23 June 2021: Human Study
Circulating Adiponectin and Resistin Levels Are Associated with Adiposity Indices and Physical Fitness in Healthy Adult Males
Syed Shahid Habib1ABCDEFG*, Mamoona Sultan2BDEF, Adeena Khan3BCDEF, Thamir Al-khlaiwi1BCDEF, Shahid Bashir4ABCDEFDOI: 10.12659/MSMBR.930322
Med Sci Monit Basic Res 2021; 27:e930322
Abstract
BACKGROUND: The aim of this study was to assess the correlation of physical fitness scores (PFS) with serum adiponectin, resistin, and adiponectin/resistin ratio (AR ratio) in relation to body adiposity indices in healthy adult males.
MATERIAL AND METHODS: This cross-sectional study was conducted at the Clinical Physiology Unit, Physiology Department, King Saud University Medical City, King Saud University, Riyadh, from March 2017 to April 2018. We included 125 healthy adult males. Serum samples were obtained after overnight fasting. Analysis was performed for fasting blood glucose (FBG), glycosylated hemoglobin (HbA1c), basal insulin, lipid profile, resistin, and adiponectin. Bioimpedance analysis (BIA) was used to assess body composition. Based on ideal body composition, PFS were computed as previously published for all subjects and compared with serum markers.
RESULTS: There was a positive correlation of adiponectin with PFS (r=.218, p=0.015) and an inverse correlation with obesity degree (OD), OD (r=-.239, p=0.001), body mass index (BMI) (r=-.244, p=0.001), and waist/hip ratio (WHR) WHR (r=-.296, p=0.001). Moreover, it was correlated negatively with basal insulin (r=-.211, p=0.009) and homeostatic insulin resistance model (HOMA-IR) HOMA-IR (r=-.221, p=0.013). Resistin was correlated negatively with PFS (r=-.203, p=0.023), while its correlation with OD, BMI, WHR, and HOMA-IR was not significant. AR ratio was positively correlated with PFS (r=.286, p=0.001) and negatively with OD (r=-.210, p=0.019), BMI (r=-.222, p=0.013), WHR (r=-.308, p=0.001) and basal insulin (r=-.237, p=0.008). In linear regression analysis, the relationship of PFS was significant with adiponectin (r=.218, p=0.015), resistin (r=-.203, p=0.023) and AR ratio (r=.286, p=0.001). ROC curve analysis showed that individually the values of adiponectin and resistin were not significantly correlated with PFS, but they were significant with the combined AR ratio with AUC 64.6% (p=0.029).
CONCLUSIONS: Serum adiponectin was positively correlated and resistin was negatively correlated with physical fitness scores based on healthy body composition with low proportion of body adiposity and a higher proportion of fat-free mass. However, the combined effect of adiponectin/resistin ratio is an even better predictor of physical fitness. Moreover, the adiponectin/resistin ratio is even more highly associated with physical fitness than adiponectin or resistin alone.
Keywords: Adiponectin, Body Composition, Body Mass Index, Physical Fitness, Resistin, Adiposity, Cross-Sectional Studies, Insulin Resistance, Insulins, Obesity
Background
Physical inactivity is the fourth leading risk factor for stroke, cardiovascular diseases, and diabetes, with the highest epidemiological impact on general population health globally, as estimated by the Global Burden of Disease Study [1].
The Global Action Plan for Physical Activity and Fitness, initiated in 2018 by the World Health Organization, with the notion of “more active people for a healthier world”, addresses physical activity with a ‘global’ and ‘whole-of-system’ approach for effective promotion of physical activity at the national and subnational levels [2]. Obesity and its repercussions, including cardiovascular disease, cancers, sleep apnea, fatty liver, and knee osteoarthritis, is a major health problem with high risk of morbidity, decreased quality of life, and decreased life expectancy [3]. Adipose tissue not only acts as a depot of energy storage, but also acts a complex endocrine organ that releases certain hormones that play a potential role in the pathogenesis of insulin resistance, type 2 diabetes (T2DM), and obesity [4]. It is well recognized that obesity and, most importantly, visceral adipose tissue accumulation, lead to an increased tendency to develop T2DM [5,6].
Many researchers have identified a substantial list of adipokine proteins, including leptin, adiponectin, resistin, and acylation-stimulating protein, involved in the regulation of glucose, lipid metabolism, and insulin resistance (IR) in obesity and diabetes [7,8]. Therefore, high visceral fat accumulation leading to high insulin resistance is independently related to a prediabetic state and T2DM [9]. Dysregulation of adipose tissue is associated with ectopic fat accumulation in the liver and other abdominal visceral organs [10,11]. Furthermore, it is characterized by a proinflammatory state and adipokine dysregulation along with high IR. All these factors collectively lead to a high risk of T2DM development compared to total body fat mass alone.
Resistin and adiponectin are important adipokines that regulate insulin sensitivity. The cardioprotective adipokine adiponectin, which plays an important protective role in inflammatory mechanisms, increases insulin sensitivity and improves lipid profile, which act together to markedly reduce the atherosclerotic risk in diabetes subjects. Plasma adiponectin concentrations are reported to be decreased in patients with obesity and coronary artery disease (CAD) [12,13]. Resistin is considered an important link between obesity and T2DM since its levels are positively correlated with IR both in vitro and in vivo [14].
Since both adiponectin and resistin have important biological activities in glucose and lipid metabolism, the comparison of the effects of these adipokines on glucometabolic control needs further research. The present study aimed to assess the correlation of physical fitness scores (PFS) computed from body composition indices with blood circulating levels of adiponectin, resistin, and adiponectin/resistin ratio.
Material and Methods
STATISTICAL ANALYSIS:
Data analysis was performed using SPSS (IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY: IBM Corp.). Descriptive data are expressed as mean± SD along with range (minimum and maximum). Kolmogorov-Smirnov an dShapiro-Wilk tests were performed for normality. Skewed data were analyzed by non-parametric Mann-Whitney test. Normally distributed continuous data groups were compared by the
Results
Table 1 shows the demographic data of all subjects. Table 2 expresses biochemical and adipokine profile data of all study participants. Table 3 shows the correlation of adiponectin, resistin, and AR ratio with adiposity and insulin resistance indices. Adiponectin was correlated positively with PFS (r=.218) and negatively with adiposity indices of OD (r=−.239), BMI (r=−.244,), and WHR (r=−.296). Additionally, it was correlated negatively with basal insulin (r=−.211) and HOMA-IR (r=−.221), with p for all values <0.05. Resistin was correlated negatively with PFS (r=−.203) and positively with WHR (r=0.176). AR ratio was correlated positively with PFS (r=.286) and negatively with OD (r=−.210), BMI (r=−.222), WHR (r=−.308), and basal insulin (r=−.237). Linear regression analysis was performed to assess the relationship of fitness score as the dependent variable with (a) adiponectin, (b) resistin, and (c) AR ratio (Figure 1). The relationship was significant for the 3 parameters [adiponectin (r=.218, p=0.015), resistin (r=−.203, p=0.023), and AR ratio (r=.286, p=0.001), respectively]. The participants were dichotomized into 2 groups based on PFS of good and poor scores from our previous research [16,17]. ROC curve analysis was performed to assess the predictive value of fitness scores of adiponectin, resistin, and AR ratio (Figure 2). It was observed that individually the value of adiponectin and resistin was nonsignificant, but when AR ratio was taken, the value became significant. Therefore, AR ratio was observed to be a better predictor for PFS, with AUC 64.6% (p=0.029).
Discussion
To the best of our knowledge, the present study is the first to determine the correlation of physical fitness assessed by PFS with circulating adipokines adiponectin, resistin, and AR ratio in relation to body adiposity indices. Measures that could increase adiponectin levels and lower resistin levels might be valuable future targets for decreasing the higher coronary artery disease (CAD) risk in people with diabetes. This study highlights the importance of exercise and better physical fitness to achieve a better biochemical blood profile that can lower the cardiovascular risk. Moreover, the relative role of the adipokines resistin and adiponectin is reported. Lower levels of adiponectin in obese subjects are inversely related to higher levels of resistin and are considered to be involved in the development of insulin resistance and accelerated atherogenesis [20]. Plasma adiponectin levels are negatively correlated with adiposity. Moreover, low serum adiponectin titers and increased WHR are independently associated with high sensitivity C reactive protein (hsCRP) concentrations in normoglycemic individuals [21–23]. Since there are gender differences between men and women with regard to adipokine levels, we included only men in the current study to avoid the possibility of the confounding effect of gender [24].
Nayak et al showed that serum adiponectin levels decrease with a rise in adiposity and insulin resistance regardless of diabetes status. Among non-obese subjects, adiponectin was negatively correlated with TG, IL-6, and HOMA-IR and positively correlated with HDL. Diabetes status, tumor necrosis factor-α (TNF-α), and BMI were identified as independent predictors of adiponectin levels. Glucose and adiponectin are useful indicators of T2DM. Moreover, insulin-mediated glucose turnover is significantly affected by adiponectin and TNF-α [25]. The inverse association of serum adiponectin levels with cardiovascular risk determined by CRP has been reported in African Americans. These results also support our data [26]. Adiponectin was negatively correlated with BMI after adjustment for age, gender, and glycemic state. In an interesting study, Lau et al proposed a novel index of adiponectin and resistin levels combined as an adiponectin/resistin (AR) index and an insulin resistance (IRAR) index. The predictive value of this index was better than adiponectin and resistin levels alone for metabolic dysregulation and disorders. IRAR proved to be a better diagnostic indicator of insulin activity [27].
Different adipocytokines that have been implicated in metabolic syndrome pathogenesis, including leptin, interleukin-6 (IL-6), resistin, TNF-α, angiotensinogen, and plasminogen activator inhibitor-1 (PAI-1) [28]. The present study also reveals that resistin might play an important role in the pathogenic mechanisms leading to high adiposity and insulin resistance. We reported previously that higher resistin levels in T2DM have a significant relationship with body fat mass [29]. Adiponectin is a cardioprotective adipokine; therefore, circulating adiponectin levels are lower in obese individuals [7]. The therapeutic effects of metformin are associated with the upregulation of adiponectin and suppression of leptin and resistin in blood [30].
Possible limitations of the present study are its cross-sectional design, from which a strong causative conclusion cannot be derived. Secondly, we tried to homogenize the group by studying only one gender with lower cardiovascular disease (CVD) risk since they all were normoglycemic. Therefore, we need more studies including females and different ethnicities to explore the contributions of adipokines in obesity and diabetes pathogenesis. Large-scale prospective studies are required to further explore the true homeostatic roles of adiponectin and resistin in relation to obesity and diabetes mellitus. Since these adipokines are related to glucose and lipid metabolism, it would be worth studying them with an integrated approach with prospective studies in relation to different pharmacological interventions, and exercise training programs. This might determine the true role of integrated biomarkers in prediction of metabolic dysfunctions and high cardiovascular risk in obesity.
Conclusions
RECOMMENDATIONS:
We recommend further research to explore the relationships of adipokines with cardiovascular risk markers, including both traditional and nontraditional risk markers.
Figures
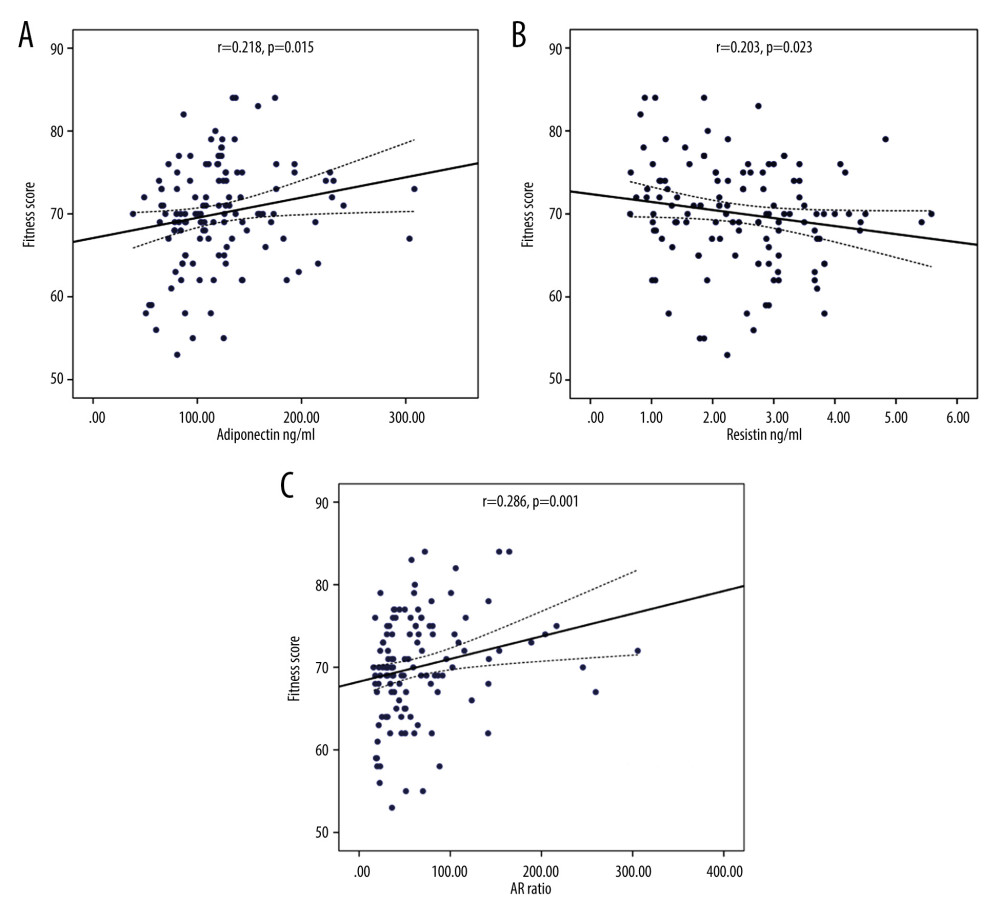 Figure 1. Linear regression analysis of physical fitness scores as dependent variable on (A) adiponectin, (B) resistin, and (C) AR ratio.
Figure 1. Linear regression analysis of physical fitness scores as dependent variable on (A) adiponectin, (B) resistin, and (C) AR ratio. 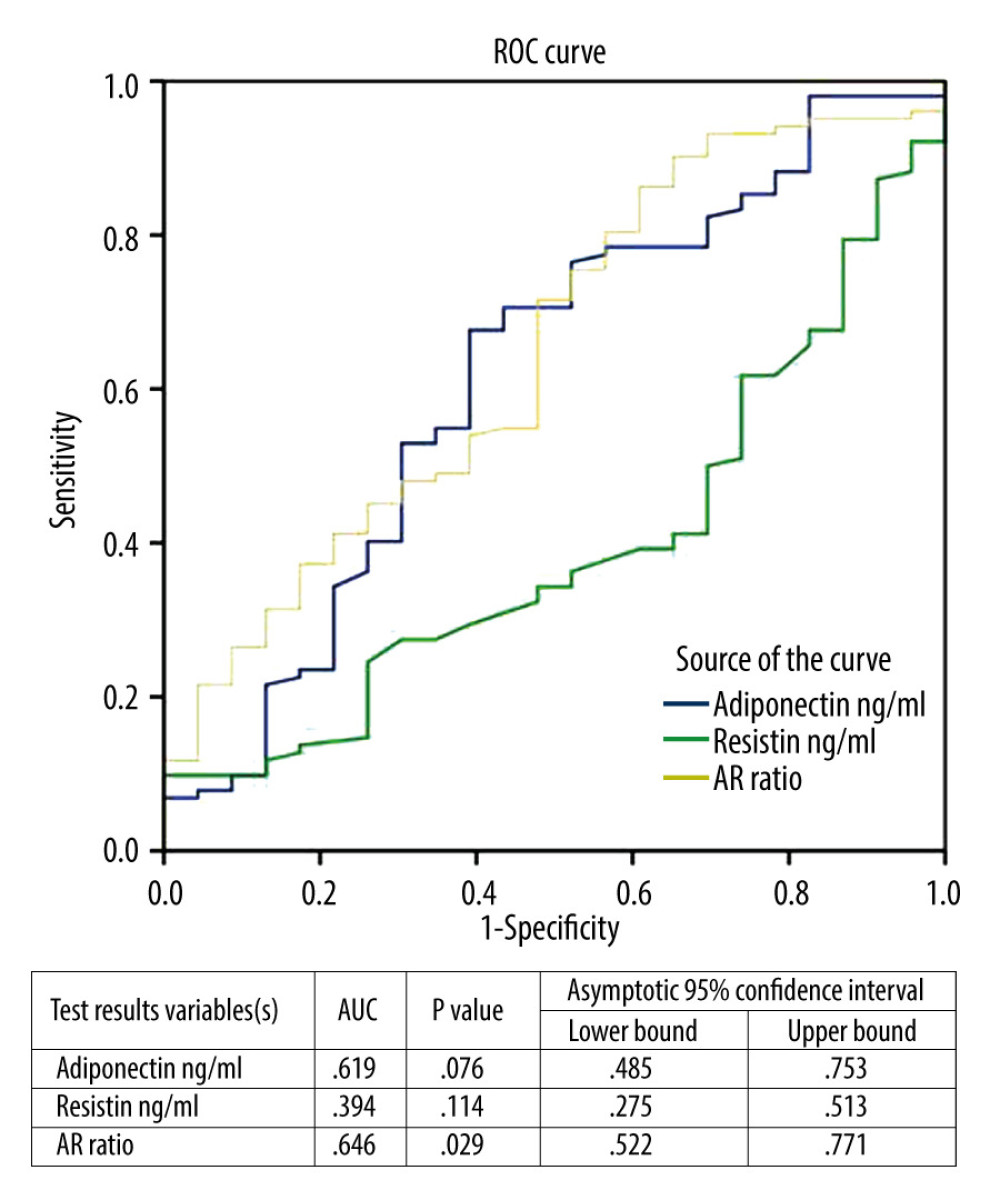 Figure 2. Linear regression analysis of physical fitness scores as dependent variable and its relationship with adiponectin, resistin, and adiponectin/resistin ratio.
Figure 2. Linear regression analysis of physical fitness scores as dependent variable and its relationship with adiponectin, resistin, and adiponectin/resistin ratio. References
1. Lavie CJ, Ozemek C, Carbone S, Sedentary behavior, exercise, and cardiovascular health: Circ Res, 2019; 124(5); 799-815
2. Scatigna M, D’Eugenio S, Cesarini VWorking Group Doping Prevention Project, Physical activity as a key issue for promoting human health on a local and global scale: Evidences and perspectives: Ann Ig, 2019; 31(6); 595-613
3. Abdelaal M, le Roux CW, Docherty NG, Morbidity and mortality associated with obesity: Ann Transl Med, 2017; 5(7); 161
4. Su X, Peng D, Emerging functions of adipokines in linking the development of obesity and cardiovascular diseases: Mol Biol Rep, 2020; 47(10); 7991-8006
5. Jensen MD, Role of body fat distribution and the metabolic complications of obesity: J Clin Endocrinol Metab, 2008; 93; S57-63
6. Booth A, Magnuson A, Foster M, Detrimental and protective fat: body fat distribution and its relation to metabolic disease: Horm Mol Biol Clin Investig, 2014; 17(1); 13-27
7. Ahima RS, Central actions of adipocyte hormones: Trends Endocrinol Metab, 2005; 16; 307-13
8. Kojta I, Chacińska M, Błachnio-Zabielska A, Obesity, bioactive lipids, and adipose tissue inflammation in insulin resistance: Nutrients, 2020; 12(5); 1305
9. Neeland IJ, Turer AT, Ayers CR, Dysfunctional adiposity and the risk of prediabetes and type 2 diabetes in obese adults: JAMA, 2012; 308(11); 1150-59
10. McLaughlin T, Lamendola C, Liu A, Abbasi F, Preferential fat deposition in subcutaneous versus visceral depots is associated with insulin sensitivity: J Clin Endocrinol Metab, 2011; 96(11); E1756-60
11. Preis SR, Massaro JM, Robins SJ, Abdominal subcutaneous and visceral adipose tissue and insulin resistance in the Framingham Heart Study: Obesity (Silver Spring), 2010; 18(11); 2191-98
12. Arita Y, Kihara S, Ouchi N, Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity 1999: Biochem Biophys Res Commun, 2012; 425(3); 560-64
13. Achari AE, Jain SK, Adiponectin, a therapeutic target for obesity, diabetes, and endothelial dysfunction: Int J Mol Sci, 2017; 18(6); 1321
14. Hjort R, Ahlqvist E, Andersson T, Physical activity, genetic susceptibility, and the risk of latent autoimmune diabetes in adults and type 2 diabetes: J Clin Endocrinol Metab, 2020; 105(11); dgaa549
15. Habib SS, Alkahtani S, Alhussain M, Aljuhani O, Sarcopenia coexisting with high adiposity exacerbates insulin resistance and dyslipidemia in Saudi adult men: Diabetes Metab Syndr Obes, 2020; 13; 3089-97
16. Iqbal M, Al-Regaiey KA, Ahmad S, Body composition analysis to determine gender specific physical fitness equations in a cohort of Saudi population: Pak J Med Sci, 2014; 30(4); 798-903
17. Habib SS, Body composition analysis and estimation of physical fitness by scoring grades in Saudi adults: J Pak Med Assoc, 2013; 63(10); 1285-89
18. Jensky-Squires NE, Dieli-Conwright CM, Rossuello A, Validity and reliability of body composition analysers in children and adults: Br J Nutr, 2008; 100(4); 859-65
19. Ramírez-Vélez R, Tordecilla-Sanders A, Correa-Bautista JE, Validation of multi-frequency bioelectrical impedance analysis versus dual-energy X-ray absorptiometry to measure body fat percentage in overweight/obese Colombian adults: Am J Hum Biol, 2018; 30(1); ajhb23071
20. Lau WB, Ohashi K, Wang Y, Role of adipokines in cardiovascular disease: Circ J, 2017; 81(7); 920-28
21. Habib SS, Al Regaiey KA, Al Dokhi L, Assessment of adipokines relationships with cardiovascular risk Markers in relation to body indices in normoglycemic males: Pak J Med Sci, 2013; 29(1); 21-26
22. Diwan AG, Kuvalekar AA, Dharamsi S, Correlation of serum adiponectin and leptin levels in obesity and type 2 diabetes mellitus: Indian J Endocrinol Metab, 2018; 22(1); 93-99
23. Harke SM, Khadke SP, Ghadge AA, Adipocytokines and anthropometric measures in type 2 diabetics: Diabetes Metab Syndr, 2017; 11(Suppl 1); S273-76
24. Doumatey AP, Lashley KS, Huang H, Relationships among obesity, inflammation, and insulin resistance in African Americans and West Africans: Obesity, 2010; 18; 598-603
25. Nayak BS, Ramsingh D, Gooding S, Plasma adiponectin levels are related to obesity, inflammation, blood lipids and insulin in type 2 diabetic and non-diabetic Trinidadians: Prim Care Diabetes, 2010; 4(3); 187-92
26. Abraham PA, Attipoe S, Kazman JB, Role of plasma adiponectin/C-reactive protein ratio in obesity and type 2 diabetes among African Americans: Afr Health Sci, 2017; 17(1); 99-107
27. Lau CH, Muniandy S, Novel adiponectin-resistin (AR) and insulin resistance (IRAR) indexes are useful integrated diagnostic biomarkers for insulin resistance, type 2 diabetes and metabolic syndrome: a case control study: Cardiovasc Diabetol, 2011; 10(1); 8
28. Derosa G, Catena G, Gaudio G, Adipose tissue dysfunction and metabolic disorders: Is it possible to predict who will develop type 2 diabetes mellitus? Role of markErs in the progreSsion of dIabeteS in obese paTIeNts (The RESISTIN trial): Cytokine, 2020; 127; 154947
29. Habib SS, Serum resistin levels in patients with type 2 diabetes mellitus and its relationship with body composition: Saudi Med J, 2012; 33(5); 495-99
30. Dludla PV, Nkambule BB, Mazibuko-Mbeje SE, Adipokines as a therapeutic target by metformin to improve metabolic function: A systematic review of randomized controlled trials: Pharmacol Res, 2020; 163; 105219
Figures
 Figure 1. Linear regression analysis of physical fitness scores as dependent variable on (A) adiponectin, (B) resistin, and (C) AR ratio.
Figure 1. Linear regression analysis of physical fitness scores as dependent variable on (A) adiponectin, (B) resistin, and (C) AR ratio. Figure 2. Linear regression analysis of physical fitness scores as dependent variable and its relationship with adiponectin, resistin, and adiponectin/resistin ratio.
Figure 2. Linear regression analysis of physical fitness scores as dependent variable and its relationship with adiponectin, resistin, and adiponectin/resistin ratio. Tables
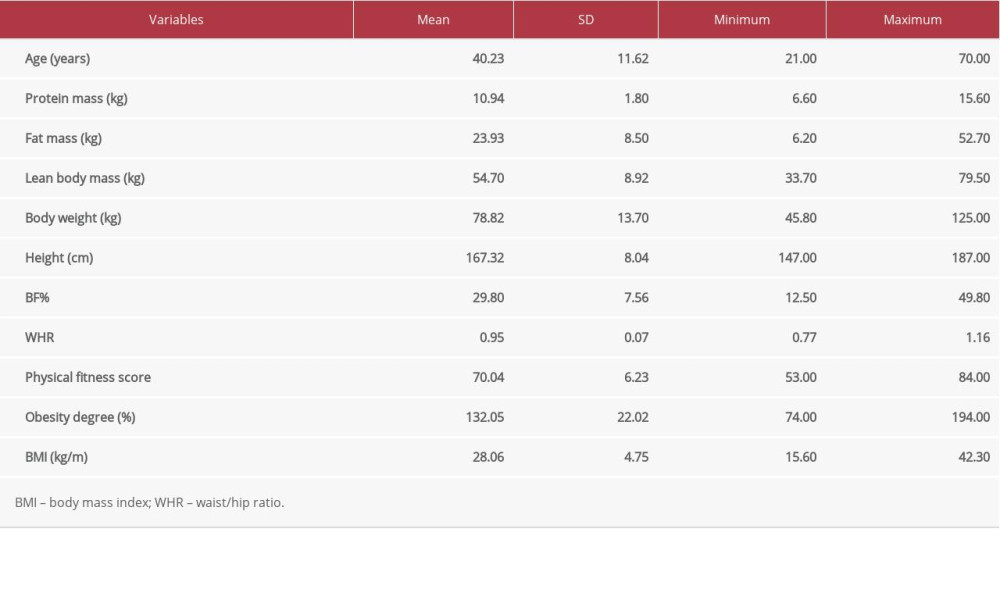 Table 1. Demographic and clinical data of all study participants.
Table 1. Demographic and clinical data of all study participants.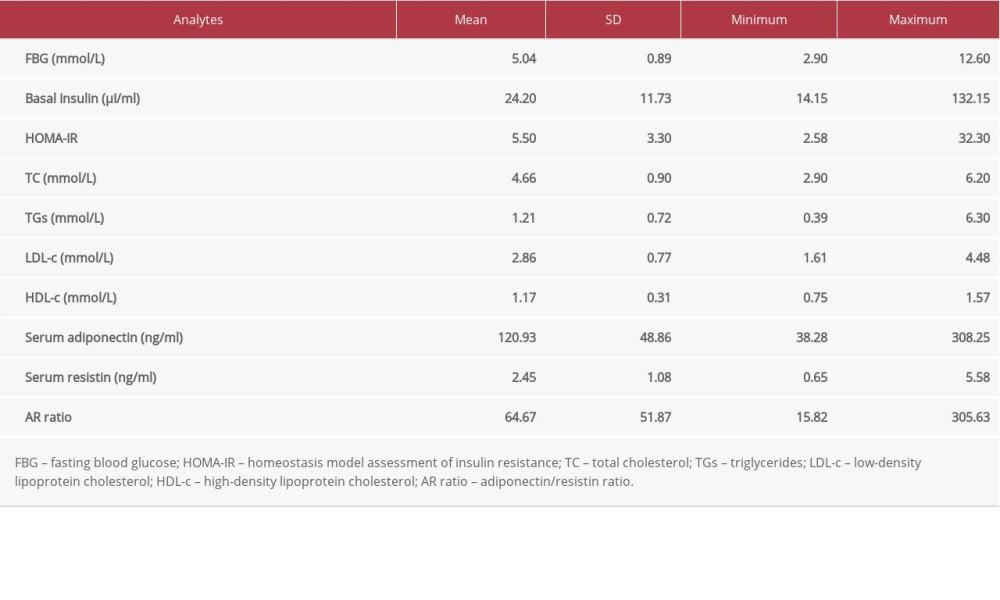 Table 2. Lipid profile, insulin indices, and adipokine levels in all study participants.
Table 2. Lipid profile, insulin indices, and adipokine levels in all study participants.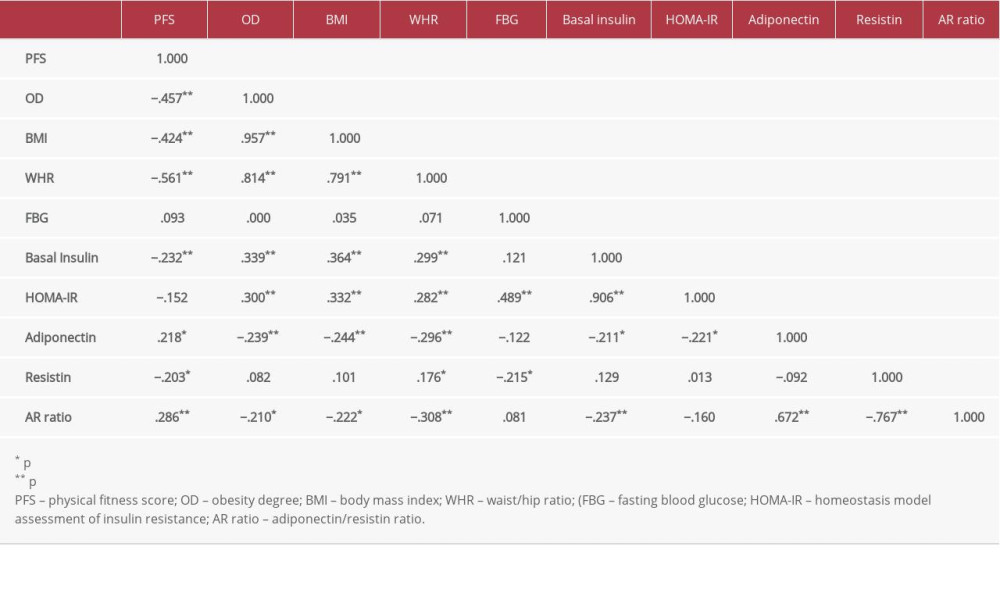 Table 3. Pearson’s Correlation coefficients between adiponectin, resistin, AR ratio, adiposity indices, and insulin resistance index.
Table 3. Pearson’s Correlation coefficients between adiponectin, resistin, AR ratio, adiposity indices, and insulin resistance index. Table 1. Demographic and clinical data of all study participants.
Table 1. Demographic and clinical data of all study participants. Table 2. Lipid profile, insulin indices, and adipokine levels in all study participants.
Table 2. Lipid profile, insulin indices, and adipokine levels in all study participants. Table 3. Pearson’s Correlation coefficients between adiponectin, resistin, AR ratio, adiposity indices, and insulin resistance index.
Table 3. Pearson’s Correlation coefficients between adiponectin, resistin, AR ratio, adiposity indices, and insulin resistance index. Most Viewed Current Articles
15 Jun 2022 : Clinical Research
Evaluation of Apical Leakage After Root Canal Obturation with Glass Ionomer, Resin, and Zinc Oxide Eugenol ...DOI :10.12659/MSMBR.936675
Med Sci Monit Basic Res 2022; 28:e936675
07 Jul 2022 : Laboratory Research
Cytotoxicity, Apoptosis, Migration Inhibition, and Autophagy-Induced by Crude Ricin from Ricinus communis S...DOI :10.12659/MSMBR.936683
Med Sci Monit Basic Res 2022; 28:e936683
01 Jun 2022 : Laboratory Research
Comparison of Sealing Abilities Among Zinc Oxide Eugenol Root-Canal Filling Cement, Antibacterial Biocerami...DOI :10.12659/MSMBR.936319
Med Sci Monit Basic Res 2022; 28:e936319
08 Dec 2022 : Original article
Use of Estimated Glomerular Filtration Rate and Urine Albumin-to-Creatinine Ratio Based on KDIGO 2012 Guide...DOI :10.12659/MSMBR.938176
Med Sci Monit Basic Res 2022; 28:e938176








