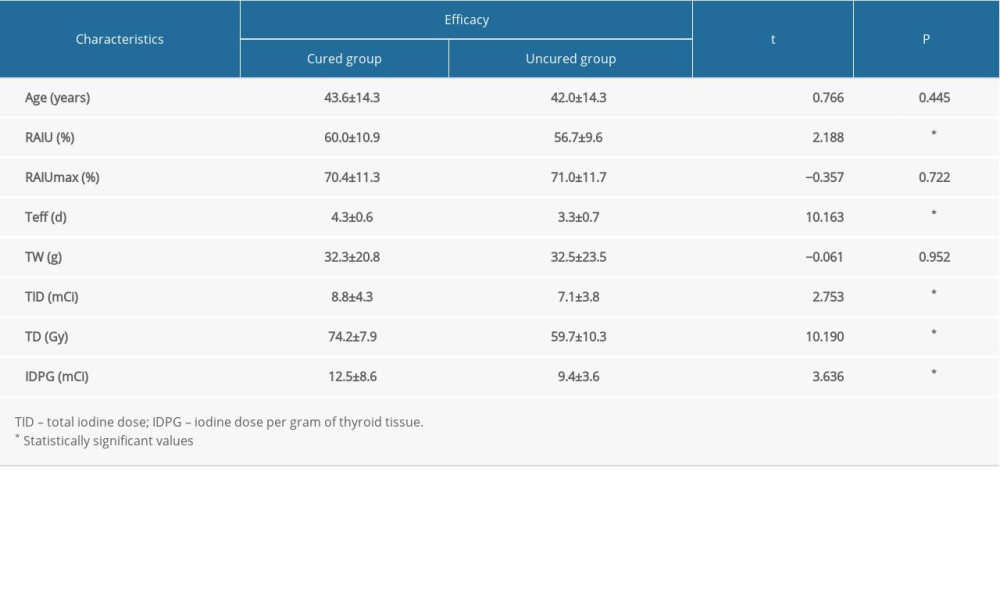 Table 2. Univariate analyses for the continuous variables.
Table 2. Univariate analyses for the continuous variables.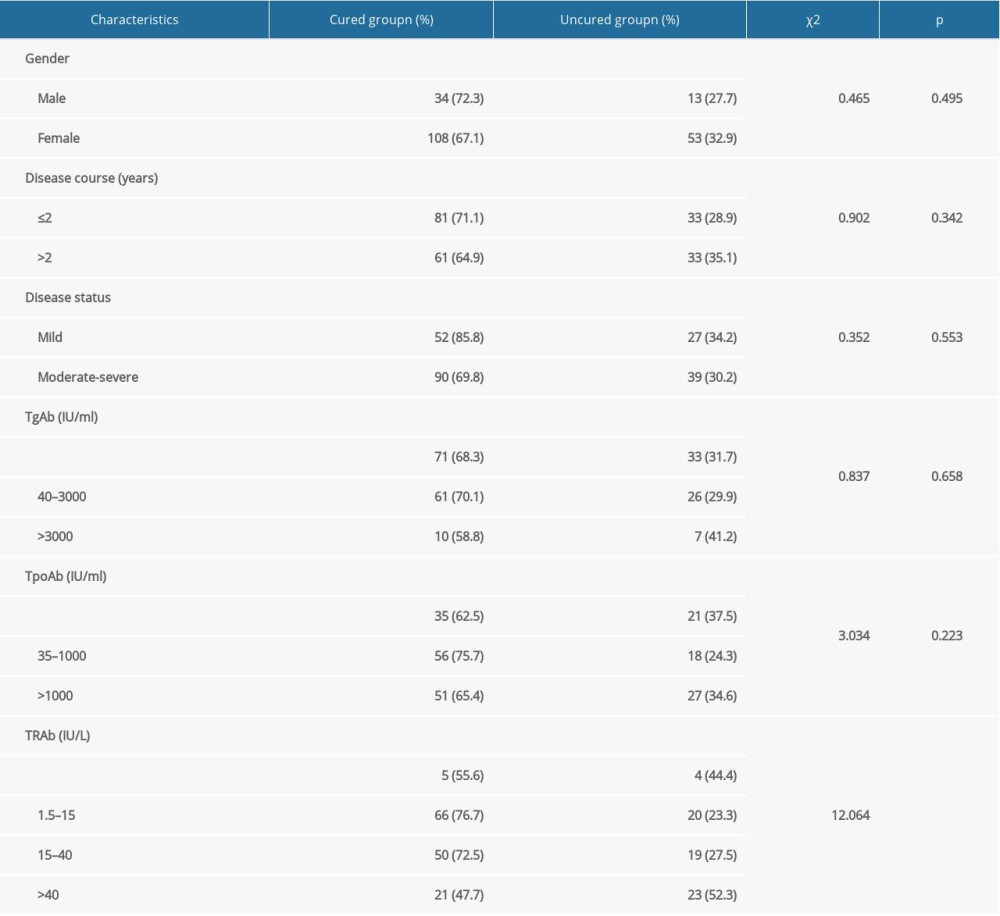 Table 3. Univariate analyses for the categorical variables.
Table 3. Univariate analyses for the categorical variables.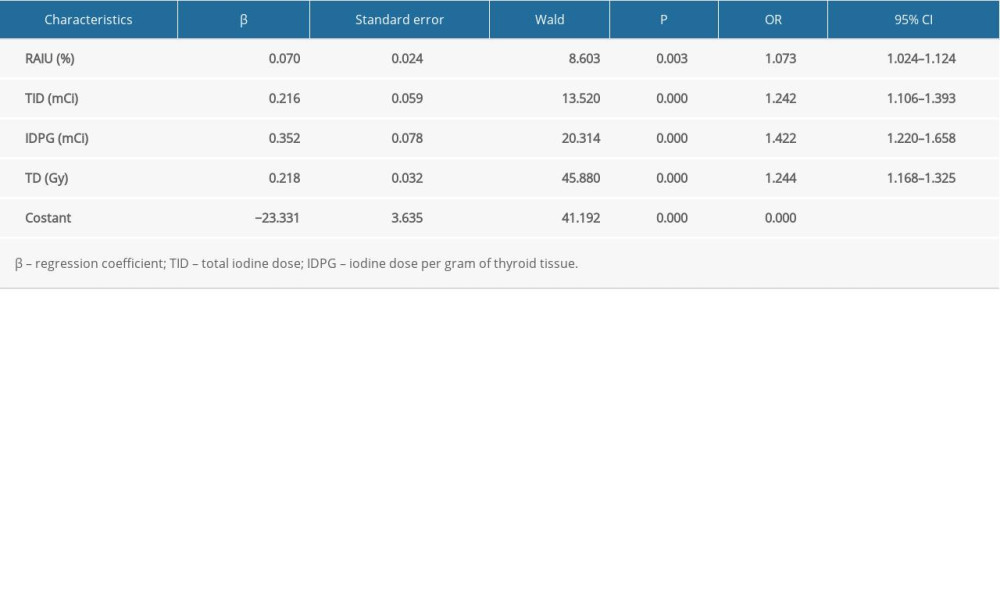 Table 4. Multivariate analyses for the variables using binary logistic regression.
Table 4. Multivariate analyses for the variables using binary logistic regression. Table 1. The efficacy after a single RIT for GD patients with shorter Teff.
Table 1. The efficacy after a single RIT for GD patients with shorter Teff. Table 2. Univariate analyses for the continuous variables.
Table 2. Univariate analyses for the continuous variables. Table 3. Univariate analyses for the categorical variables.
Table 3. Univariate analyses for the categorical variables. Table 4. Multivariate analyses for the variables using binary logistic regression.
Table 4. Multivariate analyses for the variables using binary logistic regression. In Press
06 Mar 2024 : Clinical Research
Prevalence and Variation of Medical Comorbidities in Oral Surgery Patients: A Retrospective Study at Jazan ...Med Sci Monit In Press; DOI: 10.12659/MSM.943884
08 Mar 2024 : Clinical Research
Evaluation of Foot Structure in Preschool Children Based on Body MassMed Sci Monit In Press; DOI: 10.12659/MSM.943765
15 Apr 2024 : Laboratory Research
The Role of Copper-Induced M2 Macrophage Polarization in Protecting Cartilage Matrix in OsteoarthritisMed Sci Monit In Press; DOI: 10.12659/MSM.943738
07 Mar 2024 : Clinical Research
Knowledge of and Attitudes Toward Clinical Trials: A Questionnaire-Based Study of 179 Male Third- and Fourt...Med Sci Monit In Press; DOI: 10.12659/MSM.943468
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








