28 October 2021: Clinical Research
Adverse Side Effects Associated with Corticosteroid Therapy: A Study in 39 Patients with Generalized Myasthenia Gravis
Stephen Johnson1ABCDEF, Nakul Katyal2DEF, Naureen Narula3DEF, Raghav Govindarajan2ABCDEF*DOI: 10.12659/MSM.933296
Med Sci Monit 2021; 27:e933296
Abstract
BACKGROUND: The tolerability of high-dose oral corticosteroids in patients with generalized myasthenia gravis (gMG) has not been systematically assessed. We evaluated adverse side effects (ASEs) of corticosteroid treatment in patients with gMG.
MATERIAL AND METHODS: Retrospective analysis was conducted of ASEs reported as being related to corticosteroid treatment in 39 patients with gMG who were treated with oral corticosteroids for ≥1 year.
RESULTS: Median (interquartile range [IQR]) age was 60 (21) years, 53.8% of patients were women, and 66.7% were aged ≤65 years. Median (IQR) prednisone treatment duration was 14 (2) months; median (IQR) daily dose was 40 (15) mg. The median number of ASEs reported as corticosteroid-related was 2/patient (IQR, 1). Pre-diabetes and weight gain were most common (each 43.6% of patients). Bruising, insomnia, and osteoporosis were more prevalent in patients aged >65 years, while irritability, osteopenia, and pre-diabetes were more common in patients aged £65 years, although differences were not statistically significant. Irritability and weight gain were more prevalent in women (P=0.010 for irritability); osteoporosis and pre-diabetes more common in men (P=0.015 for osteoporosis). ASEs were generally more common in the high-dose prednisone group (>30 mg/day), but were only statistically significant for irritability (P=0.001).
CONCLUSIONS: Corticosteroid-related ASEs were common in patients with gMG. Some of these ASEs can have serious medical consequences, and certain ASEs appeared to be associated with specific patient characteristics. Demographics and comorbidities of patients with gMG must be carefully considered before corticosteroid initiation. Potential ASEs, such as unanticipated osteoporosis in men, require extra vigilance.
Keywords: Myasthenia Gravis, Prednisone, Risk Factors, Safety, Steroids, Adrenal Cortex Hormones, Affect, Aged, 80 and over, Female, Humans, Male, Osteoporosis, Prediabetic State, Weight Gain, young adult
Background
Myasthenia gravis (MG) is a rare autoimmune disorder caused by autoantibodies that target elements of the neuromuscular junction [1,2]. The resulting muscle weakness can be localized (ocular or oculobulbar), but in most cases (approximately 85%) it is generalized, affecting the trunk and proximal muscles of the extremities [2]. Most patients with MG have autoantibodies against the acetylcholine receptor (AChR) [1]. Other autoantibodies involved in this condition include those targeting muscle-specific tyrosine kinase, agrin, and low-density lipoprotein receptor-related protein 4 [1,3]. The estimated prevalence and incidence of MG are 78 cases per million persons and 5.3 cases per million person-years, respectively, although results vary considerably between studies [4].
Acetylcholinesterase (AChE) inhibitors are the standard of care for the symptomatic treatment for MG and are supplemented with immunomodulating agents as necessary [3]. The latter include corticosteroids, azathioprine, cyclosporine, methotrexate, mycophenolate mofetil, and tacrolimus [3]. More recent additions include the monoclonal antibodies rituximab (anti-CD20 antibody) and eculizumab (a terminal complement inhibitor) [5–7]. Plasma exchange and intravenous immunoglobulin are frequently used to manage acute exacerbations of MG [3]. Of all these treatments, only eculizumab [8] and pyridostigmine (an AChE inhibitor) [9] are licensed in the United States to treat MG.
Corticosteroids have been used for their anti-inflammatory and immunosuppressant effects in a wide range of conditions since the 1940s [10]. A number of small studies in the 1950s and 60s described their beneficial effects in patients with MG, and their use was associated with a reduction in MG-related mortality to less than 10% from the 1960s onward, compared with 30% in 1955 [11,12]. Steroids’ immunosuppressive mechanism of action, relatively fast onset of action (compared with the slow onset of alternative immunosuppressants used in this setting), wide availability, low cost, and, as highlighted above, extensive track record across disciplines account for their continued place in therapy [10,13–15]. However, there are very few controlled studies evaluating their efficacy in MG [13]. Despite this, international consensus guidelines for the management of MG advocate the use of corticosteroids (or non-steroidal immunosuppressants) in all patients who do not meet treatment goals after an adequate trial of the AChE inhibitor pyridostigmine [16]. They also recommend gradual tapering of the corticosteroid dose once treatment goals are reached, noting that long-term use of low-dose corticosteroids can be appropriate in some patients [16].
Given their widespread use, the side effect profile of corticosteroids is well established. Adverse side effects (ASEs) range from mild to serious and affect a diverse range of body systems, including musculoskeletal (eg, osteoporosis and myopathy), metabolic and endocrine (eg, hyperglycemia, Cushing syndrome, weight gain, and hirsutism), immunologic (eg, infections), cardiovascular (eg, hypertension and arrhythmias), gastrointestinal (eg, gastric ulcers and bleeding), neuropsychiatric (eg, altered mood and psychosis), ophthalmologic (eg, glaucoma and cataract) and laboratory abnormalities (eg, hypokalemia) [15,17]. Corticosteroid-related ASEs, particularly in patients on high-dose and/or long-term regimens, can have a negative impact on patients’ quality of life [12] and require monitoring for the duration of steroid treatment [15]. Age, comorbidities, and concomitant use of immunosuppressants are other risk factors for corticosteroid-related ASEs [15].
Despite, or perhaps as a consequence of, the well-established ASE profile of corticosteroids, there is a lack of systematic evaluation of their tolerability in patients with MG. In retrospective long-term studies conducted in the 1980s and 90s [18–21], at least one ASE was observed in 52% of patients with MG treated with corticosteroids, including osteoporosis, diabetes mellitus, infection, and gastric ulcers [13]. More recently, small studies have evaluated the safety profile of corticosteroids in patients with ocular MG [22,23]. One of these followed 83 patients for a median of 58 months and documented a low rate of serious complications [22]; however, the median dose was tapered to 5 mg/day following initial treatment with higher doses. The authors highlighted that patients should be monitored to detect “relatively common but less serious complications” [22]. Another recent, large retrospective study that evaluated infections in patients with neuromuscular autoimmune conditions – including 358 corticosteroid-treated patients with MG – found a significant association between infection and corticosteroid use in a multivariate analysis, as well as an infection rate of 19.3% in patients with MG [24].
The objectives of the current study were to describe ASEs reported as being corticosteroid-related in a cohort of patients with MG and to determine potential risk factors for these ASEs.
Material and Methods
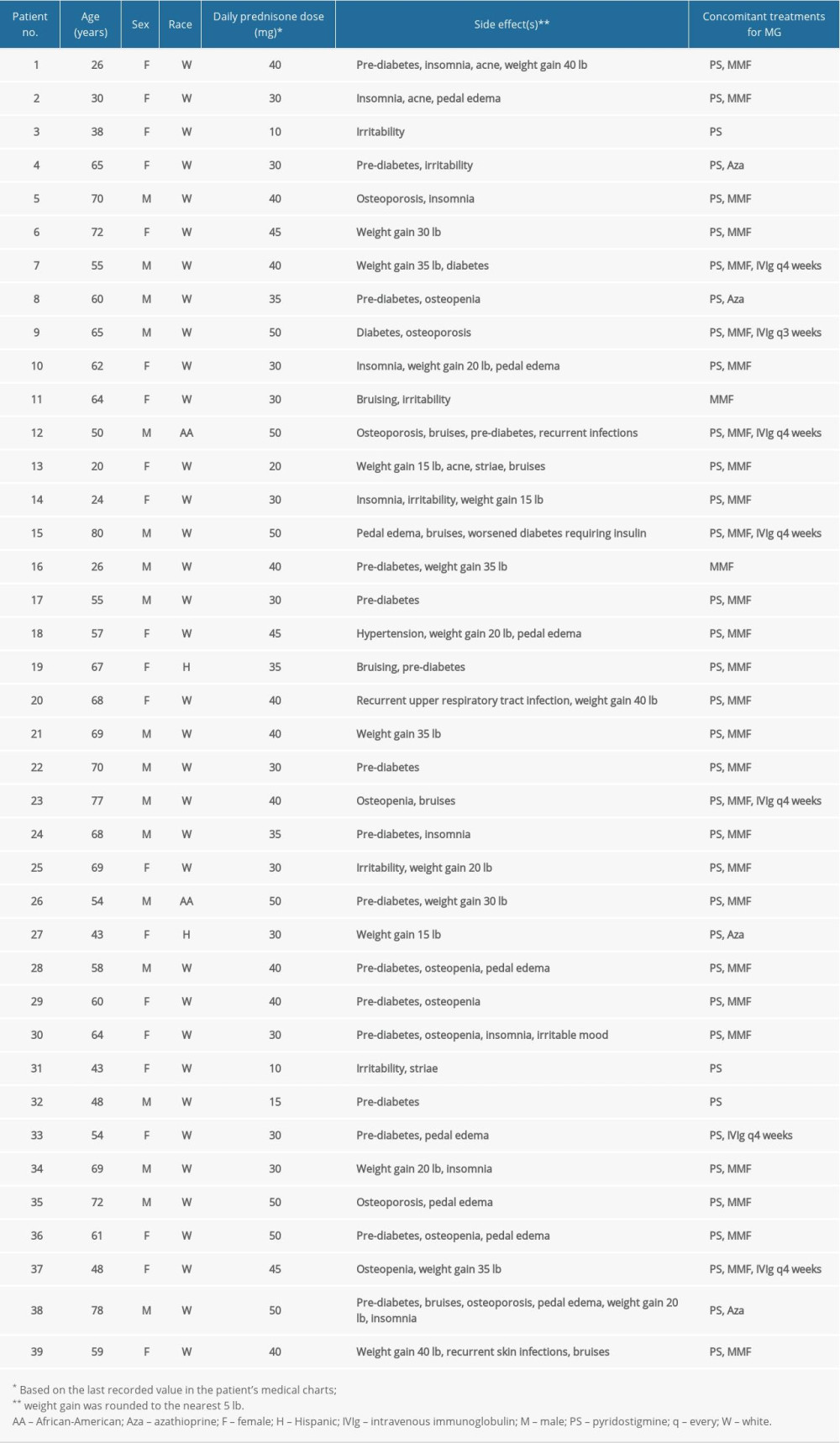
This was a retrospective analysis of data from patients with MG (aged ≥18 years) who were treated at a single center in the United States between January 2014 and December 2015. Patients were eligible for inclusion if they had been diagnosed with generalized MG (gMG), namely weakness of non-ocular muscles with or without ocular muscle weakness (Myasthenia Gravis Foundation of America [MGFA] Classes 2–4), had been treated with oral corticosteroids for their gMG symptoms for ≥1 year, and had ≥1 year of follow-up. They also had to have a decremental response on low-frequency repetitive nerve stimulation or abnormal jitter on single-fiber electromyography. Patients with muscle-specific tyrosine kinase antibodies were not included, as prednisone is not routinely the treatment of choice for these patients at our clinic.
The following information was extracted from each patient’s electronic medical record: sex, age, and race; MGFA class; pre-existing comorbidities; prednisone dose; ASEs that the treating physician judged to be steroid related (based on their own clinical experience and knowledge); other medications for MG; and Myasthenia Gravis – Activities of Daily Living (MG-ADL) scores. Non-fixed variables were based on the last recorded value in the patient’s chart. Differences in steroid doses according to sex (male vs female) and age (≤65 vs >65 years) were analyzed using a Mann-Whitney test.
Steroid-related ASEs were summarized in aggregate and subdivided according to patient age (≤65 vs >65 years), sex (male vs female), and steroid dose [25] (moderate [≤30 mg/day] vs high [>30 mg/day]). As the prednisone dose was not normally distributed, differences in the incidence of each ASE according to age, sex, and steroid dose were analyzed using Fisher’s exact test. No adjustments were made for multiplicity.
The study was approved by the University of Missouri Independent Review Board (approval number 2019523 MU). Patient consent was not required for this retrospective chart review.
Results
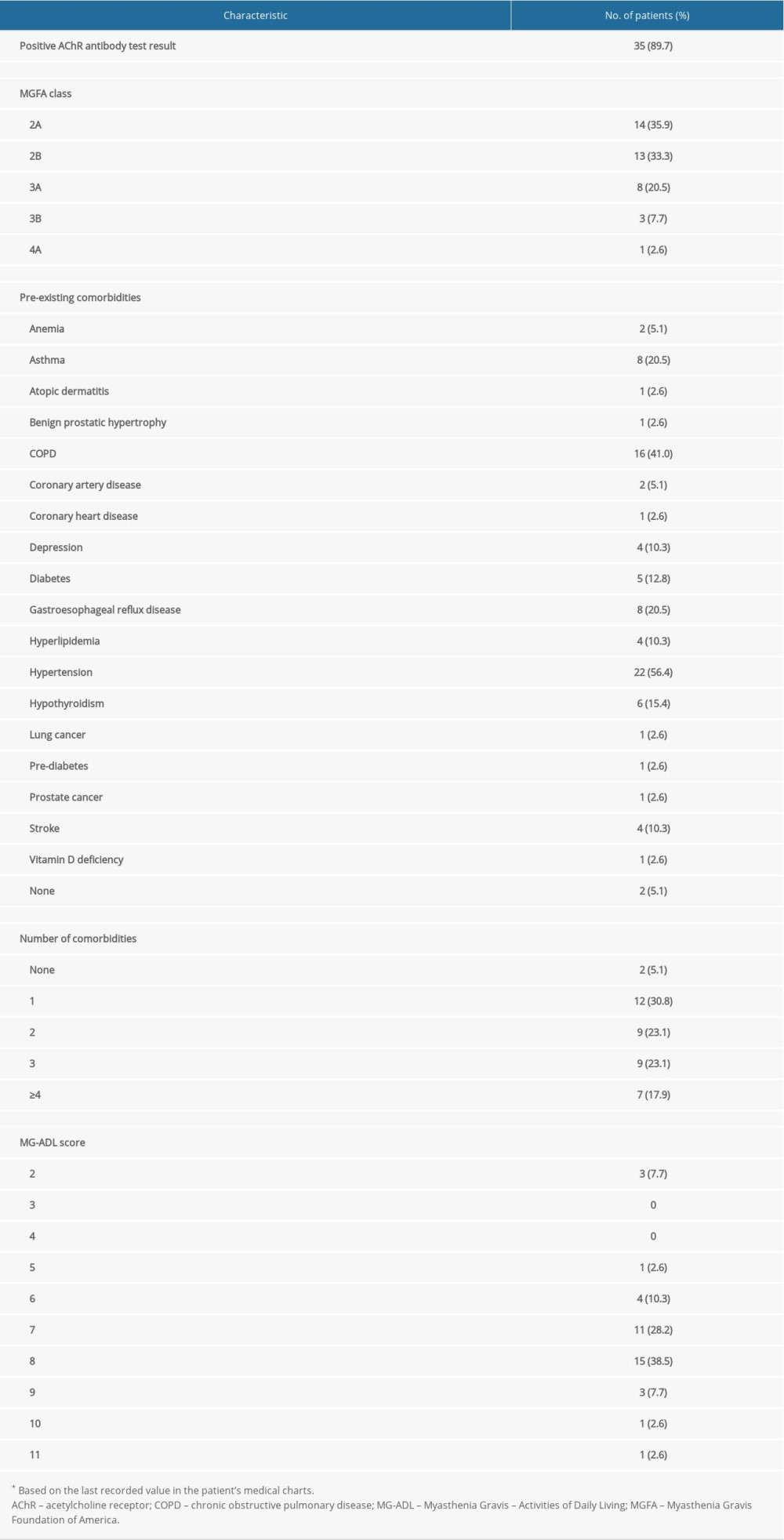
Data were obtained from 39 patients, of whom 21 (53.8%) were female; the median age (interquartile range [IQR]) was 60 (21) years, 26 (66.7%) patients were aged ≤65 years, and 35 (89.7%) were white. Individual patient data are provided in Table 1, and a summary of patients’ clinical characteristics is presented in Table 2. Overall, 35 patients (89.7%) had AChR antibody-positive MG and at the time of data collection, over 60% were categorized as MGFA disease class 2A or 2B (ie, they had mild weakness of the non-ocular muscles ± ocular weakness of any severity). Overall, 37 patients (94.9%) had comorbid conditions and 25 (64.1%) had ≥2 comorbidities (Table 2). The most common comorbid conditions were hypertension and chronic obstructive pulmonary disease. The median (IQR) MG-ADL score was 7 (1) and the range was 2 to 11 (out of a maximum possible score of 24).
The mean duration of prednisone treatment was 14.3 months (median, 14 months; IQR, 2 months) and the mean daily dose was 36.0 mg (median, 40 mg; range 10–50 mg; IQR 15 mg). Median (IQR) daily doses in non-elderly (≤65 years) and elderly (>65 years) patients were 32.5 (10) mg and 40 (10) mg, respectively (
Corticosteroid-related ASEs in the overall patient population are summarized in Figure 1. All patients experienced at least one corticosteroid-related ASE; the mean number of ASEs per patient was 2.3 (median, 2; IQR, 1); the greatest number of ASEs experienced by an individual patient was six. ASEs were reported at all prednisone doses, including 10 mg. Pre-diabetes (ie, elevated glucose concentration, but not high enough to be diagnosed with diabetes) was one of the most common ASEs, reported in 17 patients (43.6%); in addition, diabetes was reported in two patients, and worsening diabetes (monitored via HbA1c concentrations) was reported in one patient. Weight gain was also reported in 17 patients (43.6%); in over half of these cases, weight gain was ≥30 pounds (13.6 kg).
Corticosteroid-related ASEs according to age, sex, and prednisone dose are shown in Figure 2A–2C. Bruising, insomnia, and osteoporosis were more prevalent in elderly patients (those aged >65 years), while irritability, osteopenia, and pre-diabetes were more prevalent in younger patients (aged ≤65 years), although the differences were not statistically significant. In women, the incidence of irritability was significantly higher than in men (
Discussion
Although the general ASE profile of corticosteroids is well established, there is a lack of published information on their tolerability in patients with MG. The current study provides some interesting insights into corticosteroid-related ASEs in a cohort of patients with gMG treated in routine clinical practice. All patients experienced at least one ASE commonly associated with corticosteroids; the mean number of such ASEs per patient was 2.3, with one patient having six ASEs. The ubiquitous nature of ASEs in this population indicates that healthcare providers should be mindful when prescribing steroids for prolonged periods. It is also interesting that infection, a well-recognized potential consequence of corticosteroid treatment, was reported in only 3/39 patients (7.7%), a much lower incidence than that observed in the retrospective study by Prior et al (69/358, 19.3%), which was specifically designed to evaluate infection over a 10-year period [24].
Age is a risk factor for corticosteroid-related ASEs and in the current study, some ASEs (bruising, insomnia, and osteoporosis) were numerically more common in older (>65 years) than in younger patients (≤65 years). However, other ASEs (irritability, osteopenia, and pre-diabetes) were numerically more common in the younger subgroup. Whether these differences relate to the median prednisone dose being numerically higher in the older subgroup (40 mg vs 32.5 mg in younger patients) is difficult to say. Perhaps one of the most unexpected findings was the difference in the type of corticosteroid-related ASEs between men and women. Osteoporosis was reported only in men, whereas irritability was reported only in women. Pre-diabetes was numerically more common in men than women, while weight gain was numerically more common in women than men. The finding that osteoporosis was reported as a corticosteroid-related ASE in men but not in women is interesting. It may reflect an ASE attribution bias, but warrants further investigation in a larger study in which other possible confounding factors are controlled for. If supported, it would suggest the need to maintain a higher index of suspicion for osteoporosis in male patients treated with corticosteroids. Most patients in the study were treated with high prednisone doses (≥30 mg/day), and, as expected, the frequency of most ASEs was numerically greater in these patients than in those treated with lower doses. Exceptions were insomnia and irritability; the reasons for this are unclear, although for irritability, it may reflect the preponderance in younger patients, who were treated with lower doses. The possibility of confounding inter-relationships between age, sex, and dose was not controlled for in the statistical analysis. The relatively small number of patients in the current study means that the results should be interpreted with caution. The small sample number may also have compromised the ability to detect statistically significant differences between subgroups.
It is anticipated that some of the ASEs identified in the current study (eg, insomnia, irritability, and weight gain) could affect patients’ quality of life [26–28]. In an online survey of approximately 600 patients being treated with glucocorticoids for various conditions, weight gain, insomnia, and ‘moon face’ (a symptom of Cushing syndrome) were ranked as the most important ASEs [29]. Other ASEs identified in the present study could have potentially serious consequences (eg, weight gain, pre-diabetes/diabetes, and osteoporosis) [30–32]. Weight gain and pre-diabetes are of particular concern given their prevalence in the current sample, the magnitude of the observed weight gain, pre-existing risk factors, including hypertension, hyperlipidemia, and coronary artery disease, in some of the patients, and their well-recognized complications [33,34].
Various strategies have been used to manage the ASEs of corticosteroids. These include alternate-day treatment [12,13], dose tapering/de-escalation [12,16], and concomitant use of steroid-sparing treatments [12,14,35]. In a small, single-blind, 2-year study in patients with gMG, methotrexate had greater steroid-sparing effects than did azathioprine [35], but it is notable that methotrexate failed to show a steroid-sparing effect in a double-blind, placebo-controlled, 1-year study [36], although the lack of statistical power and short duration of the study have led to the conclusions being questioned [37]. Results of small case series suggest that eculizumab [38] and cyclosporine [39] may also have steroid-sparing effects. The beneficial effects of eculizumab have been confirmed in a recent analysis of data from the open-label extension (OLE) of the phase 3 REGAIN study, in which 117 patients received eculizumab for up to 4 years. At the OLE baseline, most patients (90 [76.9%]) were receiving prednisone; at the last assessment, there was a significant reduction in prednisone dose (
The primary limitations of this study include the relatively small sample size and limitations associated with retrospective chart-based data extraction. In addition, all patients were receiving at least one other medication to treat gMG, and it is possible that these contributed to the ASEs observed. Nevertheless, the results may provide useful information for clinicians who manage patients with gMG in terms of dosing, counseling, and monitoring according to patient age and sex.
Conclusions
In this study of patients being treated with prednisone for gMG, corticosteroid-related ASEs were ubiquitous, some with potentially serious medical consequences, and certain ASEs appeared to be associated with patient characteristics. Although steroids have a role to play in managing MG, these results highlight the importance of considering patients’ demographic and comorbid conditions before initiating treatment and the need to consider the various strategies for monitoring and managing corticosteroid-related ASEs.
Figures
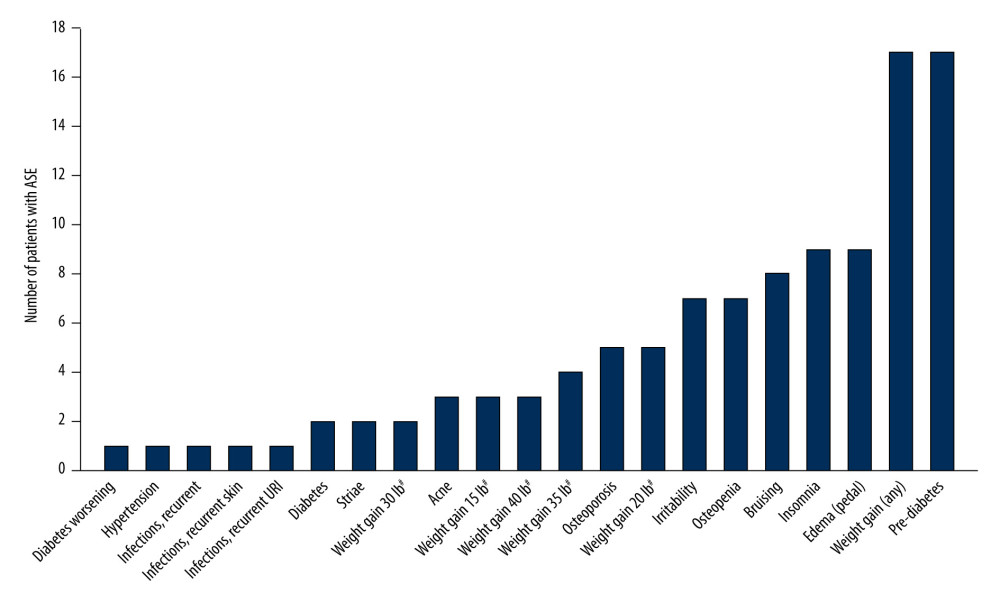 Figure 1. Corticosteroid-related ASEs reported in patients with myasthenia gravis (n=39). # Weight gain was rounded to the nearest 5 lb. ASE – adverse side effect; URI – upper respiratory tract infection. Figure was created using Adobe Illustrator version 25.3.1 (Adobe, Inc.).
Figure 1. Corticosteroid-related ASEs reported in patients with myasthenia gravis (n=39). # Weight gain was rounded to the nearest 5 lb. ASE – adverse side effect; URI – upper respiratory tract infection. Figure was created using Adobe Illustrator version 25.3.1 (Adobe, Inc.). 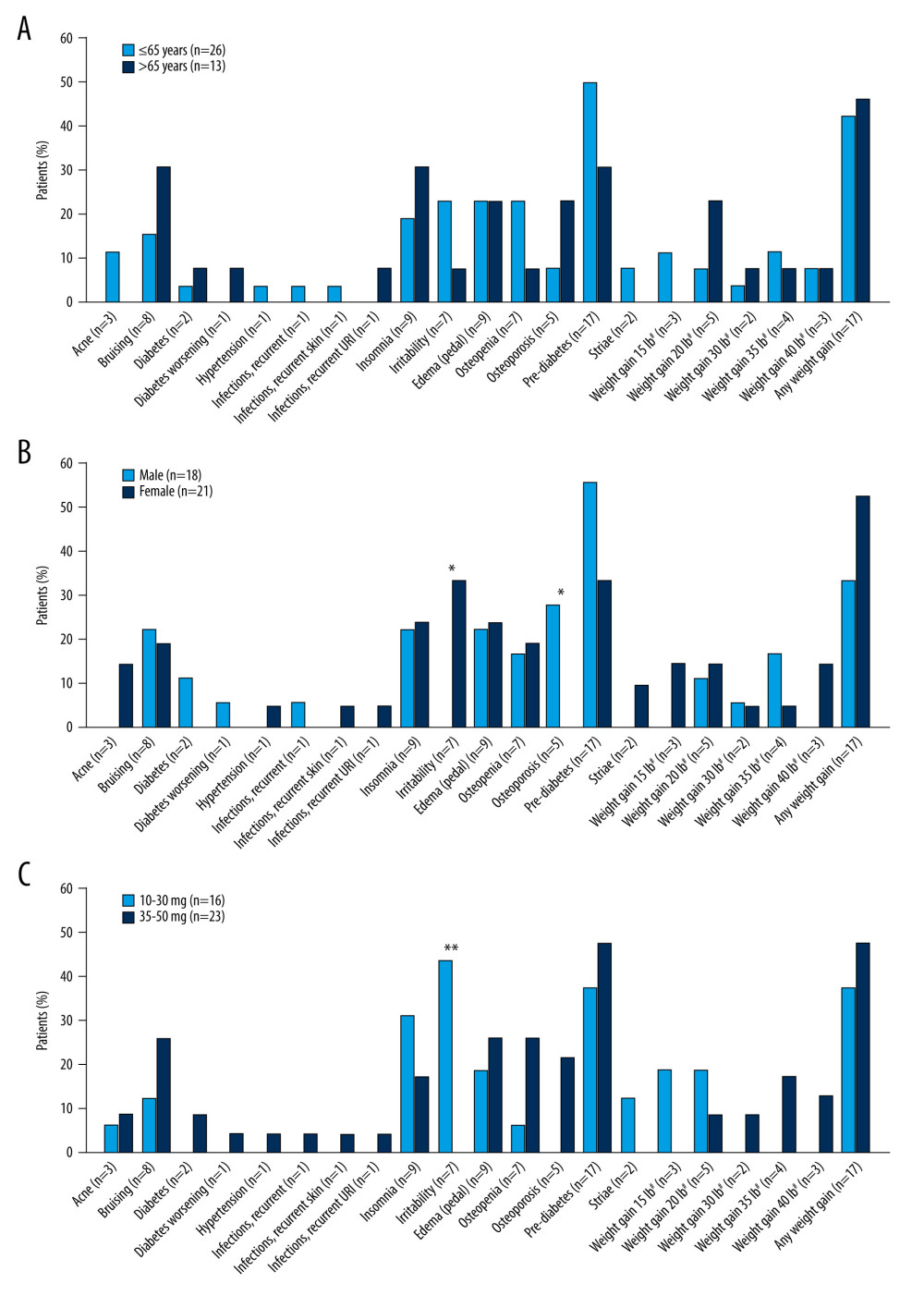 Figure 2. Corticosteroid-related adverse side effects in patients with myasthenia gravis according to (A) age, (B) sex, and (C) prednisone daily dose. n values on x axis represent the number of events reported; most patients reported more than 1 event. * P<0.05, ** P<0.01. # Weight gain was rounded to the nearest 5 lb. URI – upper respiratory tract infection. Figure was created using Adobe Illustrator version 25.3.1 (Adobe, Inc.).
Figure 2. Corticosteroid-related adverse side effects in patients with myasthenia gravis according to (A) age, (B) sex, and (C) prednisone daily dose. n values on x axis represent the number of events reported; most patients reported more than 1 event. * P<0.05, ** P<0.01. # Weight gain was rounded to the nearest 5 lb. URI – upper respiratory tract infection. Figure was created using Adobe Illustrator version 25.3.1 (Adobe, Inc.). References
1. Gilhus NE, Skeie GO, Romi F, Myasthenia gravis – autoantibody characteristics and their implications for therapy: Nat Rev Neurol, 2016; 12; 259-68
2. Gilhus NE, Tzartos S, Evoli A, Myasthenia gravis: Nat Rev Dis Primers, 2019; 5; 30
3. Beloor Suresh A, Asuncion RMD, Myasthenia gravis: StatPearls, 2021, Treasure Island (FL), StatPearls Publishing LLC [cited 2021 Feb 3]. Available from: URL: https://www.ncbi.nlm.nih.gov/books/NBK559331/
4. Carr AS, Cardwell CR, McCarron PO, McConville J, A systematic review of population based epidemiological studies in myasthenia gravis: BMC Neurol, 2010; 10; 46
5. Dhillon S, Eculizumab: A review in generalized myasthenia gravis: Drugs, 2018; 78; 367-76
6. Souto EB, Lima B, Campos JR, Myasthenia gravis: State of the art and new therapeutic strategies: J Neuroimmunol, 2019; 337; 577080
7. Stieglbauer K, Topakian R, Schäffer V, Aichner FT, Rituximab for myasthenia gravis: three case reports and review of the literature: J Neurol Sci, 2009; 280; 120-22
8. Alexion Pharmaceuticals Inc, 2020 [cited 2021 Feb 3]. Available from: URL: https://alexion.com/Documents/Soliris_USPI.pdf
9. ICN Pharmaceuticals Inc: Mestinon PI, 2001 [cited 2021 Feb 3]. Available from: URL: https://www.accessdata.fda.gov/drugsatfda_docs/label/2001/15193s18lbl.pdf
10. Coutinho AE, Chapman KE, The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights: Mol Cell Endocrinol, 2011; 335; 2-13
11. Grob D, Brunner N, Namba T, Pagala M, Lifetime course of myasthenia gravis: Muscle Nerve, 2008; 37; 141-49
12. Imai T, Suzuki S, Nagane Y, Reappraisal of oral steroid therapy for myasthenia gravis: Front Neurol, 2020; 11; 868
13. Schneider-Gold C, Gajdos P, Toyka KV, Hohlfeld RR, Corticosteroids for myasthenia gravis: Cochrane Database Syst Rev, 2005(2); CD002828
14. Tannemaat MR, Verschuuren J, Emerging therapies for autoimmune myasthenia gravis: Towards treatment without corticosteroids: Neuromuscul Disord, 2020; 30; 111-19
15. Yasir M, Goyal A, Bansal P, Sonthalia S, Corticosteroid adverse effects: StatPearls, 2021, Treasure Island (FL), StatPearls Publishing LLC [cited 2021 Feb 3]. Available from: URL: https://www.ncbi.nlm.nih.gov/books/NBK531462/
16. Sanders DB, Wolfe GI, Benatar M, International consensus guidance for management of myasthenia gravis: Executive summary: Neurology, 2016; 87; 419-25
17. Moghadam-Kia S, Werth VP, Prevention and treatment of systemic glucocorticoid side effects: Int J Dermatol, 2010; 49; 239-48
18. Cosi V, Citterio A, Lombardi M, Effectiveness of steroid treatment in myasthenia gravis: A retrospective study: Acta Neurol Scand, 1991; 84; 33-39
19. Evoli A, Batocchi AP, Palmisani MT, Long-term results of corticosteroid therapy in patients with myasthenia gravis: Eur Neurol, 1992; 32; 37-43
20. Pascuzzi RM, Coslett HB, Johns TR, Long-term corticosteroid treatment of myasthenia gravis: rReport of 116 patients: Ann Neurol, 1984; 15; 291-98
21. Sghirlanzoni A, Peluchetti D, Mantegazza R, Myasthenia gravis: Prolonged treatment with steroids: Neurology, 1984; 34; 170-74
22. Bruce BB, Kupersmith MJ, Safety of prednisone for ocular myasthenia gravis: J Neuroophthalmol, 2012; 32; 212-15
23. Lee YG, Kim US, Efficacy and safety of low-to-moderate dose oral corticosteroid treatment in ocular myasthenia gravis: J Pediatr Ophthalmol Strabismus, 2018; 55; 339-42
24. Prior DE, Nurre E, Roller SL, Infections and the relationship to treatment in neuromuscular autoimmunity: Muscle Nerve, 2018; 57; 927-31
25. Buttgereit F, da Silva JA, Boers M, Standardised nomenclature for glucocorticoid dosages and glucocorticoid treatment regimens: Current questions and tentative answers in rheumatology: Ann Rheum Dis, 2002; 61; 718-22
26. Barahmand U, Haji A, The impact of intolerance of uncertainty, worry and irritability on quality of life in persons with epilepsy: Irritability as mediator: Epilepsy Res, 2014; 108; 1335-44
27. Idzikowski C, Impact of insomnia on health-related quality of life: Pharmacoeconomics, 1996; 10(Suppl 1); 15-24
28. Allison DB, Mackell JA, McDonnell DD, The impact of weight gain on quality of life among persons with schizophrenia: Psychiatr Serv, 2003; 54; 565-67
29. Costello R, Patel R, Humphreys J, Patient perceptions of glucocorticoid side effects: A cross-sectional survey of users in an online health community: BMJ Open, 2017; 7; e014603
30. Tune JD, Goodwill AG, Sassoon DJ, Mather KJ, Cardiovascular consequences of metabolic syndrome: Transl Res, 2017; 183; 57-70
31. Mykyta LJ, The consequences of osteoporosis in the elderly: Aust Fam Physician, 1997; 26; 115-21
32. Banu J, Causes, consequences, and treatment of osteoporosis in men: Drug Des Devel Ther, 2013; 7; 849-60
33. Kinlen D, Cody D, O’Shea D, Complications of obesity: QJM, 2018; 111; 437-43
34. Papatheodorou K, Banach M, Bekiari E, Complications of diabetes 2017: J Diabetes Res, 2018; 2018; 3086167
35. Heckmann JM, Rawoot A, Bateman K, A single-blinded trial of methotrexate versus azathioprine as steroid-sparing agents in generalized myasthenia gravis: BMC Neurol, 2011; 11; 97
36. Pasnoor M, He J, Herbelin L, A randomized controlled trial of methotrexate for patients with generalized myasthenia gravis: Neurology, 2016; 87; 57-64
37. Jones LA, Robertson NP, An update on treatments in myasthenia gravis: J Neurol, 2017; 264; 205-7
38. Datta S, Singh S, Govindarajan R, Retrospective analysis of eculizumab in patients with acetylcholine receptor antibody-negative myasthenia gravis: A case series: J Neuromuscul Dis, 2020; 7; 269-77
39. Nakamura S, Kaneko S, Shinde A, Prednisolone-sparing effect of cyclosporin A therapy for very elderly patients with myasthenia gravis: Neuromuscul Disord, 2013; 23; 176-79
40. Nowak RJ, Muppidi S, Beydoun SR, Concomitant immunosuppressive therapy use in eculizumab-treated adults with generalized myasthenia gravis during the REGAIN open-label extension study: Front Neurol, 2020; 11; 556104
Figures
 Figure 1. Corticosteroid-related ASEs reported in patients with myasthenia gravis (n=39). # Weight gain was rounded to the nearest 5 lb. ASE – adverse side effect; URI – upper respiratory tract infection. Figure was created using Adobe Illustrator version 25.3.1 (Adobe, Inc.).
Figure 1. Corticosteroid-related ASEs reported in patients with myasthenia gravis (n=39). # Weight gain was rounded to the nearest 5 lb. ASE – adverse side effect; URI – upper respiratory tract infection. Figure was created using Adobe Illustrator version 25.3.1 (Adobe, Inc.). Figure 2. Corticosteroid-related adverse side effects in patients with myasthenia gravis according to (A) age, (B) sex, and (C) prednisone daily dose. n values on x axis represent the number of events reported; most patients reported more than 1 event. * P<0.05, ** P<0.01. # Weight gain was rounded to the nearest 5 lb. URI – upper respiratory tract infection. Figure was created using Adobe Illustrator version 25.3.1 (Adobe, Inc.).
Figure 2. Corticosteroid-related adverse side effects in patients with myasthenia gravis according to (A) age, (B) sex, and (C) prednisone daily dose. n values on x axis represent the number of events reported; most patients reported more than 1 event. * P<0.05, ** P<0.01. # Weight gain was rounded to the nearest 5 lb. URI – upper respiratory tract infection. Figure was created using Adobe Illustrator version 25.3.1 (Adobe, Inc.). In Press
05 Mar 2024 : Clinical Research
Role of Critical Shoulder Angle in Degenerative Type Rotator Cuff Tears: A Turkish Cohort StudyMed Sci Monit In Press; DOI: 10.12659/MSM.943703
06 Mar 2024 : Clinical Research
Comparison of Outcomes between Single-Level and Double-Level Corpectomy in Thoracolumbar Reconstruction: A ...Med Sci Monit In Press; DOI: 10.12659/MSM.943797
21 Mar 2024 : Meta-Analysis
Economic Evaluation of COVID-19 Screening Tests and Surveillance Strategies in Low-Income, Middle-Income, a...Med Sci Monit In Press; DOI: 10.12659/MSM.943863
10 Apr 2024 : Clinical Research
Predicting Acute Cardiovascular Complications in COVID-19: Insights from a Specialized Cardiac Referral Dep...Med Sci Monit In Press; DOI: 10.12659/MSM.942612
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952