25 March 2021: Lab/In Vitro Research
Knockdown of Chitinase 3-Like-1 Inhibits Cell Proliferation, Promotes Apoptosis, and Enhances Effect of Anti-Programmed Death Ligand 1 (PD-L1) in Diffuse Large B Cell Lymphoma Cells
Xiao Yang1AC, Dong Fang2B, Ming Li1F, Jiayi Chen1B, Yuanbo Cheng1F, Jianming Luo1DE*DOI: 10.12659/MSM.929431
Med Sci Monit 2021; 27:e929431
Abstract
BACKGROUND: Enzymatically inactive chitinase-like protein CHI3L1 is overexpressed in diffuse large B cell lymphoma (DLBCL) patients with PD-L1 imbalance and promotes tumor progression in the microenvironment. Based on this, we investigated how CHI3L1 acts on the proliferation and apoptosis of DLBCL and whether there is a synergy of CHI3L1 in combination with anti-PD-L1 antibodies in vivo.
MATERIAL AND METHODS: CHI3L1 was detected by quantitative real-time PCR (RT-PCR) and western blot (WB) in B-lymphoma cell lines. CHI3L1 interference plasmids were constructed, and the levels of proliferation, cell cycle, apoptosis, and cell survival were examined in vitro in B-lymphoma cell lines and in vivo in a murine xenograft model by RT-PCR, WB, CCK-8, and flow cytometry.
RESULTS: CHI3L1 was significantly expressed in SU-DHL-4 cells. CHI3L1-interfered RNA ShRNA-CHI3L1-1 was chosen to be used in the next experiment because it had a better interference effect. Dampened cell proliferation level, arrested cell cycle, reduced protein expressions of cyclin D1 and cyclin D2, and promoted cell apoptosis level were observed after SU-DHL-4 was transfected with ShRNA-CHI3L1-1. Furthermore, we also noticed increased expression of Bcl-2, decreased expressions of bax, cleaved caspase 3 and cleaved PARP, promoted cell survival-related protein p53, and reduced survivin.
CONCLUSIONS: This study demonstrated that knockdown of CHI3L1 inhibits cancer cell proliferation by regulating cell cycles, promotes cancer cell apoptosis, and enhances the pro-apoptotic effect of anti-PD-L1 antibody both in vivo and in vitro in DLBCL.
Keywords: Antibody Affinity, Antibodies, Blocking, B-Lymphocytes, B7-H1 Antigen, Chitinase-3-Like Protein 1, Immune Tolerance, Lymphoma, Large B-Cell, Diffuse, Mutation, Proto-Oncogene Proteins c-bcl-2, RNA, Small Interfering, tumor microenvironment, Tumor Suppressor Protein p53
Background
Diffuse large B cell lymphoma (DLBCL) is the most common type of aggressive non-Hodgkin lymphoma. Patients often present with a rapidly growing tumor mass in single or multiple, nodal or extranodal sites and treated with chemotherapy which can achieve high response rate and bring significant improvements in the overall survival rate [1,2]. However, 10% to 15% of DLBCLs are refractory, and 20% to 25% of patients experience relapse after an initial response, posing clinical challenges [3]. DLBCL is also characterized by its great biological heterogeneity, which not only is caused by tumor cells themselves, but is also dependent on the tumor microenvironment [4,5].
Programmed death 1 (PD-1), a member of the CD28 family, is an inhibitory receptor expressed on the surface of T cells that functions to physiologically limit T cell activation and proliferation [6]. Its ligand, programmed death ligand 1 (PD-L1), is expressed by 20% to 57% of systemic DLBCLs [7]. Binding of PD-L1 to PD-1 inhibits the proliferation of activated T cells, which is often regarded to as the main therapeutic mechanism of PD-1/PD-L1 blockade [8]. A study confirmed the oncogenic potential of miR155 in DLBCL by modulating tumor microenvironment, which indicated the sensitivity of B-lymphoma cells to anti-PD-L1 antibody [9]. The beneficial antitumoral activity of PD-1 and PD-L1 has been thoroughly demonstrated in certain metastatic malignancies (eg, melanoma, non-small cell lung cancer, renal cell carcinoma); however, its therapeutic role in lymphoid cancers is complex [10].
Chitinase 3-like-1 (CHI3L1, also called YKL-40 in humans and BRP-39 in mice) is a member of the glycosyl hydrolase gene family 18 which contains true chitinases and chitinase-like proteins that bind to chitin without degrading it. CHI3L1 is expressed in a vast array of cells, including neutrophils, macrophages, fibroblasts, vascular smooth cells, endothelial cells, and tumor cells [11]. The prognosis of PD-1+/PD-L1+ patients with DLBCL is poor, and gene profiling in the unbalanced microenvironment of PD-L1 revealed that the expression of CHI3L1 gene increased unfavorably in those patients [12]. Increased CHI3L1 was also observed in hepatocellular carcinoma and gastric cancer [13,14]. According to the GTEx database, the expression of CHI3L1 gene is increased in DLBC tumor tissue. Therefore, the present study mainly explored the inhibitory effect of CHI3L1 on DLBCL and whether it can strengthen the effect of anti-PD-L1 on aggressive B cell lymphoma cells.
Material and Methods
HUMAN CELL CULTURE:
Human DLBCL cell lines, including Farage, germinal center B cells (SU-DHL-4 and CRL-2631), and human B lymphocyte (GM12878) cells, were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA). The cells were cultured in complete medium of DMEM containing 10% FBS, 1% streptomycin, and amphotericin. The cells were incubated at 37°C in a 5% CO2 incubator with ddH2O to maintain humidity.
CELL TRANSFECTION:
Cells were prepared in 6-wells plates 24 h before transfection. SU-DHL-4 cells was transfected with the short hairpin RNA (shRNA) CHI3L1-1, shRNA CHI3L1-2, and non-target shRNA (Sangon Biotech, Shanghai, China) using lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) when the confluence of cells reached 80–90%. Cells were harvested and reseeded 24 h after transfection.
REVERSE TRANSCRIPTION PCR (RT-PCR):
Total RNA was extracted form cell lines using TRIzol (Invitrogen, California, USA). RNA was collected and then reverse-transcribed in enzyme-free PCR tubes with 2 μg of RNA to denature by using a Reverse Transcriptase Kit (M-MLV) (Promega, Wisconsin, USA). Detection of CHI3L1 mRNA levels in cell lines using the BIO-RAD Real-Time detection system. The FastStart Universal SYBR Green Master (ROX) (Roche, Basel, Switzerland) was used to configure a total volume of 15 ug of the cycling system, including 10ul of Mix and 0.5ul of each primer; the PCR cycle was set to 10 min at 95°C, and 37 cycles of reaction were performed. including 10 s at 95°C, 10 s at 60°C, and 10 s at 72°C. The relative mRNA expression levels of CHI3L1 were analyzed using the 2−ΔCt method.
WESTERN BLOT ANALYSIS:
Total protein was extracted from tissues and cells using RIPA buffer (Invitrogen, California, USA). The protein concentration was determined using the BCA protein assay kit (Invitrogen, California, USA). An equal amount (25 μg) of total protein per well was loaded into the wells of SDS-PAGE, and after 1.5 h of electrophoresis, constant-pressure electrotransfer was performed to transfer the proteins to PVDF membranes, then we blocked it in Tris-buffered saline (TBS) containing 0.1% Tween-20 (TBST) with 5% skimmed milk at room temperature for 1 h. Antibodies used in western blot analysis included CHI3L1, GAPDH (Abcam, Shanghai, China), cyclinD1, cyclinD2, bax, cleaved caspase3, cleaved PARP, p53, survivin, and Bcl2 (Cell signaling, Shanghai, China).
CELL PROLIFERATION ASSAY:
The Cell Counting Kit-8 (CCK8) assay was used to detect the effect of ShRNA-CHI3L1 on the proliferation of SU-DHL-4 cells, all according to the instructions. Briefly, 1×104 cells per well were seeded in 24-well plates and cultured in serum-free medium containing different concentrations of SU-DHL-4 cells, and the absorbance at 450 nm per well was measured at 0, 24, 48, and 72 h.
FLOW CYTOMETRY ASSAY:
Briefly, cells were seeded into 6-well plates and placed in an incubator for 12 h, followed by another 48 h after transfection. Then, transfected cells were digested using trypsin without EDTA, followed by resuspension in 500 μl flow cytometry binding buffer. Then, cells were stained with 5 μl Annexin V/FITC and 5 μl propidium iodide (PI) at room temperature in the dark for 15 min. The apoptotic cells were detected by FACS Calibur flow cytometer (BD Biosciences, CA, USA) with the excitation wavelength of Ex=488 nm and emission wavelength of Em=530 nm.
We used the Univariate Model for G0/G1 peak analysis, S phase, and G2/M peak, excluding cellular debris and fixation artifact calculation. The percentage of cells in the G0/G1, S, and G2/M phases of the cell cycle was ascertained from 3 separate experiments.
MURINE MODEL:
To further assess the correlation between CHI3L1-1 and PD-L1 in vivo, BALB/c nude mice (6 weeks-old, obtained from Shanghai Laboratory Animal Center, Shanghai, China) were injected with 1×107 ShRNA-NC and ShRNA-CHI3L1 cells into the right flank. The mice were housed under a 12-h light-dark cycle in a temperature-controlled environment, and were allowed free access to water and food. All animal procedures were approved by the First Affiliated Hospital of Guangxi Medical University. Anti-mouse PD-L1 antibody Invivomab was injected at a dose of 200 μg per mouse 3 times a week for 2 weeks. The tumor volume was calculated as 0.5×a (length)×b (width)2.
STATISTICAL ANALYSIS:
Statistical analysis was performed with GraphPad Prism 7 software. All data were expressed as the means ± Standard Deviation (SD) and ANOVA (analysis of variance) was performed. After the determination of significance by ANOVA, post hoc testing of differences between groups was performed. The comparison between the 2 groups was conducted using the
Results
CHI3L1 IS SIGNIFICANTLY ELEVATED IN DLBCL CELL LINES:
To gain deeper insight into the molecular mechanistic basis of the facilitative effect of CHI3L1 on tumors in DLBCL, we selected the most suitable cell line for our study. We examined the expression of CHI3L1 in human B lymphocyte (GM12878), Farage, SU-DHL-4, and CRL-2631 by WB (Figure 1A) and RT-PCR (Figure 1B). It was found that both the RNA expression and the protein expression of CHI3L1 were significantly increased in DLBCL cell lines, in which SU-DHL-4 exhibited the most prominent change. Therefore, the SU-DHL-4 cell line was selected for the next experiment.
KNOCKDOWN OF CHI3L1 INHIBITS THE CELL PROLIFERATION IN DLBCL CELLS:
To study the function of CHI3L1, CHI3L1 stable knockdown cell lines were used. Quantitative real-time PCR (qPCR) and WB were performed to select the best shRNA from 2 selected putative shRNAs (ShRNA-CHI3L1-1 and ShRNA-CHI3L1-2). ShRNA-CHI3L1-1 had the highest knockdown efficiency, as demonstrated by the fact that SU-DHL-4 has a suppression rate of over 50% (Figure 2A, 2B).
CCK8 assay was performed for cell proliferation detection after transfection of ShRNA-CHI3L1-1 in SU-DHL-4. The results showed that at 24, 48, and 72 h after transfection, the OD value of the cells in the ShRNA-CHI3L1-1 group was much lower than that in the control and ShRNA-NC groups, suggesting that the proliferation rate of ShRNA-CHI3L1-1 was significantly slower than that of the control and ShRNA-NC cells 48 h after injection (p<0.05) (Figure 2C). The markedly slower cell growth of SU-DHL-4 by ShRNA-CHI3L1-1 transfection suggested the inhibitory effect of CHI3L1knockdown on the proliferation of SU-DHL-4 cells.
To determine if cell cycle changes are associated with CHI3L1 expression levels, flow cytometry was used to analyze cell cycle changes. We found that reduction of CHI3L1 expression significantly increased the number of cells in the G0/G1 stage and significantly decreased the number of cells in the G2/M stage in SU-DHL-4 (p<0.05) (Figure 2D). Next, the protein expression of cyclinD1 and cyclinD2 was analyzed by western blotting. The levels of cyclinD1 and cyclinD2 in ShRNA- CHI3L1-1 group were conspicuously lower than in the control group and ShRNA-NC group (p<0.05) (Figure 2E). These results suggested that knockdown of CHI3L1 can promote cell cycle arrest.
KNOCKDOWN OF CHI3L1 PROMOTES THE APOPTOSIS IN DLBCL CELLS:
To further assess the effect of CHI3L1 on DLBCL, cell apoptosis level was determined by flow cytometry. The reduction of the CHI3L1 significantly decreased the percentages of apoptotic cells compared to the control group and the shRNA-NC group (Figure 3A). The expressions of apoptosis-related proteins were detected by western blotting. Compared with that in the control group and the shRNA-NC group, bax, cleaved caspase3, and cleaved PARP were significantly increased in the ShRNA- CHI3L1-1 group while bcl-2 was significantly decreased (Figure 3B). The expressions of cell survival-related proteins were also detected. It was found that p53 was greatly increased while survivin was greatly decreased in the ShRNA- CHI3L1-1 group compared to the control group and the shRNA-NC group (Figure 3C). The results suggested that knockdown of CHI3L1 promotes the apoptosis in DLBCL.
KNOCKDOWN OF CHI3L1 ENHANCES THE EFFECT OF ANTI-PD-L1 IN THE DLBCL MOUSE MODEL:
A murine xenograft model was established with subcutaneous injection of 1×107 cells stably transfected with ShRNA-NC or ShRNA-CHI3L1-1. Then, subcutaneous tumor growth was continuously observed and recorded in the 5 groups. As shown in Figure 4A, 4B, the amount of tumor growth formed by ShRNA-CHI3L1-1-transfected cells was significantly inhibited compared to the control group; compared with anti-PD-L1 tumors, the tumors formed by the anti-PD-L1 antibody treatment with ShRNA-CHI3L1-1 grew significantly slower. At termination of the experiment, the net weights of the corresponding tumors were also markedly reduced compared to the control weights (Figure 4C–4E). The apoptosis and cell survival-related proteins were analyzed in tumors by western blotting, showing that the expression of Bcl2 was lower in tumors formed by CHI3L1 knockout cells than those formed by the control cells and anti-PD-L1 antibodies, among which treatment with ShRNA-CHI3L1-1 exhibited the lowest Bcl2 expression. The expressions of Bax, cleaved caspase3, and cleaved PARP had contrary trends (Figure 5A). P53 in the tumors of the ShRNA-CHI3L1-1 group was significantly increased compared with the control group and the ShRNA-NC group, which was expressed more in the anti-PD-L1 group and the most in the Anti-PD-L1+ShRNA-CHI3L1-1 group; survivin showed the opposite trend (Figure 5B). These results suggested that knockdown of CHI3L1 enhances the effect of anti-PD-L1 in the DLBCL mouse model.
Discussion
DLBCL is a common type of non-Hodgkin’s lymphoma, accounting for about 1/3 of all cases, and is clinically characterized by high invasiveness and moderate to high deterioration [15]. The standard treatment for DLBCL, combination immunochemotherapy R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone), is associated with a high complete response rate of up to 80%. However, some of DLBCLs are refractory [16].
The pathogenesis of malignant lymphoma is complex, and studies have shown that CHI3L1 is critically involved in B cell lymphoma progression [17]. CHI3L1 has been reported to be positively correlated with the depth of gastric cancer invasion, lymph node status, and tumor stage [14]. CHI3L1 plays a role in inducing pro-inflammatory/pro-tumorigenic and angiogenic factors that promote tumor growth and metastasis [18]. It has been confirmed that increased CHI3L1 levels are correlated with poor prognosis and decreased survival rates of cancer patients [19,20]. Thus, CHI3L1 is considered a potential diagnostic standard and therapeutic target for cancer [21]. Serum YKL-40 and IL-6 levels were found to be increased in untreated Hodgkin lymphoma patients [22]. In the microenvironment of PD-L1 dysregulation, a study performing gene profiling and using the GTEx database found that the expression of CHI3L1 gene was increased in DLBC tumor tissue [12]. These results suggest that CHI3L1 is primarily involved in the process of DLBCL development. However, these previous studies did not analyze the underlying mechanism of CHI3L1 in DLBCL. Th present study focused on the effects of CHI3L1 on the proliferation and apoptosis of human DLBCL cell line SUDHL-4 cells in vitro and its synergistic effect with anti-PD-L1 antibody in vivo, for the purpose of providing a theoretical basis for DLBCL treatment.
Our study observed elevated expression of CHI3L1 in DLBCL cell lines, especially in SU-DHL-4 cells (Figure 1). Therefore, we transfected shRNA into SU-DHL-4 cells to study the effect of knocking down CHI3L1 on DLBCL cells. The CCK8 (Figure 2C) results showed that the proliferation ability of SUDHL-4 cells decreased after CHI3L1-1 knockdown. We further accessed the levels of cell cycle and the expression of cyclin proteins, showing that CHI3L1 promoted cancer cell cycles (Figure 2). Apoptosis is the process of maintaining a stable environment and actively removing damaged and infected cells in the body. Many studies have shown that not only do cancer cells proliferate rapidly, but the process of apoptosis is also affected [23]. We found that the apoptosis rate of ShRNA-CHI3L1-1 group cells was significantly higher than that of the cells in the control and ShRNA-NC group after 48 h of transfection, suggesting that knockdown of CHI3L1-1 can promote the process of apoptosis. The pro-apoptotic gene Bax and the anti-apoptotic gene Bcl-2 belong to the Bcl family, and the ratio of the 2 determines the cell’s response to apoptosis signals [24]. We noticed that the expression of Bax, cleaved caspase3, and cleaved PARP in the ShRNA-CHI3L1-1 group was significantly higher than that in the control group and ShRNA-NC group, and that the expression of Bcl-2 was lower than that in the control group and ShRNA-NC group, suggesting that CHI3L1 inhibits SUDHL-4 cell apoptosis by affecting the key factors of the signaling pathway. Mutations in the key tumor suppressor p53 are common in cancer, often leading to an anti-apoptotic phenotype, which promotes cell survival [25]. Survivin is an anti-apoptotic protein which inhibits caspase activation [26]. It was found in our experiments that compared to the control and ShRNA-NC group, the expression of P53 was significantly higher and the expression of survivin was much lower in the ShRNA-CHI3L1-1 group (Figure 3). These data indicate that CHI3L1 knockdown promotes cancer cell apoptosis.
The level of PD-L1 directly reflects the immune function of the body. At present, anti-PD-1 and anti-PD-L1 immunotherapy have become important in human cancer immunotherapy [27]. Anti-PD-L1 antibody can inhibit the growth of lung adenocarcinoma CMT167 and LLC cell transplantation tumors, in addition to which, PD-1 blockade monotherapies showed promising efficacy in treating relapsed/refractory DLBCL in phase I clinical trials [28]. We further investigated the correlation between CHI3L1-1 and PD-L1 in a lymphoma-bearing nude mouse model. This model mimics aspects of DLBCL in humans [29]. It showed that treatments with ShRNA-CHI3L1-1 and anti-PD-L1 antibodies can significantly inhibit tumor growth in vivo, and the combination of the 2 displayed a better effect of inhibiting the tumor volume and weight (Figure 4). In addition, we examined the role of ShRNA-CHI3L1-1 and anti-PD-L1 antibodies in tumors by detecting the expression of the apoptosis-related protein and the survival-associated protein, and found that compared to the control group and ShRNA-NC group, knockdown of CHI3L1 and treatment with anti-PD-L1 antibody promote cancer cell apoptosis and that ShRNA-CHI3L1-1 enhances the pro-apoptotic effect of anti-PD-L1 antibody on DLBCL (Figure 5). These results suggest that CH3L1 combined with anti-PD-L1 produces a synergistic effect in DLBCL. Here, we identified both in vivo and intro that knockdown of CHI3L1 inhibits cancer cell proliferation by regulating cell cycles, promotes cancer cell apoptosis, and enhances the pro-apoptotic effect of anti-PD-L1 antibody in DLBCL.
Conclusions
In summary, we demonstrated that knockdown of CHI3L1 inhibits cell proliferation via cell cycle regulation and promotes cell apoptosis in vitro and in vivo. Moreover, treatment with anti-PD-L1 antibody effectively inhibits DLBCL tumor growth, and its combination with CHI3L1-1 knockdown has a synergistic effect.
We propose that more research should be carried out based on the potential of CHI3L1-1 in controlling DLBCL disease progression. The findings of the present study may inspire a novel immunotherapeutic strategy for the treatment of DLBCL.
Figures
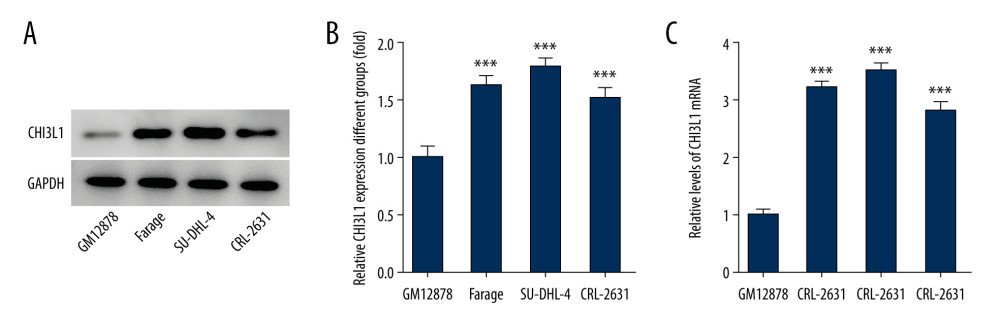 Figure 1. CHI3L1 is highly expressed in SU-DHL-4. (A) Western blot analysis of CHI3L1 protein expression in various DLBCL cell lines. (B) RT-PCR analysis of CHI3L1 protein expression in various DLBCL cell lines. Data are presented as mean±SD. Results shown here are the representative of 3 independent experiments. *** p<0.005 vs GM12878.
Figure 1. CHI3L1 is highly expressed in SU-DHL-4. (A) Western blot analysis of CHI3L1 protein expression in various DLBCL cell lines. (B) RT-PCR analysis of CHI3L1 protein expression in various DLBCL cell lines. Data are presented as mean±SD. Results shown here are the representative of 3 independent experiments. *** p<0.005 vs GM12878. 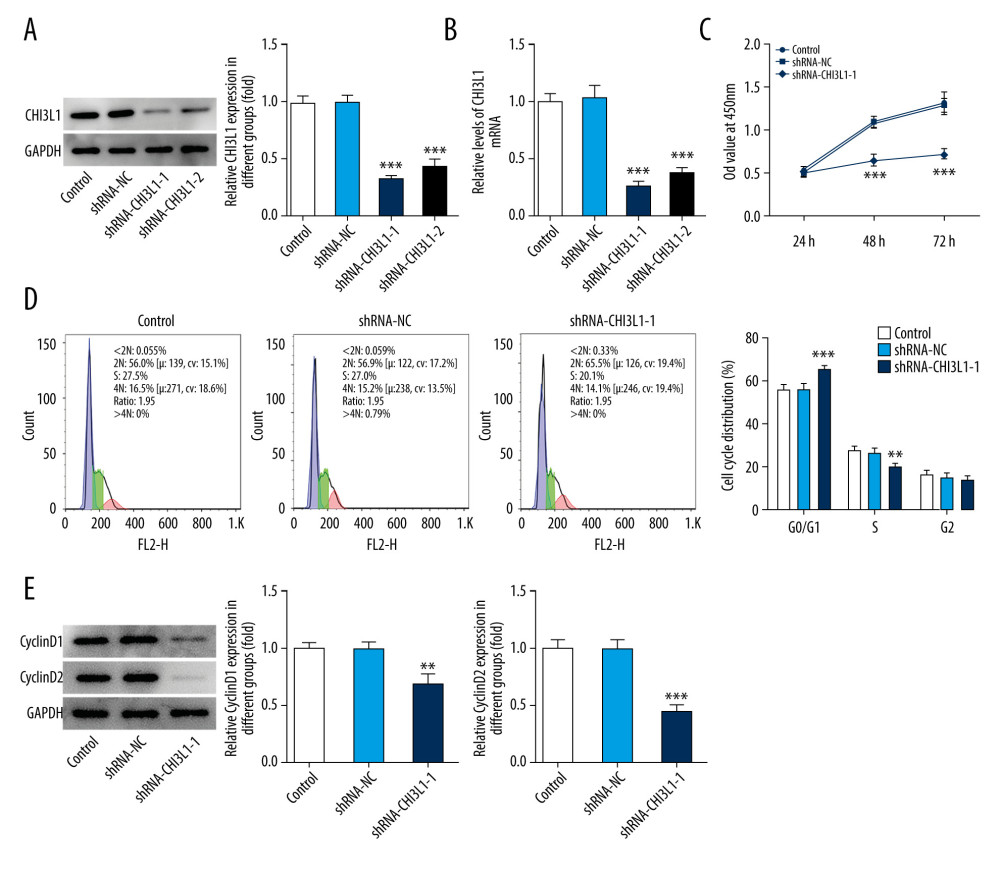 Figure 2. Knockdown of CHI3L1 reduces cell proliferation and cell cycle arrest in vitro. (A) Western blot analysis of CHI3L1 protein expression in SU-DHL-4. (B) RT-PCR analysis of CHI3L1 expression in SU-DHL-4. (C) ShRNA-CHI3L1-1 cells, ShRNA-NC cells, and the control cells were seeded into a 96-well plate. Cells were treated with CCK8, and the CCK8 absorbance value was measured 24 h, 48 h, and 72 h after cell seeding. (D) The effects of ShRNA-CHI3L1-1 on SU-DHL-4 cell proliferation were measured by CCK8 assay. (E) Western blot analysis of cyclinD1 and cyclinD2 expression in SU-DHL-4. Data are presented as mean±SD. Results shown here are the representative of 3 independent experiments. ** p<0.01, *** p<0.005 vs Control.
Figure 2. Knockdown of CHI3L1 reduces cell proliferation and cell cycle arrest in vitro. (A) Western blot analysis of CHI3L1 protein expression in SU-DHL-4. (B) RT-PCR analysis of CHI3L1 expression in SU-DHL-4. (C) ShRNA-CHI3L1-1 cells, ShRNA-NC cells, and the control cells were seeded into a 96-well plate. Cells were treated with CCK8, and the CCK8 absorbance value was measured 24 h, 48 h, and 72 h after cell seeding. (D) The effects of ShRNA-CHI3L1-1 on SU-DHL-4 cell proliferation were measured by CCK8 assay. (E) Western blot analysis of cyclinD1 and cyclinD2 expression in SU-DHL-4. Data are presented as mean±SD. Results shown here are the representative of 3 independent experiments. ** p<0.01, *** p<0.005 vs Control. 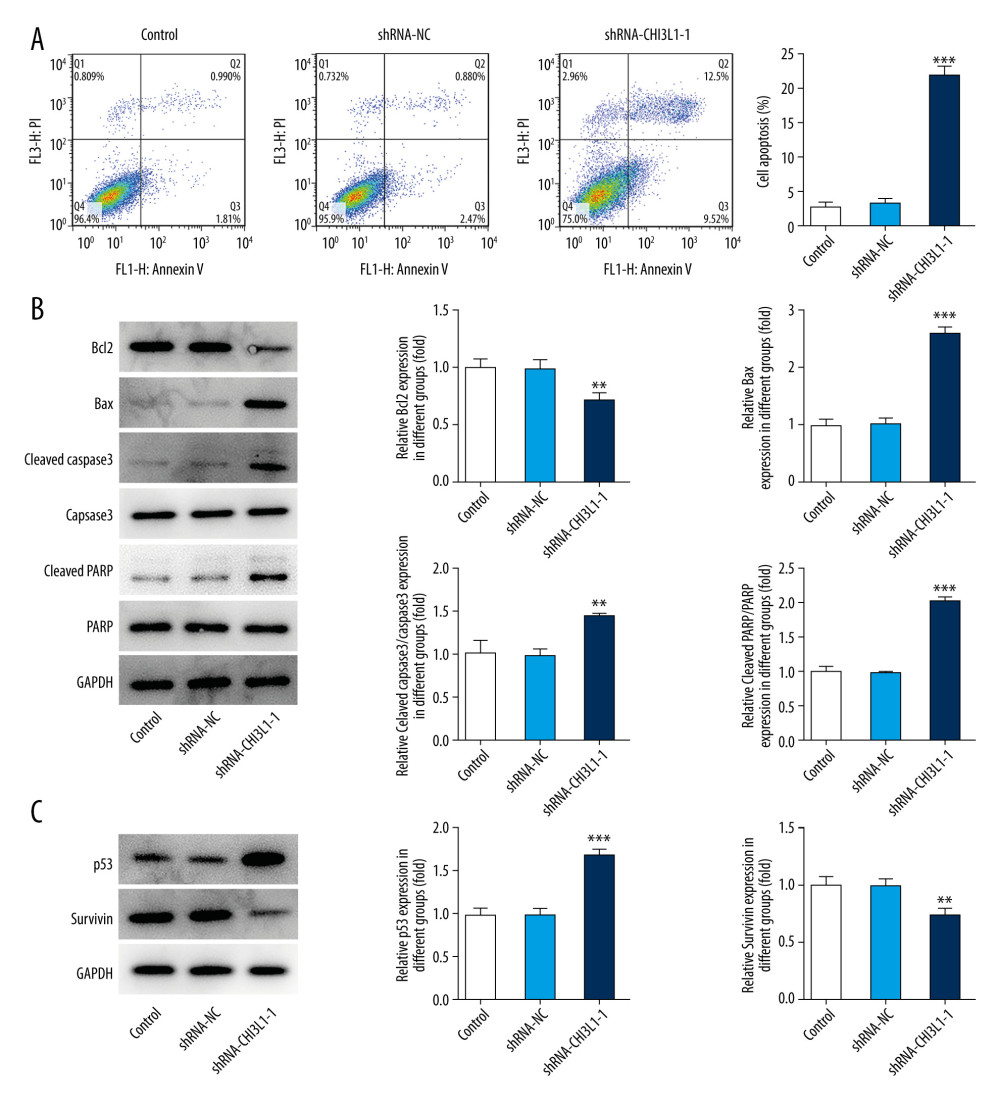 Figure 3. Knockdown of CHI3L1 promotes cell apoptosis and cell survival-related proteins. (A) The effects of ShRNA- CHI3L1-1 on SU-DHL-4 cells apoptosis were measured by flow cytometry. (B) Western blot analysis of bax, cleaved caspase3, and cleaved PARP expression in SU-DHL-4. (C) Western blot analysis of p53 and survivin expression in SU-DHL-4. Data are presented as mean±SD. Results shown here are the representative of 3 independent experiments. ** p<0.01, *** p<0.005 vs Control.
Figure 3. Knockdown of CHI3L1 promotes cell apoptosis and cell survival-related proteins. (A) The effects of ShRNA- CHI3L1-1 on SU-DHL-4 cells apoptosis were measured by flow cytometry. (B) Western blot analysis of bax, cleaved caspase3, and cleaved PARP expression in SU-DHL-4. (C) Western blot analysis of p53 and survivin expression in SU-DHL-4. Data are presented as mean±SD. Results shown here are the representative of 3 independent experiments. ** p<0.01, *** p<0.005 vs Control. 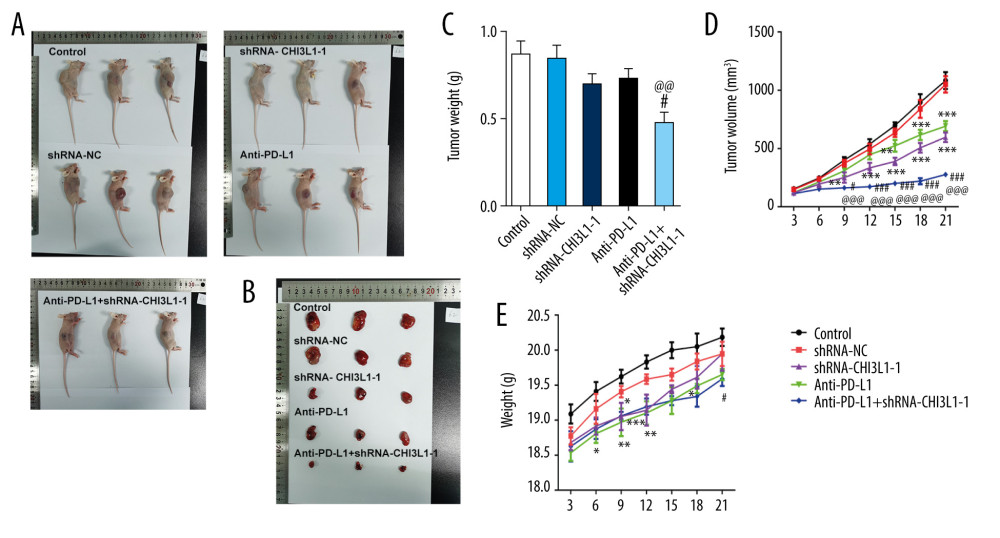 Figure 4. knockdown of CHI3L1 promotes the effect of anti-PD-L1 antibody in the mouse model. (A–C) Representative images, growth, and weight of tumors following subcutaneous injection of the ShRNA-NC, ShRNA-CHI3L1-1, anti-PD-L1, anti-PD-L1+ ShRNA-CHI3L1-1, or control cells. (D, E) tumor volume and weight were measured at 3,5, 7, 9, 11, 13, and 15 days after injection of ShRNA-NC, ShRNA-CHI3L1-1, anti-PD-L1, anti-PD-L1+ ShRNA-CHI3L1-1, or control cells. Data are presented as mean±SD. Results shown here are the representative of 3 independent experiments. * p<0.05, ** p<0.01, *** p<0.005 vs Control; # p<0.05, ### p<0.005 vs shRNA-CHI3L1-1; @@ p<0.01, @@@ p<0.005 vs Anti-PD-L1.
Figure 4. knockdown of CHI3L1 promotes the effect of anti-PD-L1 antibody in the mouse model. (A–C) Representative images, growth, and weight of tumors following subcutaneous injection of the ShRNA-NC, ShRNA-CHI3L1-1, anti-PD-L1, anti-PD-L1+ ShRNA-CHI3L1-1, or control cells. (D, E) tumor volume and weight were measured at 3,5, 7, 9, 11, 13, and 15 days after injection of ShRNA-NC, ShRNA-CHI3L1-1, anti-PD-L1, anti-PD-L1+ ShRNA-CHI3L1-1, or control cells. Data are presented as mean±SD. Results shown here are the representative of 3 independent experiments. * p<0.05, ** p<0.01, *** p<0.005 vs Control; # p<0.05, ### p<0.005 vs shRNA-CHI3L1-1; @@ p<0.01, @@@ p<0.005 vs Anti-PD-L1. 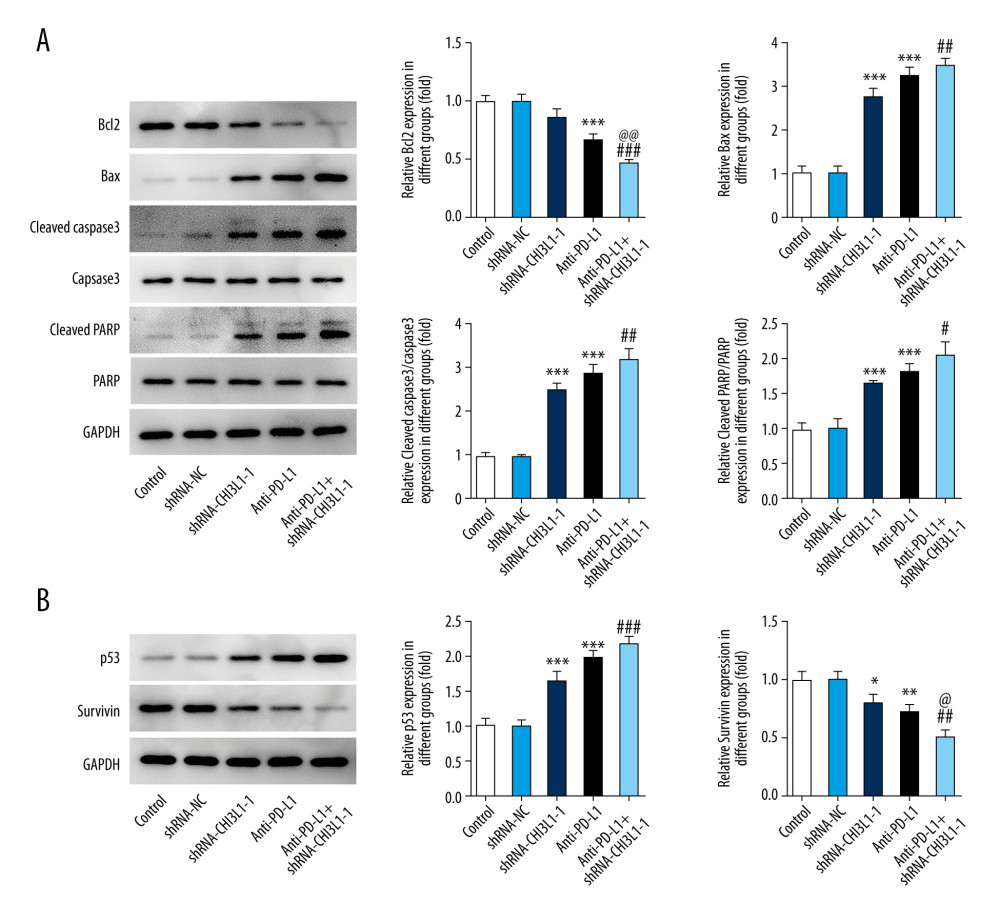 Figure 5. knockdown of CHI3L1 promotes the effect of anti-PD-L1 antibody and cell proliferation and cell cycle arrest in vivo. (A) Western blot analysis of bax, cleaved caspase3, and cleaved PARP expression in vivo. (B) Western blot analysis of p53 and survivin expression in vivo. Data are presented as mean±SD. Results shown here are representative of 3 independent experiments. * p<0.05, ** p<0.01, *** p<0.005 vs Control; # p<0.05, ## p<0.01, ### p<0.005 vs shRNA-CHI3L1-1; @ p<0.05, @@ p<0.01 vs Anti-PD-L1.
Figure 5. knockdown of CHI3L1 promotes the effect of anti-PD-L1 antibody and cell proliferation and cell cycle arrest in vivo. (A) Western blot analysis of bax, cleaved caspase3, and cleaved PARP expression in vivo. (B) Western blot analysis of p53 and survivin expression in vivo. Data are presented as mean±SD. Results shown here are representative of 3 independent experiments. * p<0.05, ** p<0.01, *** p<0.005 vs Control; # p<0.05, ## p<0.01, ### p<0.005 vs shRNA-CHI3L1-1; @ p<0.05, @@ p<0.01 vs Anti-PD-L1. References
1. Goy A, Succeeding in breaking the R-CHOP ceiling in DLBCL: Learning from negative trials: J Clin Oncol, 2017; 35(31); 3519-22
2. Li S, Young KH, Medeiros LJ, Diffuse large B-cell lymphoma: Pathology, 2018; 50(1); 74-87
3. Sehn LH, Gascoyne RD, Diffuse large B-cell lymphoma: Optimizing outcome in the context of clinical and biologic heterogeneity: Blood, 2015; 125(1); 22-32
4. Keane C, Gould C, Jones K, The T-cell receptor repertoire influences the tumor microenvironment and is associated with survival in aggressive B-cell lymphoma: Clin Cancer Res, 2017; 23(7); 1820-28
5. Beielstein AC, Pallasch CP, Tumor metabolism as a regulator of tumor-host interactions in the B-cell lymphoma microenvironment-fueling progression and novel brakes for therapy: Int J Mol Sci, 2019; 20(17); 4158
6. Keir ME, Butte MJ, Freeman GJ, Sharpe AH, PD-1 and its ligands in tolerance and immunity: Annu Rev Immunol, 2008; 26; 677-704
7. Mitteldorf C, Berisha A, Pfaltz MC, Tumor microenvironment and checkpoint molecules in primary cutaneous diffuse large B-cell lymphoma-new therapeutic targets: Am J Surg Pathol, 2017; 41(7); 998-1004
8. Sun C, Mezzadra R, Schumacher TN, Regulation and function of the PD-L1 checkpoint: Immunity, 2018; 48(3); 434-52
9. Zheng Z, Sun R, Zhao HJ, MiR155 sensitized B-lymphoma cells to anti-PD-L1 antibody via PD-1/PD-L1-mediated lymphoma cell interaction with CD8+T cells: Mol Cancer, 2019; 18(1); 54
10. Juárez-Salcedo LM, Sandoval-Sus J, Sokol L, The role of anti-PD-1 and anti-PD-L1 agents in the treatment of diffuse large B-cell lymphoma: The future is now: Crit Rev Oncol Hematol, 2017; 113; 52-62
11. Lee CG, Da Silva CA, Dela Cruz CS, Role of chitin and chitinase/chitinase-like proteins in inflammation, tissue remodeling, and injury: Annu Rev Physiol, 2011; 73; 479-501
12. Xu-Monette ZY, Xiao M, Au Q, Immune profiling and quantitative analysis decipher the clinical role of immune-checkpoint expression in the tumor immune microenvironment of DLBCL: Cancer Immunol Res, 2019; 7(4); 644-57
13. Qiu QC, Wang L, Jin SS, CHI3L1 promotes tumor progression by activating TGF-β signaling pathway in hepatocellular carcinoma: Sci Rep, 2018; 8(1); 15029
14. Geng B, Pan J, Zhao T, Chitinase 3-like 1-CD44 interaction promotes metastasis and epithelial-to-mesenchymal transition through β-catenin/Erk/Akt signaling in gastric cancer: J Exp Clin Cancer Res, 2018; 37(1); 208
15. Sinha M, Rao CR, Premalata CS, Plasma Epstein-Barr virus and hepatitis B virus in non-Hodgkin lymphomas: Two lymphotropic, potentially oncogenic, latently occurring DNA viruses: Indian J Med Paediatr Oncol, 2016; 37(3); 146-51
16. Zhang J, Medeiros LJ, Young KH, Cancer immunotherapy in diffuse large B-cell lymphoma: Front Oncol, 2018; 8; 351
17. Tun HW, Personett D, Baskerville KA, Pathway analysis of primary central nervous system lymphoma: Blood, 2008; 111(6); 3200-10
18. Libreros S, Garcia-Areas R, Iragavarapu-Charyulu V, CHI3L1 plays a role in cancer through enhanced production of pro-inflammatory/pro-tumorigenic and angiogenic factors: Immunol Res, 2013; 57(1–3); 99-105
19. Høgdall EV, Johansen JS, Kjaer SK, High plasma YKL-40 level in patients with ovarian cancer stage III is related to shorter survival: Oncol Rep, 2003; 10(5); 1535-38
20. Johansen JS, Christensen IJ, Riisbro R, High serum YKL-40 levels in patients with primary breast cancer is related to short recurrence free survival: Breast Cancer Res Treat, 2003; 80(1); 15-21
21. Kzhyshkowska J, Yin S, Liu T, Role of chitinase-like proteins in cancer: Biol Chem, 2016; 397(3); 231-47
22. Biggar RJ, Johansen JS, Smedby KE, Serum YKL-40 and interleukin 6 levels in Hodgkin lymphoma: Clin Cancer Res, 2008; 14(21); 6974-78
23. Baar MP, Brandt RMC, Putavet DA, Targeted apoptosis of senescent cells restores tissue homeostasis in response to chemotoxicity and aging: Cell, 2017; 169(1); 132-147.e16
24. Andreu-Fernández V, Sancho M, Genovés A, Bax transmembrane domain interacts with prosurvival Bcl-2 proteins in biological membranes [Erratum in: Proc Natl Acad Sci USA, 2017;114(8):E1574]: Proc Natl Acad Sci USA, 2017; 114(2); 310-15
25. Vousden KH, Lane DP, p53 in health and disease: Nat Rev Mol Cell Biol, 2007; 8(4); 275-83
26. Andersen MH, Svane IM, Becker JC, Straten PT, The universal character of the tumor-associated antigen survivin: Clin Cancer Res, 2007; 13(20); 5991-94
27. Godfrey J, Tumuluru S, Bao R, PD-L1 gene alterations identify a subset of diffuse large B-cell lymphoma harboring a T-cell-inflamed phenotype: Blood, 2019; 133(21); 2279-90
28. Lesokhin AM, Ansell SM, Armand P, Nivolumab in patients with relapsed or refractory hematologic malignancy: Preliminary results of a phase Ib study: J Clin Oncol, 2016; 34(23); 2698-704
29. Passineau MJ, Siegal GP, Everts M, The natural history of a novel, systemic, disseminated model of syngeneic mouse B-cell lymphoma: Leuk Lymphoma, 2005; 46(11); 1627-38
Figures
 Figure 1. CHI3L1 is highly expressed in SU-DHL-4. (A) Western blot analysis of CHI3L1 protein expression in various DLBCL cell lines. (B) RT-PCR analysis of CHI3L1 protein expression in various DLBCL cell lines. Data are presented as mean±SD. Results shown here are the representative of 3 independent experiments. *** p<0.005 vs GM12878.
Figure 1. CHI3L1 is highly expressed in SU-DHL-4. (A) Western blot analysis of CHI3L1 protein expression in various DLBCL cell lines. (B) RT-PCR analysis of CHI3L1 protein expression in various DLBCL cell lines. Data are presented as mean±SD. Results shown here are the representative of 3 independent experiments. *** p<0.005 vs GM12878. Figure 2. Knockdown of CHI3L1 reduces cell proliferation and cell cycle arrest in vitro. (A) Western blot analysis of CHI3L1 protein expression in SU-DHL-4. (B) RT-PCR analysis of CHI3L1 expression in SU-DHL-4. (C) ShRNA-CHI3L1-1 cells, ShRNA-NC cells, and the control cells were seeded into a 96-well plate. Cells were treated with CCK8, and the CCK8 absorbance value was measured 24 h, 48 h, and 72 h after cell seeding. (D) The effects of ShRNA-CHI3L1-1 on SU-DHL-4 cell proliferation were measured by CCK8 assay. (E) Western blot analysis of cyclinD1 and cyclinD2 expression in SU-DHL-4. Data are presented as mean±SD. Results shown here are the representative of 3 independent experiments. ** p<0.01, *** p<0.005 vs Control.
Figure 2. Knockdown of CHI3L1 reduces cell proliferation and cell cycle arrest in vitro. (A) Western blot analysis of CHI3L1 protein expression in SU-DHL-4. (B) RT-PCR analysis of CHI3L1 expression in SU-DHL-4. (C) ShRNA-CHI3L1-1 cells, ShRNA-NC cells, and the control cells were seeded into a 96-well plate. Cells were treated with CCK8, and the CCK8 absorbance value was measured 24 h, 48 h, and 72 h after cell seeding. (D) The effects of ShRNA-CHI3L1-1 on SU-DHL-4 cell proliferation were measured by CCK8 assay. (E) Western blot analysis of cyclinD1 and cyclinD2 expression in SU-DHL-4. Data are presented as mean±SD. Results shown here are the representative of 3 independent experiments. ** p<0.01, *** p<0.005 vs Control. Figure 3. Knockdown of CHI3L1 promotes cell apoptosis and cell survival-related proteins. (A) The effects of ShRNA- CHI3L1-1 on SU-DHL-4 cells apoptosis were measured by flow cytometry. (B) Western blot analysis of bax, cleaved caspase3, and cleaved PARP expression in SU-DHL-4. (C) Western blot analysis of p53 and survivin expression in SU-DHL-4. Data are presented as mean±SD. Results shown here are the representative of 3 independent experiments. ** p<0.01, *** p<0.005 vs Control.
Figure 3. Knockdown of CHI3L1 promotes cell apoptosis and cell survival-related proteins. (A) The effects of ShRNA- CHI3L1-1 on SU-DHL-4 cells apoptosis were measured by flow cytometry. (B) Western blot analysis of bax, cleaved caspase3, and cleaved PARP expression in SU-DHL-4. (C) Western blot analysis of p53 and survivin expression in SU-DHL-4. Data are presented as mean±SD. Results shown here are the representative of 3 independent experiments. ** p<0.01, *** p<0.005 vs Control. Figure 4. knockdown of CHI3L1 promotes the effect of anti-PD-L1 antibody in the mouse model. (A–C) Representative images, growth, and weight of tumors following subcutaneous injection of the ShRNA-NC, ShRNA-CHI3L1-1, anti-PD-L1, anti-PD-L1+ ShRNA-CHI3L1-1, or control cells. (D, E) tumor volume and weight were measured at 3,5, 7, 9, 11, 13, and 15 days after injection of ShRNA-NC, ShRNA-CHI3L1-1, anti-PD-L1, anti-PD-L1+ ShRNA-CHI3L1-1, or control cells. Data are presented as mean±SD. Results shown here are the representative of 3 independent experiments. * p<0.05, ** p<0.01, *** p<0.005 vs Control; # p<0.05, ### p<0.005 vs shRNA-CHI3L1-1; @@ p<0.01, @@@ p<0.005 vs Anti-PD-L1.
Figure 4. knockdown of CHI3L1 promotes the effect of anti-PD-L1 antibody in the mouse model. (A–C) Representative images, growth, and weight of tumors following subcutaneous injection of the ShRNA-NC, ShRNA-CHI3L1-1, anti-PD-L1, anti-PD-L1+ ShRNA-CHI3L1-1, or control cells. (D, E) tumor volume and weight were measured at 3,5, 7, 9, 11, 13, and 15 days after injection of ShRNA-NC, ShRNA-CHI3L1-1, anti-PD-L1, anti-PD-L1+ ShRNA-CHI3L1-1, or control cells. Data are presented as mean±SD. Results shown here are the representative of 3 independent experiments. * p<0.05, ** p<0.01, *** p<0.005 vs Control; # p<0.05, ### p<0.005 vs shRNA-CHI3L1-1; @@ p<0.01, @@@ p<0.005 vs Anti-PD-L1. Figure 5. knockdown of CHI3L1 promotes the effect of anti-PD-L1 antibody and cell proliferation and cell cycle arrest in vivo. (A) Western blot analysis of bax, cleaved caspase3, and cleaved PARP expression in vivo. (B) Western blot analysis of p53 and survivin expression in vivo. Data are presented as mean±SD. Results shown here are representative of 3 independent experiments. * p<0.05, ** p<0.01, *** p<0.005 vs Control; # p<0.05, ## p<0.01, ### p<0.005 vs shRNA-CHI3L1-1; @ p<0.05, @@ p<0.01 vs Anti-PD-L1.
Figure 5. knockdown of CHI3L1 promotes the effect of anti-PD-L1 antibody and cell proliferation and cell cycle arrest in vivo. (A) Western blot analysis of bax, cleaved caspase3, and cleaved PARP expression in vivo. (B) Western blot analysis of p53 and survivin expression in vivo. Data are presented as mean±SD. Results shown here are representative of 3 independent experiments. * p<0.05, ** p<0.01, *** p<0.005 vs Control; # p<0.05, ## p<0.01, ### p<0.005 vs shRNA-CHI3L1-1; @ p<0.05, @@ p<0.01 vs Anti-PD-L1. In Press
06 Mar 2024 : Clinical Research
Comparison of Outcomes between Single-Level and Double-Level Corpectomy in Thoracolumbar Reconstruction: A ...Med Sci Monit In Press; DOI: 10.12659/MSM.943797
21 Mar 2024 : Meta-Analysis
Economic Evaluation of COVID-19 Screening Tests and Surveillance Strategies in Low-Income, Middle-Income, a...Med Sci Monit In Press; DOI: 10.12659/MSM.943863
10 Apr 2024 : Clinical Research
Predicting Acute Cardiovascular Complications in COVID-19: Insights from a Specialized Cardiac Referral Dep...Med Sci Monit In Press; DOI: 10.12659/MSM.942612
06 Mar 2024 : Clinical Research
Enhanced Surgical Outcomes of Popliteal Cyst Excision: A Retrospective Study Comparing Arthroscopic Debride...Med Sci Monit In Press; DOI: 10.12659/MSM.941102
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








