30 June 2020: Clinical Research
Application of the Molecular Adsorbent Recirculating System in Type 1 Hepatorenal Syndrome in the Course of Alcohol-Related Acute on Chronic Liver Failure
Grzegorz Kade1ABCEF, Arkadiusz Lubas1ACDEF, Sebastian Spaleniak2ADEF*, Anna Wojtecka1B, Ksymena Leśniak1B, Sławomir Literacki3BF, Stanisław Niemczyk1E, Przemysław Dyrla4BCDEDOI: 10.12659/MSM.923805
Med Sci Monit 2020; 26:e923805
Abstract
BACKGROUND: This study aimed to evaluate the Molecular Adsorbent Recirculating System (MARS) effectiveness in patients with alcohol-related acute-on-chronic liver failure (AoCLF) complicated with type 1 hepatorenal syndrome (HRS). So far, MARS efficacy and safety has been demonstrated in various acute liver failure scenarios.
MATERIAL AND METHODS: Data from 41 MARS procedures (10 patients with type 1 HRS, in the course of alcohol-related AoCLF were considered for this study. Biochemical tests of blood serum were performed before and after each procedure. The condition of patients was determined before and after the treatment with the use of the model for end-stage liver disease – sodium (MELD-Na) and the stage of encephalopathy severity based on the West Haven criteria.
RESULTS: During the observation period (20.5±13.9 days), 5 patients died, and the remaining 5 surviving patients were discharged from the hospital. In the group of 10, the 14-day survival, starting from the first MARS treatment, was 90%. The MARS procedure was associated with a 19% reduction in bilirubin (27.5±6.1 versus 22.3±4.0 mg/dL, P<0.001), 37% reduction in ammonia (44.1±22.5 versus 27.6±20.9 P<0.001), 27% reduction in creatinine (1.5±1.0 versus 1.1±0.6 mg/dL, P<0.001) and 14% reduction urea (83.8±36.1 versus 72.1±33.3, P<0.001) in blood serum samples, with stable hemodynamic parameters. In the group of patients discharged from the clinic (n=5), the MARS treatments resulted in an improvement in hepatic encephalopathy (West Haven; P=0.043), as well as a reduction in the MELD-Na score (P=0.015).
CONCLUSIONS: MARS is a hemodynamically safe method for supporting the function of the liver and the kidneys. Application of the MARS reduces the symptoms of encephalopathy in patients with alcohol-related type 1 HRS.
Keywords: Acute Kidney Injury, Dialysis, hepatorenal syndrome, Mars, Acute-On-Chronic Liver Failure, Hemoperfusion, Liver, Liver Transplantation, Sorption Detoxification
Background
Molecular Adsorbent Recirculating System (MARS) is a system of extracorporeal toxin removal using the albumin dialysis method. It was introduced into clinical practice by the German nephrologists Mitzner and Stang in 1993, in order to support the detoxification function of the liver in cases of acute liver failure as well as previously existing acute-on-chronic liver failure (AoCLF) [1]. This technique is based on extracorporeal removal of toxins associated with plasma proteins which result from liver failure. This system allows for the simultaneous elimination of water-soluble uremic and non-uremic toxins. The main goal of the MARS method is to remove small and medium molecules from the circulation. This is achieved by a specially constructed highly permeable membrane [1]. Procedures using MARS are well tolerated, safe, stabilize the function of cardiovascular system, reduce the degree of hepatic encephalopathy, and have a positive effect on selected biochemical markers, which has been confirmed in numerous studies – including the randomized trial named RELIFE [2–4].
The most common factors causing AoCLF include alcohol consumption. Although alcohol abuse and/or addiction are not a prerequisite for the development of alcoholic liver disease, they still frequently correlate with alcoholic liver disease. One of the stages of alcoholic liver disease can be liver cirrhosis. The bases for the diagnosis of alcoholic liver disease are the following: medical history, clinical symptoms of liver disease, and abnormal results of additional tests. One of the elements of the medical history review interview may be a questionnaire such as the AUDIT (alcohol use disorders identification test).
One of the most severe AoCLF complications is kidney damage. Even a slight increase of creatinine concentration in the serum indicates a significant decrease in glomerular filtration in patients with chronic liver failure and is associated with poor prognosis. The occurrence of acute kidney injury (AKI) in the form of type 1 hepatorenal syndrome (HRS) as a component of multiorgan failure in patients with AoCLF is associated with high short-term mortality (28-day mortality greater than 15%) [5]. In a prospective, randomized, controlled clinical trial, Mitzner et al. in the group of patients with type 1 HRS found that the mortality in the control group on day 7 of the study was 100%. In the MARS group, the mortality rate on day 7 was 62.5%, and on day 30, the mortality rate was 75%. The authors found that the removal of substances associated with albumins using the MARS method can contribute to improving the survival of patients with type 1 HRS [6].
The aim of this study was to evaluate the effectiveness of MARS in patients with alcohol-related AoCLF complicated with type 1 HRS.
Material and Methods
STATISTICAL ANALYSIS:
The test results were evaluated using the Statistica 12.0 software. Due to the small sample size, the basic results are presented in the form of means with standard deviations as well as in the form of medians with minimum and maximum values. Differences between the results obtained before and after the procedure were tested using the
Results
The study included 10 patients (7 males, 3 females, age 52.1±11.1 years) with alcohol-related AoCLF, without hepatitis B virus or hepatitis C virus infections. The clinical characteristics of the study group are shown in Table 1. The parameters of the performed procedures are presented in Table 2.
During the hospital observation of 20.5±13.9 days on average, covering the period from the first MARS treatment to discharge or death, 5 patients (4 males, 1 females) died, and the remaining 5 (3 males, 2 females) were discharged from the hospital. Initial sepsis was recognized in 1 survivor patient and 1 deceased patient. Concomitant bleeding complications (nasal, esophago-gastrointestinal) occurred in 1 discharged patient and 3 deceased patients, but these complications were not closely related to the MARS procedures. A comparison of baseline data of survivors and non-survivors is presented in Table 3.
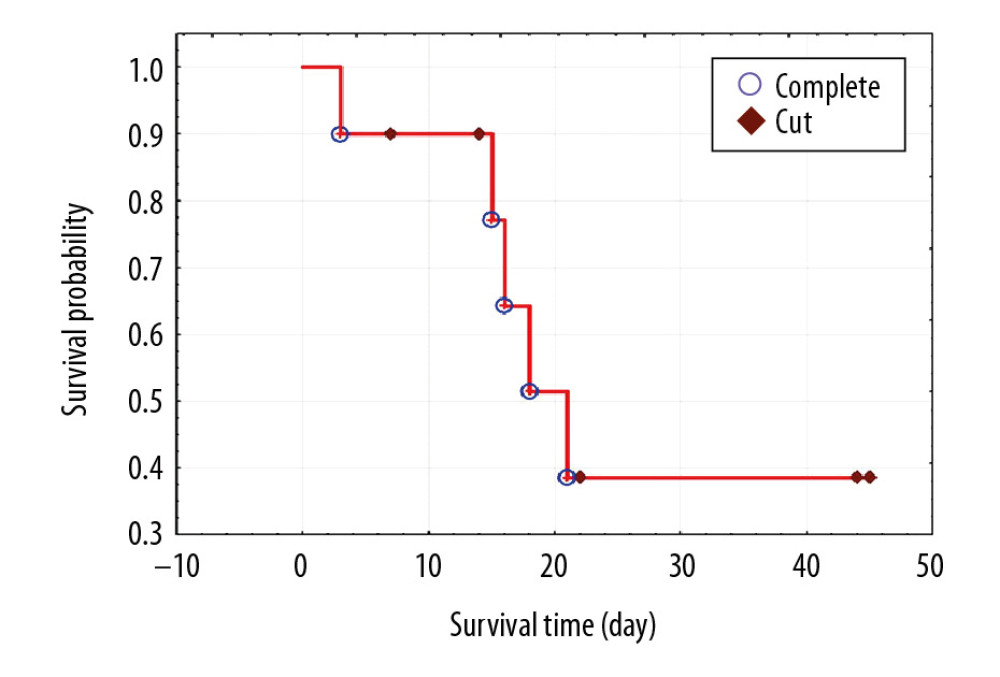
In the entire study group, the 14-day survival, starting from the first MARS treatment, was 90%, but the 21-day survival was only 39% (Figure 1).
The MARS treatments were associated with a significant reduction in blood serum of bilirubin by 18.9%, ammonia by 37.4%, creatinine by 26.7%, and urea by 14%, with stable hemodynamic parameters (Table 4).
The observed improvement of blood circulation parameters in the form of a slight increase in both systolic blood pressure (approximately 3.2%) and diastolic blood pressure (approximately 4.1%) during the procedure was not significant. Examining the entire group, we initially found no significant improvement in hepatic encephalopathy (
Discussion
Acute-on-chronic liver failure (AoCLF) was first described in 1995 by Ohnishi et al. [8]. According to the authors, this disease entity is characterized by an acute deterioration of compensated or decompensated chronic liver disease leading to multiorgan failure. AoCLF most often manifests as a hepatic coma or hepatorenal syndrome. Clinically, on the basis of the time of increase in creatinine concentration, it can be divided into type 1 HRS with a serious prognosis and high mortality, and type 2 HRS with a relatively better prognosis. Type 1 HRS is burdened with 15% mortality within 28 days [5].
The incidence of renal failure in patients with liver cirrhosis is estimated at 7% to 65% [9–12]. One of the forms of acute kidney injury in patients with liver cirrhosis is HRS [13]. The risk of development of HRS in patients with liver cirrhosis and ascites is estimated at 18% in the first year and approximately 40% after 5 years of observation [14]. For our study, patients with a positive history of alcoholic liver disease, liver cirrhosis, and ascites were qualified. All patients had HRS diagnosed on the basis of the International Ascites Club criteria [12]. In the study group treated with MARS, the 14-day survival was 90%, but the 21-day survival was only 39%. According to Moreau et al., patients with AoCLF are burdened with high mortality of 50% to 90% [15]. In order to assess the risk of death among patients and plan for their appropriate treatment, various point scales are used. One of them is the MELD scale, which was originally developed to predict the short-term survival of patients undergoing transjugular intrahepatic portosystemic shunt. Obtaining a result of over 25 points by a patient is an indication for urgent transplantation. Since January 1, 2016, the MELD scale has been used taking into account the concentration of sodium in the blood serum, the MELD-Na score [14]. In our group, the initial MELD-Na was 34.9. Paradoxically, in the group of deceased patients, the initial MELD-Na score was lower than in the group of discharged survivor patients; however, in both groups it was in the range of very high values (>30) and burdened with the highest mortality [16]. Some studies suggest that APACHE-II (Acute Physiology and Chronic Health Evaluation) is the best system of predictive assessment due to the fact that, in the case of AoCLF, prognosis depends on the degree of failure of other organs [17]. CLIF-SOFA (Chronic Liver Failure-sequencing Organ Failure Assessment) is a scale similar to APACHE-II that also accounts for multi-organ dysfunction. The scale estimates mortality based on the severity of multiorgan dysfunction in patients with AoCLF [18]. In other studies, it was found that MELD was a discriminatory factor similar to SOFA and APACHE II [19]. Data obtained from the CANONIC supported development of a simplified prognostic scale for AoCLF called the “CLIF Consortium ACLF score” (CLIF-C AoCLFs) [20,21]. The new scale shows much higher predictive accuracy of AoCLF severity than MELD and MELD-Na. On the other hand, a meta-analysis by Wu et al. showed that the scales referring to sodium concentration in the blood serum, i.e. MESO (MELD to Serum Sodium ratio) and MELD-Na had the highest accuracy in the prediction of mortality in decompensated liver cirrhosis [18].
At present, the administration of vasoconstrictor drugs (e.g., terlipressin) in combination with albumin infusions is recommended for the first-line treatment of type 1 HRS [22]. This strategy leads to improvement in kidney function in most patients, however, in more than 40% of cases it is ineffective [23,24]. The use of renal replacement therapy (RRT) in this syndrome is considered controversial, as it has not been associated with improvement in short- and long-term survival in both treated and untreated patients with RRT [25]. This method is only considered for candidates for liver transplantation (over 30% of individuals who have received RRT have had a transplantation procedure). Compared to the standard RRT, the use of the MARS system can improve the prognosis of patients with HRS by simultaneous support of kidney and liver functions, and stabilization of the circulatory system without the need for the administration of additional catecholamines.
In the only correctly planned randomized study with the acronym RELIEF, Bañares et al. [4] showed that the use of the MARS system in AoCLF was characterized by a favorable safety profile, a significant dialysis effect, and a slight impact on the improvement of severe cerebral encephalopathy (statistically not significant), which is consistent with the results of our work. As in our work, the Bañares et al. study found it was not possible to demonstrate a beneficial effect of MARS on survival [4].
Gerth et al. [26] retrospectively analyzed AoCLF patients treated with MARS in combination with standard therapy and patients only treated with standard medical treatment. Similar to our study, significant effects on bilirubin and creatinine in the serum were demonstrated in the patients treated with MARS [26]. Their study showed an increase in short-term 14-day survival in the MARS group [26], which was not observed in our study. Similar results were obtained by Heemann et al. who reported that MARS procedures in combination with standard therapy for AoCLF were associated with greater 30-day survival and a significant reduction in bilirubin in the serum [27]. In the study by Hessel et al. [28], the cumulative 3-year survival probability was shown to be higher in the MARS group treated for AoCLF. This study was created to assess the cost-effectiveness of MARS treatment in AoCLF, therefore the randomization methodology in the study was unclear [28].
The administration of MARS treatments in our group of patients with AoCLF caused a significant reduction in the concentration of renal dysfunction (as measured by creatinine and urea levels) and liver failure parameters (as measured by bilirubin and ammonia levels). Despite the declared permeability of filters used in MARS, available reports were not consistent with the significance of the reduction of these toxins during the procedure. Wolf et al. found that after 48 MARS treatments in 14 patients with AoCLF, there was a significant reduction in bilirubin, creatinine, and urea, but not ammonia [29]. Schaefer et al. did not observe a significant reduction in either bilirubin or ammonia after MARS procedures in children, in contrast to the plasmapheresis with hemodialysis (PE/HD) procedures alternating with MARS [30]. However, a significant decrease in ammonia concentration during PE/HD probably caused lower concentrations of this toxin before MARS, which affected the final lack of effectiveness of the method. On the other hand, Bourgoin et al., performing 17 albumin dialyses (MARSFLUX and MiniMARS) in 6 children, only found a significant reduction in ammonia concentration after the procedures, but without a decrease in bilirubin [31]. Undoubtedly, the results of this study were affected by premature (<50% of the assumed time) completion of almost 30% of treatments. However, MARS treatments can serve as a bridge for liver transplantation in severe poisoning in children [32]. In a recently published retrospective, multicenter study by Cisneros-Garza et al., after 79 MARS treatments in 38 patients, the authors found a significant reduction in bilirubin, transaminases, and bile acids, and a decrease in symptoms of hepatic encephalopathy, without a reduction of creatinine [33]. In contrast to our study, where all patients presented with AKI in the course of AoCLF or type 1 HRS, in the work by Cisneros-Garza et al. only 5 patients (13%) had type 1 HRS. Taking into account the aforementioned observations, our study summarizes the research performed so far and shows the significant effectiveness of MARS in the elimination of selected liver and kidney toxins. Moreover, in our study a significant reduction in ammonia was accompanied by a significant decrease in symptoms of hepatic encephalopathy. In addition, studies have shown that plasma nitric oxide and both anti-inflammatory and pro-inflammatory cytokines such as tumor necrosis factor alpha (TNF-α), interleukin (IL)-6 or IL-10, involved in the pathogenesis of encephalopathy may be removed by the MARS method [34].
In our study group, performed MARS treatments were associated with a mild improvement in hemodynamic parameters in the form of a slight increase in blood pressure. During the procedures, there were no blood pressure drops that forced the reduction of planned ultrafiltration. Our observations are consistent with the results of the study by Schmidt et al. in which the improvement of hemodynamic conditions included an increase in both systolic and diastolic blood pressure [35,36]. Similar to our study, these authors observed the constant catecholamine administration in spite of conducted ultrafiltration.
Our study documents the efficiency and safety of albumin dialysis in the form of MARS procedures in patients with type 1 HPS in the course of AoCLF. Despite promising observations, the results of our study are subject to limitations caused primarily by the small number of patients resulting from the selection of a homogeneous group of patients.
Conclusions
Albumin dialysis using the MARS method is effective at removing selected liver toxins such as bilirubin or ammonia and kidney toxins such as creatinine or urea, reducing the symptoms of encephalopathy in the course of alcohol-related type 1 HRS. MARS procedures not only allow for the removal of selected toxins, which cannot be obtained using standard conservative therapy with the administration of terlipressin, but also might have a positive effect on the concentrations of selected cytokines. Hemodynamic stability during the MARS procedure without a necessity for a modification of the initial settings makes this method safe and well tolerated. No adverse events were observed during the procedures.
Tables
Table 1. Clinical characteristics of the studied group.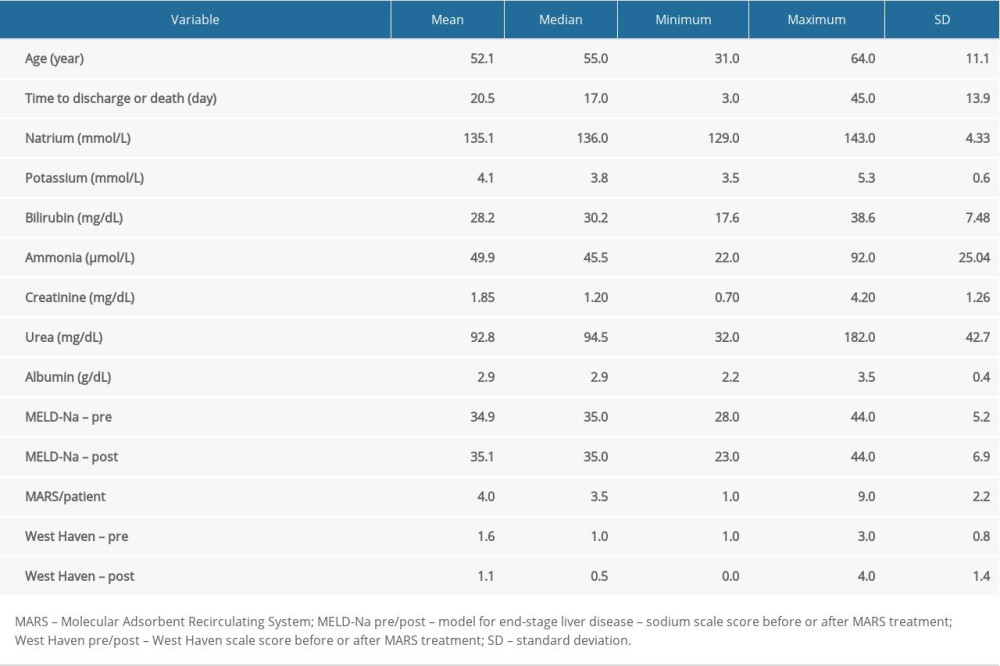 Table 2. Characteristics of MARS procedures.
Table 2. Characteristics of MARS procedures.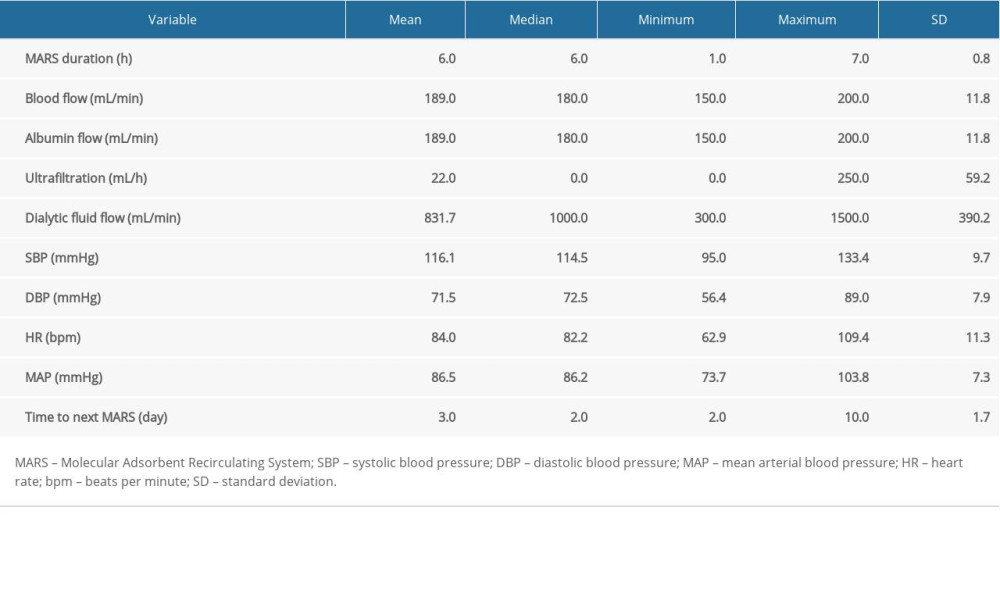 Table 3. Comparison of baseline parameters between survivors and non-survivors.
Table 3. Comparison of baseline parameters between survivors and non-survivors.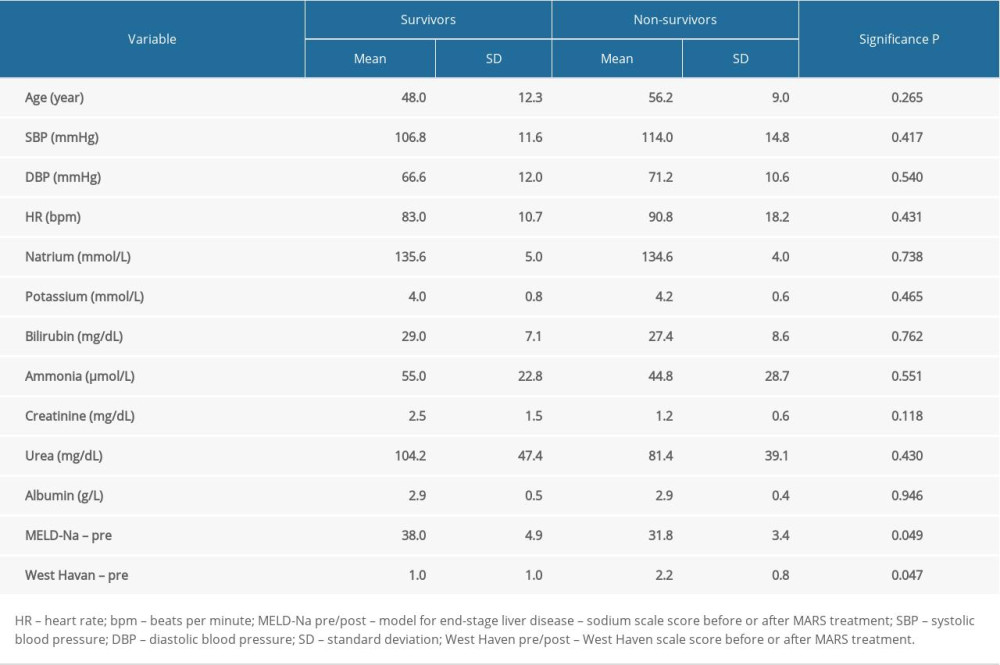 Table 4. Comparison of blood pressure and laboratory data before and after MARS treatment.
Table 4. Comparison of blood pressure and laboratory data before and after MARS treatment.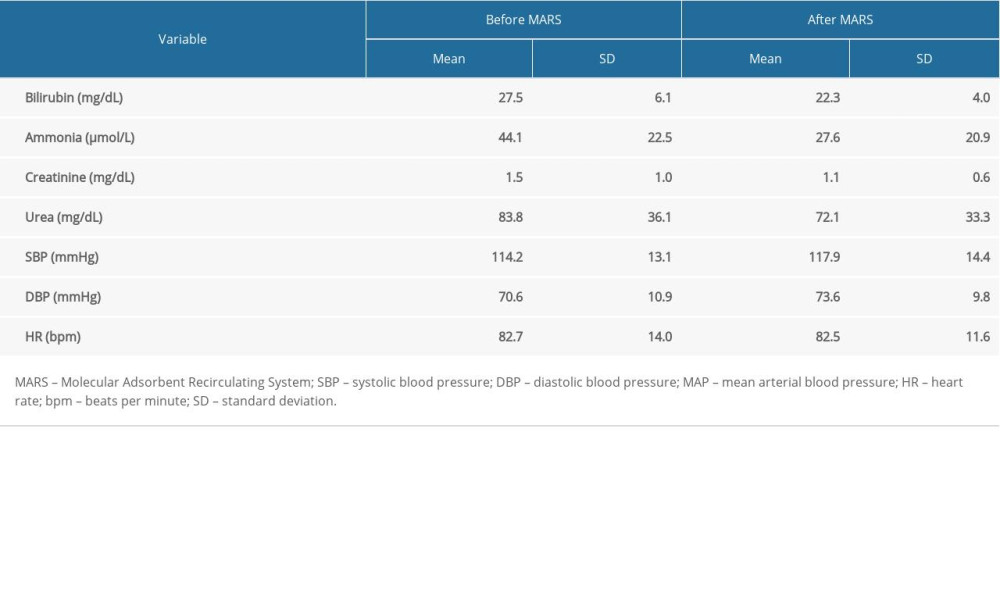 Table 5. Comparison of the effectiveness of MARS treatments in West Haven and MELD-Na score results.
Table 5. Comparison of the effectiveness of MARS treatments in West Haven and MELD-Na score results.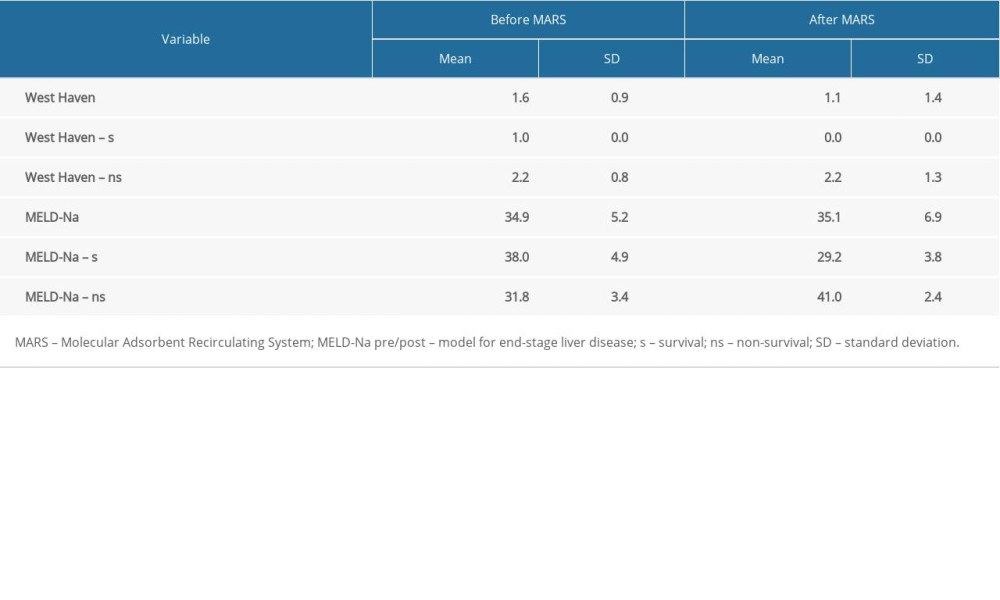
References
1. Stange J, Ramlow W, Mitzner S, Dialysis against a recycled albumin solution enables the removal of albumin-bound toxins: Artif Organs, 1993; 17(9); 809-13
2. Larsen FS, Artificial liver support in acute and acute-on-chronic liver failure: Curr Opin Crit Care, 2019; 25(2); 187-91
3. MacDonald AJ, Karvellas CJ, Emerging role of extracorporeal support in acute and acute-on-chronic liver failure: Recent developments: Semin Respir Crit Care Med, 2018; 39(5); 625-34
4. Bañares R, Nevens F, Larsen FS, Extracorporeal albumin dialysis with the molecular adsorbent recirculating system in acute-on-chronic liver failure: The RELIEF trial: Hepatology, 2013; 57(3); 1153-62
5. Piechota M, Supporting liver function: Extracorporeal blood purification in the intensive care unit, 2017; 331-48, Gdańsk, Via Medica
6. Mitzner SR, Stange J, Klammt S, Improvement of hepatorenal syndrome with extracorporeal albumin dialysis MARS: Results of a prospective, randomized, controlled clinical trial: Liver Transpl, 2000; 6(3); 277-86
7. Hydzik P, Gawlikowski T, Ciszowski K, Sułek MThe clinical experience with MARS and Prometheus procedures: Przegl Lek, 2007; 64(4–5); 352-54 [in Polish]
8. Ohnishi H, Sugihara J, Moriwaki H, Muto YAcute-on-chronic liver failure: Ryoikibetsu Shokogun Shirizu, 1995(7); 217-19 [in Japanese]
9. Wong F, Blendis L, New challenge of hepatorenal syndrome: Prevention and treatment: Hepatology, 2001; 34(6); 1242-51
10. Arroyo V, Ginès P, Gerbes AL, Definition and diagnostic criteria of refractory ascites and hepatorenal syndrome in cirrhosis. International Ascites Club: Hepatology, 1996; 23(1); 164-76
11. Crawford DH, Endre ZH, Axelsen RA, Universal occurrence of glomerular abnormalities in patients receiving liver transplants: Am J Kidney Dis, 1992; 19(4); 339-44
12. Wong F, Nadim MK, Kellum JA, Working Party proposal for a revised classification system of renal dysfunction in patients with cirrhosis: Gut, 2011; 60(5); 702-9
13. Matuszkiewicz-Rowińska J, Niemczyk SHepato-renal syndrome – a new pathophysiological paradigm: Nefrol Dial Pol, 2015; 19; 173-75 [in Polish]
14. Piechota M, Hepatic encephalopathy in the course of alcoholic liver disease – treatment options in the intensive care unit: Anaesthesiol Intensive Ther, 2014; 46(1); 34-36
15. Moreau R, Jalan R, Gines P, Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis: Gastroenterology, 2013; 144(7); 1426-37
16. Wong VW, Chim AM, Wong GL, Performance of the new MELD-Na score in predicting 3-month and 1-year mortality in Chinese patients with chronic hepatitis B: Liver Transpl, 2007; 13(9); 1228-35
17. Blasco-Algora S, Masegosa-Ataz J, Gutiérrez-García ML, Acute-on-chronic liver failure: Pathogenesis, prognostic factors and management: World J Gastroenterol, 2015; 21(42); 12125-40
18. Wu SL, Zheng YX, Tian ZW, Scoring systems for prediction of mortality in decompensated liver cirrhosis: A meta-analysis of test accuracy: World J Clin Cases, 2018; 6(15); 995-1006
19. Garg H, Kumar A, Garg V, Clinical profile and predictors of mortality in patients of acute-on-chronic liver failure: Dig Liver Dis, 2012; 44; 166-71
20. Jalan R, Saliba F, Pavesi M, Development and validation of a prognostic score to predict mortality in patients with acute-on-chronic liver failure: J Hepatol, 2014; 61; 1038-47
21. Jalan R, Pavesi M, Saliba F, The CLIF Consortium Acute Decompensation score (CLIF-C ADs) for prognosis of hospitalised cirrhotic patients without acute-on-chronic liver failure: J Hepatol, 2015; 62; 831-40
22. Colle I, Laterre P-F, Hepatorenal syndrome: The clinical impact of vasoactive therapy: Expert Rev Gastroenterol Hepatol, 2018; 12; 173-88
23. Kalambokis GN, Tsiakas I, Christaki M, Systemic hemodynamic response to terlipressin predicts development of hepatorenal syndrome and survival in advanced cirrhosis: Eur J Gastroenterol Hepatol, 2018; 30; 659-67
24. Salerno F, Navickis RJ, Wilkes MM, Albumin treatment regimen for type 1 hepatorenal syndrome: Aa dose-response meta-analysis: BMC Gastroenterol, 2015; 15; 167
25. Zhang Z, Maddukuri G, Jaipaul N, Cai CX, Role of renal replacement therapy in patients with type 1 hepatorenal syndrome receiving combination treatment of vasoconstrictor plus albumin: J Crit Care, 2015; 30; 969-74
26. Gerth HU, Pohlen M, Tholking G, Molecular adsorbent recirculating system can reduce short-term mortality among patients with acute-on-chronic liver failure-a retrospective analysis: Crit Care Med, 2017; 45(10); 1616-24
27. Heemann U, Treichel U, Loock J, Albumin dialysis in cirrhosis with superimposed acute liver injury: A prospective, controlled study: Hepatology, 2002; 36(4 Pt 1); 949-58
28. Hessel FP, Bramlage P, Wasem J, Mitzner SR, Cost-effectiveness of the artificial liver support system MARS in patients with acute-on-chronic liver failure: Eur J Gastroenterol Hepatol, 2010; 22(2); 213-20
29. Wolff B, Machill K, Schumacher D, Schulzki I, MARS dialysis in decompensated alcoholic liver disease: A single-center experience: Liver Transpl, 2007; 13(8); 1189-92
30. Schaefer B, Schaefer F, Engelmann G, Comparison of Molecular Adsorbents Recirculating System (MARS) dialysis with combined plasma exchange and hemodialysis in children with acute liver failure: Nephrol Dial Transplant, 2011; 26(11); 3633-39
31. Bourgoin P, Merouani A, Phan V, Molecular Absorbent Recirculating System therapy (MARS®) in pediatric acute liver failure: A single center experience: Pediatr Nephrol, 2014; 29(5); 901-8
32. Prokurat S, Grenda R, Lipowski D, MARS procedure as a bridge to combined liver-kidney transplantation in severe chromium-copper acute intoxication: A pediatric case report: Liver, 2002; 22(Suppl 2); 76-77
33. Cisneros-Garza LE, del Muñoz-Ramírez MR, Muñoz-Espinoza LE, The molecular adsorbent recirculating system as a liver support system: summary of Mexican experience: Ann Hepatol, 2014; 13(2); 240-47
34. Guo LM, Liu JY, Xu DZ, Application of molecular adsorbents recirculating system to remove NO and cytokines in severe liver failure patients with multiple organ dysfunction syndrome: Liver Int, 2003; 23(Suppl 3); 16-20
35. Schmidt LE, Wang LP, Hansen BA, Larsen FS, Systemic hemo-dynamic effects of treatment with the molecular adsorbents recycling system in patients with hyperacute liver failure: A prospective controlled trial: Liver Transpl, 2003; 9(3); 290-97
36. Schmidt LE, Sørensen VR, Svendsen LB, Hemodynamic changes during a single treatment with the molecular adsorbents recirculating system in patients with acute-on-chronic liver failure: Liver Transpl, 2001; 7(12); 1034-39
Tables
 Table 1. Clinical characteristics of the studied group.
Table 1. Clinical characteristics of the studied group. Table 2. Characteristics of MARS procedures.
Table 2. Characteristics of MARS procedures. Table 3. Comparison of baseline parameters between survivors and non-survivors.
Table 3. Comparison of baseline parameters between survivors and non-survivors. Table 4. Comparison of blood pressure and laboratory data before and after MARS treatment.
Table 4. Comparison of blood pressure and laboratory data before and after MARS treatment. Table 5. Comparison of the effectiveness of MARS treatments in West Haven and MELD-Na score results.
Table 5. Comparison of the effectiveness of MARS treatments in West Haven and MELD-Na score results. Table 1. Clinical characteristics of the studied group.
Table 1. Clinical characteristics of the studied group. Table 2. Characteristics of MARS procedures.
Table 2. Characteristics of MARS procedures. Table 3. Comparison of baseline parameters between survivors and non-survivors.
Table 3. Comparison of baseline parameters between survivors and non-survivors. Table 4. Comparison of blood pressure and laboratory data before and after MARS treatment.
Table 4. Comparison of blood pressure and laboratory data before and after MARS treatment. Table 5. Comparison of the effectiveness of MARS treatments in West Haven and MELD-Na score results.
Table 5. Comparison of the effectiveness of MARS treatments in West Haven and MELD-Na score results. In Press
06 Mar 2024 : Clinical Research
Comparison of Outcomes between Single-Level and Double-Level Corpectomy in Thoracolumbar Reconstruction: A ...Med Sci Monit In Press; DOI: 10.12659/MSM.943797
21 Mar 2024 : Meta-Analysis
Economic Evaluation of COVID-19 Screening Tests and Surveillance Strategies in Low-Income, Middle-Income, a...Med Sci Monit In Press; DOI: 10.12659/MSM.943863
10 Apr 2024 : Clinical Research
Predicting Acute Cardiovascular Complications in COVID-19: Insights from a Specialized Cardiac Referral Dep...Med Sci Monit In Press; DOI: 10.12659/MSM.942612
06 Mar 2024 : Clinical Research
Enhanced Surgical Outcomes of Popliteal Cyst Excision: A Retrospective Study Comparing Arthroscopic Debride...Med Sci Monit In Press; DOI: 10.12659/MSM.941102
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952