20 July 2020: Animal Study
Pyrazolo[4,3-c]pyridine-4-one (PP-4-one) Exhibits Anti-Epileptogenic Effect in Rat Model of Traumatic Epilepsy by Mammalian Target of Rapamycin (mTOR) Signaling Pathway Downregulation
Jungong Jin1BCD, Xi Shen2BCF, Lu Tian3BCD, Guoyishi He1BCD, Ying Zhang3ADEF*DOI: 10.12659/MSM.923919
Med Sci Monit 2020; 26:e923919
Abstract
BACKGROUND: Post-traumatic epilepsy (PTE) is a common type of acquired epilepsies secondary to traumatic brain injury (TBI), accounting for approximately 10–25% of patients. The present study evaluated activity of PP-4-one against mTOR signaling activation in a rat model of FeCl₂-induced post-traumatic epilepsy.
MATERIAL AND METHODS: Epilepsy in rats was induced by injecting 10 µl FeCl₂ (concentration 100 mM) at a uniform rate of 1 µl/minute. The iNOS expression was detected using a Leica microscope connected to a digital camera system. Reverse transcription polymerase chain reaction (RT‑PCR) was used for determination of NR1 mRNA expression.
RESULTS: The post-traumatic epilepsy induced neuronal degeneration in the hippocampus and frontal cortex of the rats. Treatment with PP-4-one prevented neuronal degeneration in the hippocampus and frontal cortex in rats with post-traumatic epilepsy. The data revealed markedly higher levels of p-mTOR and p-P70S6K in rat hippocampal tissues after induction of traumatic epilepsy. Treatment of post-traumatic epilepsy rats with PP-4-one significantly suppressed p-mTOR and p-P70S6K expression, and PP-4-one treatment reduced epileptic brain injury in the rats with post-traumatic epilepsy.
CONCLUSIONS: PP-4-one exhibits an anti-epileptogenic effect in the rat model of PTE by inhibiting behavioral seizures through suppression of iNOS and astrocytic proliferation. Moreover, PP-4-one treatment suppressed NR1 expression and targeted the mTOR pathway in PTE-induced rats. Thus, PP-4-one shows promise as a novel and effective therapeutic agent for treatment of epilepsy induced by PTE.
Keywords: endometrial hyperplasia, Epilepsy, Neuroprotective Agents, Anticonvulsants, Astrocytes, Brain, Brain Injuries, Brain Injuries, Traumatic, Epilepsy, Post-Traumatic, Frontal Lobe, Hippocampus, Pyrazoles, Ribosomal Protein S6 Kinases, 70-kDa, Seizures, TOR Serine-Threonine Kinases
Background
Post-traumatic epilepsy (PTE) is one of the types of epilepsies developing in ~25% of patients with traumatic brain injuries [1]. The TBI initiates various pathological changes such as neuronal death, sprouting of axons, oxidative stress, and release of neurotransmitters, which are associated with epileptogenesis [2]. PTE was reported to develop gradually following TBI during a 10-year period because of medical mis-management [3]. PTE treatment development is hindered by the lack of a clear mechanism underlying the disease pathogenesis. Thus, there remains a need for effective and novel therapy to treat PTE.
Recently, the role of the mammalian target of rapamycin (mTOR) pathway has been studied in several disorders/diseases, showing the association of the mTOR pathway with many physiological processes, including cellular proliferation, synthesis of proteins, and cortex development [4]. The involvement of the mTOR pathway has also recently been found in neurological disorders like epilepsy [5]. Activation of the mTOR pathway is involved in the pathogenesis of multiple types of epilepsy, and overexpression of p-mTOR has been reported in cortical dysplasia, which is a commonly diagnosed pediatric epilepsy [5]. The epileptogenesis of the temporal lobe, also known as adult epilepsy, is also associated with p-mTOR overexpression [6]. mTOR protein activation is induced and maintained by many pathways, including AMPK and PI3K/AKT [7]. Triggering of the mTOR pathway mediates phosphorylation of several downstream proteins, including P70S6K (p70 ribosomal S6 kinase), in human cells [7]. In a cellular model of PTE, epileptogenesis was induced by mTOR activation through the PI3K-Akt pathway [8]. More than 30% of epilepsy patients are insensitive to the available anti-epileptic drugs [9], which also have adverse effects, including weight gain, nausea, and tiredness. Compounds that can deactivate the mTOR pathway possess neuroprotective and anti-epileptic effects through targeting the mTOR pathway [5,10].
Pyrazole, a heterocyclic molecule, is the structural moiety in several pharmacological compounds and FDA-approved drug candidates [11]. Prominent compounds containing pyrazole include anti-inflammatory drugs (e.g., celecoxib and lonazolac), insecticides (e.g., fipronil), and analgesics (e.g., dipyrone [12–14]. The structure of pyrazole has received considerable interest because of its presence in compounds with a wide spectrum of pharmacological activities, such as like anti-convulsant, tranquillizing, psychoanaleptic, anti-hypotensive, and antidepressant properties [15]. The present study investigated PP-4-one derivative (Figure 1) as an anti-epileptic agent in an
Material and Methods
ANIMALS:
Fifty Sprague-Dawley rats (body weight 185–215 g) were obtained from the Animal Center belonging to Fujian University, China. The rats were housed 15 days prior to start of the experiment at 24±1˚C temperature, 60% humidity, and exposed to a 12/12 h light/dark cycle. All rats had free access to sterile water and standard food. The guidelines for Animal Use and Care issued by the Science and Technology Ministry of China were followed for all protocols. The study was approved by the Animal Care and Use Committee of Fujian Medical University.
PTE RAT MODEL ESTABLISHMENT AND MEASUREMENT OF SEIZURES:
The PTE induction in rats was achieved successfully using a previously established protocol [16,17]. The rats were put in a David Kopf small animal stereotaxic apparatus after anesthetization using 350-mg/kg chloral hydrate injections via intraperitoneal route. The pressure points were applied with 2% lidocaine ointment and a ~0.5-mm diameter burr hole was made into the left calvarium 2 mm posterior and lateral to the bregma. A 30-gauge needle was fitted to a microinjection syringe fixed in a stereotaxic micromanipulator. The needle was carefully penetrated into the cortex ~1.3 mm deep into the dura to inject 10 μl of FeCl2 (concentration 100 mM) to each rat over 5 min. The rats were assigned to 5 groups of 10 animals each: a sham group, a FeCl2-induced PTE group, and 3 PP-4-one-treated PTE groups. The rats in the sham group only had a needle inserted but were not injected with FeCl2 solution. After regaining consciousness at approximately 3 h, the rats were monitored continuously using Nihon Kohden 9200 Studio software (QP-219BK; Nihon Kohden) to record video–EEG. It was found that around 60% of rats showed induction of epilepsy. The rats after FeCl2 injections were individually housed in sterile cages at controlled temperatures and humidity. The rats in the 3 treatment groups received 2-, 4-, and 6-mg/kg doses of PP-4-one in normal saline intragastrically. The sham and vehicle treatment groups were injected with equal volumes of normal saline. After surgery the rats were killed using 350 mg/kg doses of chloral hydrate injections via intraperitoneal route. The brains from rats were carefully excised on day 28th of PTE to determined protein expression, iNOS expression, and astrocytic hyperplasia.
EPILEPTIC SEIZURE ASSESSMENT:
Epileptic seizures in the rats were observed following 1 h of epilepsy induction, and assessment of seizures was made using Racine’s scale [18]. The intensity scale ranged from stages 0 to 5, in which absence of response was Stage 0; hyperactivity and twitching was Stage 1; nodding of head and jerking was Stage 2; rearing (i.e., standing on hind legs) was Stage 4; and clonic-tonic seizures with absence of reflexes was Stage 5 [18].
SAMPLE PREPARATION:
Decapitation of the rats was followed by removal of the entire brain and its subsequent dissection on ice. Half of the hippocampal tissues were frozen under liquid nitrogen and stored for RT-PCR assay at a temperature of −80°C, and the remaining half were fixed in 4% paraformaldehyde and then embedded in paraffin. A microtome was used for slicing of embedded tissues into 2-μm sections.
HEMATOXYLIN AND EOSIN STAINING:
The hippocampus tissues were randomly selected for determination of epileptic injury using hematoxylin and eosin dye. The stained tissue sections were examined for neuronal changes under a light microscope (BX51M; Olympus, Tokyo, Japan).
IMMUNOHISTOCHEMISTRY:
The brain slices were subjected to immunostaining using the Polink-2 Plus® Detection System (PV-9001; GBI Labs, Mukilteo, WA, USA). The stained sections were dehydrated on treatment with gradient ethyl alcohol, then incubation was performed with antibodies against glial fibrillary acidic protein (GFAP). PBS washing of the samples was followed by incubation with horseradish peroxidase-conjugated secondary antibodies. The complex formed from avidin-biotin and diaminobenzidine was detected as the reaction product. The control specimens (negative control) were not incubated with primary antibodies. iNOS expression was detected in a similar manner, except that the primary antibodies used were rabbit anti-mouse antibodies (Santa Cruz Biotechnology). The sections were examined and imaged using a Leica microscope connected to a digital camera system.
REVERSE TRANSCRIPTION POLYMERASE CHAIN REACTION (RT-PCR):
The commercially available Quant One Step kit (Tiangen Biotech, Co., Beijing, China) was used for RT-PCR reaction in accordance with the supplier’s instructions. The sequence of parameters used in the thermal cycler were: 93°C for 4 min, 38 cycles at 93°C for 26 s, 56°C for 26 s, 70°C for 35 s, and 70°C for 10 min. The primer sequences used in RT-PCR were: NR1, forward: 5′-GCTGCACGCCTTTATCTG-3′ and backward 5′-TCCTACGGGCATCCTTGT-3′. The electrophoresis of products using agarose gel (2.0%) was employed for separation of PCR products. The SC-810 Gel System and Gel-Pro 3.1 software (Media Cybernetics, Inc., Bethesda, MD, USA) were used for analysis of the band intensity.
WESTERN BLOT ANALYSIS:
The hippocampus tissue lysis and subsequent homogenization was followed by protein concentration measurement using the BCA kit (Beyotime, China). Separation of proteins was made by loading equal amounts on 10% SDS-PAGE and then transferred to polyvinylidene fluoride membranes for 80 min at 100 V. The membranes were blocked for 3 h with 5% nonfat dry milk and then subjected to incubation at 4°C with primary antibodies overnight. The antibodies used were against: p-mTOR (1: 200; ab2732; Abcam, Cambridge, UK) and p-P70S6K (1: 200; 9205; Cell Signaling Technologies, Cambridge, UK). Membranes were washed in PBST, followed by incubation for 2 h with horseradish peroxidase-conjugated secondary antibodies. Blot detection was performed using the enhanced chemiluminescence kit as per the manual protocol. ImageJ software was used for determination of densities of proteins.
STATISTICAL ANALYSIS:
Data are expressed as mean±SD of triplicate measurements. Data analysis was performed using SPSS 13.0 (SPSS, Inc., Chicago, IL, USA). The data were compared statistically using the
Results
PP-4-ONE DECREASED BEHAVIORAL SEIZURES IN PTE RATS:
The average number of behavioral seizures increased to 15 in the vehicle group of PTE rats compared to zero in the sham group on day 28 of PTE (Figure 2). In PP-4-one-treated PTE rats, the number of behavioral seizures showed a significant (P<0.05) decrease in dose-dependent manner. The behavioral seizures were reduced to a minimum in the rats treated with 6 mg/kg PP-4-one on day 28 of PTE. The behavioral seizures in the 2 and 4 mg/kg PP-4-one-treated PTE rat groups were 10 and 7, respectively on day 28 of PTE.
PP-4-ONE INHIBITED INOS EXPRESSION:
The iNOS-positive cells in hippocampal tissues of PTE rats were determined by measuring light-density regions (IOD/Area, Figure 3). In vehicle-treated PTE rats, the iNOS-positive cell density was significantly (P<0.05) higher than in the sham group. The iNOS-positive cell count in the hippocampal tissues of PTE rats was significantly reduced by treatment with PP-4-one. The reduction of iNOS-positive cell count by PP-4-one treatment in the hippocampal tissues of PTE rats was the highest at 6 mg/kg dose.
INHIBITION OF ASTROCYTIC HYPERPLASIA BY PP-4-ONE:
The proportion of GFAP-positive cells showed a marked increase in the vehicle-treated PTE rat hippocampus tissues relative to the sham group (Figure 4). PP-4-one treatment significantly (P<0.05) suppressed GFAP-positive cellular count in PTE rats in a dose- dependent manner. The proportion of GFAP-positive cells in the 6 mg/kg PP-4-one treatment group was suppressed to a level very close to that of the sham control group. The PTE-mediated increase in GFAP-positive cells was also significantly alleviated in the 2 and 4 mg/kg PP-4-one treatment groups.
PP-4-ONE SUPPRESSED NR1 EXPRESSION:
The level of NR1 mRNA in vehicle-treated PTE rats was markedly higher than in the sham control group (Figure 5). However, in PP-4-one treated PTE rats, the expression of NR1 mRNA was significantly (P<0.01) suppressed in a dose- dependent manner. In the 6 mg/kg PP-4-one treatment group, the level of NR1 mRNA was suppressed to a level very close to that of the sham control group. The PTE-mediated increase in NR1 mRNA was also significantly alleviated in the 2 and 4 mg/kg PP-4-one treatment groups.
PP-4-ONE ATTENUATED EPILEPTIC INJURY:
The cells of the vehicle group PTE rats were disarranged compared to those of the sham group on day 28 of PTE (Figure 6). In PP-4-one-treated PTE rats, the disarrangement of cells showed a significant improvement in a dose-dependent manner. The PTE-induced disruption of cell arrangement was completely alleviated in the rats treated with 6 mg/kg PP-4-one. In the 2 and 4 mg/kg PP-4-one treatment groups, the PTE-mediated disruption of cell arrangement was alleviated, but less prominently than in the 6 mg/kg PP-4-one treatment group.
PP-4-ONE INHIBITED MTOR/P70S6K PHOSPHORYLATION IN PTE RATS:
In vehicle-treated rats, the activated mTOR/P70S6K expression was markedly higher in the hippocampal and frontal lobe tissues compared to the sham group (Figure 7). The expression of phosphorylated mTOR/P70S6K was suppressed in a dose- dependent manner by PP-4-one-treatment in the hippocampal and frontal lobe tissues of PTE rats. The inhibition of activated mTOR/P70S6K by PP-4-one treatment was more prominent in PTE rats treated with 6 mg/kg doses compared to the 2 and 4 mg/kg treatment groups.
Discussion
The present study demonstrated that PP-4-one treatment suppressed PTE-induced behavioral seizures and cell damage in hippocampus tissues of rats. PP-4-one targeted NR1 expression and mTOR signaling pathway to alleviate the PTE-induced epileptic injury. In animals, PTE is induced by many procedures, including the reduction of magnesium ion model [19], the regulated cortical impact model [20], and the injection of FeCl2 model [16]. The FeCl2 injection method in the frontal cortex is generally preferred because this model of PTE closely resembles TBI [17]. Moreover, the PTE model induced by FeCl2 injection has more stability, higher rate of success, and high repeatability [21]. In the current study, PTE was induced in rats by injecting FeCl2 in the frontal cortex. Among the FeCl2-injected rats, epileptogenesis developed in 87% of animals, which confirmed the high success rate of this method. The FeCl2 injection induced behavioral seizures in rats within 1 hour and was sustained for more than 1 month. The PP-4-one treatment of PTE rats significantly suppressed the number of behavioral seizures in a dose-dependent manner. The behavioral seizures were reduced to a minimum in the rats treated with 6 mg/kg PP-4-one on day 28 of PTE. In the PTE rat model, the iNOS-positive cell density was markedly increased relative to the sham group. However, the density of iNOS-positive cells was significantly lower in the hippocampal and frontal lobes of PTE rats after treatment with PP-4-one. PP-4-one treatment also reduced the GFAP-positive cellular count in the PTE rats in a dose- dependent manner. Astrocytes, the main glial cells present in the brain, are increased during epilepsy and play a major role in disease pathogenesis [22]. During epilepsy, astrocytes mediate neuronal excitation and promote formation of mossy fibers in the dentate gyrus, resulting in development of an excitability loop which causes induction of seizures [20]. The present study shows that PP-4-one suppressed astrocytic proliferation in the hippocampus of PTE rats. The astrocytic proliferation in vehicle-treated PTE rat hippocampus was markedly higher compared to the sham group. This suggests that PP-4-one inhibits behavioral seizures in rats by preventing a PTE-mediated increase in astrocytic proliferation. The activated NMDA receptors are associated with the development of seizures and binding sites of these receptors are increased during convulsions. It is reported that convulsion in animal models is inhibited by antagonists of NMDA receptors [23]. In the present study, PP-4-one treatment of PTE rats markedly downregulated NR1 expression compared to control. Studies have found involvement of the mTOR pathway in various neurological disorders, including ischemia, depression, gliomas, and epilepsy [24,25]. The reduction of behavioral seizures in animal models has been shown to be associated with downregulation of the mTOR pathway [7,17]. The present study showed a marked elevation of activated mTOR and P70S6K in the PTE rats, indicating upregulation of the mTOR pathway. In PP-4-one-treated rats, the PTE-induced activation of mTOR/P70S6K was markedly less than in the vehicle-treated group, suggesting that the anti-epileptogenic activity of PP-4-one occurs by targeting mTOR/P70S6K activation in PTE rats.
Conclusions
In summary, PP-4-one exhibits an anti-epileptogenic effect in the rat model of PTE by inhibiting behavioral seizures through suppression of iNOS and astrocytic proliferation. Moreover, PP-4-one treatment suppressed NR1 expression and targeted the mTOR pathway in PTE-induced rats. Thus, PP-4-one appears to be a novel and effective therapeutic agent for treatment of epilepsy induced by PTE. Further research using different epilepsy models is needed to elucidate the anti-epileptogenic effect of PP-4-one.
Figures
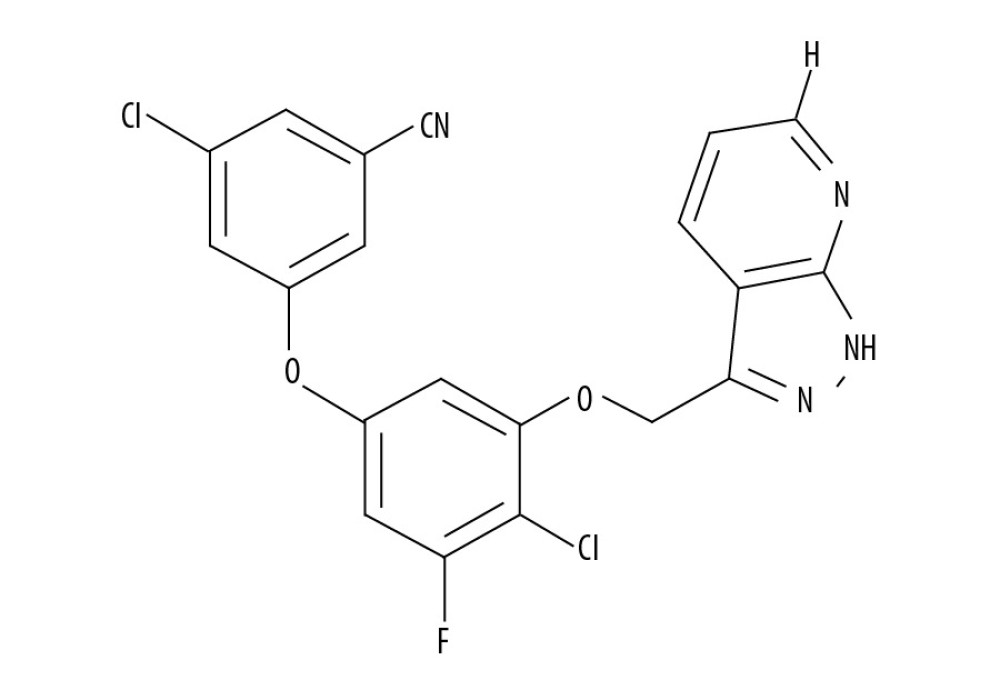 Figure 1. Chemical structure of PP-4-one.
Figure 1. Chemical structure of PP-4-one. 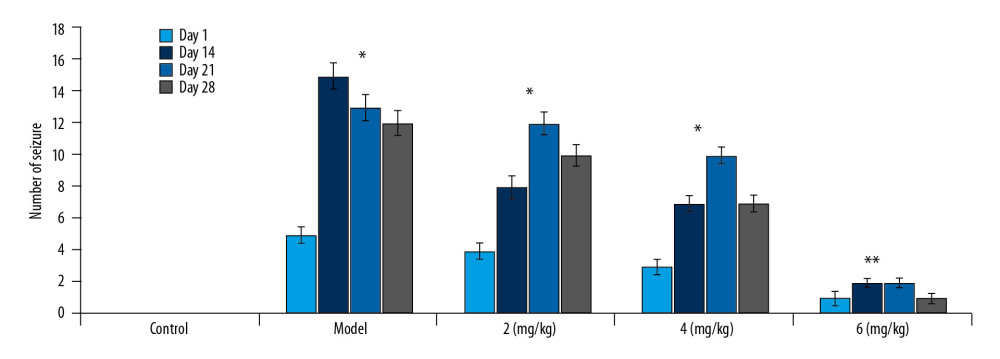 Figure 2. Effect of PP-4-one on behavioral seizures. The PTE rats treated with or without PP-4-one treatment were subjected to measurement of behavioral seizures. * P<0.02 and ** P<0.02 vs. the vehicle-treated rats.
Figure 2. Effect of PP-4-one on behavioral seizures. The PTE rats treated with or without PP-4-one treatment were subjected to measurement of behavioral seizures. * P<0.02 and ** P<0.02 vs. the vehicle-treated rats. 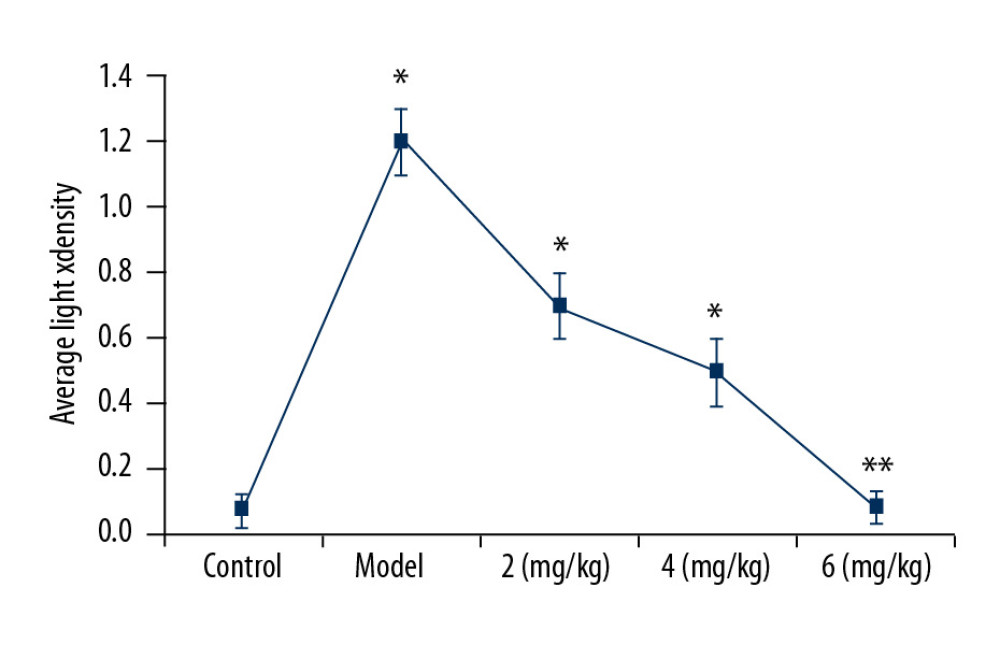 Figure 3. Effect of PP-4-one on iNOS-positive cells. We assessed average light density to determine iNOS-positive cells in PTE-induced rats treated with PP-4-one or without treatment. * P<0.02 and ** P<0.02 vs. the vehicle-treated rats.
Figure 3. Effect of PP-4-one on iNOS-positive cells. We assessed average light density to determine iNOS-positive cells in PTE-induced rats treated with PP-4-one or without treatment. * P<0.02 and ** P<0.02 vs. the vehicle-treated rats. 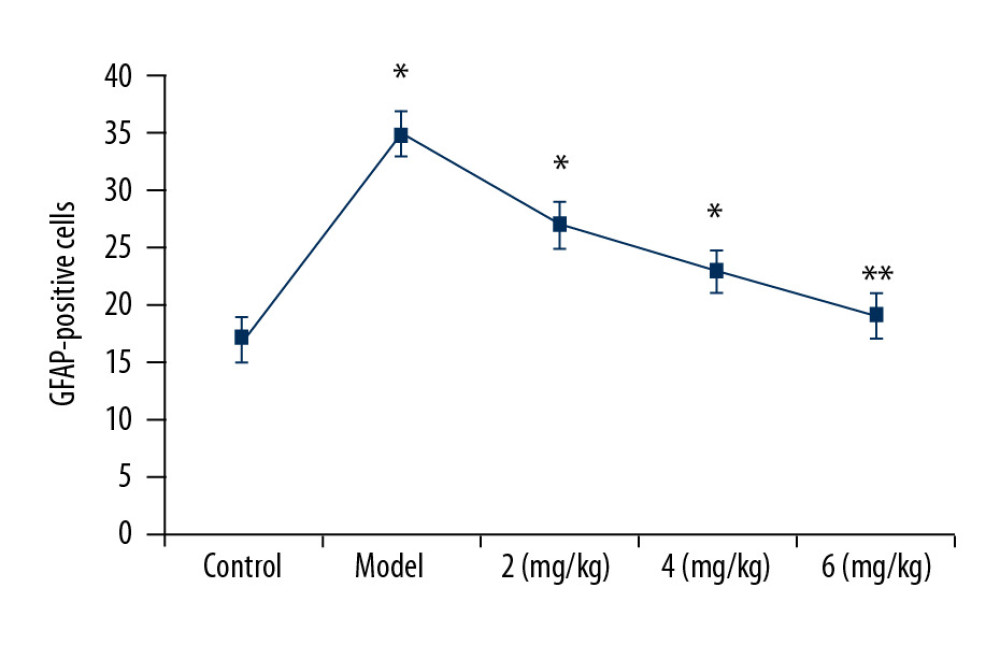 Figure 4. Effect of PP-4-one on GFAP-positive cell count. Under a microscope, we counted the numbers of GFAP-positive cells from PTE-induced rats treated with PP-4-one or without treatment. * P<0.02 and ** P<0.02 vs. the vehicle-treated rats.
Figure 4. Effect of PP-4-one on GFAP-positive cell count. Under a microscope, we counted the numbers of GFAP-positive cells from PTE-induced rats treated with PP-4-one or without treatment. * P<0.02 and ** P<0.02 vs. the vehicle-treated rats. 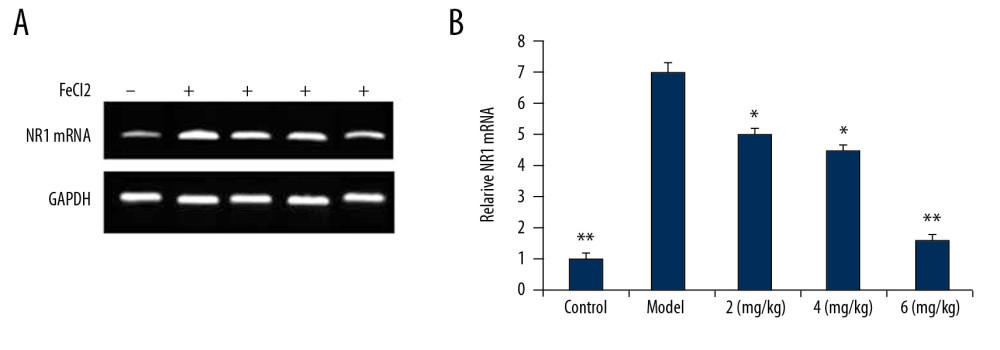 Figure 5. (A, B) Effect of PP-4-one on NR1 mRNA. We performed RT-PCR assay to assess NR1 mRNA levels in PTE-induced rats treated with PP-4-one or without treatment. * P<0.02 and ** P<0.02 vs. the vehicle-treated rats.
Figure 5. (A, B) Effect of PP-4-one on NR1 mRNA. We performed RT-PCR assay to assess NR1 mRNA levels in PTE-induced rats treated with PP-4-one or without treatment. * P<0.02 and ** P<0.02 vs. the vehicle-treated rats. 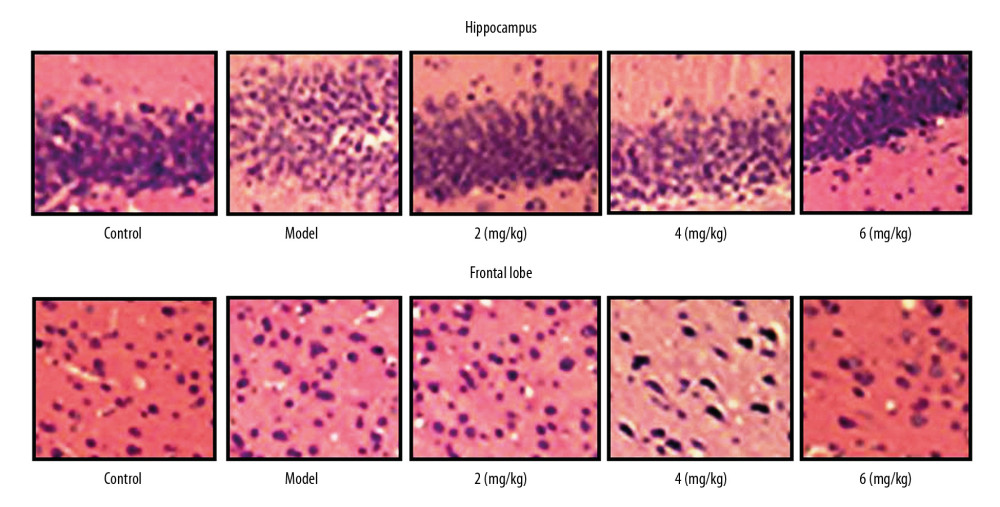 Figure 6. Effect of PP-4-one on PTE-mediated cell injury. We examined cellular impairment using immunohistochemistry in PTE rats treated with or without PP-4-one treatment (magnification ×400).
Figure 6. Effect of PP-4-one on PTE-mediated cell injury. We examined cellular impairment using immunohistochemistry in PTE rats treated with or without PP-4-one treatment (magnification ×400). 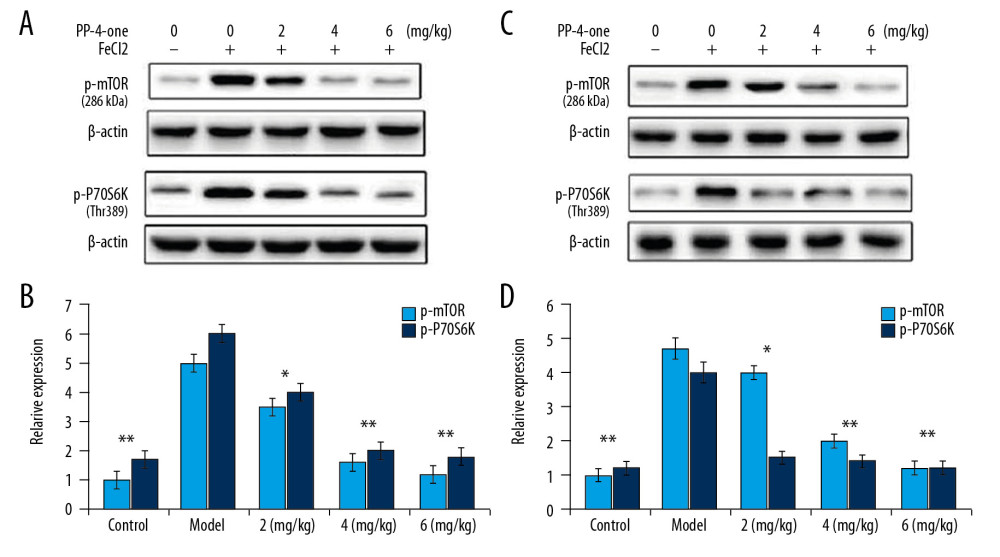 Figure 7. Effect of PP-4-one on mTOR/P70S6K activation. (A, B) The PTE rats treated with or without PP-4-one treatment were assessed for mTOR/P70S6K phosphorylation in hippocampal and frontal lobe tissues by Western blot analysis. (C, D) Quantified data for mTOR/P70S6K phosphorylation in hippocampal and frontal lobe tissues. * P<0.02 and ** P<0.02 vs. vehicle-treated rats.
Figure 7. Effect of PP-4-one on mTOR/P70S6K activation. (A, B) The PTE rats treated with or without PP-4-one treatment were assessed for mTOR/P70S6K phosphorylation in hippocampal and frontal lobe tissues by Western blot analysis. (C, D) Quantified data for mTOR/P70S6K phosphorylation in hippocampal and frontal lobe tissues. * P<0.02 and ** P<0.02 vs. vehicle-treated rats. References
1. Gupta PK, Sayed N, Ding K, Subtypes of post-traumatic epilepsy: Clinical, electrophysiological, and imaging features: J Neurotrauma, 2014; 31; 1439-43
2. Bhuyan P, Patel DC, Wilcox KS, Patel M, Oxidative stress in murine Theiler’s virus-induced temporal lobe epilepsy: Exp Neurol, 2015; 271; 329-34
3. Christensen J, Pedersen MG, Pedersen CB, Long-term risk of epilepsy after traumatic brain injury in children and young adults: A population-based cohort study: Lancet, 2009; 373; 1105-10
4. Crino PB, The mTOR signalling cascade: Paving new roads to cure neurological disease: Nat Rev Neurol, 2016; 12; 379-92
5. Nguyen LH, Brewster AL, Clark ME, mTOR inhibition suppresses established epilepsy in a mouse model of cortical dysplasia: Epilepsia, 2015; 56; 636-46
6. Sha LZ, Xing XL, Zhang D, Mapping the spatio-temporal pattern of the mammalian target of rapamycin (mTOR) activation in temporal lobe epilepsy: PLoS One, 2012; 7; e39152
7. Liu P, Yang X, Hei C, Meli Y, Niu J, Sun T, Li PA, Rapamycin reduced ischemic brain damage in diabetic animals is associated with suppressions of mTOR and ERK1/2 Signaling: Int J Biol Sci, 2016; 12; 1032-40
8. Berdichevsky Y, Dryer AM, Saponjian Y, PI3K-Akt signaling activates mTOR-mediated epileptogenesis in organotypic hippocampal culture model of post-traumatic epilepsy: J Neurosci, 2013; 33; 9056-67
9. Sancho-Rieger J, Parra-Martinez JPreventive and therapeutic attitude in post-traumatic epileptic seizures: Rev Neurol, 2002; 35(Suppl 1); S39-42 [in Spanish]
10. Talos DM, Sun H, Zhou X, The interaction between early life epilepsy and autistic-like behavioral consequences: A role for the mammalian target of rapamycin (mTOR) pathway: PLoS One, 2012; 7; e35885
11. Singh P, Bodiwala HS, Recent advances in anti-HIV natural products: Nat Prod Rep, 2010; 27; 1781-800
12. Tageldin GN, Fahmy SM, Ashour HM, Design, synthesis and evaluation of some pyrazolo[3,4-d]pyrimidines as anti-inflammatory agents: Bioorg Chem, 2018; 78; 358-71
13. Xing Y, Zuo J, Krogstad P, Jung ME, Synthesis and Atructure-Activity Relationship (SAR) studies of novel pyrazolopyridine derivatives as inhibitors of enterovirus replication: J Med Chem, 2018; 61; 1688-703
14. Nassar IF, El Farargy AF, Abdelrazek FM, Synthesis and anticancer activity of some new fused pyrazoles and their glycoside derivatives: J Heterocyclic Chem, 2018; 55; 1709-19
15. Abdel-Aziz M, Abuo-Rahma GE-DA, Hassan AA, Synthesis of novel pyrazole derivatives and evaluation of their antidepressant and anticonvulsant activities: Eur J Med Chem, 2009; 44(9); 3480-87
16. Willmore LJ, Triggs WJ, Effect of phenytoin and cortico- steroids on seizures and lipid peroxidation in experimental posttraumatic epilepsy: J Neurosurg, 1984; 60; 467-72
17. Ueda Y, Willmore J, Triggs WJ, Amygdalar injection of FeCl3 causes spontaneous recurrent seizures: Exp Neurol, 1998; 153; 123-27
18. Arida RM, Scorza FA, Peres CA, Cavalheiro EA, The course of untreated seizures in the pilocarpine model of epilepsy: Epilepsy Res, 1999; 34(2–3); 99-107
19. Gong XW, Li JB, Lu QC, Effective connectivity of hippocampal neural network and its alteration in Mg2+-free epilepsy model: PLoS One, 2014; 9; e92961
20. Butler CR, Boychuk JA, Smith BN, Effects of rapamycin treatment on neurogenesis and synaptic reorganization in the dentate gyrus after controlled cortical impact injury in mice: Front Syst Neurosci, 2015; 9; 163
21. Lloyd BA, Hake HS, Ishiwata T, Exercise increases mTOR signaling in brain regions involved in cognition and emotional behavior: Behav Brain Res, 2017; 323; 56-67
22. Lee HJ, Koh SH, Song KM, The Akt/mTOR/p70S6K pathway is involved in the neuroprotective effect of erythropoietin on hypoxic/ischemic brain injury in a neonatal rat model: Neonatology, 2016; 110; 93-100
23. Fan W, Wang W, Mao X, Elevated levels of p-Mnk1, p-eIF4E and p-p70S6K proteins are associated with tumor recurrence and poor prognosis in astrocytomas: J Neurooncol, 2017; 131; 485-93
24. Lippman-Bell JJ, Rakhade SN, Klein PM, AMPA receptor antagonist NBQX attenuates later-life epileptic seizures and autistic-like social deficits following neonatal seizures: Epilepsia, 2013; 54; 1922-32
25. Ekonomou A, Angelatou F, Upregulation of NMDA receptors in hippocampus and cortex in the pentylenetetrazol-induced ‘kindling’ model of epilepsy: Neurochem Res, 1999; 24; 1515-22
Figures
 Figure 1. Chemical structure of PP-4-one.
Figure 1. Chemical structure of PP-4-one. Figure 2. Effect of PP-4-one on behavioral seizures. The PTE rats treated with or without PP-4-one treatment were subjected to measurement of behavioral seizures. * P<0.02 and ** P<0.02 vs. the vehicle-treated rats.
Figure 2. Effect of PP-4-one on behavioral seizures. The PTE rats treated with or without PP-4-one treatment were subjected to measurement of behavioral seizures. * P<0.02 and ** P<0.02 vs. the vehicle-treated rats. Figure 3. Effect of PP-4-one on iNOS-positive cells. We assessed average light density to determine iNOS-positive cells in PTE-induced rats treated with PP-4-one or without treatment. * P<0.02 and ** P<0.02 vs. the vehicle-treated rats.
Figure 3. Effect of PP-4-one on iNOS-positive cells. We assessed average light density to determine iNOS-positive cells in PTE-induced rats treated with PP-4-one or without treatment. * P<0.02 and ** P<0.02 vs. the vehicle-treated rats. Figure 4. Effect of PP-4-one on GFAP-positive cell count. Under a microscope, we counted the numbers of GFAP-positive cells from PTE-induced rats treated with PP-4-one or without treatment. * P<0.02 and ** P<0.02 vs. the vehicle-treated rats.
Figure 4. Effect of PP-4-one on GFAP-positive cell count. Under a microscope, we counted the numbers of GFAP-positive cells from PTE-induced rats treated with PP-4-one or without treatment. * P<0.02 and ** P<0.02 vs. the vehicle-treated rats. Figure 5. (A, B) Effect of PP-4-one on NR1 mRNA. We performed RT-PCR assay to assess NR1 mRNA levels in PTE-induced rats treated with PP-4-one or without treatment. * P<0.02 and ** P<0.02 vs. the vehicle-treated rats.
Figure 5. (A, B) Effect of PP-4-one on NR1 mRNA. We performed RT-PCR assay to assess NR1 mRNA levels in PTE-induced rats treated with PP-4-one or without treatment. * P<0.02 and ** P<0.02 vs. the vehicle-treated rats. Figure 6. Effect of PP-4-one on PTE-mediated cell injury. We examined cellular impairment using immunohistochemistry in PTE rats treated with or without PP-4-one treatment (magnification ×400).
Figure 6. Effect of PP-4-one on PTE-mediated cell injury. We examined cellular impairment using immunohistochemistry in PTE rats treated with or without PP-4-one treatment (magnification ×400). Figure 7. Effect of PP-4-one on mTOR/P70S6K activation. (A, B) The PTE rats treated with or without PP-4-one treatment were assessed for mTOR/P70S6K phosphorylation in hippocampal and frontal lobe tissues by Western blot analysis. (C, D) Quantified data for mTOR/P70S6K phosphorylation in hippocampal and frontal lobe tissues. * P<0.02 and ** P<0.02 vs. vehicle-treated rats.
Figure 7. Effect of PP-4-one on mTOR/P70S6K activation. (A, B) The PTE rats treated with or without PP-4-one treatment were assessed for mTOR/P70S6K phosphorylation in hippocampal and frontal lobe tissues by Western blot analysis. (C, D) Quantified data for mTOR/P70S6K phosphorylation in hippocampal and frontal lobe tissues. * P<0.02 and ** P<0.02 vs. vehicle-treated rats. In Press
06 Mar 2024 : Clinical Research
Comparison of Outcomes between Single-Level and Double-Level Corpectomy in Thoracolumbar Reconstruction: A ...Med Sci Monit In Press; DOI: 10.12659/MSM.943797
21 Mar 2024 : Meta-Analysis
Economic Evaluation of COVID-19 Screening Tests and Surveillance Strategies in Low-Income, Middle-Income, a...Med Sci Monit In Press; DOI: 10.12659/MSM.943863
10 Apr 2024 : Clinical Research
Predicting Acute Cardiovascular Complications in COVID-19: Insights from a Specialized Cardiac Referral Dep...Med Sci Monit In Press; DOI: 10.12659/MSM.942612
06 Mar 2024 : Clinical Research
Enhanced Surgical Outcomes of Popliteal Cyst Excision: A Retrospective Study Comparing Arthroscopic Debride...Med Sci Monit In Press; DOI: 10.12659/MSM.941102
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








