26 April 2021: Clinical Research
Risk Factors for the Development of Intraoperative Hypoxia in Patients Undergoing Nonintubated Video-Assisted Thoracic Surgery: A Retrospective Study from a Single Center
Lan Lan1AE*, Yanyi Cen1BD, Long Jiang23BF, Huazhang Miao4C, Weixiang Lu23BDOI: 10.12659/MSM.928965
Med Sci Monit 2021; 27:e928965
Abstract
BACKGROUND: Nonintubated video-assisted thoracic surgery (NIVATS) has been demonstrated to be safe and effective in patients. However, the risk factors for intraoperative hypoxia are unclear. This retrospective study aimed to identify the risk factors for the development of intraoperative hypoxia in patients undergoing NIVATS.
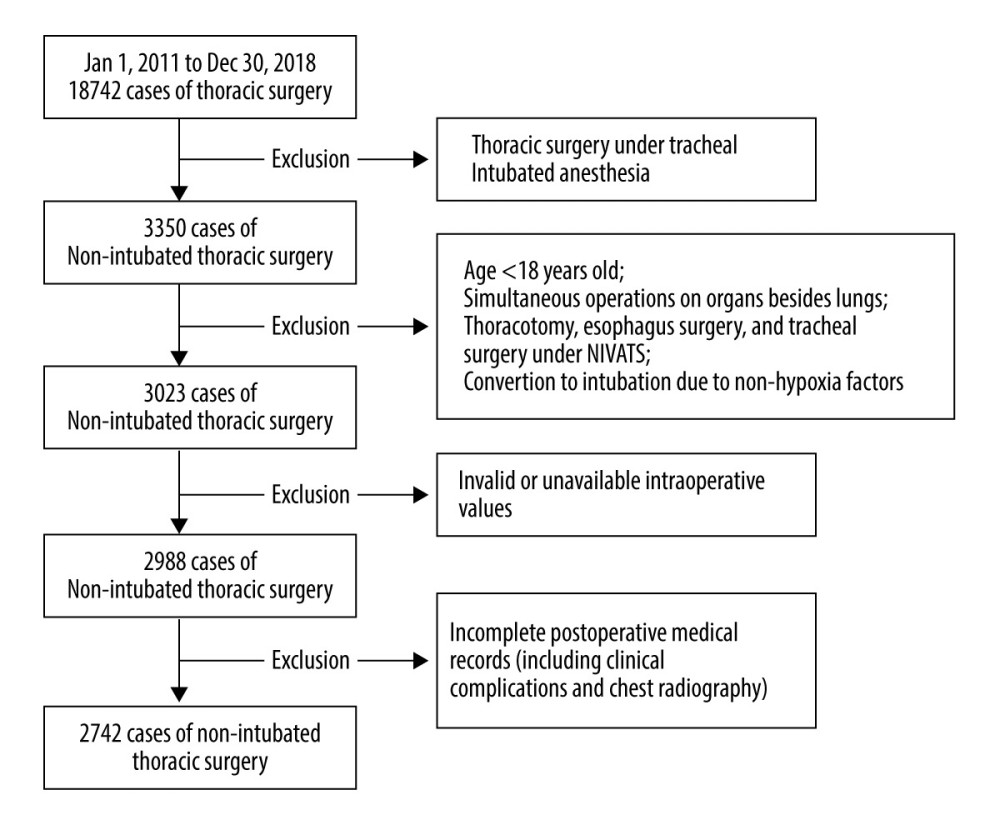
MATERIAL AND METHODS: The study included patients who underwent NIVATS between January 2011 and December 2018. Intraoperative hypoxia was defined as SpO₂ ≤93%. Risk factors for hypoxia were identified by binary logistic regression analysis, and the characteristic distribution of patients with and without hypoxia was elaborated.
RESULTS: Of 2742 included patients, age, anesthesia method, the technical level of surgeons, stair-climbing ability, and type of thoracic procedure were associated with intraoperative hypoxia (P<0.05). The characteristics of patients with hypoxia were older age (P=0.011), higher body mass index and revised cardiac risk index level (P=0.033 and P=0.031), and lower composition of stair-climbing ≥22 m (P<0.001). These patients also had more anatomical lung surgery and mediastinal mass resection (P=0.033) and more epidural anesthesia (P=0.005). The surgeries were more likely to be performed by surgeons with less than 10 years of VATS training (P=0.009) and to have increased intraoperative maximum end-expiratory carbon dioxide partial pressure (P<0.001). These patients had a longer Intensive Care Unit stay (P<0.001), duration of chest-tube drainage (P=0.019), and postoperative hospitalization (P=0.003).
CONCLUSIONS: The current study suggests that old age and stair-climbing ability of patients, anesthesia method, thoracic procedures, and surgeon experience are risk factors for intraoperative hypoxia in patients undergoing NIVATS.
Keywords: Risk Factors, Hypoxia, Brain, Intraoperative Complications, Thoracic Surgery, Video-Assisted, Adolescent, Anesthesia, Epidural, Chest Tubes, Length of Stay, operative time, Postoperative Period, young adult
Background
The successful debut of nonintubated video-assisted thoracic surgery (NIVATS) for wedge resection under thoracoscopy was first reported in 1997 [1]. With the enhancement of surgical instruments and advances in minimally invasive thoracoscopic techniques, NIVATS has been widely adapted for different types of thoracic procedures [2,3], including lobectomy [4], bullectomy [5], lung volume reduction surgery [6], and tracheal reconstruction [7], and it has even been used with a uniportal approach [8].
NIVATS is reported to shorten surgery time and hospital stay [9,10] and to enhance rehabilitation by having fewer complications [2–5,11]. The perioperative surgical outcomes of NIVATS for lobectomy have been confirmed to be comparable to those of the intubated technique [12–14]. These benefits may be due to NIVATS minimizing the adverse effects of intubation-induced airway trauma, ventilation-related lung injury, and residual muscle relaxation [3]. Use of NIVATS may also reduce postoperative nausea and vomiting and decrease the stress hormones and proinflammatory mediators related to mechanical ventilation [15]. Furthermore, patients may benefit from the low risk of intrapulmonary shunt due to the efficient contraction of the dependent hemidiaphragm and preserved hypoxic pulmonary vasoconstriction in spontaneous ventilation [16].
However, the perioperative risk factors for hypoxia in NIVATS are unclear. NIVATS requires spontaneous breathing of 1 lung and the iatrogenic pneumothorax-induced collapse of the other lung to facilitate the operation, which tends to bring about intraoperative hypoxia and hypercapnia. Severe intraoperative hypoxia can jeopardize tissue oxygenation and hemodynamic stability [17–19] and may increase the incidence of postoperative complications [20,21]. Moreover, patients may have to transfer to endotracheal intubation when the hypoxia cannot be corrected in NIVATS. Therefore, it is important to identify the preoperative risk factors for intraoperative hypoxia to exclude high-risk patients and to permit early and timely intervention in others. However, many medical centers fail to do so due to their cases being scarce.
This retrospective study aimed to identify the risk factors for the development of intraoperative hypoxia in patients who underwent NIVATS between 2011 and 2018, and it compared patients’ perioperative characteristics with nonhypoxia and hypoxia events.
Material and Methods
ANESTHESIA PROCEDURE:
All patients received thoracoscopic surgery with nonintubated anesthesia under the 3-portal approach as described previously [22,23]. Some patients received a target-controlled infusion of propofol 2–4 μg/mL and remifentanil 1–3 ng/mL and intravenous dexmedetomidine 0.5–1 μg/kg/h. A bispectral index value between 40 and 60 was maintained. Laryngeal mask airway (LMA) was placed when patients became unconscious, and end-expiratory carbon dioxide partial pressure (PETCO2) was continuously monitored via capnography. The other patients received epidural anesthesia by placing an epidural catheter into the T5–T6 or T6–T7 epidural space, and anesthesia was maintained with 0.375% ropivacaine with the anesthesia plane titrated to between the 2nd and 10th ribs. All patients received standard monitoring, but PETCO2 was not monitored in patients who received epidural anesthesia. Arterial catheterization was used for arterial pressure monitoring and to examine arterial blood gas analysis when SpO2 ≤93%, while central venous catheterization was dependent on the conditions of operation and patients.
The gas flow was inhaled at 4–5 L/min with FiO2 of 100% during the operation. Synchronous intermittent mandatory ventilation was implemented after the main procedures were completed to exhale carbon dioxide. The main procedures were defined as the removal of the lung lesion, lymph node dissection, and pleural lavage. Dopamine or norepinephrine was used to maintain mean arterial pressure >60 mmHg. Patients were transferred to the Postanesthesia Care Unit to remove the LMA or epidural catheter after the operation. Self-controlled intravenous analgesia was used for all patients after the operation. After that, patients were sent to the Intensive Care Unit or General Ward according to their conditions.
SURGICAL PROCEDURE:
The thoracoscopic procedures followed the consensus guidelines of the American Association for Thoracic Surgery [24]. Skin local anesthesia was performed with 2% lidocaine. After the pleura opening, the surface of the visceral pleura was sprayed with 5 mL of 2% lidocaine, and the intercostal nerve and left vagus nerve were infiltrated with 2.5 mL of 2% lidocaine and 2.5 mL of 0.75% ropivacaine under direct vision for inhibition of pain and the coughing reflex. Lung collapse was achieved through iatrogenic pneumothorax. The patient’s condition and surgical procedures determined whether a chest tube would be placed. All thoracic procedures were classified into 5 types: nonanatomical lung surgery, including wedge resection, bullectomy, and lung volume reduction surgery; anatomical lung surgery, including lobectomy and segmentectomy; mediastinal mass resection; bilateral sympathectomy; and other surgery, including thoracoscopic exploration, lung biopsy, pericardial cyst resection, and so forth.
The surgery time was the interval from skin incision to surgical dressing covering. Blood testing was done before the operation and on the first day after the operation. Postoperative clinical complications, including pleural effusion, dyspnea, air leakage, and reoperation, were recorded.
OUTCOME DEFINITION:
Due to the respiratory volume reduction and rebreathing effect in NIVATS [25], the primary concern is the intraoperative hypoxia values. Since SpO2 reacts to hypoxia quickly and effectively, we chose continuous monitoring of SpO2 as the main observation indicator instead of intermittent monitoring of PaO2. An intraoperative SpO2 ≤93% lasting for 5 min was taken as evidence of hypoxia [26]. Manual assisted ventilation or synchronous intermittent mandatory ventilation was initiated when SpO2 ≤90%. The intraoperative SpO2 was continuously monitored and recorded in the anesthesia record sheet.
STATISTICAL ANALYSIS:
Statistical analyses were performed with SPSS software (version 26.0, Chicago, IL, USA). Continuous variables are presented as mean±standard deviation or as median (interquartile range). Dichotomous variables are presented as percentages of the total number of data points available for that field. The variables for different patients with and without hypoxia were analyzed by an independent-samples
Results
RISK FACTORS OF INTRAOPERATIVE HYPOXIA:
Logistic regression analysis showed that age, anesthesia method, technical level of surgeons, stair-climbing ability, and types of thoracic procedures were the risk factors for intraoperative hypoxia (P<0.05). Moreover, the technical level of surgeons with VATS training ≤5 years (P=0.029) and the thoracic types of nonanatomical and anatomical lung surgery were risk factors for intraoperative hypoxia (P=0.015, and P=0.002) (Table 4).
PREOPERATIVE CHARACTERISTICS OF PATIENTS WITH AND WITHOUT HYPOXIA:
Further analysis revealed that patients with intraoperative hypoxia were older and a higher proportion were over 60 years (47.32 vs 44.48 years, P=0.011 and 27% vs 22%, P=0.046), and they had higher BMI (21.99 vs 21.55 kg/m2, P=0.033), a higher level of RCRI (P=0.031), and a higher proportion of stair-climbing ability <22 m (4 vs 1%, P<0.001). These patients were also more likely to have undergone anatomical lung surgery and mediastinal mass resection (29% vs 25% and 11% vs 7%, P=0.033) and to have received epidural anesthesia (29% vs 21%, P=0.005), and fewer of the surgeons had VATS training ≥10 years (52% vs 63%, P=0.009) (Table 1).
INTRAOPERATIVE COMPARISON OF PATIENTS WITH AND WITHOUT HYPOXIA:
The total incidence of hypoxia (SpO2 ≤ 93%) was 9% (245/2742) and only 1.2% of patients (34/2742) experienced severe hypoxia (<90%). The total incidence of hypercapnia (PETCO2 ≥46 mmHg) was 41% (882/2150), and only 3.8% of patients (83/2150) had moderate hypercapnia (PETCO2 ≥61 mmHg).
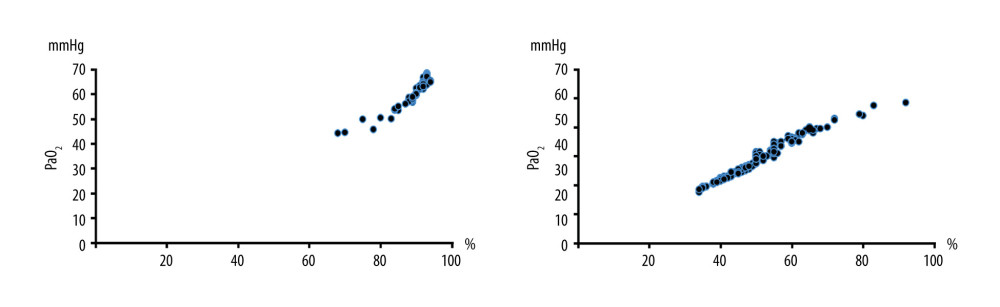
The results of propensity score-matching analysis showed that the patients with hypoxia were also combined with a higher-level proportion of PETCO2 ≥46 mmHg (P < 0.001). The intraoperative maximum PETCO2 was higher (49.90 vs 45.25 mmHg, P < 0.001) (Table 2). Arterial blood gas was analyzed when SpO2 ≤93% and the mean values of PaO2 and PaCO2 were 62.28±4.23 mmHg and 56.59 ± 12.84 mmHg in patients with hypoxia (Figure 2).
POSTOPERATIVE COMPARISON OF PATIENTS WITH AND WITHOUT HYPOXIA:
The results of propensity score-matching analysis showed that the patients who experienced intraoperative hypoxia had a longer Intensive Care Unit stay (P=0.039), but the chest-tube drainage, postoperative hospital stay, and the incidence of postoperative clinical complications were similar to those without hypoxia (P=0.077, P=0.059, and P=0.216) (Table 3).
Discussion
This retrospective study revealed that the total incidence of intraoperative SpO2 ≤93% was 9%. The main risk factors for intraoperative hypoxia were age, anesthesia method, technical level of surgeons, stair-climbing ability, and the type of thoracic procedure. The overall distribution of characteristics in this study indicated that NIVATS was mostly performed in young- to middle-aged patients with normal BMI, American Society of Anesthesiologists (ASA) level grade I or II, RCRI grade I, and normal cardiopulmonary function. Comorbidities of smoking history, cardiopulmonary disease, and a history of pulmonary operation were not absolute exclusion criteria. The procedure of NIVATS was mainly nonanatomical lung surgery, and it also had no limitation with regard to the sex of the patient or the surgical location.
NIVATS is a safe and feasible alternative procedure and improves postoperative rehabilitation by reducing postoperative complications, hospital stay, and attenuating stress and inflammatory responses [10,13,14]. However, few specific analyses of risk factors for NIVATS have been conducted even though the procedure has been used in a large number of patients [27–31]. The distribution characteristics of patients with intraoperative hypoxia revealed that older patients with higher BMI and inferior cardiopulmonary function who underwent anatomical lung surgery under epidural anesthesia by thoracic surgeons with <10 years of VATS training had a high incidence of hypoxia. A logistic regression analysis of these risk factors confirmed these inferences. As reported by the ASA, the risk of morbidity and mortality from general anesthesia is always present, and the risk of laryngotracheal injuries caused by double-lumen tube should not be underestimated [32]. Nevertheless, NIVATS was sometimes advocated for patients with inferior pulmonary function in less invasive procedures. Although the difficulty of intraoperative management increased, the patients recovered quickly after the operation, due to less sedative and analgesic drugs, and the probability of postoperative mechanical ventilation and weaning difficulty was reduced [32–35]. At present, most patients who have undergone NIVATS are successful case reports, but whether NIVATS is suitable or not still needs to be verified. Therefore, NIVATS must be carefully evaluated to attain more advantages than disadvantages for patients with inferior pulmonary function or those who cannot tolerate tracheal intubation general anesthesia.
In theory, hypoxia does not occur easily in spontaneous ventilation when an individual is in a lateral position. The lower diaphragm contracts more efficiently without obviously decreasing the functional residual capacity during spontaneous respiration [36,37], and more blood distribution occurs in the dependent lung by gravity and hypoxic pulmonary vasoconstriction [16,38], which results in a better matching of the ventilation quotient. However, hypoxia still occurred in 9% of patients undergoing NIVATS in our study. Nonintubated anesthesia cannot avoid rebreathing, which easily leads to hypercapnia. Our study also revealed that the patients with intraoperative hypoxia also had a higher proportion of hypercapnia, which reached 41%, but only 1.2% and 3.8% of patients had severe hypoxia (<90%) and moderate hypercapnia (PETCO2 ≥61 mmHg), which showed that NIVATS was safe. Complex thoracic surgery, such as anatomical lung surgery, may aggravate the extent of hypoxia. In addition, the surgeon’s familiarity with the anatomy of the endoscope view is a prerequisite for a quick and accurate operation, which decreases perioperative complications and improves recovery in NIVATS [37,38]. Therefore, to avoid increased incidence of hypoxia and hypercapnia in patients, we recommend against surgeons with <10 years of VATS training proceeding with complicated procedures in NIVATS. It is possible in our study that junior surgeons were more aggressive in manipulating lung tissues or the operations were too complicated for their experience.
The anesthesia method of target-controlled infusion combined with LMA seemed to be safer than epidural anesthesia in our study. Since the lung is innervated by the visceral nerve, the somatic nerve can be blocked under local anesthesia of the skin and intercostal nerve, and then VATS can be completed under the assistance of moderate sedation and mild analgesia [39,40]. LMA is a strong measure to ensure oxygenation in patients receiving nonintubated anesthesia, and it can decrease the incidence of hypoxia, especially in patients with a high risk of upper airway obstruction. As to the high plane block in thoracic epidural anesthesia, it decreases the inspiratory capacity as a consequence of the motor block of the intercostal muscles [41,42], which may lead to hypercapnia and hypoxia during thoracic surgery. It is possible that patients that received an epidural also had a higher RCRI and more complex resections, resulting in the epidural being seen as increasing the incidence of hypoxia. But which anesthesia method is the best suited for NIVATS? A more detailed comparison is still needed to determine the answer [43].
Furthermore, Bellucci and Qiao [20] and Cureley et al [21] proposed that intraoperative hypoxia may increase the incidence of postoperative complications. However, after the propensity score-matching analysis of preoperative basic characteristics in the current study, patients with hypoxia were associated with intraoperative hypercapnia and increased the probability of intensive care, but hypoxia did not affect the overall complications and postoperative hospital stay. This outcome may imply that the impact of intraoperative hypoxia on patients is short-term in NIVATS and has no significant impact on the overall clinical recovery after the operation.
Undoubtedly, this study design could be modified and improved. First, this is a single-center retrospective investigation with data collected over 8 years, and bias (eg, laboratory measurement bias) due to the long period of time was inevitable. In addition, the large sample of data were collected by more than one person, which inevitably led to measurement bias. However, all patients were treated at the same center and a uniform therapeutic regime may have reduced the potential bias. Second, miscellaneous types rather than a specific type of thoracic procedures were included, and cases were consequently less comparable. However, this situation presents a real-world clinical scenario. Third, patients younger than 18 years old were excluded, and pediatric patients need to be included in future studies to determine whether NIVATS is safe for young patients. Fourth, although the PaO2 can reflect the real oxygenation status of patients, a change in SpO2 is recognized much more easily and quickly. Therefore, the continuous monitoring index SpO2 ≤93%, not the PaO2, was chosen as the outcome standard. Fifth, although the propensity score-matching analysis was used to reduce the preoperative bias to the outcomes, prospective studies are still needed to confirm whether intraoperative hypoxia affects the prognosis of patients in NIVATS.
Conclusions
Rigorous preoperative evaluations should be implemented for patients who are scheduled for NIVATS. Identification of risk factors for intraoperative hypoxia in NIVATS may guide recommendations for early identification of high-risk patients and enable interventions before the operation. The incidence of intraoperative hypoxia in our study was related to the patient’s age, anesthesia method, the technical level of surgeons, the stair-climbing ability, and the type of thoracic procedure. However, even if intraoperative hypoxia occurred, it did not affect the postoperative recovery duration.
References
1. Nezu K, Kushibe K, Tojo T, Thoracoscopic wedge resection of blebs under local anesthesia with sedation for treatment of a spontaneous pneumothorax: Chest, 1997; 111; 230-35
2. Pompeo E, Mineo D, Rogliani P, Feasibility and results of awake thoracoscopic resection of solitary pulmonary nodules: Ann Thorac Surg, 2004; 78; 1761-68
3. Gonzalez-Rivas D, Bonome C, Fieira E, Non-intubated video-assisted thoracoscopic lung resections: the future of thoracic surgery?: Eur J Cardiothorac Surg, 2016; 49; 721-31
4. Chen JS, Cheng YJ, Hung MH, Nonintubated thoracoscopic lobectomy for lung cancer: Ann Surg, 2011; 254; 1038-43
5. Pompeo E, Tacconi F, Frasca L, Awake thoracoscopic bullaplasty: Eur J Cardiothorac Surg, 2011; 39; 1012-17
6. Pompeo E, Rogliani P, Tacconi F, Randomized comparison of awake nonresectional versus nonawake resectional lung volume reduction surgery: J Thorac Cardiovasc Surg, 2012; 143; 47-54
7. Li S, Liu J, He J, Video-assisted transthoracic surgery resection of a tracheal mass and reconstruction of trachea under non-intubated anesthesia with spontaneous breathing: J Thorac Dis, 2016; 8; 575-85
8. Gonzalez-Rivas D, Fernandez R, de la Torre M, Single-port thoracoscopic lobectomy in a nonintubated patient: The least invasive procedure for major lung resection?: Interact Cardiovasc Thorac Surg, 2014; 19; 552-55
9. Deng HY, Zhu ZJ, Wang YC, Non-intubated video-assisted thoracoscopic surgery under loco-regional anesthesia for thoracic surgery: A meta-analysis: Interact Cardiovasc Thorac Surg, 2016; 23; 31-40
10. Zhang K, Chen HG, Wu WB, Non-intubated video-assisted thoracoscopic surgery vs intubated video-assisted thoracoscopic surgery for thoracic disease: A systematic review and meta-analysis of 1,684 cases: J Thorac Dis, 2019; 11; 3556-68
11. Li S, Jiang L, Ang KL, New tubeless video-assisted thoracoscopic surgery for small pulmonary nodules: Eur J Cardiothorac Surg, 2017; 51; 689-93
12. AlGhamdi ZM, Lynhiavu L, Moon YK, Comparison of non-intubated versus intubated video-assisted thoracoscopic lobectomy for lung cancer: J Thorac Dis, 2018; 10; 4236-43
13. Yu MG, Jing R, Mo YJ, Non-intubated anesthesia in patients undergoing video-assisted thoracoscopic surgery: A systematic review and meta-analysis: PLoS One, 2019; 14; e0224737
14. Wen Y, Liang H, Qiu G, Non-intubated spontaneous ventilation in video-assisted thoracoscopic surgery: A meta-analysis: Eur J Cardiothorac Surg, 2020; 57; 428-37
15. Tacconi F, Pompeo E, Sellitri F, Surgical stress hormones response is reduced after awake video thoracoscopy: Interact Cardio Vasc Thorac Surg, 2010; 10; 666-71
16. Güldner A, Braune A, Carvalho N, Higher levels of spontaneous breathing induce lung recruitment and reduce global stress/strain in experimental lung injury: Anesthesiology, 2014; 120; 673-82
17. Brown SJ, Barnes MJ, Mundel T, Effects of hypoxia and hypercapnia on human HRV and respiratory sinus arrhythmia: Acta Physiol Hung, 2014; 101; 263-72
18. Kiely DG, Cargill RI, Lipworth BJ, Effects of hypercapnia on hemodynamic, inotropic, iusitropic, and electrophysiologic indices in humans: Chest, 1996; 109; 1215-21
19. Steinback CD, Saizer D, Medeiros PJ, Hypercapnic vs hypoxic control of cardiovascular, cardiovagal, and sympathetic function: Am J Physiol Regul Integr Comp Physiol, 2009; 296; R402-10
20. Bellucci B, Qiao R, A 47-year-old woman with hypercapnia and altered mental status: Clin Respir J, 2018; 12; 331-33
21. Cureley G, Contreras MM, Nichol AD, Hypercapnia and acidosis in sepsis: A double-edged sword?: Anesthesiology, 2010; 112; 462-72
22. Lan L, Cen Y, Zhang C, A propensity score-matched analysis for non-intubated thoracic surgery: Med Sci Monit, 2018; 24; 8081-87
23. Dong Q, Liang L, Li Y, Anaesthesia with nontracheal intubation in thoracic surgery: J Thorac Dis, 2012; 4; 126-30
24. Svensson LG, Gillinov AM, Weisel RD, The American Association for Thoracic Surgery consensus guidelines: Reasons and purpose: J Thorac Cardiovasc Surg, 2016; 151; 935-39e1
25. Kregenow DA, Swenson ER, The lung and carbon dioxide: Implications for permissive and therapeutic hypercapnia: Eur Respir J, 2002; 20; 6-11
26. Seo DE, Shin SD, Song KJ, Effect of hypoxia on mortality and disability in traumatic brain injury according to shock status: A cross-sectional analysis: Am J Emerg Med, 2019; 37; 1709-15
27. Hung MH, Chan KC, Liu YJ, Nonintubated thoracoscopic lobectomy for lung cancer using epidural anesthesia and intercostal blockade: A retrospective cohort study of 238 cases: Medicine (Baltimore), 2015; 94; e727
28. Klijian AS, Gibbs M, Andonian NT, AVATS: Awake video-assisted thoracic surgery – extended series report: J Cardiothorac Surg, 2014; 9; 149
29. Chen KC, Cheng YJ, Hung MH, Nonintubated thoracoscopic lung resection: A 3-year experience with 285 cases in a single institution: J Thorac Dis, 2012; 4; 347-51
30. Chen KC, Cheng YJ, Hung MH, Nonintubated thoracoscopic surgery using regional anesthesia and vagal block and targeted sedation: J Thorac Dis, 2014; 6; 31-36
31. Hung MH, Hsu HH, Chan KC, Non-intubated thoracoscopic surgery using internal intercostal nerve block, vagal block and targeted sedation: Eur J Cardiothoracic Surg, 2014; 46; 620-25
32. Migliore M, Giuliano R, Aziz T, Four-step local anesthesia and sedation for thoracoscopic diagnosis and management of pleural diseases: Chest, 2002; 121; 2032-35
33. Rusch VW, Mountain C, Thoracoscopy under regional anesthesia for the diagnosis and management of pleural disease: Am J Surg, 1987; 154; 274-78
34. Migliore M, Deodato G, A single trocar technique for minimally invasive surgery of the chest: Surg Endosc, 2001; 13; 899-901
35. Pompeo E, Tacconi F, Mineo TC, Comparative results of non-resectional lung volume reduction performed by awake or non-awake anesthesia: Eur J Cardiothorac Surg, 2011; 39; e51-58
36. Noda M, Okada Y, Maeda S, Successful thoracoscopic surgery for intractable pneumothorax after pneumonectomy under local and epidural anesthesia: J Thorac Cardiovasc Surg, 2011; 141; 1545-47
37. Liu YJ, Hung MH, Hsu HH, Effects on respiration of nonintubated anesthesia in thoracoscopic surgery under spontaneous ventilation: Ann Transl Med, 2015; 3; 107
38. Hales CA, Ahluwalia B, Kazemi H, Strength of pulmonary vascular response to regional alveolar hypoxia: J Appl Physiol, 1975; 38; 1083-87
39. Katlic MR, Video-assisted thoracic surgery utilizing local anesthesia and sedation: How I teach it: Ann Thorac Surg, 2017; 104; 727-30
40. Katlic MR, Facktor MA, Video-assisted thoracic surgery utilizing local anesthesia and sedation: 384 consecutive cases: Ann Thorac Surg, 2010; 90; 240-45
41. Sundberg A, Wattwil M, Arvill A, Respiratory effects of high thoracic epidural anaesthesia: Acta Anaesthesiol Scand, 1986; 30; 215-17
42. Groeben H, Epidural anesthesia and pulmonary function: J Anesth, 2006; 20; 290-99
43. Umari M, Falini S, Segat M, Anesthesia and fast-track in video-assisted thoracic surgery (VATS): From evidence to practice: J Thorac Dis, 2018; 10; S542-54
Figures
Tables
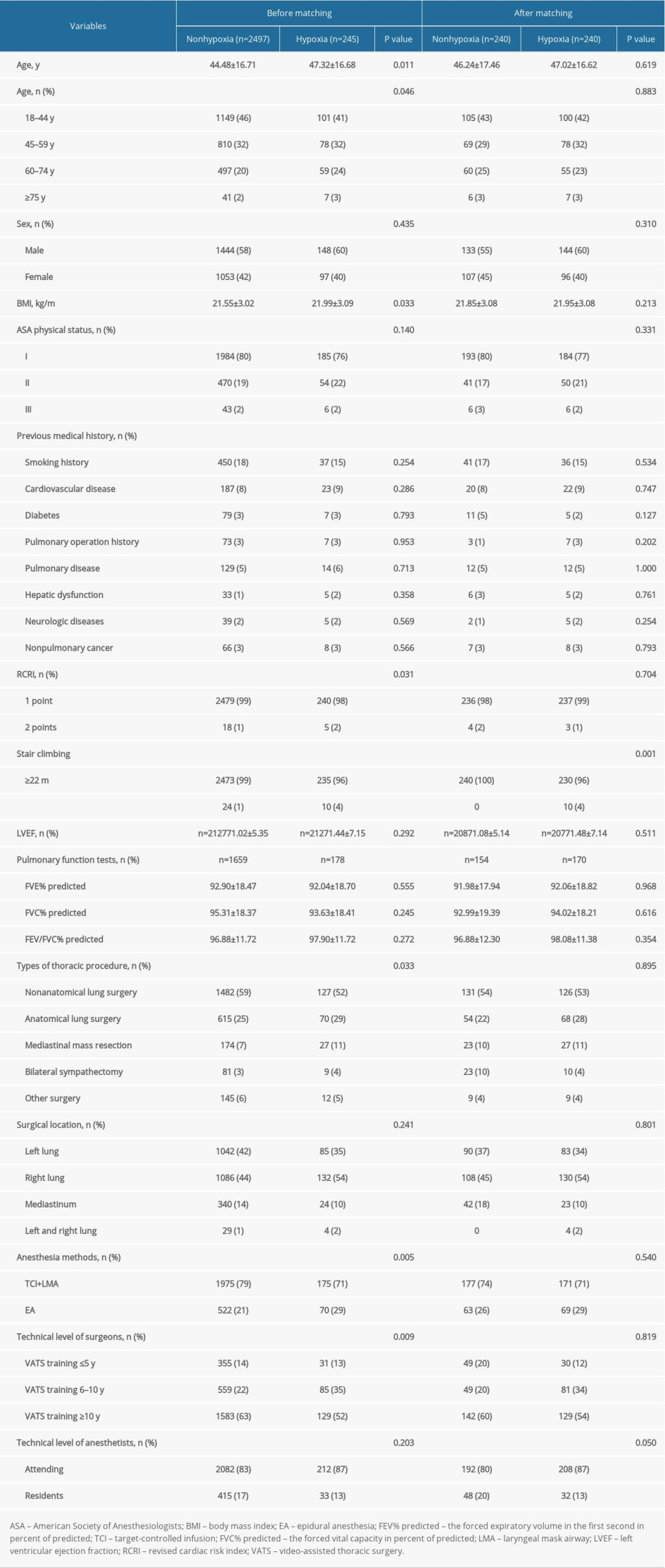 Table 1. Preoperative characteristics of patients with or without hypoxia.
Table 1. Preoperative characteristics of patients with or without hypoxia.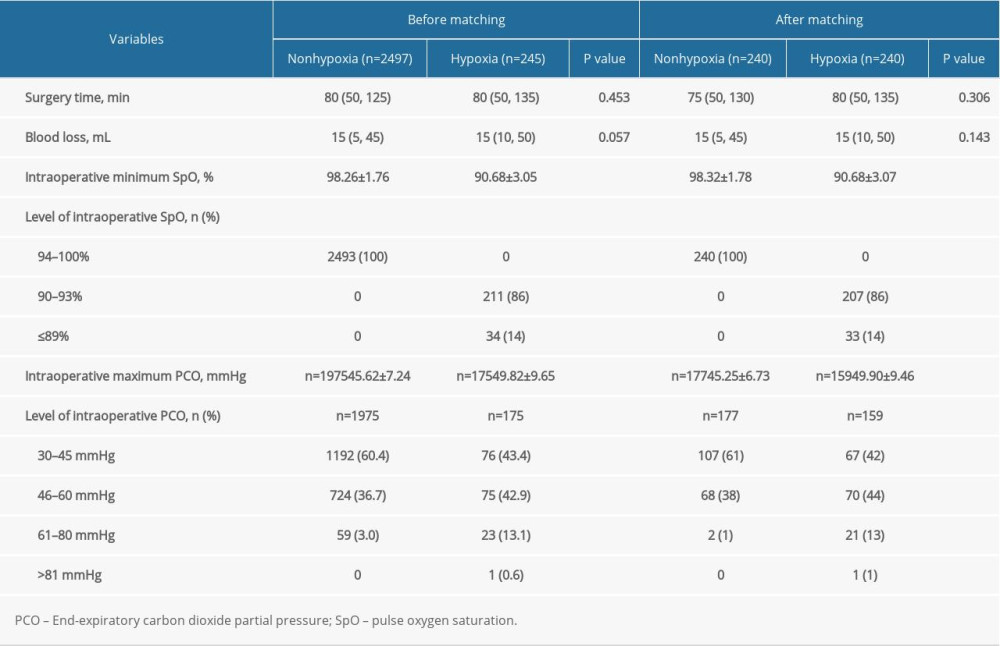 Table 2. Intraoperative comparisons of patients with or without hypoxia.
Table 2. Intraoperative comparisons of patients with or without hypoxia.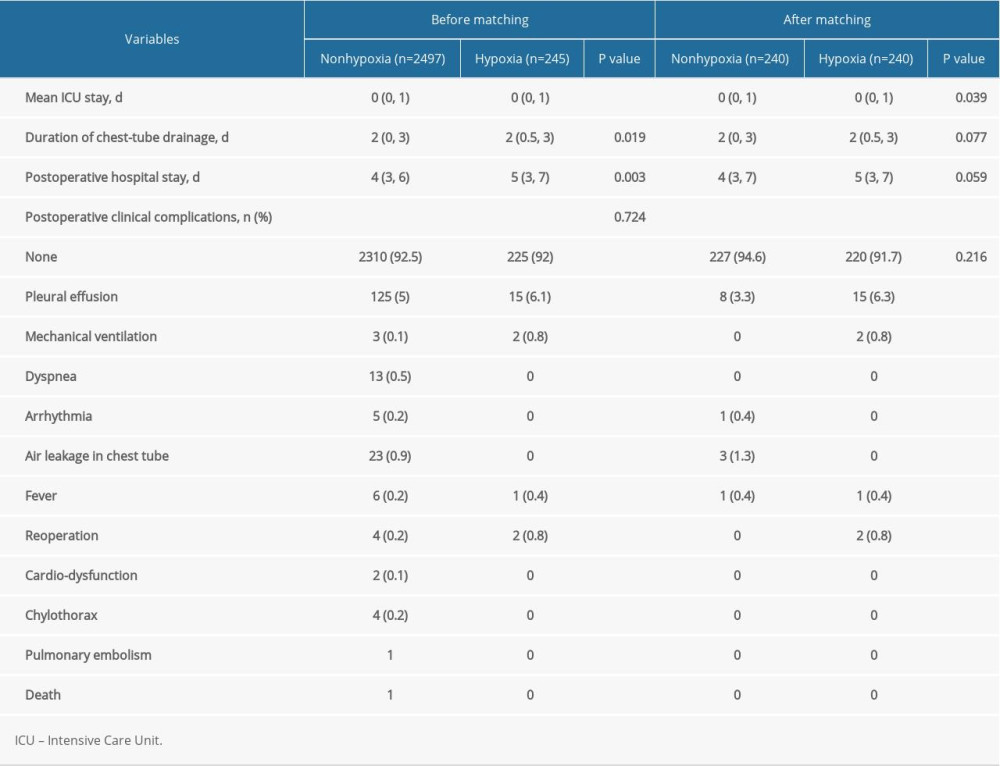 Table 3. Postoperative comparisons of patients with or without hypoxia.
Table 3. Postoperative comparisons of patients with or without hypoxia.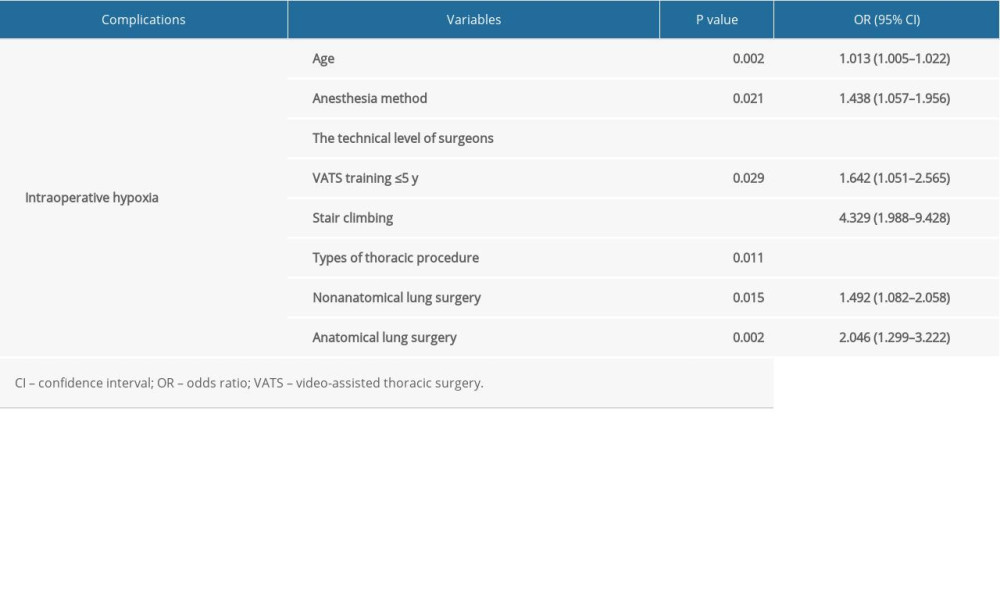 Table 4. Risk factors of intraoperative hypoxia.
Table 4. Risk factors of intraoperative hypoxia. Table 1. Preoperative characteristics of patients with or without hypoxia.
Table 1. Preoperative characteristics of patients with or without hypoxia. Table 2. Intraoperative comparisons of patients with or without hypoxia.
Table 2. Intraoperative comparisons of patients with or without hypoxia. Table 3. Postoperative comparisons of patients with or without hypoxia.
Table 3. Postoperative comparisons of patients with or without hypoxia. Table 4. Risk factors of intraoperative hypoxia.
Table 4. Risk factors of intraoperative hypoxia. In Press
05 Mar 2024 : Clinical Research
Role of Critical Shoulder Angle in Degenerative Type Rotator Cuff Tears: A Turkish Cohort StudyMed Sci Monit In Press; DOI: 10.12659/MSM.943703
06 Mar 2024 : Clinical Research
Comparison of Outcomes between Single-Level and Double-Level Corpectomy in Thoracolumbar Reconstruction: A ...Med Sci Monit In Press; DOI: 10.12659/MSM.943797
21 Mar 2024 : Meta-Analysis
Economic Evaluation of COVID-19 Screening Tests and Surveillance Strategies in Low-Income, Middle-Income, a...Med Sci Monit In Press; DOI: 10.12659/MSM.943863
10 Apr 2024 : Clinical Research
Predicting Acute Cardiovascular Complications in COVID-19: Insights from a Specialized Cardiac Referral Dep...Med Sci Monit In Press; DOI: 10.12659/MSM.942612
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952