21 April 2021: Animal Study
Short-Term High-Intensity Treadmill Exercise Promotes Ceramide-Dependent Extracellular Vesicle Secretion in the Central Nervous System of Mice
Rui Zhang12ABCEF, Xiaoyan Liang1BC, Shi Tang1BC, Lin Song1CD, Jing Zhang1CF, Yifeng Du1AG*DOI: 10.12659/MSM.929609
Med Sci Monit 2021; 27:e929609
Abstract
BACKGROUND: A lack of physical exercise, a critical aspect of a healthy lifestyle, contributes to several cerebral diseases, such as cognitive impairment, Parkinson disease (PD), and Alzheimer disease (AD). The purpose of the present study was to evaluate the effect of physical exercise on cerebral disease via released extracellular vesicles (EVs).
MATERIAL AND METHODS: Short-term high-intensity treadmill exercise was applied to assess the effect of physical activity on EVs in the serum and brain tissue. Immunofluorescence staining and western blot analysis were used to analyze biomarkers of EVs, including TSG101, HSC70, and CD63. Nanoparticle tracking analysis (NTA) was used to analyze the size and concentration of EVs.
RESULTS: Short-term high-intensity exercise increased the number of neuronal EVs in the brain. In the peripheral blood serum, the level of HSC70 showed a temporary increase after exercise and quickly returned to the normal level, whereas the levels of CD63 and TSG101 showed no obvious change in response to physical exercise. In brain tissue, the levels of HSC70 and TSG101 increased dramatically after exercise, while the level of CD63 remained unchanged. The concentration of EVs was significantly increased after exercise, while the mean diameter of the EVs showed no significant change. The levels of ceramide were significantly increased after exercise, and quickly returned to normal levels.
CONCLUSIONS: These data suggest that the secretion of EVs in the brain and blood is a transitory response to physical exercise and is dependent on ceramide synthesis.
Keywords: Central Nervous System, Ceramides, Exercise, exosomes, Brain Diseases, DNA-Binding Proteins, Endosomal Sorting Complexes Required for Transport, Exercise Test, Extracellular Vesicles, HSC70 Heat-Shock Proteins, Models, Animal, Physical Conditioning, Animal, Tetraspanin 30, Transcription Factors
Background
Physical exercise, especially regular aerobic exercise, is a healthy and non-pharmacological activity that is widely recognized to lower the risk of aging-related diseases and chronic metabolic disorders such as cardiovascular disease, cancer, cognitive impairment, and stroke [1,2]. However, the mechanism by which physical exercise affects physiological and pathological processes still requires in-depth analysis, and understanding the differences in the effects of short-term and high-intensity exercise and those of traditional aerobic exercise on physiological function needs further exploration.
Exercise stimulates the release of a broad range of molecules, including proteins, enzymes, and nucleic acid, participating in the communication between cells [3,4]. Numerous tissues, such as skeletal muscle and brain and adipose tissue, secrete quantities of proteins involved in physical adaptations during exercise [5,6]. Physical exercise exerts positive biological effects in both physiological and pathological conditions. Abundant studies have shown that exercise can ameliorate depression and sleep disturbances in Alzheimer disease (AD) [7]. The mechanism by which exercise contributes to neurodegenerative diseases involves angiogenesis, neurogenesis, synaptic plasticity, and energy metabolism [8]. Extracellular vesicles (EVs) play a central role in communication between cells. EVs are small vesicles containing complex RNAs and proteins ranging in diameter from 40 to 200 nm. EVs are actively secreted from cells after multivesicular body fusion with the plasma membrane. Recently, mild to moderate exercise was demonstrated to alter the miR-31 profile of circulating EVs [9]. The neuro-vascular unit (NVU) corresponds to the assembly of neurons, astrocytes, oligodendrocytes, pericytes, microglia, and extracellular matrix [10]. The NVU has the ability to influence blood-brain barrier (BBB) physiology. Neurons, glia, extracellular matrix, and neurovascular unit biology have been shown to be related to CNS homeostasis. The relationship between EV secretion and the NVU could hasten the link between exercise and CNS regulation [10]. Extracellular vesicles (EVs) have beneficial effects on neurodegenerative diseases [11]. The levels of CD63, a tetraspanin, which is a type of membrane-specific protein, are decreased in aged rats, indicating that CD63 plays a beneficial role in aging and in neurodegenerative diseases [12]. The release of HSC70 into the human circulation is associated with exosomes [13]. Functional depletion of TSG101 attenuates ER stress and perturbs the structure, mobility, and function of the ER, all of which are closely associated with neurodegenerative diseases [14]. Thus, we hypothesized that there is a close relationship between exercise and the role of EVs in the crosstalk between the brain and peripheral blood.
Ceramides, a family of membrane lipids, have been demonstrated to play a role in exosome formation [15,16]. Ceramide also accelerates exosome release from neuronal cells. Neutral sphingomyelinase 2, an enzyme that can produce ceramide from sphingomyelin, increases exosome release [17].
The present study explored the alteration of EVs in the serum and brain tissue during short-term high-intensity exercise. Our data indicate that short-term high-intensity exercise affects the release of EVs through the synthesis of ceramide.
Material and Methods
ANIMALS:
Male C57BL/6 mice were first obtained from the Jackson Laboratory and housed in a room on a 12-h dark/12-h light cycle with a constant temperature (24°C) in the Experimental Animal Centre of Shandong Provincial Hospital. Four-month-old mice (25–30 g) were randomly divided into 2 groups (non-exercise control group and high-intensity exercise group, n=8 per group). All animal experiments were approved by the Animal Care and Utilization Committee of Shandong Provincial Hospital Affiliated with Shandong University (no. 2017-015).
HIGH-INTENSITY TREADMILL PROGRAM:
After exposure to a 10-min adaptive training protocol, the mice in the high-intensity exercise group underwent incremental treadmill exercise for 30 min according to a previously reported protocol [18] (starting velocity of 5 m/min for 2 min followed by 10 m/min for 2 min, 15 m/min for 2 min, and 20 m/min for 24 min). The mice in the non-exercise control group were allowed to freely run on a treadmill without effort, and there was no significant difference in age or weight between the control and high-intensity exercise groups. The mice were sacrificed 0 min or 90 min after the short-term high-intensity treadmill program.
ISOLATION OF EVS FROM PERIPHERAL BLOOD SERUM:
For serum EV isolation, the mouse whiskers were cut off, the eyeballs were removed, and blood was taken from the ophthalmic artery. The blood was then rapidly centrifuged at 3000 rpm for 10 min. A total of 400 μl of serum per mouse was diluted with an equal volume of PBS and centrifuged at 2000×g at 4°C for 30 min. The supernatant then was transferred to ultracentrifuge tubes and centrifuged at 110 000×g at 4°C for 2 h. The pellets were resuspended and centrifuged twice for 70 min at 110 000×g at 4°C. Finally, the pellets were resuspended in 100 μl of PBS and prepared for western blot analysis.
ISOLATION OF EVS FROM BRAIN TISSUES:
After blood collection, the mice were anesthetized with 0.1% pentobarbital sodium and perfused with 0.9% saline through cardiac perfusion. The cerebral hemispheres were removed from the skull. Next, brain EVs were prepared from the brain based on the method reported by Muraoka et al [19] with some modifications. First, the brain was minced and transferred into a 15-ml conical tube with 3.5 ml papain solution in Hibernate A, maintained at 37°C, and then incubated for 20 min. Next, 6.5 ml ice-cold Hibernate A supplemented with protease inhibitors was added. The homogenate was centrifuged at 300×g for 10 min at 4°C. Then, the supernatant was collected, centrifuged at 2000×g for 10 min at 4°C, and transferred into a high-speed centrifugation polycarbonate tube. PBS was added until the volume was 60 ml. Then, the tubes were ultracentrifuged for 30 min at 10 000×g at 4°C using a fixed-angle rotor, and the supernatant was filtered into another polycarbonate tube. The tube was then filled with PBS up to 60 ml and then ultracentrifuged for 70 min at 100 000×g at 4°C. The supernatant was discarded, and the pellet was resuspended and ultracentrifuged for 70 min at 100 000×g at 4°C. The supernatant was discarded again, and the pellet was resuspended, followed by ultracentrifugation at 200 000×g at 4°C for 16 h using a swinging-bucket rotor. The supernatant was discarded, and the pellet was resuspended. Finally, the tubes were ultracentrifuged at 100 000×g at 4°C for 1 h, and the pellets were collected with PBS.
WESTERN BLOT ANALYSIS:
Equal volumes of EV pellets were separated by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and then blotted onto PVDF membranes. The membranes were blocked with 5% milk for 1.5 h and incubated at 4°C overnight with primary antibodies: mouse anti-TSG101 (1: 1000, Abcam), rabbit anti-HSP70 (1: 1000, Abcam), mouse anti-CD63 (1: 1000, Abcam), and mouse anti-β-actin (1: 2000, Zsbio). The membranes were washed 3 times and then incubated with anti-rabbit or anti-mouse IgG antibodies (1: 4000, Zsbio) conjugated with peroxidase at room temperature for 1.5 h. The protein bands were visualized by enhanced chemiluminescence and were scanned using a luminescent image analyzer (GE Healthcare Bio-Sciences AB, Amersham Imager 600).
IMMUNOFLUORESCENCE:
Mice were anesthetized with 0.1% pentobarbital sodium and intracardially perfused with 4% PFA and 0.9% saline. Next, the whole brain was immersed and fixed in 4% paraformaldehyde to prepare for paraffin-embedding [20]. Hippocampal coronal sections (4-um thick) were cut from the paraffin-embedded brain tissue. The sections were deparaffinized, hydrated, and boiled in citrate buffer under high pressure for antigen retrieval. Then, the sections were incubated with the primary antibodies at 4°C overnight and incubated with the secondary antibodies at 37°C for 1 h. The primary antibodies used were mouse anti-TSG101 (1: 200, Abcam), rabbit anti-HSP70 (1: 200, Abcam), and mouse anti-CD63 (1: 200, Abcam). The sections were then incubated with an Alexa Fluor 488-conjugated goat anti-rabbit antibody (1: 1000, Invitrogen) and an Alexa Fluor 594-conjugated goat anti-mouse antibody (1: 1000, Invitrogen). Immunofluorescence was captured using an Olympus DP73 microscope and manually assessed using ImageJ software by a researcher blinded to the identity of groups.
NANOPARTICLE TRACKING ANALYSIS (NTA):
To detect particle size, we performed NTA with a NanoSight 3.2 Dev Build 3.2.16 instrument equipped with NTA 3.2 analytical software. The exosomal suspension was isolated and purified from each group of brain tissues. All samples were diluted with pure water at a 1: 100 dilution and were detected in triplicate. The particle size distribution in a typical experiment was as follows: D10, 58.4 nm; D50, 117.3 nm; and D90, 211.2 nm.
CERAMIDE ANALYSIS:
For ceramide analysis, lipids were extracted from brain tissues using the Folch extraction method with 2: 1 chloroform: methanol, and thin-layer chromatography (TLC) was used to detect ceramide release [21]. After being dried in a nitrogen atmosphere, the lipids extracted from brain tissues were then resolubilized with 1: 1 chloroform: methanol. The TLC plates were loaded for normalization of lipid levels to protein or cholesterol levels as indicated, developed using chloroform: methanol: glacial acetic acid (95: 4.5: 0.5), and visualized by charring with 3% cupric acetate (w/v) in 8% phosphoric acid (v/v).
STATISTICAL ANALYSIS:
The results were graphed in GraphPad Prism and are presented as the mean±SD. The unpaired
Results
SHORT-TERM HIGH-INTENSITY EXERCISE ALTERED LEVEL OF EVS IN THE BRAIN:
Immunofluorescence staining of the brain tissues was used to investigate the effect of short-term exercise on EV release in the hippocampus and their colocalization with Tuj1+ neurons. The expression of HSC70 and TSG101, which are selective exosome markers [21], were detected in neuronal exosomes in this study. Specifically, there were significant differences in the proportions of TSG101+Tuj1+ cells and HSC70+Tuj1+ cells among total Tuj1+ cells in the hippocampus between the non-exercise group and the group assessed immediately after exercise (TSG101: 282.4±98.0 vs 100±9.4, P<0.01; HSC70: 215.4±65.5 vs 100±11.4, P<0.05) (Figure 1). These results suggest that there were more EVs in the hippocampus neurons immediately after exercise than before exercise.
SHORT-TERM HIGH-INTENSITY EXERCISE SIGNIFICANTLY INFLUENCED RELEASE OF SERUM EVS IN C57BL/6 MICE:
To investigate the relationship between high-intensity exercise and the release of EVs in the serum, mice in the exercise group performed incremental treadmill exercise for 30 min. After EVs were purified from peripheral blood serum, western blot was used to analyze EV markers TSG101, CD63, and HSC70 expression (Figure 2A). The protein bands revealed that the levels of HSC70 rapidly increased just after high-intensity exercise (0 min) (144.4±25.0 vs 100±14.4, P<0.01) and quickly returned to the original level after exercise (90 min) (111.5±13.0 vs 100±14.4, P>0.05) (Figure 2D). In contrast, there were no significant differences in the levels of TSG101 or CD63 before and after exercise (TSG101 117.0±17.4 vs 100±12.0; CD63 103.7±14.6 vs 100±27.9; p>0.05) (Figure 2B, 2C).
SHORT-TERM HIGH-INTENSITY EXERCISE INCREASED RELEASE OF EVS IN BRAIN TISSUE OF C57BL/6 MICE:
To investigate the relationship between high-intensity exercise and the release of EVs in the brain, mice in the exercise group performed incremental treadmill exercise for 30 min. We collected brain tissue from mice before exercise, immediately after exercise (0 min), and 90 min after exercise (90 min), and analyzed EV secretion by western blot (Figure 3A–3D). Western blot analysis of EV proteins confirmed that the release of EVs immediately after exercise at 0 min was significantly greater than that before exercise. Moreover, the effect of short-term and high-intensity exercise was more evident in HSC70- and TSG101-positive vesicles (HSC70 124.3±13.3 vs 100±3.5; TSG101 152.7±21.1 vs 100±17.5; P<0.05), whereas there was no significant change in CD63-positive vesicles after exercise (102.8±6.2 vs 100±21.4; P>0.05). Western blot analysis also revealed that the levels of TSG101 and HSC70 almost returned to baseline levels 90 min after exercise (HSC70: 106.7±11.1 vs 100±3.5; TSG101: 123.3±17.7 vs 100±17.5; P>0.05) (Figure 3A–3D).
Furthermore, EV secretion from mice before exercise, immediately after exercise (0 min), and 90 min after exercise (90 min) was further confirmed by NTA. The concentration of EVs (×108 particles/ml) at 0 min was higher than that before exercise (13.2±7.2 vs 2.9±2.1, P<0.01) and had returned to baseline levels at 90 min after exercise (2.2±0.7 vs 2.9±2.1; P>0.05) (Figure 3E), while the mean diameter of EVs showed no significant change before and after exercise (150±42.2 nm vs 148±22.4 nm; P>0.05) (Figure 3F).
SHORT-TERM HIGH-INTENSITY EXERCISE SIGNIFICANTLY INFLUENCED RELEASE OF CERAMIDE IN BRAIN TISSUES OF C57BL/6 MICE:
To determine whether exosome secretion is dependent on ceramide synthesis, we measured ceramide secretion in brain tissues by TLC (Figure 4A). The results showed that the release of ceramide at 0 min after short-term high-intensity exercise was significantly greater than that before exercise (150.4±15.0 vs 100±4.4, P<0.05) and almost returned to baseline levels at 90 min after exercise (122.8±12.2 vs 100±4.4; P>0.05) (Figure 4B).
Discussion
In our study, we delineated the relationship between short-term high-intensity exercise and the secretion of EVs into serum and brain tissue. These interesting findings demonstrate that aerobic exercise increases the release of EVs into peripheral circulation [13,22]. Accordingly, we found that the concentration of EVs immediately after exercise was higher than that found in animals in the non-exercise group.
Physical exercise is well established as a non-pharmacological method to improve human health [22]. Short-term high-intensity exercise, which is characterized by short periods of intense activity, is a highly effective method of enhancing metabolic function and aerobic capacity. Importantly, short-term high-intensity exercise has been shown to be more efficacious than moderate endurance exercise [23]. The purpose of this study was to explore whether there is a change in the release of EVs at 0 min and 90 min after short-term high-intensity exercise. In our study, EVs were generally cleared during the early recovery period within 90 min after exercise, which is consistent with a previous study by Frühbeis et al, who showed that EVs are rapidly released after exercise in humans [13]. In another study, Karine Bertoldi et al showed that exercise has prolonged effects on EVs 18 h after the last exercise session in young adult and aged Wistar rats [12].
Many cells secrete EVs under psychological and pathological conditions [24]. EVs are mainly derived from multivesicular bodies formed by intracellular lysosomal microparticles and are released into the extracellular matrix through fusion of the outer membrane of the multivesicles with the cell membrane. Among 4000 different types of proteins identified from purified exosomes, the most common proteins are tetraspanins (CD63, CD81, and CD9), HSP 70, and TSG101 [24]. We then examined the expression of the EV biomarkers HSC70, TSG101, and CD63 and found that the levels of HSC70 and TSG101 were clearly increased after short-term high-intensity exercise. However, the levels of CD63 responded differently to this exercise procedure. CD63 levels did not show an obvious increase immediately after exercise, which is in contrast to a previous report showing that exercise has transitory and delayed effects on serum CD63 levels in young adult and aged Wistar rats [12]. We hypothesized that the different exercise protocols or sources of EVs may contribute to these differences in results. CD63 is derived from platelets and basophils under physiological conditions and plays a role in the activation of platelets and immune reactions. However, HSC70 and TSG101 both exert an effect on the transport of proteins [25]. Together, our results suggest that short-term high-intensity exercise increases the release of EVs into circulation. Moreover, HSC70-positive EVs are more prevalent immediately after short-term high-intensity exercise. Studies have indicated that HSC70 is a reliable marker of the acute stress response and that its presence in circulation increases in response to exercise [13]. Exercise-induced elevation of cardiac HSC70 levels can induce cardioprotection in rats subjected to ischemia and reperfusion (I/R) injury [26,27]. Internal and external changes in the environment can impact the components and secretion of EVs. In vitro experiments have shown that hypoxia affects the components secreted in EVs, thereby promoting the repair of damaged tissues. Our results provide a new perspective on the mechanism of exercise intervention. EVs in circulation are closely associated with multiple systems during physical activity and may include stem cell-derived vesicles, platelet-derived vesicles, pro-coagulant EVs, and cardiovascular-associated vesicles [21,28-30]. Further studies examining exercise-mediated EV release and EV-mediated signaling pathways may be useful for exploring new avenues for chronic disease treatment and clinical diagnosis [6].
There is an explicit relationship between EVs and neurodegenerative diseases such as AD, Parkinson disease (PD), Huntington disease (HD), multiple sclerosis (MS), and amyotrophic lateral sclerosis (ALS). The release of APP and Aβ, which is associated with AD, is related to exosomes [31–33]. Exosomal surface proteins such as PrPC can sequester aggregates of synapto-toxic Aβ [34,35]. The exosomal alpha-synuclein level in the cerebrospinal fluid may be a useful diagnostic marker for PD. Cerebrospinal fluid exosomes in PD patients contain a-synuclein, a pathogenic species that can cause soluble a-synuclein oligomerization and induce PD symptoms [36]. In an in vitro HD model, exosomes released from adipose-derived stem cells were shown to downregulate the expression of apoptotic proteins and suppress cell apoptosis and mitochondrial dysfunction, thus decreasing the number of HTT aggregates in R6/2 mouse-derived neuronal cells [37]. In MS patients, platelet-derived MVs associated with endothelial-derived MVs can increase endothelial layer permeability, indicating that EVs are involved in BBB disruption [38–40]. Exosome secretion enhances the neuronal clearance ability of pathological TDP-43 [41]. Incubation with the cerebrospinal fluid of ALS-FTD patients increases the number of exosomes in cultured U251 cells at different stages, resulting in TDP-43 aggregate propagation [42].
Different mechanisms are utilized to sort specific molecules into exosomes. The sorting mechanisms include ESCRT-dependent, lipid-dependent, and specific mechanisms involving the loading of RNA into exosomes [43]. As some positive exosome secretion is dependent on ceramide, we measured ceramide secretion in this study and found that the levels of ceramide were clearly increased after short-term high-intensity exercise. Several studies have assessed the role of ceramide in exosome release [15,17]. Inhibition of neutral SMase was shown to decrease exosome secretion [17]. These studies clearly indicate that the production of ceramide through complex sphingolipids hydrolysis stimulates exosome formation and release. Ceramides promote endosome budding by inducing separation of lateral phase and spontaneous curvature of membranes [15]. Based on these data, we propose that short-term high-intensity exercise stimulates the release of EVs from the cell through increasing ceramide synthesis.
The functional ability and integrity of nerves require the interactive exchange of information among motor neurons, neurons, and glial cells [44]. The secretion of EVs into the extracellular environment can result in changes in the physiological or pathological processes of the recipient cells [45]. In addition, EVs also carry biomolecules associated with their source cells, which facilitate their recognition, stimulate signal cascades by interacting with receptors, interact with specific target cells, and participate in intercellular communication [7]. Short-term high-intensity exercise increases the activity of neurons, which can lead to EV secretion or promote the transfer of EVs, thereby elevating the level of EVs in the brain. Several studies suggest that EVs are involved in the mutual signaling between myelin glial cells and neurons to promote neuronal survival, the immune response, synaptic assembly, and plasticity mediated by microglia [46]. The systemic benefits of exercise may be regulated by EVs in an autocrine, paracrine, or endocrine manner. In response to exercise, the concentration of EVs in the circulation may increase. Previous studies also illustrate that EVs are able to cross the BBB [47]. Each cell can produce EVs to communicate between cells across short or long distances. The interactions between EVs and endothelial cells mediated by surface markers allow EVs to cross the BBB, indicating that EVs allow NVU cells to respond to a variety of environmental stimuli [10]. Further understanding of the molecular mechanism underlying the effect of physical exercise may help promote exercise efficiency and provide new methods to treat related chronic diseases.
Conclusions
Our study indicates that short-term high-intensity exercise triggers an increase in the total number of EVs in serum and brain tissue via the synthesis of ceramide. However, as the regulatory activity of elements during EV marker upregulation is uncertain, more light needs to be shed on the specific receptors involved in EV transport as well as the interaction of EVs with target cells or tissues. Further understanding of this response may lead to the development of new strategies for the treatment and diagnosis of diseases affecting multiple systems.
Figures
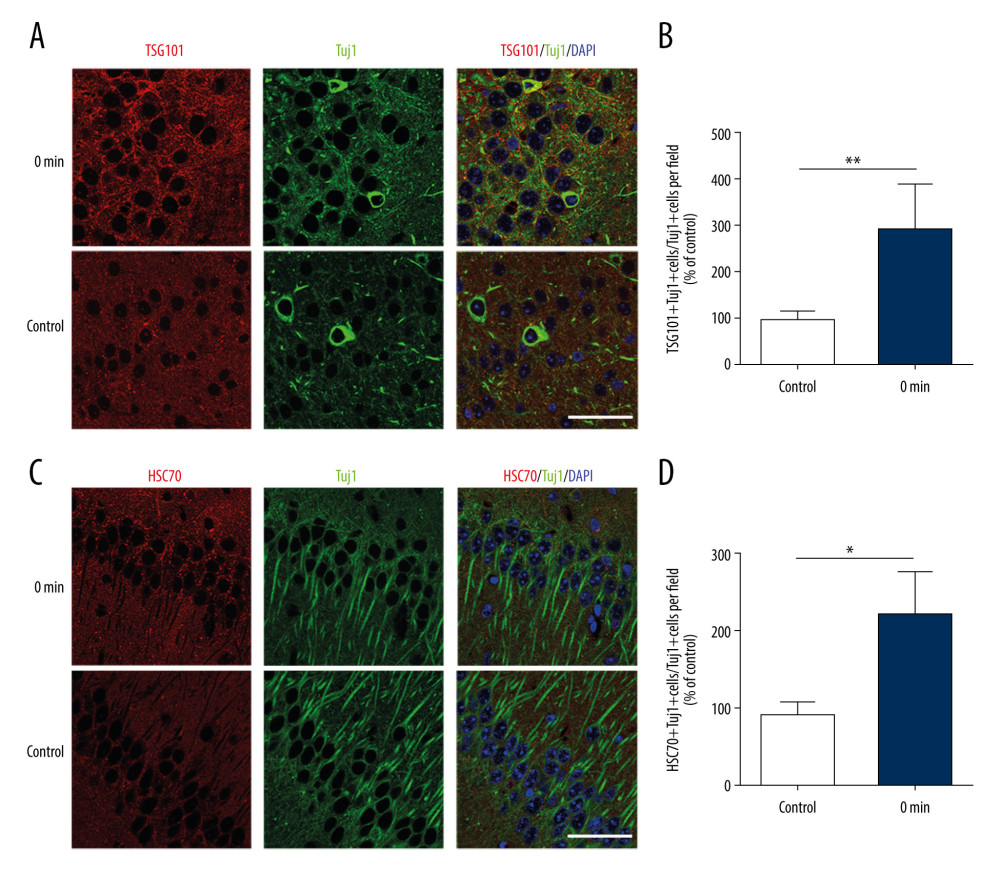 Figure 1. Short-term high-intensity exercise increased the levels of EV markers in the mouse hippocampus. (A) A paraffin section of mouse hippocampus obtained immediately after exercise is co-stained with anti-TSG101 and anti-Tuj1; Scale bar: 50 μm. (B) Mice in the exercise group show higher hippocampal neuron levels of TSG101 compared with the non-exercise group; n=8/group. Data are expressed as the mean±SD; ** p<0.01 using the t test. (C) A paraffin section of the hippocampus from mice after exercise was co-stained using anti-HSC70 and anti-Tuj1; Scale bar: 50 μm. (D) Mice in the exercise group showed higher hippocampal neuron levels of HSC70 compared with the non-exercise group; n=8/group. Data are the mean±SD; * p<0.05 using the t test.
Figure 1. Short-term high-intensity exercise increased the levels of EV markers in the mouse hippocampus. (A) A paraffin section of mouse hippocampus obtained immediately after exercise is co-stained with anti-TSG101 and anti-Tuj1; Scale bar: 50 μm. (B) Mice in the exercise group show higher hippocampal neuron levels of TSG101 compared with the non-exercise group; n=8/group. Data are expressed as the mean±SD; ** p<0.01 using the t test. (C) A paraffin section of the hippocampus from mice after exercise was co-stained using anti-HSC70 and anti-Tuj1; Scale bar: 50 μm. (D) Mice in the exercise group showed higher hippocampal neuron levels of HSC70 compared with the non-exercise group; n=8/group. Data are the mean±SD; * p<0.05 using the t test. 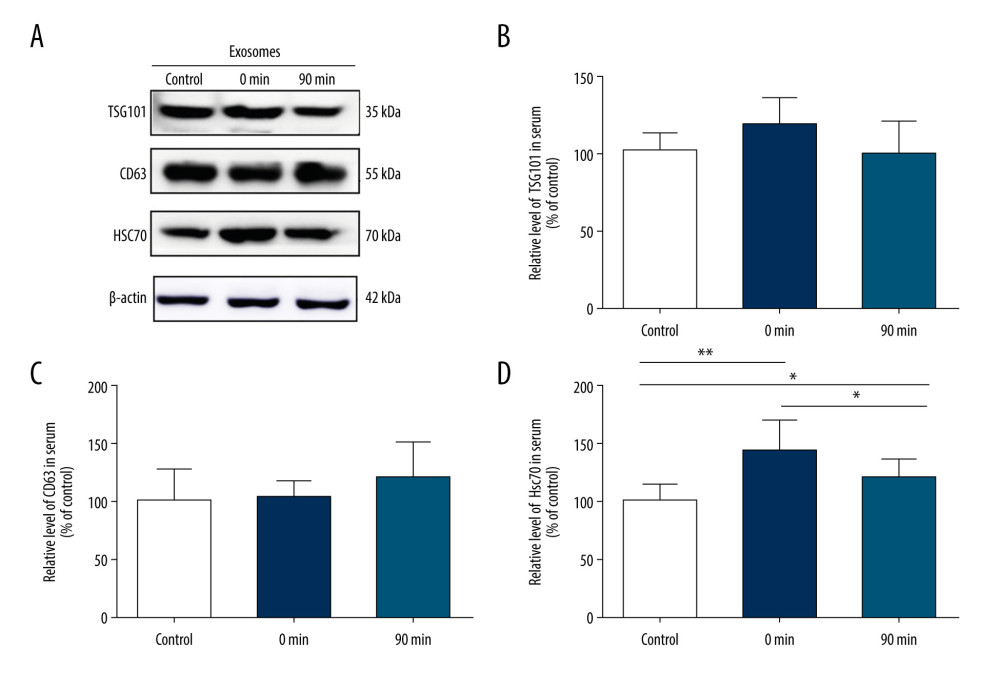 Figure 2. Short-term high-intensity exercise increased the levels of EV biomarkers in the mouse serum. (A) Western blot shows the indicated biomarkers in serum from mice sacrificed before exercise (control), immediately after exercise (0 min) and 90 min after exercise (90 min). (B) The serum level of TSG101 showed no significant increase (P>0.05). (C) The serum level of CD63 showed no apparent changes (P>0.05). (D) The HSC70 level was markedly higher after exercise than before exercise (p<0.01); n=8/group. Data are expressed as the mean±SD; * P<0.05, ** P<0.01 using one-way ANOVA with the Bonferroni post-test.
Figure 2. Short-term high-intensity exercise increased the levels of EV biomarkers in the mouse serum. (A) Western blot shows the indicated biomarkers in serum from mice sacrificed before exercise (control), immediately after exercise (0 min) and 90 min after exercise (90 min). (B) The serum level of TSG101 showed no significant increase (P>0.05). (C) The serum level of CD63 showed no apparent changes (P>0.05). (D) The HSC70 level was markedly higher after exercise than before exercise (p<0.01); n=8/group. Data are expressed as the mean±SD; * P<0.05, ** P<0.01 using one-way ANOVA with the Bonferroni post-test. 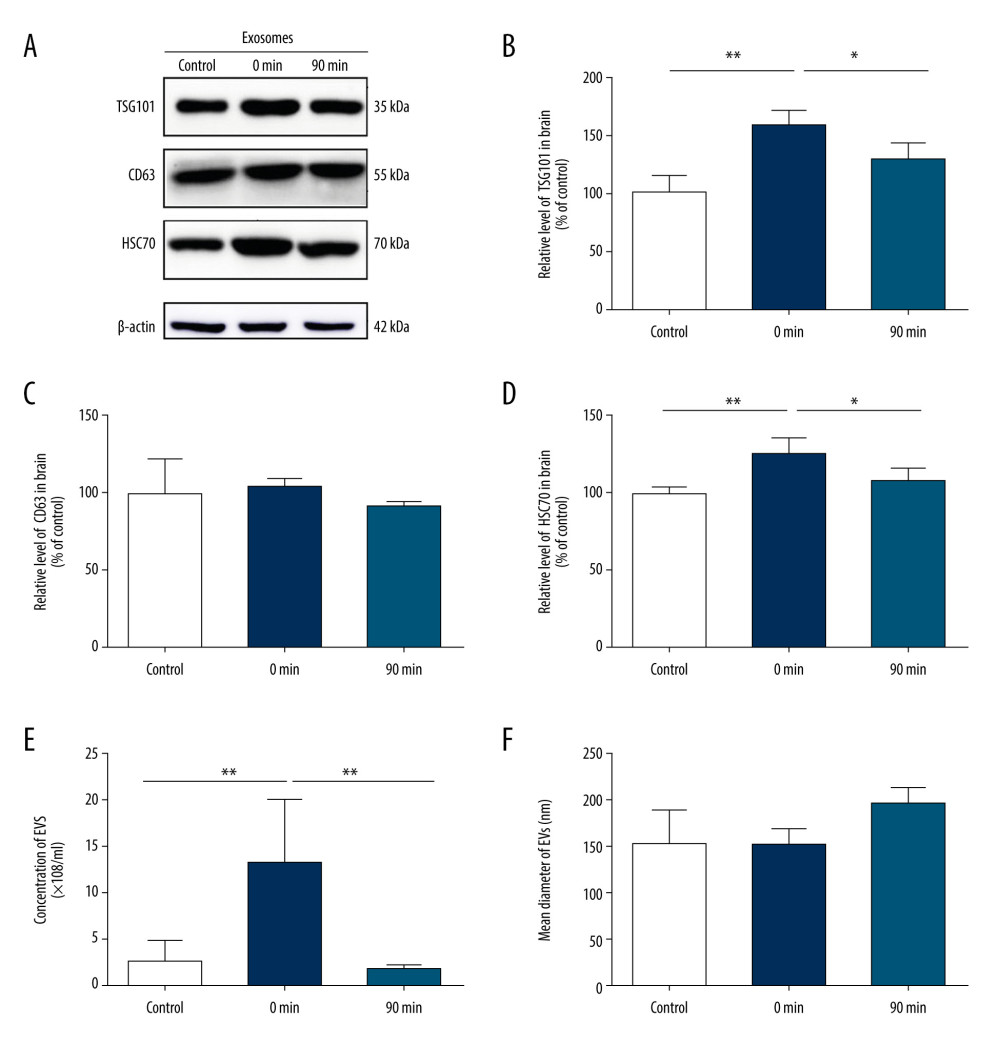 Figure 3. Short-term high-intensity exercise increased the levels of EV biomarkers in the mouse brain. (A) Western blot shows immunoreactivity for TSG101, HSC70 and CD63 from mice sacrificed before exercise (control), immediately after exercise (0 min) and 90 min after exercise (90 min). (B) The TSG101 level showed a significant increase after exercise (p<0.01) and almost returned to baseline within 90 min (P<0.05). (C) The level of CD63 in brain tissue showed no significant changes (P>0.05). (D) The HSC70 level was higher after exercise than before exercise (P<0.01) and almost returned to baseline within 90 min (P<0.05). (E, F) NTA of EVs from the brain tissues of mice sacrificed before exercise (control), immediately after exercise (0 min) and 90 min after exercise (90 min) is shown. The concentration of EVs (E) and the mean diameter of EVs (F) in the brain tissues were determined; n=8/group. Data are expressed as the mean±SD, ** P<0.01 using one-way ANOVA with the Bonferroni post-test.
Figure 3. Short-term high-intensity exercise increased the levels of EV biomarkers in the mouse brain. (A) Western blot shows immunoreactivity for TSG101, HSC70 and CD63 from mice sacrificed before exercise (control), immediately after exercise (0 min) and 90 min after exercise (90 min). (B) The TSG101 level showed a significant increase after exercise (p<0.01) and almost returned to baseline within 90 min (P<0.05). (C) The level of CD63 in brain tissue showed no significant changes (P>0.05). (D) The HSC70 level was higher after exercise than before exercise (P<0.01) and almost returned to baseline within 90 min (P<0.05). (E, F) NTA of EVs from the brain tissues of mice sacrificed before exercise (control), immediately after exercise (0 min) and 90 min after exercise (90 min) is shown. The concentration of EVs (E) and the mean diameter of EVs (F) in the brain tissues were determined; n=8/group. Data are expressed as the mean±SD, ** P<0.01 using one-way ANOVA with the Bonferroni post-test. 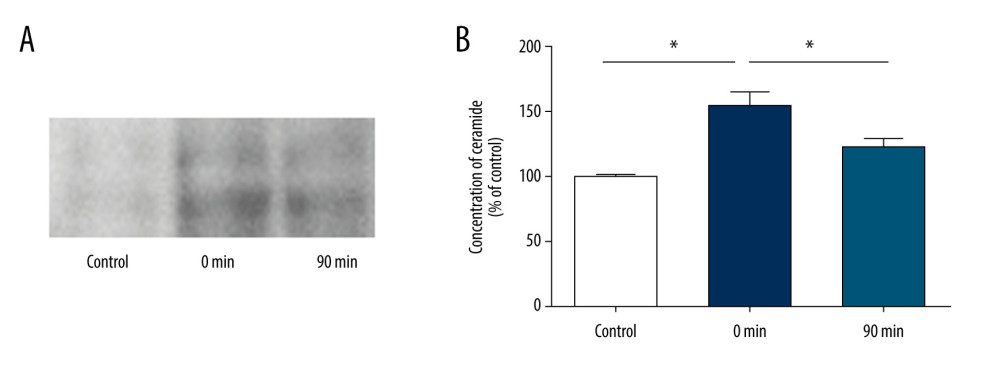 Figure 4. Short-term high-intensity exercise increase ceramide levels in the mouse brain. (A) Ceramide levels in the brain tissues of mice sacrificed before exercise (control), immediately after exercise (0 min) and 90 min after exercise (90 min) are shown. Lipids were isolated and separated by TLC. (B) Quantification of ceramide levels as shown in A. n=8/group. Data are expressed as the mean±SD, * P<0.05 using one-way ANOVA with the Bonferroni post-test.
Figure 4. Short-term high-intensity exercise increase ceramide levels in the mouse brain. (A) Ceramide levels in the brain tissues of mice sacrificed before exercise (control), immediately after exercise (0 min) and 90 min after exercise (90 min) are shown. Lipids were isolated and separated by TLC. (B) Quantification of ceramide levels as shown in A. n=8/group. Data are expressed as the mean±SD, * P<0.05 using one-way ANOVA with the Bonferroni post-test. References
1. Zafonte RD, Shih SL, Iaccarino MA, Tan CO, Neurologic benefits of sports and exercise: Hand Clin Neurol, 2018; 158; 463-71
2. Halabchi F, Alizadeh Z, Sahraian MA, Abolhasani M, Exercise prescription for patients with multiple sclerosis; potential benefits and practical recommendations: BMC Neurol, 2017; 17; 185
3. Brown CD, Muniz M, Kauvar DS, Response of the popliteal artery to treadmill exercise and stress positioning in patients with and without exertional lower extremity symptoms: J Vasc Surg, 2019; 69; 1545-51
4. Hammeren J, Powers S, Lawler J, Exercise training-induced alterations in skeletal muscle oxidative and antioxidant enzyme activity in senescent rats: Int J Sports Med, 1992; 13; 412-16
5. Keller S, Sanderson MP, Stoeck A, Exosomes: from biogenesis and secretion to biological function: Immunol Lett, 2006; 107; 102-8
6. Whitham M, Parker BL, Friedrichsen M, Extracellular vesicles provide a means for tissue crosstalk during exercise: Cell Metab, 2018; 27; 237-51.e4
7. Veronese N, Solmi M, Basso C, Role of physical activity in ameliorating neuropsychiatric symptoms in Alzheimer disease: A narrative review: Int J Geriatr Psychiatry, 2019; 34; 1316-25
8. Tari AR, Norevik CS, Scrimgeour NR, Are the neuroprotective effects of exercise training systemically mediated?: Prog Cardiovasc Dis, 2019; 62; 94-101
9. Lovett JAC, Durcan PJ, Myburgh KH, Investigation of circulating extracellular vesicle microRNA following two consecutive bouts of muscle-damaging exercise: Front Physiol, 2018; 9; 1149
10. Saint-Pol J, Gosselet F, Duban-Deweer S, Targeting and crossing the blood–brain barrier with extracellular vesicles: Cells, 2020; 9; 851
11. Croese T, Furlan R, Extracellular vesicles in neurodegenerative diseases: Mol Aspects Med, 2018; 60; 52-61
12. Bertoldi K, Cechinel LR, Schallenberger B, Circulating extracellular vesicles in the aging process: impact of aerobic exercise: Mol Cell Biochem, 2018; 440; 115-25
13. Frühbeis C, Helmig S, Tug S, Physical exercise induces rapid release of small extracellular vesicles into the circulation: J Extracell Vesicles, 2015; 4; 28239
14. Kaul Z, Mookherjee D, Das S, Loss of tumor susceptibility gene 101 (TSG101) perturbs endoplasmic reticulum structure and function: Biochim Biophys Acta Mol Cell Res, 2020; 1867; 118741
15. Yuyama K, Takahashi K, Usuki S, Plant sphingolipids promote extracellular vesicle release and alleviate amyloid-β pathologies in a mouse model of Alzheimer’s disease: Sci Rep, 2019; 9; 16827
16. Trajkovic K, Hsu C, Chiantia S, Ceramide triggers budding of exosome vesicles into multivesicular endosomes: Science, 2008; 319; 1244-47
17. Yuyama K, Sun H, Mitsutake S, Sphingolipid-modulated exosome secretion promotes clearance of amyloid-β by microglia: J Biol Chem, 2012; 287; 10977-89
18. Baker EJ, Gleeson TT, The effects of intensity on the energetics of brief locomotor activity: The J Exp Biol, 1999; 202; 3081-87
19. Pérez-González R, Gauthier SA, Kumar A, A method for isolation of extracellular vesicles and characterization of exosomes from brain extracellular space: Methods Mol Biol, 2017; 1545; 139-51
20. Folch J, Lees M, Sloane SGH, A simple method for the isolation and purification of total lipides from animal tissues: J Biol Chem, 1957; 226; 497-509
21. Kalluri R, LeBleu VS, The biology function and biomedical applications of exosomes: Science, 2020; 367; eaau6977
22. Oliveira GP, Porto WF, Palu CC, Effects of acute aerobic exercise on rats serum extracellular vesicles diameter, concentration and small RNAs content: Front Physiol, 2018; 9; 532
23. Burgomaster KA, Heigenhauser GJ, Gibala MJ, Effect of short-term sprint interval training on human skeletal muscle carbohydrate metabolism during exercise and time-trial performance: J Appl Physiol (1985), 2006; 100; 2041-47
24. Wu Q, Sun S, Li Z, Breast cancer-released exosomes trigger cancer-associated cachexia to promote tumor progression: Adipocyte, 2019; 8; 31-45
25. Hurwitz SN, Cheerathodi MR, Nkosi D, Tetraspanin CD63 bridges autophagic and endosomal processes to regulate exosomal secretion and intracellular signaling of Epstein-Barr virus LMP1: J Virol, 2018; 92; 1969-71
26. Li Y, Han C, Wang J, Exosomes mediate the beneficial effects of exercise: Adv Exp Med Biol, 2017; 1000; 333-53
27. Esposito F, Ronchi R, Milano G, Myocardial tolerance to ischemia-reperfusion injury, training intensity and cessation: Eur J Applied Physiol, 2011; 111; 859-68
28. Ferreira PM, Bozbas E, Tannetta SD, Mode of induction of platelet-derived extracellular vesicles is a critical determinant of their phenotype and function: Sci Rep, 2020; 10; 18061
29. Plasín-Rodríguez MA, Patricio P, Monteagudo J, Procoagulant microparticles are associated with arterial disease in patients with systemic lupus erythematosus: J Thromb Thrombolysis, 2020 [Online ahead of print]
30. Osman A, Benameur T, Korashy HM, Interplay between endoplasmic reticulum stress and large extracellular vesicles (microparticles) in endothelial cell dysfunction: Biomedicines, 2020; 8; 409
31. Perez-Gonzalez R, Gauthier SA, Kumar A, The exosome secretory pathway transports amyloid precursor protein carboxyl-terminal fragments from the cell into the brain extracellular space: J Biol Chem, 2012; 287; 43108-15
32. Rajendran L, Honsho M, Zahn TR, Alzheimer’s disease beta-amyloid peptides are released in association with exosomes: Proc Natl Acad Sci USA, 2006; 103; 11172-77
33. Vingtdeux V, Hamdane M, Loyens A, Alkalizing drugs induce accumulation of amyloid precursor protein by-products in luminal vesicles of multivesicular bodies: J Biol Chem, 2007; 282; 18197-205
34. An K, Klyubin I, Kim Y, Exosomes neutralize synaptic-plasticity-disrupting activity of Aβ assemblies in vivo: Mol Brain, 2013; 6; 47
35. Yuyama K, Sun H, Usuki S, A potential function for neuronal exosomes: sequestering intracerebral amyloid-β peptide: FEBS Lett, 2015; 589; 84-88
36. Stuendl A, Kunadt M, Kruse N, Induction of α-synuclein aggregate formation by CSF exosomes from patients with Parkinson’s disease and dementia with Lewy bodies: Brain, 2016; 139; 481-94
37. Lee ST, Im W, Ban JJ, Exosome-based delivery of miR-124 in a Huntington’s disease model: J Mov Disord, 2017; 10; 45-52
38. Alexander JS, Chervenak R, Weinstock-Guttman B, Blood circulating microparticle species in relapsing-remitting and secondary progressive multiple sclerosis. A case-control, cross sectional study with conventional MRI and advanced iron content imaging outcomes: J Neurol Sci, 2015; 355; 84-89
39. Marcos-Ramiro B, Oliva Nacarino P, Serrano-Pertierra E, Microparticles in multiple sclerosis and clinically isolated syndrome: effect on endothelial barrier function: BMC Neurosci, 2014; 15; 110
40. Colombo E, Borgiani B, Verderio C, Microvesicles: Novel biomarkers for neurological disorders: Front Physiol, 2012; 3; 63
41. Iguchi Y, Eid L, Parent M, Exosome secretion is a key pathway for clearance of pathological TDP-43: Brain, 2016; 139; 3187-201
42. Ding X, Ma M, Teng J, Exposure to ALS-FTD-CSF generates TDP-43 aggregates in glioblastoma cells through exosomes and TNTs-like structure: Oncotarget, 2015; 6; 24178-91
43. Record M, Carayon K, Poirot M, Exosomes as new vesicular lipid transporters involved in cell-cell communication and various pathophysiologies: Biochim Biophys Acta, 2014; 1841; 108-20
44. Shin MS, Park HK, Kim TW, Neuroprotective effects of bone marrow stromal cell transplantation in combination with treadmill exercise following traumatic brain injury: Int Neurourol J, 2016; 20; S49-56
45. Sim YJ, Treadmill exercise alleviates impairment of spatial learning ability through enhancing cell proliferation in the streptozotocin-induced Alzheimer’s disease rats: J Exerc Rehabil, 2014; 10; 81-88
46. Li H, Luo Y, Zhu L, Glia-derived exosomes: Promising therapeutic targets: Life Sci, 2019; 239; 116951
47. Lakhal S, Wood MJ, Exosome nanotechnology: an emerging paradigm shift in drug delivery: exploitation of exosome nanovesicles for systemic in vivo delivery of RNAi heralds new horizons for drug delivery across biological barriers: BioEssays, 2011; 33; 737-41
Figures
 Figure 1. Short-term high-intensity exercise increased the levels of EV markers in the mouse hippocampus. (A) A paraffin section of mouse hippocampus obtained immediately after exercise is co-stained with anti-TSG101 and anti-Tuj1; Scale bar: 50 μm. (B) Mice in the exercise group show higher hippocampal neuron levels of TSG101 compared with the non-exercise group; n=8/group. Data are expressed as the mean±SD; ** p<0.01 using the t test. (C) A paraffin section of the hippocampus from mice after exercise was co-stained using anti-HSC70 and anti-Tuj1; Scale bar: 50 μm. (D) Mice in the exercise group showed higher hippocampal neuron levels of HSC70 compared with the non-exercise group; n=8/group. Data are the mean±SD; * p<0.05 using the t test.
Figure 1. Short-term high-intensity exercise increased the levels of EV markers in the mouse hippocampus. (A) A paraffin section of mouse hippocampus obtained immediately after exercise is co-stained with anti-TSG101 and anti-Tuj1; Scale bar: 50 μm. (B) Mice in the exercise group show higher hippocampal neuron levels of TSG101 compared with the non-exercise group; n=8/group. Data are expressed as the mean±SD; ** p<0.01 using the t test. (C) A paraffin section of the hippocampus from mice after exercise was co-stained using anti-HSC70 and anti-Tuj1; Scale bar: 50 μm. (D) Mice in the exercise group showed higher hippocampal neuron levels of HSC70 compared with the non-exercise group; n=8/group. Data are the mean±SD; * p<0.05 using the t test. Figure 2. Short-term high-intensity exercise increased the levels of EV biomarkers in the mouse serum. (A) Western blot shows the indicated biomarkers in serum from mice sacrificed before exercise (control), immediately after exercise (0 min) and 90 min after exercise (90 min). (B) The serum level of TSG101 showed no significant increase (P>0.05). (C) The serum level of CD63 showed no apparent changes (P>0.05). (D) The HSC70 level was markedly higher after exercise than before exercise (p<0.01); n=8/group. Data are expressed as the mean±SD; * P<0.05, ** P<0.01 using one-way ANOVA with the Bonferroni post-test.
Figure 2. Short-term high-intensity exercise increased the levels of EV biomarkers in the mouse serum. (A) Western blot shows the indicated biomarkers in serum from mice sacrificed before exercise (control), immediately after exercise (0 min) and 90 min after exercise (90 min). (B) The serum level of TSG101 showed no significant increase (P>0.05). (C) The serum level of CD63 showed no apparent changes (P>0.05). (D) The HSC70 level was markedly higher after exercise than before exercise (p<0.01); n=8/group. Data are expressed as the mean±SD; * P<0.05, ** P<0.01 using one-way ANOVA with the Bonferroni post-test. Figure 3. Short-term high-intensity exercise increased the levels of EV biomarkers in the mouse brain. (A) Western blot shows immunoreactivity for TSG101, HSC70 and CD63 from mice sacrificed before exercise (control), immediately after exercise (0 min) and 90 min after exercise (90 min). (B) The TSG101 level showed a significant increase after exercise (p<0.01) and almost returned to baseline within 90 min (P<0.05). (C) The level of CD63 in brain tissue showed no significant changes (P>0.05). (D) The HSC70 level was higher after exercise than before exercise (P<0.01) and almost returned to baseline within 90 min (P<0.05). (E, F) NTA of EVs from the brain tissues of mice sacrificed before exercise (control), immediately after exercise (0 min) and 90 min after exercise (90 min) is shown. The concentration of EVs (E) and the mean diameter of EVs (F) in the brain tissues were determined; n=8/group. Data are expressed as the mean±SD, ** P<0.01 using one-way ANOVA with the Bonferroni post-test.
Figure 3. Short-term high-intensity exercise increased the levels of EV biomarkers in the mouse brain. (A) Western blot shows immunoreactivity for TSG101, HSC70 and CD63 from mice sacrificed before exercise (control), immediately after exercise (0 min) and 90 min after exercise (90 min). (B) The TSG101 level showed a significant increase after exercise (p<0.01) and almost returned to baseline within 90 min (P<0.05). (C) The level of CD63 in brain tissue showed no significant changes (P>0.05). (D) The HSC70 level was higher after exercise than before exercise (P<0.01) and almost returned to baseline within 90 min (P<0.05). (E, F) NTA of EVs from the brain tissues of mice sacrificed before exercise (control), immediately after exercise (0 min) and 90 min after exercise (90 min) is shown. The concentration of EVs (E) and the mean diameter of EVs (F) in the brain tissues were determined; n=8/group. Data are expressed as the mean±SD, ** P<0.01 using one-way ANOVA with the Bonferroni post-test. Figure 4. Short-term high-intensity exercise increase ceramide levels in the mouse brain. (A) Ceramide levels in the brain tissues of mice sacrificed before exercise (control), immediately after exercise (0 min) and 90 min after exercise (90 min) are shown. Lipids were isolated and separated by TLC. (B) Quantification of ceramide levels as shown in A. n=8/group. Data are expressed as the mean±SD, * P<0.05 using one-way ANOVA with the Bonferroni post-test.
Figure 4. Short-term high-intensity exercise increase ceramide levels in the mouse brain. (A) Ceramide levels in the brain tissues of mice sacrificed before exercise (control), immediately after exercise (0 min) and 90 min after exercise (90 min) are shown. Lipids were isolated and separated by TLC. (B) Quantification of ceramide levels as shown in A. n=8/group. Data are expressed as the mean±SD, * P<0.05 using one-way ANOVA with the Bonferroni post-test. In Press
05 Mar 2024 : Clinical Research
Role of Critical Shoulder Angle in Degenerative Type Rotator Cuff Tears: A Turkish Cohort StudyMed Sci Monit In Press; DOI: 10.12659/MSM.943703
06 Mar 2024 : Clinical Research
Comparison of Outcomes between Single-Level and Double-Level Corpectomy in Thoracolumbar Reconstruction: A ...Med Sci Monit In Press; DOI: 10.12659/MSM.943797
21 Mar 2024 : Meta-Analysis
Economic Evaluation of COVID-19 Screening Tests and Surveillance Strategies in Low-Income, Middle-Income, a...Med Sci Monit In Press; DOI: 10.12659/MSM.943863
10 Apr 2024 : Clinical Research
Predicting Acute Cardiovascular Complications in COVID-19: Insights from a Specialized Cardiac Referral Dep...Med Sci Monit In Press; DOI: 10.12659/MSM.942612
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








