18 August 2021: Clinical Research
Effects of Doxofylline Combined with Ceftazidime on Clinical Efficacy, Drug Safety, and Prognosis in Patients with Chronic Obstructive Pulmonary Disease Complicated with Infection
Ying WangEFGDOI: 10.12659/MSM.930494
Med Sci Monit 2021; 27:e930494
Abstract
BACKGROUND: The aim of this study was to investigate the effects of doxofylline combined with ceftazidime on clinical efficacy, drug safety, and prognosis in patients with COPD complicated with infection.
MATERIAL AND METHODS: A total of 450 patients admitted to the Inner Mongolia BaoGang Hospital for treatment of COPD from January 2017 to December 2019 were selected to participate. All patients were randomly divided into control and observation groups, with 225 patients in each group. In addition, patients with COPD in the remission stage were matched by sex and age for a blank control group. The control group was treated with doxofylline, and the observation group was treated with ceftazidime and doxofylline. No drug intervention was given to the blank control group. Short-term efficacy, pulmonary ventilation function, patient quality of life, peripheral blood TNF-α and PGDF-B levels, and adverse drug reactions were observed.
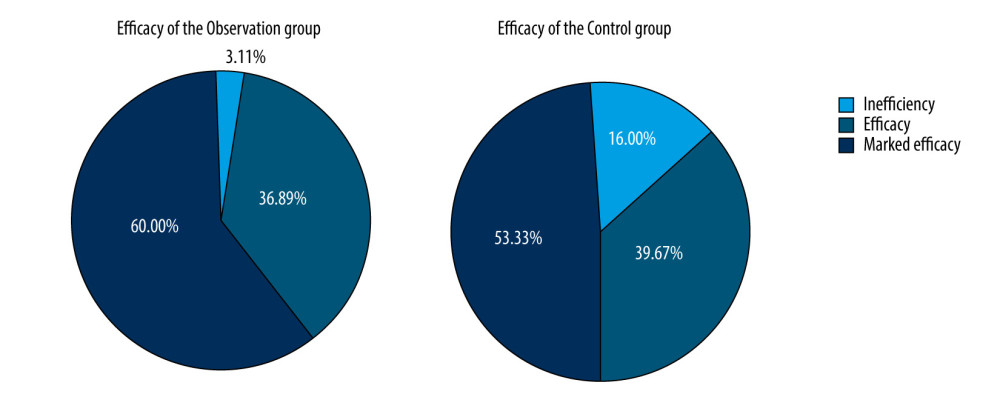
RESULTS: The effective treatment rate in the observation group was 96.89%, which was significantly higher than that in the control group (84.00%). Measures of pulmonary ventilation function and patient quality of life in the observation group were significantly higher than those in the control group. Levels of TNF-α and PDGF-B in the observation group were significantly lower than those in the control group. There were no significant differences in the above indicators between the blank control group and the observation group.
CONCLUSIONS: Doxofylline combined with ceftazidime effectively treated patients with COPD complicated with infection. These results provide a reference for clinical treatment.
Keywords: Adenophorea Infections, Ceftazidime, pulmonary emphysema, Drug Therapy, Combination, Forced Expiratory Volume, infections, Lung, Pulmonary Disease, Chronic Obstructive, Quality of Life, Theophylline
Background
Chronic obstructive pulmonary disease (COPD) is a chronic lung disease that mostly affects the elderly. Characterized by airway obstruction, COPD is usually caused by exposure to cigarette smoke, particulates, and other noxious gases. The clinical symptoms include chest congestion, cough, expectoration, and dyspnea [1,2]. As the fourth leading cause of death currently in the United States, COPD is expected to be the third leading cause of death worldwide by 2030 [3]. Patients who are elderly and have weakened immunity and physical limitations are prone to have complications of lung infection [4], and their condition can easily worsen. Therefore, timely and effective treatment of patients with COPD and lung infection is vital to improving patients’ health [5].
In general, improving airway symptoms is the priority of COPD treatment. Doxofylline is a derivative of methylxanthine, and its main mechanism is relaxing the smooth muscle of the bronchus through the inhibition of phosphodiesterase activities in smooth muscle cells. Consequently, doxofylline can alleviate cough and facilitate airway ventilation [6,7]. Also, compared with traditional respiratory stimulants, doxofylline has fewer adverse effects and reduces the impact on the nervous, gastrointestinal, and cardiovascular systems [8]. Cephalosporin antibiotics are spectrometric antibacterial agents that have been applied widely clinically. Their high resistance to acids, enzymes, and bacteria makes cephalosporin antibiotics increasingly common in clinical application [9].
The oxidative stress reaction and inflammatory mechanism play vital roles in the onset and development of COPD [11]. Platelet-derived growth factor (PDGF-B) lies downstream of the oxidative stress reaction, and tumor necrosis factor-α (TNF-α) is an important factor in the inflammatory reaction. Clinical studies show that PDGF-B and TNF-α play critical roles in the onset and development of COPD and are of great significance in the progression and prognosis of the disease [12,13].
In this study, 450 patients admitted to the Inner Mongolia BaoGang Hospital for the treatment of COPD from January 2017 to December 2019 were selected as research participants. The aim of this study was to investigate the effects of doxofylline combined with ceftazidime on clinical efficacy, drug safety, and prognosis in patients with COPD complicated with infection.
Material and Methods
GENERAL PATIENT INFORMATION:
In this study, 450 patients admitted to the Inner Mongolia BaoGang Hospital for treatment of COPD from January 2017 to December 2019 were selected to participate. All 450 patients were randomly allocated into an observation group or control group, with 225 patients in each group. In addition, patients with COPD in the remission stage, with matching sex and age, were selected as a blank control group. There were no significant differences in sex, age, disease course, and other general information among these 3 groups (P>0.05, Table 1). Patients in all groups were diagnosed with COPD after X-ray or chest computed tomography (CT) scanning. No patients had a history of drug allergy or nervous system disease. All patients gave their informed consent to participate in the study.
PATHOLOGICAL INCLUSION AND EXCLUSION CRITERIA:
The inclusion criteria were as follows: (1) patients who met the diagnostic criteria of COPD [14]; (2) patients aged between 40 and 80 years; and (3) patients who gave informed consent. The exclusion criteria were as follows: (1) patients with allergy to the medication used in this study; (2) patients with severe heart, liver, or lung dysfunction; (3) patients with malignant tumors or systemic immune diseases; (4) patients with a recent history of surgery; and (5) patients taking medication that was different from the study medication.
TREATMENT:
Patients in the observation group and control group received conventional COPD treatment including nutrition support, oxygen inhalation, and fluid infusion. No drug intervention was given to the blank control group. The patients in the control group were treated with doxofylline (A&Z Pharmaceutical Inc., SFDA approval no. H20052247) as follows: 0.5 g doxofylline was dissolved in 250 mL glucose for intravenous injection once per day. The patients in the observation group were treated with doxofylline, as above, combined with ceftazidime with 1 g ceftazidime (Shanghai New Asia Pharmaceutical Co., Ltd., SFDA approval no. H20084054) dissolved in 250 mL glucose injection for intravenous injection once per day.
MEASUREMENT OF SHORT-TERM EFFICACY:
Short-term efficacy was observed after 2 courses of treatment, and the criteria for efficacy were as follows: (1) inefficacy: patients’ clinical symptoms were not notably improved or were aggravated after treatment; (2) efficacy: patients’ clinical symptoms were notably improved but did not resolve after treatment; and (3) marked efficacy: patients’ clinical symptoms were notably improved or even resolved after treatment and had no further impact on daily life.
MEASUREMENT OF PULMONARY VENTILATION FUNCTION:
The recovery of the pulmonary function of patients in the observation and control groups was observed and compared, including the peak expiratory flow rate, maximal mid-expiratory flow curve, and the forced expiratory volume in 1 s.
MEASUREMENT OF PATIENTS’ QUALITY OF LIFE:
Data on patients’ quality of life, including physiological function, social function, role restriction, and overall health, were collected, and the scores were compared between the groups.
MEASUREMENT OF PERIPHERAL BLOOD TNF-α AND PGDF-B LEVELS:
Fasting venous blood samples were collected in the early morning before the treatment and after 2 courses of treatment. The samples were then centrifuged to separate the serum, and the TNF-α and PGDF-B levels were tested by enzyme-linked immunosorbent assay. The diagnostic kits were provided by Shanghai Jianglai Biotechnology Co., Ltd.
MEASUREMENT OF ADVERSE DRUG REACTIONS:
Data on the adverse drug reactions between the observation and control groups were collected and compared.
STATISTICAL ANALYSIS:
In this study, all experimental data were statistically analyzed and processed by SPSS software version 19.0. The measurement data were tested by the
Results
COMPARISON OF EFFICACY:
Results of measurements on the short-term efficacy of the observation and control groups showed the effective rate of treatment in the observation group was 96.89%, which was significantly higher than that in the control group (84.00%). The difference was statistically significant (chi-square=5.997, P<0.05) (Table 2, Figure 1).
COMPARISON OF PULMONARY FUNCTION:
In the observation group, the peak expiratory flow rate was 0.54±0.29L/min; the volume of 1 s forced expiratory respiration was 1.59±0.68 L; and the maximal mid-expiratory flow curve was 0.46±0.30 mL/s. In the control group, the peak expiratory flow rate was 0.28±0.12 L/min; the volume of 1 s forced expiratory respiration was 1.08±0.37 L; and the maximal mid-expiratory flow curve was 0.19±0.12 mL/s. The differences between the observation and control groups were statistically significant for the 3 variables (P<0.05, Table 3). In the blank control group, the peak expiratory flow rate was 0.55±0.14 L/min; the volume of 1 s forced expiratory respiration was 1.61±0.53 L; and the maximal mid-expiratory flow curve was 0.47±0.22 mL/s. There were no significant differences between the blank control group and the observation group (P>0.05, Table 3).
COMPARISON OF PATIENT QUALITY OF LIFE:
In the observation group, the physiological function score of treated patients was 76.10±8.70; the social function score was 79.59±10.49; the role restriction score was 79.99±11.72; and the overall health score was 84.23±11.89. In the control group, the physiological function score was 63.28±7.89; the social function score was 63.59±0.49; the role restriction score was 65.19±11.37; and the overall health score was 65.87±9.86. The above indicators in the blank group were 77.19±8.21; 81.58±11.27; 82.18±12.29; and 85.28±13.94, respectively, and there were no statistically significant differences between the blank group and the observation group (Table 4).
COMPARISON OF PERIPHERAL BLOOD TNF-α AND PGDF-B LEVELS:
The peripheral blood TNF-α and PGDF-B levels of the observation and control groups were significantly lower after treatment. Also, the levels of TNF-α and PDGF-B in the observation group were significantly lower than those in the control group after treatment (P<0.05). TNF-α and PGDF-B levels in the blank group were 17.78±2.36 and 123.89±16.28, respectively, and there was no statistically significant difference between the blank group and the observation group after treatment (P>0.05, Table 5).
COMPARISON OF ADVERSE DRUG REACTIONS:
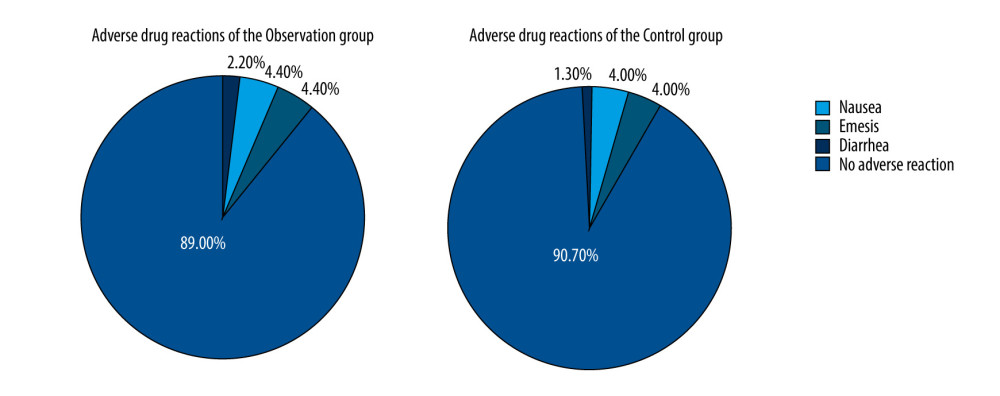
In the course of treatment, there were 5 cases of nausea, 10 cases of emesis, and 10 cases of diarrhea in the observation group, with a rate of adverse drug reactions of 11.11%. In the control group, there were 3 cases of nausea, 9 cases of emesis, and 9 cases of diarrhea, with a rate of adverse drug reactions of 9.33%. There was no significant difference between the groups (chi-square=0.10, P=0.750, Figure 2).
Discussion
COPD is a respiratory disease characterized by pathologic changes in the respiratory system. Clinically, it includes localized obstructive emphysema and diffuse obstructive emphysema [15]. The main pathologic changes are increased residual volume and sustained expansion of pulmonary tissue on terminal bronchioles along with the destruction of the alveolar septum and reduced elasticity of pulmonary tissue, leading to increased volume [16–18]. Studies have shown that
This study investigated the effects of doxofylline combined with ceftazidime on the clinical efficacy, drug safety, and prognosis of patients with COPD complicated with infection. It showed that the efficacy rate of doxofylline combined with ceftazidime was significantly higher than that of treatment with doxofylline alone. The rates of efficacy and marked efficacy in the observation group were significantly higher than that in the control group, indicating that treatment using doxofylline combined with ceftazidime was more effective than treatment with doxofylline alone.
In general, improving airway symptoms is the priority of COPD treatment. In the present study, the treated patients in the observation group had larger increases in peak expiratory flow rate, maximal mid-expiratory flow curve, and forced expiratory respiration volume of 1 s than did the control group, and there were no significant differences between the observation group and the blank control group. The results further indicated that treatment using doxofylline combined with ceftazidime was significantly more effective than the treatment using doxofylline alone. Also, the quality-of-life scores, including physiological function, social function, role restriction, and overall health, indicated that the treatment using doxofylline combined with ceftazidime was significantly more effective than the treatment of doxofylline. There were no significant differences in quality-of-life scores in the blank control group. Patients with COPD usually have long-term low oxygen levels and chronic inflammation, which encourage the secretion of inflammatory factors [22–25]. Our results showed that the levels of TNF-α and PDGF-B in the observation group were significantly lower those in the control group, and there were no significant differences in levels between the observation group and the blank control group.
Conclusions
In this study, doxofylline combined with ceftazidime effectively treated patients with COPD complicated with infection. The results of this study provide a reference for the clinical treatment of COPD.
References
1. Rabe KF, Watz H, Chronic obstructive pulmonary disease: Lancet, 2017; 389(10082); 1931-40
2. Duffy SP, Criner GJ, Chronic obstructive pulmonary disease: Evaluation and management: Med Clin North Am, 2019; 103(3); 453-61
3. Vogelmeier CF, Criner GJ, Martínez FJ, global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report: GOLD executive summary: Arch Bronconeumol, 2017; 53(3); 128-49
4. Giusti M, Blasi F, Iori I, Prulifloxacin vs levofloxacin for exacerbation of COPD after failure of other antibiotics: COPD, 2016; 13(5); 555-60
5. Lin TH, Chen SI, Su YC, Conventional western treatment combined with Chinese herbal medicine alleviates the progressive risk of lung cancer in patients with chronic obstructive pulmonary disease: A nationwide retrospective cohort study: Front Pharmacol, 2019; 10; 987
6. Chen S, Kwong JSW, Zheng R, Normative application of xiyanping injection: A systematic review of adverse case reports: Evid Based Complement Alternat Med, 2018; 2018; 4013912
7. Cazzola M, Matera MG, The effect of doxofylline in asthma and COPD: Respir Med, 2020; 164; 105904
8. Lal D, Manocha S, Ray A, Comparative study of the efficacy and safety of theophylline and doxofylline in patients with bronchial asthma and chronic obstructive pulmonary disease: J Basic Clin Physiol Pharmacol, 2015; 26(5); 443-51
9. Gudiol C, Cuervo G, Shaw E: Expert Opin Pharmacother, 2017; 18(18); 1947-63
10. Torres A, Chalmers JD, Dela Cruz CS, Challenges in severe community-acquired pneumonia: A point-of-view review: Intensive Care Med, 2019; 45(2); 159-71
11. McGuinness AJ, Sapey E, Oxidative stress in COPD: Sources, markers, and potential mechanisms: J Clin Med, 2017; 6(2); 21
12. Sun Q, Liu L, Mandal J, PDGF-BB induces PRMT1 expression through ERK1/2 dependent STAT1 activation and regulates remodeling in primary human lung fibroblasts: Cell Signal, 2016; 28(4); 307-15
13. Qian CL, Fan R, Effect of Pingchuan Guben decoction on patients with chronic obstructive pulmonary disease: Results from a randomized comparative effectiveness research trial: Exp Ther Med, 2017; 14(4); 3915-25
14. Franssen FM, Alter P, Bar N, Personalized medicine for patients with COPD: Where are we?: Int J Chron Obstruct Pulmon Dis, 2019; 14; 1465-84
15. Peepratoom B, Low G, Malathum P, A structural equation model of health-related quality of life among Thai men with chronic obstructive pulmonary disease: J Clin Nurs, 2020; 29(13–14); 2638-51
16. Martinez-Garcia MA, Miravitlles M, Bronchiectasis in COPD patients: More than a comorbidity?: Int J Chron Obstruct Pulmon Dis, 2017; 12; 1401-11
17. Burtin C, Hebestreit H, Rehabilitation in patients with chronic respiratory disease other than chronic obstructive pulmonary disease: Exercise and physical activity interventions in cystic fibrosis and non-cystic fibrosis bronchiectasis: Respiration, 2015; 89(3); 181-89
18. Scheeren TWL, Welte T, Saulay M, Early improvement in severely ill patients with pneumonia treated with ceftobiprole: A retrospective analysis of two major trials: BMC Infect Dis, 2019; 19(1); 195
19. Tantucci C, Modina D, Lung function decline in COPD: Int J Chron Obstruct Pulmon Dis, 2012; 7; 95-99
20. André S, Conde B, Fragoso E, COPD and cardiovascular disease: Pulmonology, 2019; 25(3); 168-76
21. Itoh M, Tsuji T, Nemoto K, Undernutrition in patients with COPD and its treatment: Nutrients, 2013; 5(4); 1316-35
22. Smith MP, Diagnosis and management of bronchiectasis: CMAJ, 2017; 189(24); E828-35
23. Zhao P, Li J, Yang L, Integration of transcriptomics, proteomics, metabolomics and systems pharmacology data to reveal the therapeutic mechanism underlying Chinese herbal Bufei Yishen formula for the treatment of chronic obstructive pulmonary disease: Mol Med Rep, 2018; 17(4); 5247-57
24. Caram LM, Ferrari R, Naves CR, Risk factors for cardiovascular disease in patients with COPD: Mild-to-moderate COPD versus severe-to-very severe COPD: J Bras Pneumol, 2016; 42(3); 179-84
25. Jones PW, Harding G, Berry P, Development and first validation of the COPD Assessment Test: Eur Respir J, 2009; 34(3); 648-54
Figures
Tables
 Table 1. Comparison of patients’ general information.
Table 1. Comparison of patients’ general information. Table 2. Comparison of efficacy.
Table 2. Comparison of efficacy.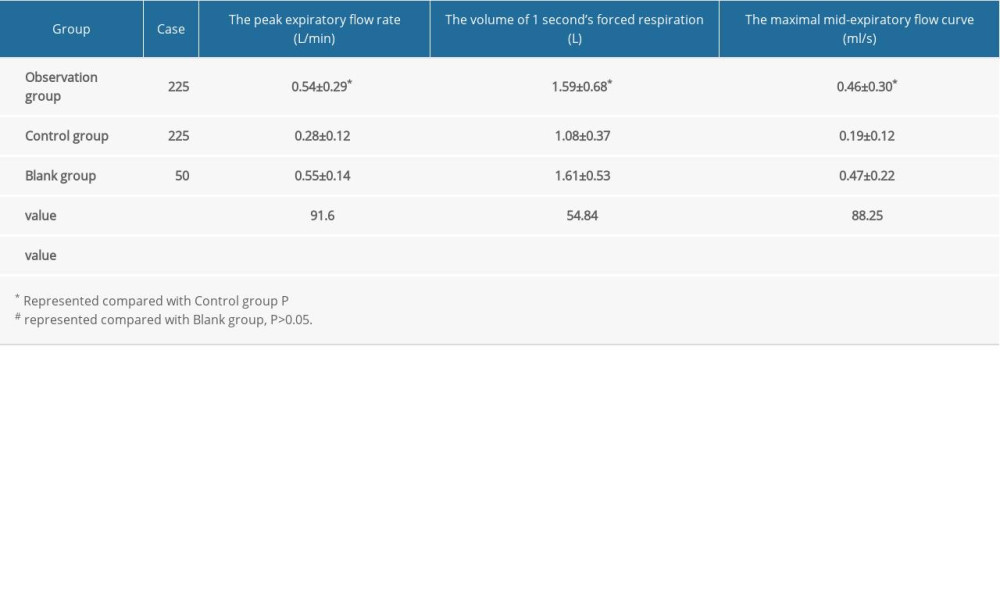 Table 3. Comparison of pulmonary function.
Table 3. Comparison of pulmonary function.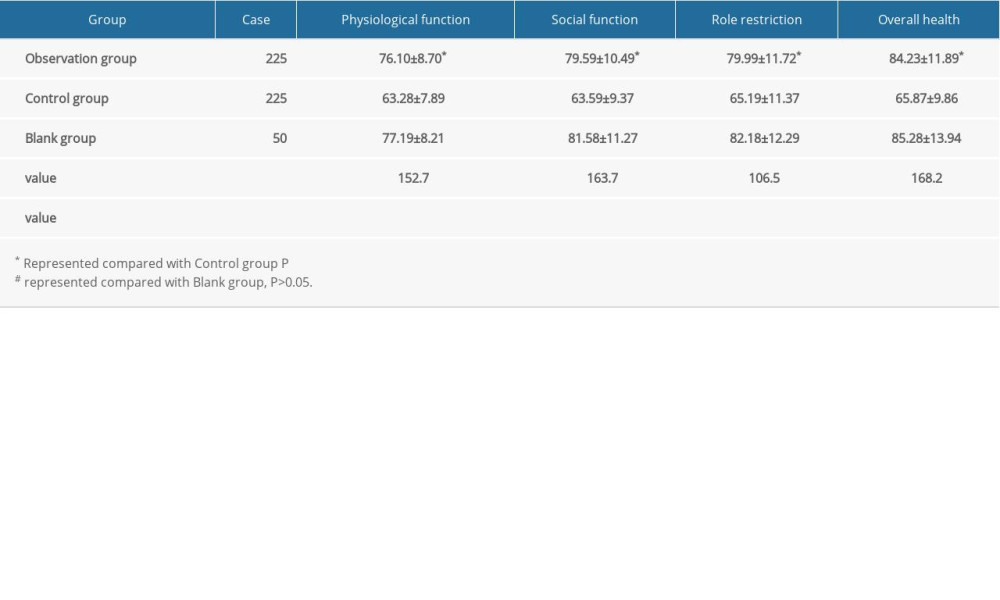 Table 4. Comparison of patients’ quality-of-life scores.
Table 4. Comparison of patients’ quality-of-life scores.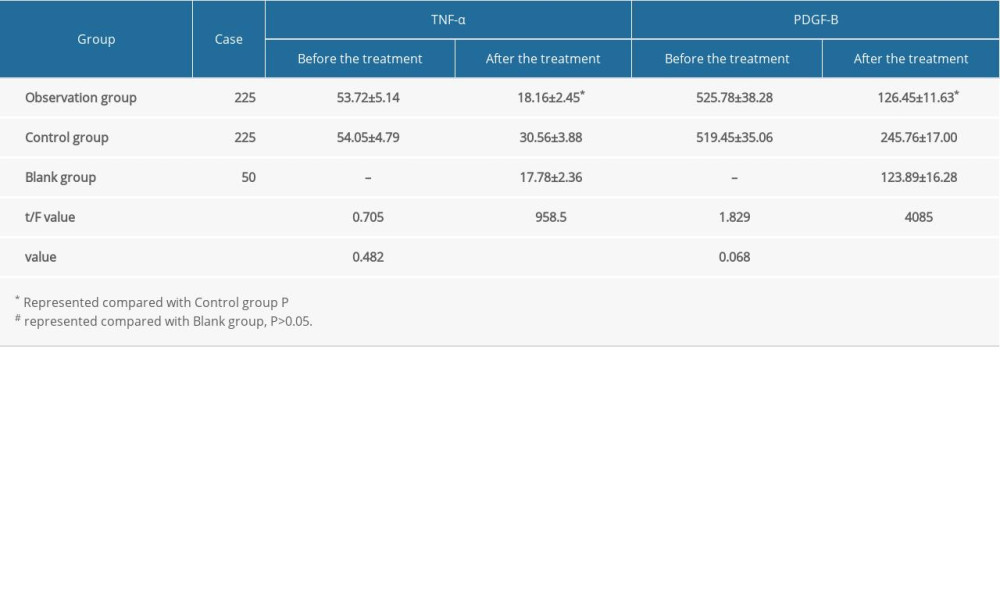 Table 5. Comparison of peripheral blood TNF-α and PGDF-B levels.
Table 5. Comparison of peripheral blood TNF-α and PGDF-B levels. Table 1. Comparison of patients’ general information.
Table 1. Comparison of patients’ general information. Table 2. Comparison of efficacy.
Table 2. Comparison of efficacy. Table 3. Comparison of pulmonary function.
Table 3. Comparison of pulmonary function. Table 4. Comparison of patients’ quality-of-life scores.
Table 4. Comparison of patients’ quality-of-life scores. Table 5. Comparison of peripheral blood TNF-α and PGDF-B levels.
Table 5. Comparison of peripheral blood TNF-α and PGDF-B levels. In Press
06 Mar 2024 : Clinical Research
Comparison of Outcomes between Single-Level and Double-Level Corpectomy in Thoracolumbar Reconstruction: A ...Med Sci Monit In Press; DOI: 10.12659/MSM.943797
21 Mar 2024 : Meta-Analysis
Economic Evaluation of COVID-19 Screening Tests and Surveillance Strategies in Low-Income, Middle-Income, a...Med Sci Monit In Press; DOI: 10.12659/MSM.943863
10 Apr 2024 : Clinical Research
Predicting Acute Cardiovascular Complications in COVID-19: Insights from a Specialized Cardiac Referral Dep...Med Sci Monit In Press; DOI: 10.12659/MSM.942612
06 Mar 2024 : Clinical Research
Enhanced Surgical Outcomes of Popliteal Cyst Excision: A Retrospective Study Comparing Arthroscopic Debride...Med Sci Monit In Press; DOI: 10.12659/MSM.941102
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952