01 March 2021: Human Study
Role of Calcium/Creatinine Ratio in Urine Compared with Proteinuria and Uric Acid in Predicting Preeclampsia: A Study from Kosovo
Lindita Ibrahimi1ABCDEF, Myrvete Paçarada1BCD, Syheda Latifi Hoxha1CD, Astrit Bimbashi2CD, Vlora Ademi Ibishi1CDF*DOI: 10.12659/MSMBR.929845
Med Sci Monit Basic Res 2021; 27:e929845
Abstract
BACKGROUND: Preeclampsia is a common complication of pregnancy and a major cause of morbidity and mortality of mothers and babies worldwide. This study aimed to explore what the role of calcium/creatinine ratio is in urine compared with proteinuria and uric acid in predicting preeclampsia.
MATERIAL AND METHODS: In this prospective case-control study, 200 pregnant women who participated in the study were consecutively divided into 3 groups: a group of 59 women with preeclampsia, 61 women with pregnancy-induced hypertension, and a control group of 80 normotensive pregnant women. A 24-h urine sample was collected for estimation of calcium/creatinine ratio and proteinuria and a blood sample for estimation of uric acid at a gestational age of 24-34 weeks of pregnancy.
RESULTS: The study found that the sensitivity of proteinuria as a predictor of preeclampsia was 96.6% (P=0.000) and specificity was 21.3%. The sensitivity of uric acid as a predictor was 96.6% (P=0.000) and the specificity was 48.8%; whereas for the 24-h urine calcium/creatinine ratio, the sensitivity was 87.9% (P=0.000) and the specificity 40.7%, which corresponds to a value of 0.105 (cutoff). Women with a calcium/creatinine ratio <0.105 have a higher risk of developing preeclampsia (87.9% confidence interval, P=0.000).
CONCLUSIONS: The role of the calcium/creatinine ratio in urine is inferior to proteinuria and uric acid in predicting preeclampsia.
Keywords: Pre-Eclampsia, Proteinuria, Uric Acid, Calcium, Case-Control Studies, Creatinine, Female, Humans, Infant, Kosovo, Pregnancy, Sensitivity and Specificity
Background
Preeclampsia is a common complication of pregnancy and a major cause of morbidity and mortality of mothers and babies worldwide [1]. Studying normal and complicated pregnancies with preeclampsia provides important, useful information to find a potential predictor of preeclampsia. Identification of this clinical entity and effective management play an important role in the outcome of pregnancy for both mother and baby. In developing countries, in pregnancies with insufficient care, preeclampsia persists and many cases remain undiagnosed until major complications occur. Predictor identification would help provide closer case surveillance and reduce the negative consequences of preeclampsia.
Although the exact cause of preeclampsia remains unclear, many theories indicate that the disease starts at placental implantation and the level of trophoblastic invasion [2,3]. One of the most stringent pathophysiological changes is the strong systemic vasospasm, which is responsible for the diminished perfusion of almost all organ systems [4]. Perfusion also decreases because of vascular hemoconcentration and fluid discharge from the intravascular space. Also, preeclampsia is associated with an inflammatory response and inadequate activation of the endothelium [2]. Extensive changes are seen in the renal system in preeclampsia. As a part of the end-organ pathology, preeclamptic glomeruli undergo structural changes with pronounced endothelial vacuolization and hypertrophy of the cytoplasmic organelles, first defined as glomerular endothelins. Activation of the coagulation cascade and the formation of microthrombi result in further compromise of blood flow to organs [4].
Creatinine clearance is an indicator of kidney damage in pregnancy with hypertension. The lower the creatinine clearance, the more severe the kidney disease. This is parallel to the decrease of urinary calcium in preeclampsia, even before the clinical signs and symptoms appear. Although the precise reason for this phenomenon is not clear, it has been speculated that renal changes in preeclampsia are the basis for using urinary calcium/creatinine ratio as a screening test [5].
Renal changes in preeclampsia may be caused by severe internal vasospasm and this may cause glomerular filtration rate decline in some cases, leading to hypocalcemia [6]. Proteinuria occurs because of glomerular renal endothelins, a manifestation of widespread endothelial damage [7,8]. Many diagnostic studies have determined that there is a link between increasing proteinuria levels and maternal and fetal complications from preeclampsia [9–11].
Prospective randomized studies are suggesting that uric acid inhibits trophoblastic invasion in the muscular layer of the coil arteries [12] and inhibits the intake of amino acids from the placental system [13]. Also, uric acid can be directly involved in preeclampsia pathogenesis by promoting inflammation, oxidative stress, and endothelial dysfunction [14,15]. Therefore, uric acid in the blood during pregnancy may not only be a biomarker for preeclampsia but may also have a contributing role in the pathogenesis of maternal and fetal manifestations [3,11].
The aim of the present study was to determine the predictive value of the calcium/creatinine ratio concerning proteinuria and uric acid in the 24-h urine sample in the prediction of preeclampsia in pregnant women. Another goal of this study is developing a test that will identify pregnant women likely to develop hypertensive diseases in pregnancy such as preeclampsia, and to determine the seriousness and the progression of the disease to be able to provide adequate treatment before the disease becomes serious. This test should be easy to apply at a reasonable cost.
Material and Methods
INCLUSION CRITERIA:
Case group: Groups with preeclampsia and PIH between 24 and 34 weeks of pregnancy formed the case group. From this group we excluded multiple pregnancies and pregnancies resulting from artificial reproductive technology.
We excluded pregnancies resulting from in vitro fertilization because neonatal outcomes are generally different. Neonates of women whose pregnancies were complicated by hypertension diseases had a higher birth weight and longer gestational age, whereas high rates of intrauterine growth restriction (5.9%) and preterm births (6.0%) occurred among pregnancies resulting from in vitro fertilization [16].
Control group: In this group were normotensive pregnant women between 24 and 34 weeks of gestation without any of the risk factors for preeclampsia such as prima gravida, preeclampsia in previous births, multiple pregnancies, family history of preeclampsia (mothers, sisters), being with a partner less than 1 year, body mass index more than 35, use of any anti-eclampsia drug, or are on any diuretic.
From both groups we excluded patients with hypertension before pregnancy, patients with diabetes and other endocrine diseases, a history suggesting thrombophilia or coagulopathy, urinary tract infection, patients with renal disease, and other chronic diseases.
All women were clinically evaluated to eliminate exclusion criteria and then underwent routine laboratory testing and clinical examination such as history (name, surname, age, family history, history of other diseases), general examination for edema (especially in the face and upper extremities), nausea, vomiting, headache, visual disturbances, upper right quadrant pain, convulsions, blood pressure >140/90 mmHg or a sudden 30-mmHg increase in systolic or 15-mmHg diastolic blood pressure (measured twice in 6 h) associated with both edema and proteinuria or only one of them; 24-h urine samples collected for calcium, creatinine, and protein evaluation; hematologic effects: hematocrit, hemoglobin, number of platelets; kidney function tests: uric acid and creatinine in serum; liver function tests: serum transaminases: aspartate transaminase, alanine transaminase, and lactate dehydrogenase; mode of delivery, gestational age, newborn weight, Apgar score at the first and fifth minute, and eventual fetal and maternal complications.
Proteinuria, urinary calcium, and creatinine were measured from a 24-h carefully collected urine sample and the calcium/creatinine ratio was determined. The calcium/creatinine ratio was used in this study to compare whether it was a practical test compared with proteinuria and uric acid in predicting preeclampsia.
ETHICAL CONSIDERATION:
The study protocol was reviewed and approved by the Ethics Committee of the Medical Faculty of the University of Pristina (ref. number: 10389 dt. 09/12/2014), and informed consent was obtained from all patients.
ANALYTICAL METHODS:
The experiment was done over approximately 8 months and was organized at the PROLAB Diagnostic Laboratory Center in Pristina and at the laboratory of the Biochemistry Clinic at the University Clinical Center of Kosovo. The ILAB ARIES Biochemical Random Discrete Automatic Access Analyzer from Instrumentation Laboratory (Bedford, MA, USA) was used.
Under aseptic conditions, 7 mL of venous blood were collected, with 5 mL placed in a simple sterile bottle and 2 mL in an oxalate bottle. Urine samples (24-h) were collected from the test group and brought to the laboratory. For the determination of urine calcification, the sample was acidified to pH 2 to dissolve calcium oxalate crystals in urine. An unidentified sample was used for creatinine and protein determination.
Urine protein determination was performed using the reagent from Analyticon® Biotechnologies AG (Lichtenfels, Germany) and Fluitest U/CSF ULTRASENSITIVE PROTEIN. The calibrators and controls are from BIOCAL. Low-level and high-level (abnormal) urine control sets were used.
Creatinine determination in urine was performed using Instrumentation Laboratory creatinine Jaffe reagent. Urine calcium was determined by calcium arsenazo (Instrumentation Laboratory). The calibrators and controls for creatine and calcium were also from Instrumentation Laboratory. The ReferIL G multiparameter calibrator was used for the calibrators, whereas the SeraCHEM level 1 and SeraCHEM level 2 products were used for the controls.
For all tests, calibration was performed once per series and in triplicate; the control was tested at least daily or as needed.
STATISTICAL ANALYSIS:
Statistical programs Stat Soft STATISTICA 10.0 and SPSS 20.0 were used for the statistical analysis; collected data were processed using the following statistical methods. A database was formed by using specific computer software and was processed by using standard descriptive and analytical methods. Statistical data were analyzed by determination of rates and percents and statistical significance between detected differences (difference test). Quantitative data were analyzed by measuring the central tendency and probability distribution (mean and standard deviation). In numerical series with 2 variables, the significance of the difference was tested by the
Results
The subjects of the study were divided into 3 groups (Table 1): group I included normotensive pregnancies; group II included patients with PIH; group III included patients with preeclampsia.
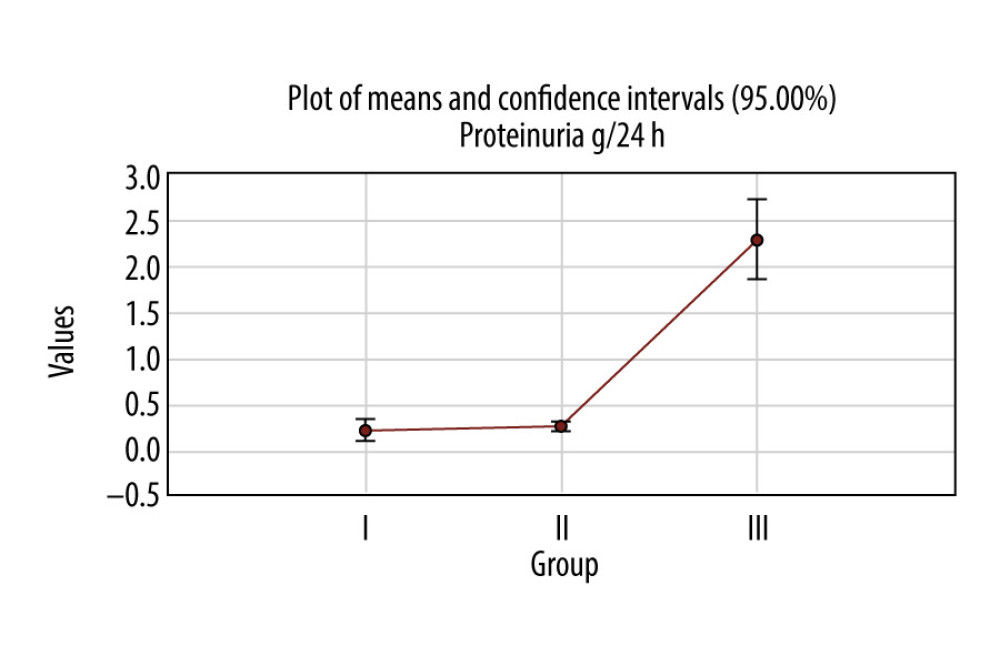
In the first group, the average of proteinuria was 0.2±0.4 g/24 h; in the second group it was 0.25±0.1 g/24 h, and in the third group 2.28±1.7 g/24 h. The average values in all third groups were over the normal range of values (<0.15 g/24 h).
The difference between average values of proteinuria among the 3 groups was statistically significant between the first and the third groups (
About 48.75% of the patients in the first group had a proteinuria value over 0.15 g/24 h, 88.5% were over 0.15 g/24 h in the second group, and 98.3% of the patients’ values in the third group were over 0.15 g/24 h (Figure 1). There was a statistically significant difference among the third groups (Pearson chi-square: 56.4462, df=2,
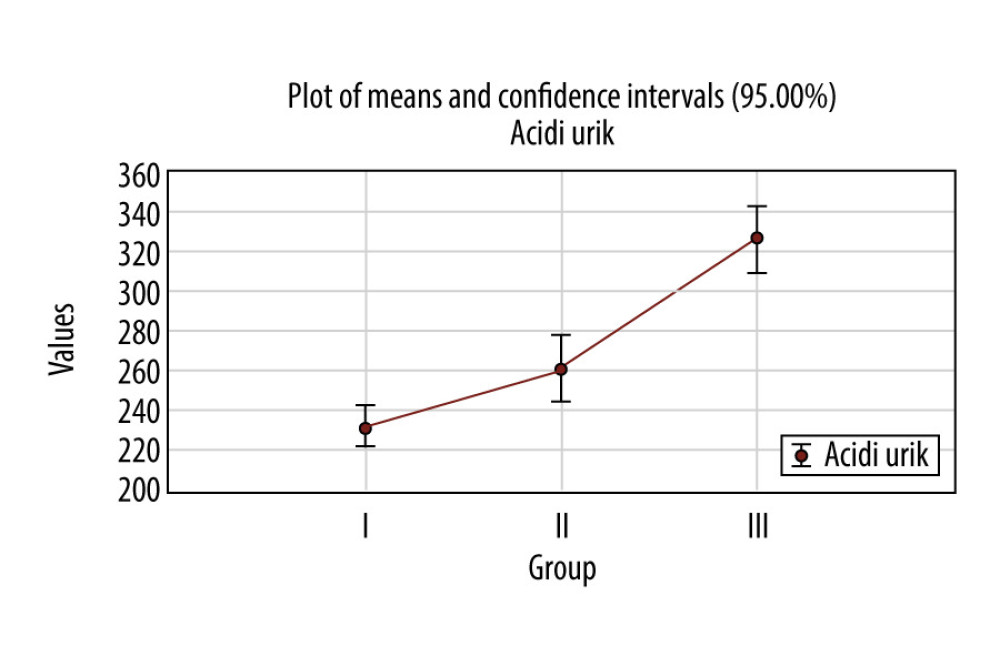
In the first group, the average of uric acid was 232.6±44.3 μmol/L, in the second group 263.3±60.3 μmol/L, and in the third group is 326.1±64.3 μmol/L (the highest) (Figure 2). The average values in the third group were in the range of normal values, 155.0–430.0 μmol/L.
The difference between the average value of uric acid among the 3 groups was statistically significant between the first group and the second group, the first group and the third group, and the second group and the third group (
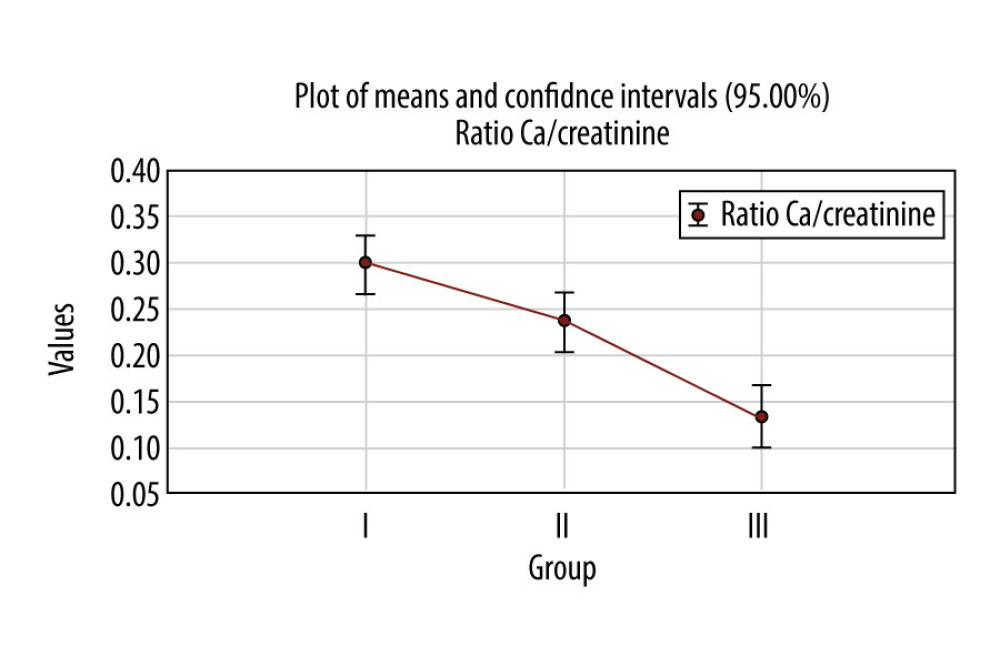
In the first group, the average urinary calcium/creatinine ratio was 0.3±0.1, in the second group 0.2±0.1, and in the third group 0.1±0.1 (Figure 3). The difference between average values of urinary calcium/creatinine ratio among the 3 groups was statistically significant between the first group and the second group, the first group and the third group, and the second group and the third group (
The average urinary calcium/creatinine ratio in the third group – mild preeclampsia – was 0.14±013 and in severe preeclampsia was 0.08±0.09; according to the
In the first group, the average urinary calcium/creatinine ratio was 0.3±0.1, in the second group 0.2±0.1, and in the third group 0.1±0.1 (Figure 3). The difference between the average values of urinary calcium/creatinine ratio among the 3 groups was statistically significant for
According to the post hoc Tukey HSD test, there were statistically significant differences between the first group and the second group, the first group and the third group, and the second group and the third group (
The ratio of urinary calcium/creatinine in both groups, the case group and controls, was calculated after measuring this ratio at the beginning of the study. The ROC curve was used and from this, the calcium/creatinine ratio limit value of 0.105 was taken as the value for predicting preeclampsia. However, according to the dynamics index, the rate of decline for the normotensive group of patients was about 7.5% by the time of birth. At the same time in this group during follow-up there were 4 patients with PIH and 2 patients with preeclampsia (Table 2). According to the dynamics index, the decline rate for the group of patients with PIH was about 38.6% by the time of birth. At the same time in this group during follow-up there were 17 patients with preeclampsia (15 with mild type and 2 severe types) (Table 2).
The number of patients diagnosed with preeclampsia at the beginning of the study did not change during the study (until birth). However, we recorded the change in the number of types of preeclampsia: there was a decrease of mild preeclampsia by 72.9% and an increase of severe preeclampsia by 31.8% during postpartum follow-up.
ROC analysis indicates that proteinuria contributes to the prediction of preeclampsia (96.6%;
Uric acid contributes to prediction of preeclampsia (96.6%;
Calcium/creatinine ratio contributes to the prediction of preeclampsia (87.9%;
Discussion
Preeclampsia can cause changes in many organs and organ systems such as the heart, kidneys, blood, and immune system. It is therefore very important to detect it early to reduce morbidity and mortality from this disease. On the basis of the importance of predicting this disease, we have focused this study to show the role of the calcium/creatinine ratio compared with proteinuria and uric acid in predicting preeclampsia. The ROC curve was used to determine the limit value of the calcium/creatinine ratio of 0.105, which was taken as the value for predicting preeclampsia.
Of a total of 200 patients included in the study, the group of normotensive patients at the beginning of the study (~7.5%) remained with such a diagnosis at the time of parturition. In this group there were 4 patients with PIH and 2 patients with preeclampsia. Of the group with PIH at the beginning of the study, about 38.6% remained with such a diagnosis until parturition. In this group there were 17 patients with preeclampsia (15 with mild type and 2 severe types).
The number of patients diagnosed with preeclampsia at the beginning of the study did not change during the study (until birth). However, we recorded the difference in the number of types of preeclampsia. Mild preeclampsia decreased by 72.9% and severe preeclampsia increased by 31.8% at postpartum follow-up.
In women with preeclampsia, hypocalciuria may be due to decreased secretion of calcium from the renal tubules and increased tubular absorption [17]. Regardless of the pathophysiological mechanism, hypocalciuria is a pathological sign to distinguish preeclampsia from other forms of hypertension in pregnancy. Various studies have shown that in a normal pregnancy, there may be a decrease or increase in urinary calcium secretion [18].
In a cross-sectional study, the link between urinary calcium secretion values and creatinine clearance in late pregnancy was shown [17]. In preeclampsia, it has been observed that there is a decrease in urinary calcium secretion due to increased uptake by the fetoplacental unit and increased uptake by the distal tubules of the kidney [18,19].
In this study, in a normotensive group of 80 patients, 4 patients developed PIH and 2 patients developed preeclampsia (Table 2). The PIH group had 61 patients; 17 developed preeclampsia (15 with mild type and 2 with severe type).
Statistical analysis of the selected parameters showed that the greatest impact on the differences in preeclampsia was protein, which contributes to the diagnosis with 96.3%, and acidic urine, which contributes to the diagnosis with 85.7%. Also, a good divider in preeclampsia difference was calcium/creatinine ratio, which contributes to an accuracy of 80.4%.
According to the ROC curve coordinates for the calcium/creatinine ratio, 87.9% were sensitive (correctly identified positively); the specificity was 40.7%, corresponding to 0.105 (cutoff). The sensitivity of this parameter as a predictor is 87.9% and the specificity is 40.7%.
The sensitivity of proteinuria as a predictor of preeclampsia is 96.6% and the specificity is 21.3%, whereas the sensitivity of uric acid is 96.6% and the specificity is 48.8%.
Conclusions
On the basis of these results, we conclude that proteinuria is a very useful predictor of preeclampsia, certifying a 96.6% reliability in predicting this pregnancy disorder. Nevertheless, uric acid provided the same reliability, 96.6%. The calcium/creatinine ratio contributes to the prediction of preeclampsia with an accuracy of 87.9%. Women with a calcium/creatinine ratio <0.105 have a higher risk of developing preeclampsia (87.9% CI,
The role of the calcium/creatinine ratio in 24-h urine samples is inferior to protein and uric acid in predicting preeclampsia; however, this ratio can be used to predict the development of preeclampsia in asymptomatic patients. Therefore, it can be used as a screening test for preeclampsia to identify the population at risk for developing this disease.
References
1. Cunningham FG, Leveno KJ, Bloom SL: Williams obstetrics Hypertensive disorders of pregnancy, 2014; 729-45, New York, NY, McGraw-Hill Education/Medical
2. Dekker GA, Sibai BM, Etiology and pathogenesis of preeclampsia: Current concepts: Am J Obstet Gynecol, 1998; 179; 1359-75
3. Postovit LM, Adams MA, Graham CH, Does nitric oxide play a role in the etiology of pre-eclampsia?: Placenta, 2001; 22(Suppl A); S51-55
4. Roberts JM, Cooper DW, Pathogenesis and genetics of pre-eclampsia: Lancet, 2001; 357(9249); 53-56
5. David A, Padmaja P, Calcium-to-creatinine ratio in a spot sample of urine, for early prediction of hypertensive disorders of pregnancy: A prospective study: J Obstet Gynecol India, 2016; 66; 94-97
6. Dasgupta M, Adhikari S, Sanghamita M, Urinary calcium levels in pre-eclampsia: J Obstet Gynecol India, 2008; 58(4); 312
7. Barnas U, Schmidt A, Hs M, Parameters associated with chronic renal transplant failure: Nephrol Dial Transplant, 1997; 12(Suppl 2); 82-85
8. Ruggenenti P, Perna A, Mosconi L, Urinary protein excretion rate is the best independent predictor of ESRF in non-diabetic proteinuric chronic nephropathies: Kidney Int, 1998; 53(5); 1209-16
9. ACOG Committee on Obstetric Practice, ACOG practice bulletin. Diagnosis and management of preeclampsia and eclampsia: Int J Gynaecol Obstet, 2002; 77; 67-75
10. Ferazzani S, Caruso A, De Carolis S, Proteinuria and outcome of 444 pregnancies complicated by hypertension: Am J Obstet Gynecol, 1990; 162; 366-71
11. Kang DH, Finch J, Nakagawa T, Uric acid, endothelial dysfunction and pre-eclampsia: Searching for a pathogenetic link: J Hypertens, 2004; 22; 229-35
12. Bainbridge SA, Roberts JM, von Versen-Höynck F, Uric acid attenuates trophoblast invasion and integration into endothelial cell monolayers: Am J Physiol Cell Physiol, 2009; 297; C440-50
13. Bainbridge SA, von Versen-Hoynck F, Roberts JM, Uric acid inhibits placental system amino acid uptake: Placenta, 2009; 30; 195-200
14. Duley L, The global impact of pre-eclampsia and eclampsia: Semin Perinatol, 2009; 33; 130-37
15. North RA, McCowan LM, Dekker GA, Clinical risk prediction for pre-eclampsia in nulliparous women: Development of model in international prospective cohort: Br Med J, 2011; 342; d1875
16. Vuniqi-Krasniqi M, Paçarada M, Daka Q, Hypertensive disorders of in vitro fertilization pregnancies: A study from Kosovo: Int J Reprod Biomed, 2018; 16(2); 77-82
17. Howarth AT, Morgan DB, Payne RB, Urinary excretion of calcium in late pregnancy and its relation to creatinine clearance: Am J Obstet Gynecol, 1977; 129; 499-502
18. Pitkin RM, Calcium metabolism in pregnancy and the perinatal period: A review: Am J Obstet Gynecol, 1985; 151(1); 99-109
19. Taufield PA, Ales KL, Resnick LM, Hypocalciuria in preeclampsia: N Eng J Med, 1987; 316; 715-18
Figures
Tables
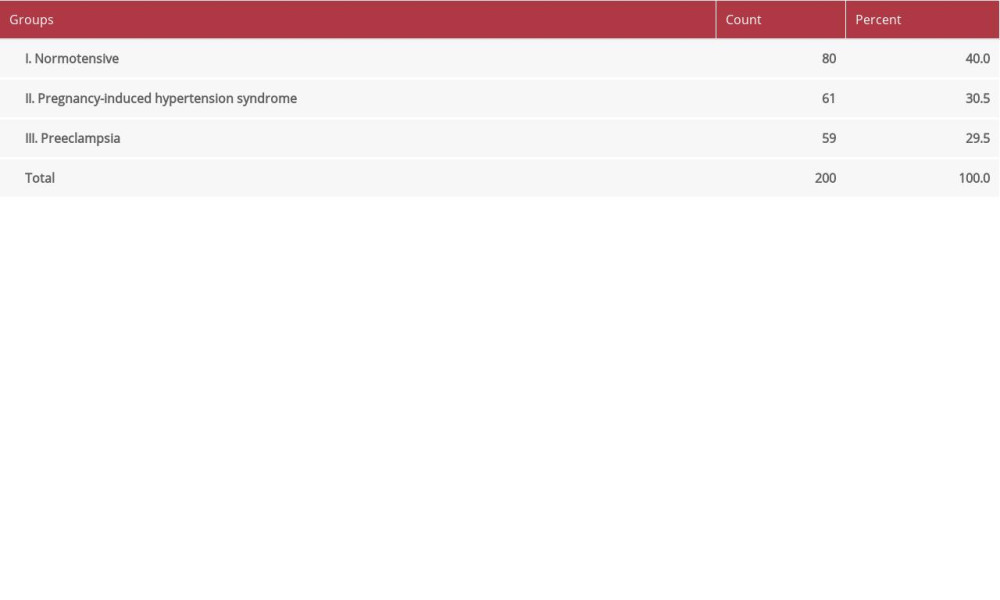 Table 1. Distribution of the patients according to group.
Table 1. Distribution of the patients according to group.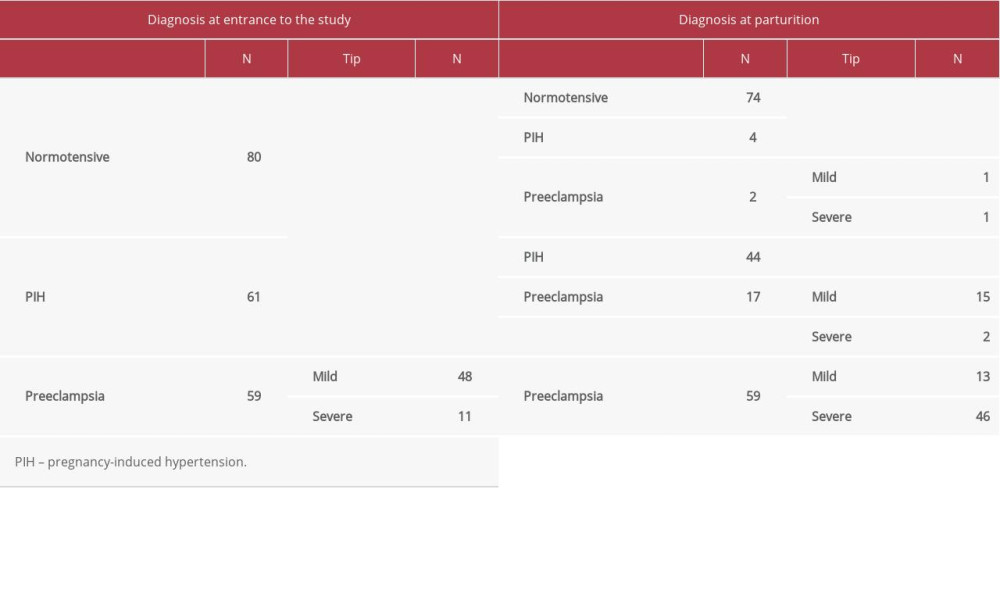 Table 2. Number of patients with diagnosis at the beginning of the study and diagnosis in parturition.
Table 2. Number of patients with diagnosis at the beginning of the study and diagnosis in parturition.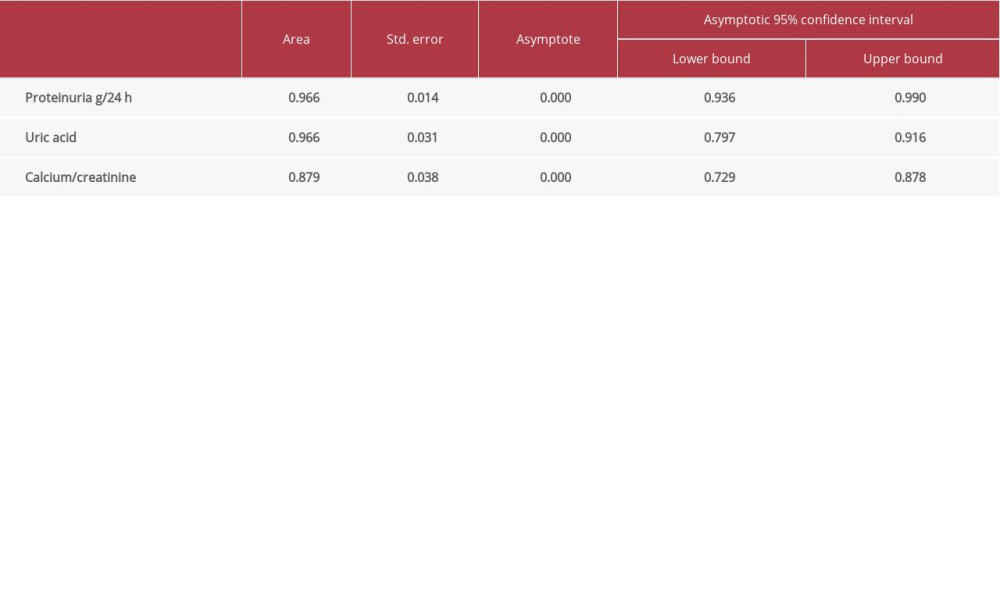 Table 3. Area under the curve.
Table 3. Area under the curve. Table 1. Distribution of the patients according to group.
Table 1. Distribution of the patients according to group. Table 2. Number of patients with diagnosis at the beginning of the study and diagnosis in parturition.
Table 2. Number of patients with diagnosis at the beginning of the study and diagnosis in parturition. Table 3. Area under the curve.
Table 3. Area under the curve. Most Viewed Current Articles
15 Jun 2022 : Clinical Research
Evaluation of Apical Leakage After Root Canal Obturation with Glass Ionomer, Resin, and Zinc Oxide Eugenol ...DOI :10.12659/MSMBR.936675
Med Sci Monit Basic Res 2022; 28:e936675
07 Jul 2022 : Laboratory Research
Cytotoxicity, Apoptosis, Migration Inhibition, and Autophagy-Induced by Crude Ricin from Ricinus communis S...DOI :10.12659/MSMBR.936683
Med Sci Monit Basic Res 2022; 28:e936683
01 Jun 2022 : Laboratory Research
Comparison of Sealing Abilities Among Zinc Oxide Eugenol Root-Canal Filling Cement, Antibacterial Biocerami...DOI :10.12659/MSMBR.936319
Med Sci Monit Basic Res 2022; 28:e936319
08 Dec 2022 : Original article
Use of Estimated Glomerular Filtration Rate and Urine Albumin-to-Creatinine Ratio Based on KDIGO 2012 Guide...DOI :10.12659/MSMBR.938176
Med Sci Monit Basic Res 2022; 28:e938176