13 June 2021: Clinical Research
Hemorheological Alteration in Patients with Cirrhosis Clinically Diagnosed with Portal Vein System Thrombosis After Splenectomy
Long Huang1ABCEF*, Qingsheng Yu1AEF, Hui Peng1CDEDOI: 10.12659/MSM.931157
Med Sci Monit 2021; 27:e931157
Abstract
BACKGROUND: Portal vein system thrombosis (PVST) is a common and serious complication after splenectomy. Key factors, including wider diameters of the portal vein, decreased liver function, and high flow volume of portosystemic collateral vessel, are recognized PVST risks. Relationships between PVST and altered hemorheology, including increased plasma viscosity, remain unclear. We investigated hemorheological alterations and explored risk factors of PVST in patients with cirrhosis after splenectomy.
MATERIAL AND METHODS: Data on patients with cirrhosis who underwent splenectomy were collected retrospectively from January 2018 to June 2020. Color Doppler ultrasonography was performed after splenectomy. Hemorheological indexes were compared between groups. Receiver operating characteristic (ROC) analysis was conducted to analyze risk factor cutoff values. Univariate and multivariate analyses were conducted to explore risk factors of PVST.
RESULTS: A total of 50 patients were divided into a PVST group (n=30) and control group (n=20). Hemorheological indexes of activated partial thromboplastin time, fibrinogen degradation products (FDP), D-dimer, middle shear rates 50 and 30, low shear rates 5 and 1, and hematocrit in the PVST group were significantly higher than those of the control group (P<0.05). FDP and low shear rate 1 were found to be risk factors of PVST after splenectomy by multivariate analysis. ROC analysis showed that the cutoff points for FDP and low shear rate 1 were ≥38.6 ug/mL and ≥16.855 mPa.s, respectively.
CONCLUSIONS: PVST after splenectomy is closely related to hemorheological alteration. FDP and low shear rate 1 may be valuable markers of PVST.
Keywords: Hemorheology, Liver Cirrhosis, Portal System, Hypertension, Portal, Laparoscopy, Portal Vein, Postoperative Complications, Risk Factors, Splenectomy, Venous Thrombosis
Background
As an important organ in hemorheology, the liver synthesizes many crucial components of the body, including plasma proteins, lipids, and coagulation factors. The liver also controls the cell composition of the blood, having an important and decisive role in the changes of hemorheology. Liver function in patients with chronic liver disease is impaired to varying degrees, especially in cirrhosis, which leads to hemorheological alteration [1]. Portal vein system thrombosis (PVST) is a common and serious complication with different causes, including decreased antithrombin III activity, large splenic vein diameter, wider diameters of the portal vein, splenic sequela of babesiosis, and hemorheological abnormalities [2,3]. Although previous studies have comprehensively explored the risk factors of PVST [4–7], the effects of hemorheological alterations on thrombosis, including plasma viscosity, shear rate, erythrocyte deformation index, and erythrocyte rigidity index, have not been reported.
In liver cirrhosis, the chemical and physical properties of blood is changed, including changes in the deformation of erythrocytes and plasma protein abnormalities, which together lead to hemorheological abnormalities. With the progression of liver disease, the abnormality of blood composition becomes more serious.
Blood viscosity refers to the resistance formed by the friction between 2 adjacent parallel fluid layers when the blood flows. It is affected by hematocrit, deformation and aggregation of erythrocytes, plasma viscosity, and plasma proteins, including fibrinogen, globulins, and albumin. Furthermore, patients with cirrhosis have lower plasma viscosity owing to the lower concentration of fibrinogen, albumin, and other larger proteins in the body, which show less viscosity resistance [8]. In contrast, splenectomy can increase the viscosity of whole blood, increase platelets, and reduce the deformation of erythrocytes, thus affecting the changes in blood rheology [9,10].
PVST is a common and serious complication after splenectomy, which can aggravate portal hypertension, jaundice, hepatic encephalopathy, liver failure, and other serious complications. However, PVST forms easily in patients with cirrhosis and hypersplenism after splenectomy, which can be related to the increased plasma viscosity, recovery of coagulation function, and sharply elevated platelets [11]. Therefore, the prevention of PVST after splenectomy is important.
Currently, a few key factors, including wider diameters of the portal vein, worse liver function, and high flow volume of portosystemic collateral vessel, have been recognized as risk factors of PVST [12–15]. Although the risk factors of PVST have been studied, some novel factors regarding hemorheological alterations have not. Hemorheological disorders are often secondary in patients who have concomitant cirrhosis and hypersplenism after splenectomy [16]. Furthermore, there are presently few studies on the relationship between hemorheology and PVST in patients with cirrhosis after splenectomy.
PVST is recognized as a serious and life-threatening complication after splenectomy [17,18]; however, the relationship between risk factors of PVST and hemorheology remains unclear [19,20]. Because of the severity of PVST and its associated risks, predicting the occurrence of PVST by monitoring the changes of hemorheology values is important [21]. Therefore, an optimal critical value of hemorheological changes that can be used as an important indicator of PVST should be determined. The present study aimed to explore the value of hemorheology in the portal vein thrombosis of patients with cirrhosis after splenectomy by comparing the hemorheological indexes between patients with PVST and patients without PVST. Further, we aimed to determine comprehensive hemorheological indicators to explore risk factors of PVST after splenectomy.
Material and Methods
SURGERY:
All included patients received endotracheal intubation and intravenous anesthesia for traditional splenectomy. Surgery was performed with patients in the supine position, and a paramedian incision was made on the left upper abdomen. The splenic artery was fully exposed for ligation and autologous splenic blood was transfused. With splenic vessels ligated by bundling, the spleen was removed and a latex drainage tube was placed in the splenic fossa for drainage effusion. The drainage tube was used to monitor postoperative bleeding and pancreatic leakage. The drainage tube was removed when there was only a small amount drainage fluid and the amylase level of the drainage fluid was normal. Five staff surgeons performed all of the operations. Postoperative complications, including bleeding, abdominal infection, abdominal collection, PVST, and hepatic failure, were routinely monitored for 2 weeks.
COLOR DOPPLER ULTRASOUND DETECTION:
Color Doppler ultrasound detection was performed by a single experienced examiner with a color Doppler ultrasound system (ACUSON S2000, Siemens, USA) and a broadband convex array probe (3–5 MHz). In general, all patients received routine ultrasound examination at admission and every 7 days after surgery to monitor for the occurrence of PVST.
LABORATORY TESTS:
Preoperative laboratory examinations were done on admission. Coagulation and hemorheological parameters were collected on postoperative day 2. Coagulation function was detected by a hemagglutination analyzer, and the indexes of blood rheology were detected by an automatic hemorheology analyzer.
STATISTICAL ANALYSIS:
A receiver operating characteristic (ROC) curve was analyzed to determine the optimal cutoff values of each hemorheological indicator. The association between the hemorheological indicators and influencing factors of PVST was assessed using univariate analyses, and those variables showing statistical significance (
Results
OUTCOMES AND COMPLICATIONS:
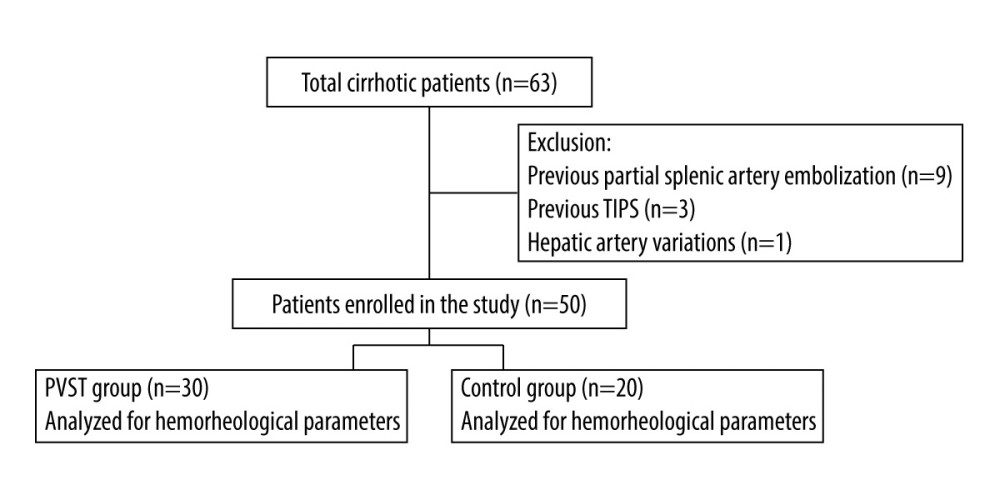
Splenectomy was performed successfully in all included patients. No patient died after surgery. Two patients had abdominal hemorrhage after surgery and received blood transfusions. Five patients who had ascites received diuretic therapy, and 2 patients developed incisional infections. A total of 30 patients had PVST 7 days after surgery and received heparin therapy. According to the location of the thrombus, the PVST group was divided into the following 5 subgroups: (1) main portal vein/portal vein branch (n=13); (2) splenic vein (n=4); (3) main portal vein/portal vein branch+superior mesenteric vein (n=2); (4) main portal vein/portal vein branch+splenic vein (n=5); and (5) main portal vein/portal vein branch+superior mesenteric vein+splenic vein (n=6). All patients fully recovered after medical treatment. Patient outcomes and complications are listed in Table 1.
HEMORHEOLOGY INDEXES:
The coagulation and hemorheological indexes between the 2 groups on the second day after surgery are compared in Table 2. In comparison with the control group, the PVST group revealed significant differences in activated partial thromboplastin time, fibrinogen degradation products (FDP), D-dimer, middle shear rate 50, middle shear rate 30, low shear rate 5, low shear rate 1, and hematocrit level (P<0.05).
RISK FACTORS OF PVST:
FDP >38.6 ug/mL, middle shear rate 50 >3.75 mPa.s, middle shear rate 30 >4.165 mPa.s, low shear rate 5 >6.705 mPa.s, low shear rate 1 >16.855 mPa.s, and erythrocyte sedimentation rate (ESR) >9.0 mm/L were determined as the optimal cutoff values by ROC curve analysis. The comparison of sensitivity and 1-specificity are shown in Table 3.
When the coagulation and hemorheological indexes were assessed by univariate analysis to determine their relationship to PVST, statistical significance was detected for FDP, middle shear rate 50, middle shear rate 30, low shear rate 5, low shear rate 1, and ESR (P<0.05). The FDP and low shear rate 1 were determined as independent risk factors of PVST by multivariate logistic analysis. The comparison of univariate and multivariate logistic analysis of risk factors of PVST in patients is shown in Table 4.
Discussion
In the continuous progression of cirrhosis, splenomegaly and hypersplenism can occur, which can cause hemorheological alterations. Splenectomy can effectively control the symptoms of hypersplenism; however, the blood rheology of patients after splenectomy is characterized by increased blood viscosity and a hypercoagulable state [27,28]. Furthermore, complications such as mesentery and portal vein thrombosis can easily occur with a rebounded platelet count, seriously affecting patient prognosis. Hemorheological alterations are mainly related to the changes of blood fluidity and blood viscosity. Splenomegaly can reduce the blood viscosity of patients and increase blood viscosity after splenectomy [29]. At the same time, the intima of blood vessels is damaged and platelet adhesion and aggregation occur, thereby allowing portal vein or mesentery thrombosis to easily form [30]. The process of thrombosis is closely related to changes in blood rheology; therefore, the relationship between splenectomy and hemorheological alterations should be given increased attention. Although some risk factors of PVST, including a large main portal vein diameter, large splenic vein diameter, low white blood cell count, and decreased antithrombin III activity, have been reported in the literature, the hemorheological factors are rarely involved. The present study mainly focused on hemorheological alterations regarding PVST, including plasma viscosity, shear rate, FDP, erythrocyte deformation index, and erythrocyte rigidity index, which had not been reported before. At present, there is little research on the relationship between thrombosis and hemorheological alterations after splenectomy. Therefore, the study of changes of blood rheology in patients with cirrhosis and hypersplenism during the perioperative period of splenectomy can provide an important theoretical basis of the prevention of thrombosis in patients with liver cirrhosis. Therefore, we performed a comprehensive study of hemorheological alterations in patients with cirrhosis and investigated the associations between risk factors of PVST after splenectomy and hemorheological indicators to better prevent the occurrence of PVST.
Patients with cirrhosis have lower plasma viscosity than do people without cirrhosis due to the lower concentration of fibrinogen, albumin, and other larger proteins in the body, which show less viscosity resistance [31]. However, the elevated plasma viscosity in patients with cirrhosis after splenectomy can be related to the increase in fibrinogen, which is increased from the inflammatory reaction and other elevated macromolecular proteins. Splenectomy alone will lead to an obvious inflammatory reaction in the body, thereby increasing the fibrinogen and the plasma viscosity of the body after splenectomy [32]. Blood viscosity refers to the resistance formed by the friction between 2 adjacent parallel fluid layers when the blood flows. It is a physical property of blood and represents the inherent resistance of blood against the flow within blood vessels [31]. Blood viscosity is influenced by factors such as hematocrit, fibrinogen, albumin, deformation and aggregation of erythrocytes, and plasma viscosity. Patients with cirrhosis have adapted to the low plasma viscosity over an extended time; therefore, the sudden increase in plasma viscosity after splenectomy, together with elevated platelets, can easily lead to a coagulation function disorder and PVST.
It is well known that the liver synthesizes many crucial components of the body, including plasma proteins, lipids, and coagulation factors, and meanwhile controls the cell composition of the blood. All of these processes are crucial influencing factors of hemorheology; therefore, the liver has a critical role in hemorheology [33–35]. Studies on hemorheology mainly involve the changes of blood fluidity, coagulation, and viscosity [36,37]. In the present study, we found that elevated blood viscosity easily formed PVST after splenectomy and affected the prognosis of patients at the same time. Therefore, the process of PVST is closely related to hemorheological alteration. After splenectomy, PVST is prone to occur in patients with cirrhosis with splenomegaly, which may be related to the increase in blood viscosity and changes of erythrocyte membrane structure. At present, there are few clinical studies focused on the relationship between PVST and hemorheological alteration after splenectomy in patients with cirrhosis.
FDP and low shear rate 1 were recognized as independent risk factors for PVST after splenectomy in our study (
Ordinarily, the blood viscosity of patients with cirrhosis increases after splenectomy and the platelet count temporarily increases, which leads to the formation of PVST [41,42]. The present study can aid future studies on the mechanism of PVST by comparing changes in blood rheology and D-dimer between the PVST group and control group. We discovered that middle shear rate 50, middle shear rate 30, low shear rate 5, low shear rate 1, and hematocrit were higher in the PVST group. At the same time, activated partial thromboplastin time, FDP, and D-dimer were also higher in the PVST group, and the differences were statistically significant (
Spleen autotransplantation has been recognized as a simple and effective method to compensate for splenic function after total splenectomy. A recent study showed that spleen autotransplantation could be useful to preserve the filtration function and alter red blood cell aggregation [45]. Because the influence of spleen resection on erythrocyte aggregation parameters in animal models is controversial, our hemorheological changes after splenectomy may help to improve the hemodynamic effects of spleen autotransplantation.
There are several limitations in the present study. First, this study was a retrospective and single-center investigation, which means it had a limited number of patients and contained accidental errors and biases. Second, the number of patients with cirrhosis was limited; therefore, data bias will appear in the results. These limitations will be considered in our future prospective studies, which will be improved over the present study if we conduct a randomized controlled trial with additional centers, thereby providing more data for further research.
Conclusions
In conclusion, hypersplenism after splenectomy in patients with cirrhosis can lead to hemorheological alterations and the easy formation of PVST. FDP and low shear rate 1 were independent risk factors for PVST after splenectomy in our study.
Tables
Table 1. Clinical characteristics of included patients, n (%).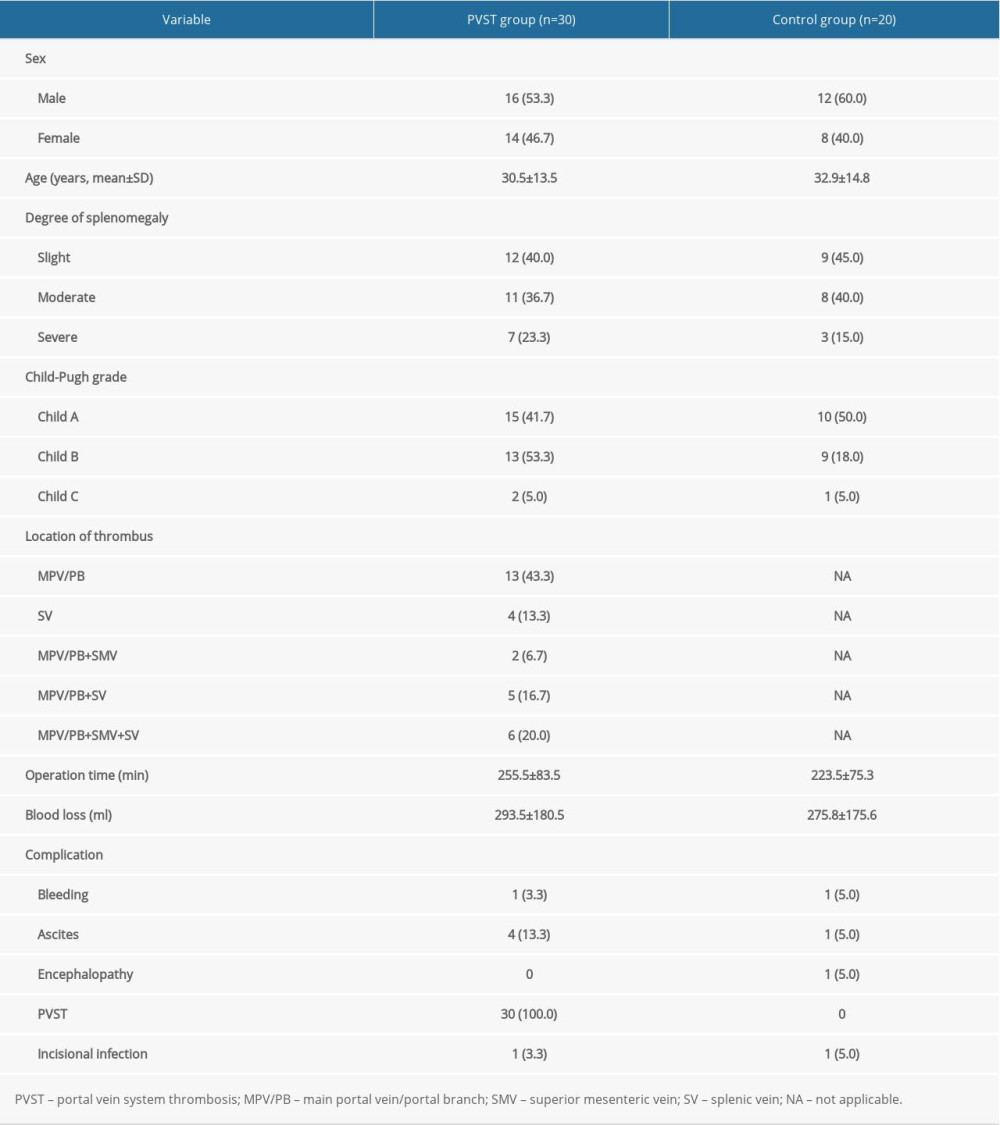 Table 2. Comparison of coagulation function and hemorheology between the portal vein system thrombosis (PVST) group and control group (n=50, mean±standard deviation).
Table 2. Comparison of coagulation function and hemorheology between the portal vein system thrombosis (PVST) group and control group (n=50, mean±standard deviation).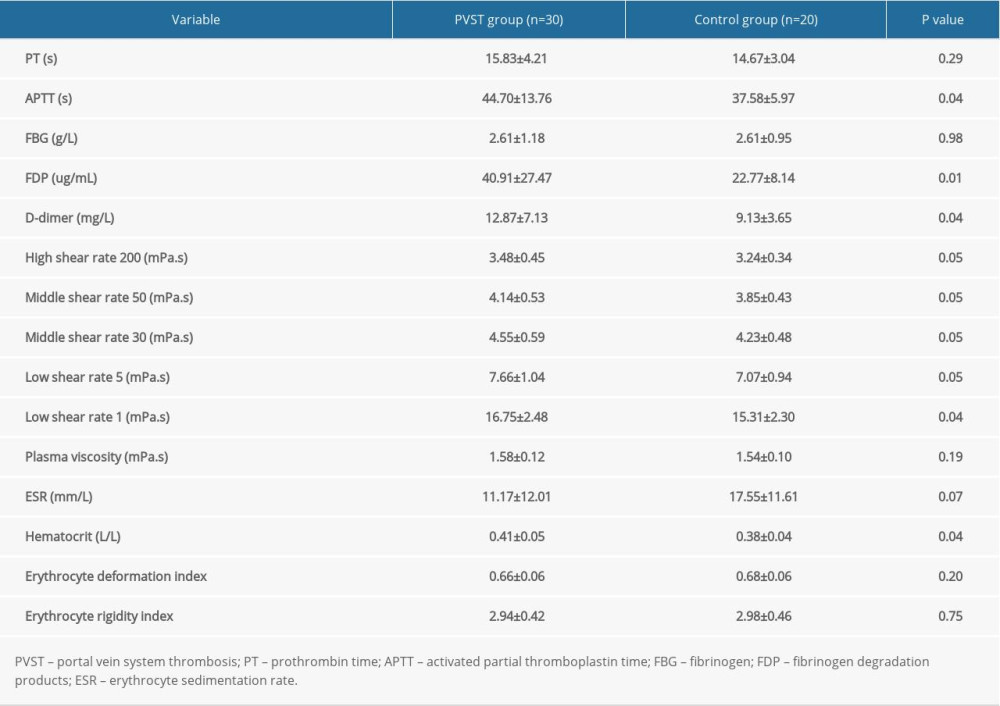 Table 3. Receiver operating characteristic curve analysis for identifying the optimal cutoff values of influencing factors of portal vein system thrombosis after splenectomy.
Table 3. Receiver operating characteristic curve analysis for identifying the optimal cutoff values of influencing factors of portal vein system thrombosis after splenectomy.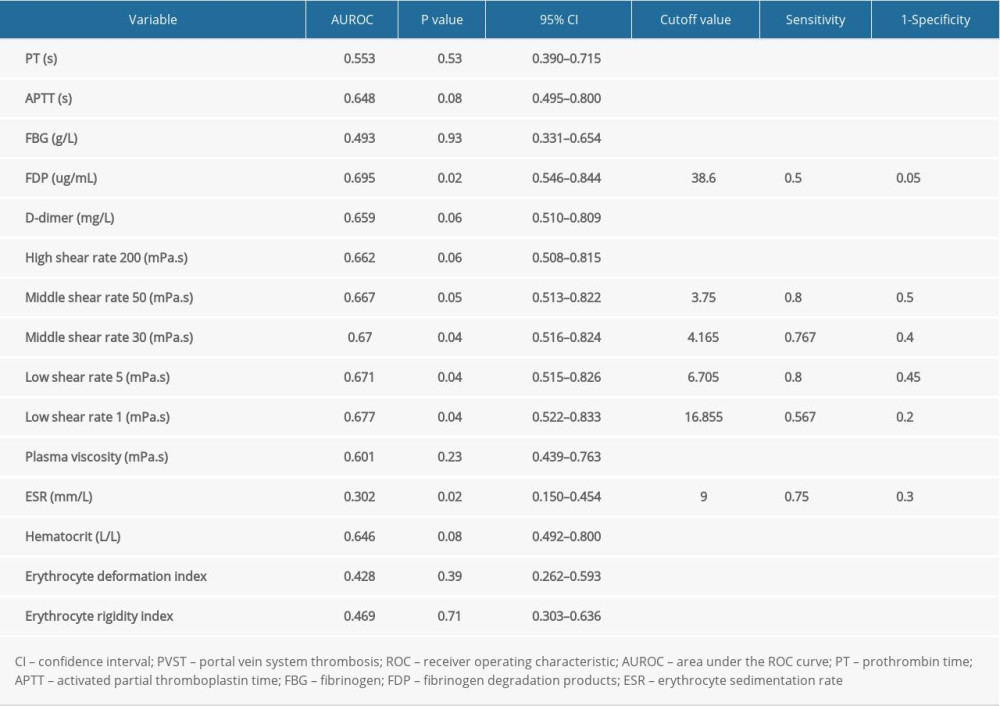 Table 4. Univariate and multivariate analysis of risk factors of portal vein system thrombosis observed by logistic regression.
Table 4. Univariate and multivariate analysis of risk factors of portal vein system thrombosis observed by logistic regression.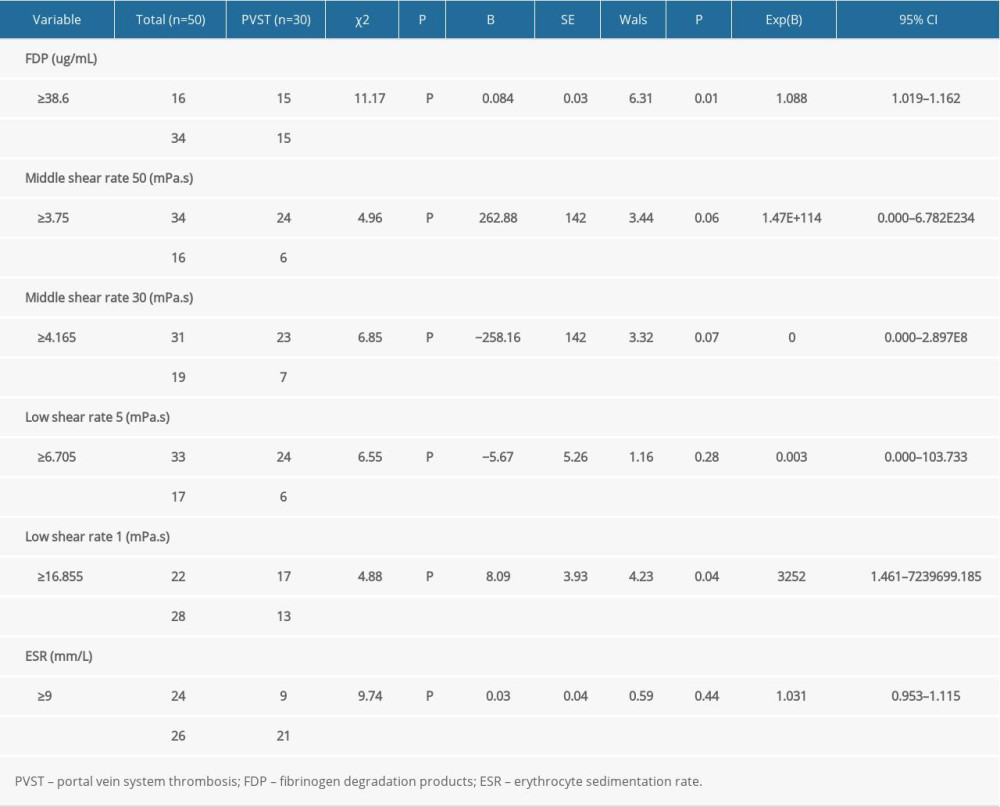
References
1. Liu TT, Wong WJ, Hou MC, Hemorheology in patients with liver cirrhosis: Special emphasis on its relation to severity of esophageal variceal bleeding: J Gastroenterol Hepatol, 2006; 21(5); 908-13
2. Sahu KK, Petrou N, Cohn Z, Bathini V, Splenic sequela of babesiosis: QJM, 2020; 113(8); 577-78
3. Denninger MH, Chaït Y, Casadevall N, Cause of portal or hepatic venous thrombosis in adults: The role of multiple concurrent factors: Hepatology, 2000; 31(3); 587-91
4. Kinjo N, Kawanaka H, Akahoshi T, Risk factors for portal venous thrombosis after splenectomy in patients with cirrhosis and portal hypertension: Br J Surg, 2010; 97(6); 910-16
5. Kawanaka H, Akahoshi T, Itoh S, Optimizing risk stratification in portal vein thrombosis after splenectomy and its primary prophylaxis with antithrombin III concentrates and danaparoid sodium in liver cirrhosis with portal hypertension: J Am Coll Surg, 2014; 219(5); 865-74
6. Ruiz-Tovar J, Priego P, Portal vein thrombosis after splenic and pancreatic surgery: Adv Exp Med Biol, 2017; 906; 241-51
7. Targarona EM, Portal vein thrombosis after laparoscopic splenectomy: The size of the risk: Surg Innov, 2008; 15(4); 266-70
8. Robertson DA, Simpson FG, Losowsky MS, Blood viscosity after splenectomy: Br Med J (Clin Res Ed), 1981; 283(6291); 573-75
9. Miko I, Nemeth N, Sogor V, Comparative erythrocyte deformability investigations by filtrometry, slit-flow and rotational ektacytometry in a long-term follow-up animal study on splenectomy and different spleen preserving operative techniques: Partial or subtotal spleen resection and spleen autotransplantation: Clin Hemorheol Microcirc, 2017; 66(1); 83-96
10. Shiraishi K, Tsuruya K, Anzai K, Effects of ethanol and acetaldehyde load on erythrocyte deformability in healthy subjects and patients with liver cirrhosis: Nihon Arukoru Yakubutsu Igakkai Zasshi, 2015; 50(1); 13-18
11. Huang L, Yu Q, Wang J, association between changes in splanchnic hemodynamics and risk factors of portal venous system thrombosis after splenectomy with periesophagogastric devascularization: Med Sci Monit, 2018; 24; 4355-62
12. Kuroki T, Kitasato A, Tokunaga T, Predictors of portal and splenic vein thrombosis after laparoscopic splenectomy: A retrospective analysis of a single-center experience: Surg Today, 2018; 48(8); 804-9
13. Qi X, Li H, Liu X, Novel insights into the development of portal vein thrombosis in cirrhosis patients: Expert Rev Gastroenterol Hepatol, 2015; 9(11); 1421-32
14. Dai J, Qi X, Peng Y, Association between D-dimer level and portal venous system thrombosis in liver cirrhosis: A retrospective observational study: Int J Clin Exp Med, 2015; 8(9); 15296-301
15. Zhang X, Wang Y, Yu M, effective prevention for portal venous system thrombosis after splenectomy: A meta-analysis: J Laparoendosc Adv Surg Tech A, 2017; 27(3); 247-52
16. Kumsishvili T, Varazashvili M, McHedlishvili G, Local hemorheological disorders during chronic inflammation: Clin Hemorheol Microcirc, 2004; 30(3–4); 427-29
17. Stamou KM, Toutouzas KG, Kekis PB, Prospective study of the incidence and risk factors of postsplenectomy thrombosis of the portal, mesenteric, and splenic veins: Arch Surg, 2006; 141(7); 663-69
18. Pietrabissa A, Moretto C, Antonelli G, Thrombosis in the portal venous system after elective laparoscopic splenectomy: Surg Endosc, 2004; 18(7); 1140-43
19. Chawla YK, Bodh V, Portal vein thrombosis: J Clin Exp Hepatol, 2015; 5(1); 22-40
20. Qi X, Portal vein thrombosis: Recent advance: Adv Exp Med Biol, 2017; 906; 229-39
21. Sharma AM, Zhu D, Henry Z, Portal vein thrombosis: When to treat and how?: Vasc Med, 2016; 21(1); 61-69
22. Xu XY, Ding HG, Li WG, Chinese guidelines on the management of liver cirrhosis (abbreviated version): World J Gastroenterol, 2020; 26(45); 7088-103
23. Angeli P, Bernardi M, Villanueva C, EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis: J Hepatol, 2018; 69(2); 406-60
24. European Association for Study of Liver, EASL Clinical Practice Guidelines: Wilson’s disease: J Hepatol, 2012; 56(3); 671-85
25. Ikegami T, Soejima Y, Taketomi A, Hypersplenism after living donor liver transplantation: Hepatogastroenterology, 2009; 56; 778-82
26. Parikh S, Shah R, Kapoor P, Portal vein thrombosis: Am J Med, 2010; 123(2); 111-19
27. Tikhomirova I, Petrochenko E, Malysheva Y, Interrelation of blood coagulation and hemorheology in cancer: Clin Hemorheol Microcirc, 2016; 64(4); 635-44
28. Hayashi Y, Brun MA, Machida K, Simultaneous assessment of blood coagulation and hematocrit levels in dielectric blood coagulometry: Biorheology, 2017; 54(1); 25-35
29. Ha YR, Kang YJ, Lee SJ, In vivo study on splenomegaly inhibition by genistein in Plasmodium berghei-infected mice: Parasitol Int, 2015; 64(5); 369-76
30. Harding DJ, Perera MT, Chen F, Portal vein thrombosis in cirrhosis: Controversies and latest developments: World J Gastroenterol, 2015; 21(22); 6769-84
31. Jang B, Han JW, Sung PS, Hemorheological alteration in patients clinically diagnosed with chronic liver diseases: J Korean Med Sci, 2016; 31(12); 1943-48
32. Liu TT, Wong WJ, Hou MC, Hemorheology in patients with liver cirrhosis: Special emphasis on its relation to severity of esophageal variceal bleeding: J Gastroenterol Hepatol, 2006; 21(5); 908-13
33. Tennent GA, Brennan SO, Stangou AJ, Human plasma fibrinogen is synthesized in the liver: Blood, 2007; 109(5); 1971-74
34. Tamer S, Cefle K, Gokkusu C, Comparison of rheological parameters in patients with post hepatitic and alcoholic cirrhosis: Clin Hemorheol Microcirc, 2007; 36; 247-52
35. Anwar MA, Rampling MW, Abnormal hemorheological properties in patients with compensated and decompensated hepatic cirrhosis: Clin Hemorheol Microcirc, 2003; 29; 95-101
36. Tripodi A, Mannucci PM, The coagulopathy of chronic liver disease: N Engl J Med, 2011; 365; 147-56
37. Baskurt OK, Meiselman HJ, Blood rheology and hemodynamics: Semin Thromb Hemost, 2003; 29; 435-50
38. Byers JM, Rudolf Virchow – father of cellular pathology: Am J Clin Pathol, 1989; 92(4 Suppl 1); S2-S8
39. Kwon J, Koh Y, Yu SJ, Yoon JH, Low-molecular-weight heparin treatment for portal vein thrombosis in liver cirrhosis: Efficacy and the risk of hemorrhagic complications: Thromb Res, 2018; 163; 71-76
40. Tripodi A, Primignani M, Braham S, Coagulation parameters in patients with cirrhosis and portal vein thrombosis treated sequentially with low molecular weight heparin and vitamin K antagonists: Dig Liver Dis, 2016; 48(10); 1208-13
41. Dai J, Qi X, Li H, Guo X, Role of D-dimer in the development of portal vein thrombosis in liver cirrhosis: A meta-analysis: Saudi J Gastroenterol, 2015; 21(3); 165-74
42. Dai J, Qi X, Peng Y, Association between D-dimer level and portal venous system thrombosis in liver cirrhosis: A retrospective observational study: Int J Clin Exp Med, 2015; 8(9); 15296-301
43. Stringer MD, Lucas N, Thrombocytosis and portal vein thrombosis after splenectomy for paediatric haemolytic disorders: How should they be managed?: J Paediatr Child Health, 2018; 54(11); 1184-88
44. Buzelé R, Barbier L, Sauvanet A, Fantin B, Medical complications following splenectomy: J Visc Surg, 2016; 153(4); 277-86
45. Kinjo N, Kawanaka H, Akahoshi T, Risk factors for portal venous thrombosis after splenectomy in patients with cirrhosis and portal hypertension: Br J Surg, 2010; 97(6); 910-16
Tables
 Table 1. Clinical characteristics of included patients, n (%).
Table 1. Clinical characteristics of included patients, n (%). Table 2. Comparison of coagulation function and hemorheology between the portal vein system thrombosis (PVST) group and control group (n=50, mean±standard deviation).
Table 2. Comparison of coagulation function and hemorheology between the portal vein system thrombosis (PVST) group and control group (n=50, mean±standard deviation). Table 3. Receiver operating characteristic curve analysis for identifying the optimal cutoff values of influencing factors of portal vein system thrombosis after splenectomy.
Table 3. Receiver operating characteristic curve analysis for identifying the optimal cutoff values of influencing factors of portal vein system thrombosis after splenectomy. Table 4. Univariate and multivariate analysis of risk factors of portal vein system thrombosis observed by logistic regression.
Table 4. Univariate and multivariate analysis of risk factors of portal vein system thrombosis observed by logistic regression. Table 1. Clinical characteristics of included patients, n (%).
Table 1. Clinical characteristics of included patients, n (%). Table 2. Comparison of coagulation function and hemorheology between the portal vein system thrombosis (PVST) group and control group (n=50, mean±standard deviation).
Table 2. Comparison of coagulation function and hemorheology between the portal vein system thrombosis (PVST) group and control group (n=50, mean±standard deviation). Table 3. Receiver operating characteristic curve analysis for identifying the optimal cutoff values of influencing factors of portal vein system thrombosis after splenectomy.
Table 3. Receiver operating characteristic curve analysis for identifying the optimal cutoff values of influencing factors of portal vein system thrombosis after splenectomy. Table 4. Univariate and multivariate analysis of risk factors of portal vein system thrombosis observed by logistic regression.
Table 4. Univariate and multivariate analysis of risk factors of portal vein system thrombosis observed by logistic regression. In Press
05 Mar 2024 : Clinical Research
Role of Critical Shoulder Angle in Degenerative Type Rotator Cuff Tears: A Turkish Cohort StudyMed Sci Monit In Press; DOI: 10.12659/MSM.943703
06 Mar 2024 : Clinical Research
Comparison of Outcomes between Single-Level and Double-Level Corpectomy in Thoracolumbar Reconstruction: A ...Med Sci Monit In Press; DOI: 10.12659/MSM.943797
21 Mar 2024 : Meta-Analysis
Economic Evaluation of COVID-19 Screening Tests and Surveillance Strategies in Low-Income, Middle-Income, a...Med Sci Monit In Press; DOI: 10.12659/MSM.943863
10 Apr 2024 : Clinical Research
Predicting Acute Cardiovascular Complications in COVID-19: Insights from a Specialized Cardiac Referral Dep...Med Sci Monit In Press; DOI: 10.12659/MSM.942612
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952