22 October 2020: Clinical Research
Embryological Characteristics and Clinical Outcomes of Oocytes with Heterogeneous Zona Pellucida During Assisted Reproduction Treatment: A Retrospective Study
Chengshuang Pan1BCEF, Huan Zhang1ADF*DOI: 10.12659/MSM.924316
Med Sci Monit 2020; 26:e924316
Abstract
BACKGROUND: The condition of the zona pellucida can be used to predict human oocyte quality. This study investigated the embryological characteristics and clinical outcomes of oocytes with heterogeneous zona pellucida (HZP) during in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI).
MATERIAL AND METHODS: This was a retrospective study of IVF and ICSI cycles undertaken at The First Affiliated Hospital of Wenzhou Medical University between June 2006 and March 2016. Cycles involving oocytes with HZP (HZP group) were compared with those involving non-HZP oocytes retrieved on the same day (non-HZP group). Embryological characteristics and clinical outcomes were compared.
RESULTS: There were 29 IVF and 46 ICSI cycles in the HZP group, and 521 IVF and 206 ICSI cycles in the non-HZP group. In ICSI cycles, the rates of MII oocyte and high-quality embryo were lower in the HZP group (p<0.05 vs. non-HZP). In IVF cycles, the MII oocyte (p<0.001), normal fertilization (p<0.001), and cleavage (p<0.001) rates were lower, while the abandoned transfer rate (p<0.001) was higher in the HZP group compared with the non-HZP group. The positive human chorionic gonadotropin (HCG), implantation, pregnancy, and miscarriage rates were similar between groups. Multivariate analysis revealed that the woman’s age (OR=0.916 95% CI 0.873–0.962; p<0.001) and the number of D3 high-quality embryos (OR=1.120 95% CI 1.004–1.249; p=0.043) were associated with pregnancy in IVF cycles, but no significant factors were found in ICSI cycles.
CONCLUSIONS: ICSI may help increase the number of viable embryos in cycles with oocytes showing HZP. However, both IVF and ICSI cycles can achieve pregnancy.
Keywords: Fertilization, Oocytes, Pregnancy, Zona Pellucida, Abortion, Spontaneous, Age Factors, Chorionic Gonadotropin, Pregnancy Outcome, Pregnancy Rate, Sperm Injections, Intracytoplasmic
Background
The use of assisted reproduction technologies accounts for over 1 million births each year worldwide [1]. The morphological assessment of oocytes and subsequent embryos is a crucial part of assisted reproduction techniques such as
The human zona pellucida (ZP) is an extracellular glycoprotein matrix that is synthesized and secreted by oocytes during follicular development. The ZP is made of 4 glycosylated proteins (ZP1, ZP2, ZP3, and ZP4) [4,5]. The human ZP is vital for specific sperm-oocyte binding, acrosome reaction induction, and polyspermy prevention [6]. In addition,
The ZP shows different morphologies among oocytes of women undergoing assisted reproductive technology treatment. Abnormal ZP morphology can be observed in 2–5% of all oocytes [9]. It is likely that any abnormality in composition, shape, color, and thickness of the ZP may affect the outcomes of IVF. Oocytes with an oval-shaped ZP have a high risk of abnormal embryo cleavage and are associated with lower rates of implantation and pregnancy after IVF [10,11]. The fertilization rate and clinical outcomes may depend upon variations in ZP thickness [12,13]. In contrast, the fertilization or clinical outcomes seems unaffected by a dark appearance of the ZP [14–16], but contradictory results have been reported [17]. Oocytes with heterogeneous zona pellucida (HZP) have a bright vitreous appearance with an irregular outer edge. A study with a limited sample size found that HZP is associated with reduced oocyte maturity and reduced rates of fertilization and high-quality embryos [18]. Further investigation into the outcome of oocytes with HZP is needed.
Therefore, this study investigated the embryological characteristics and clinical outcomes of oocytes with HZP during IVF and ICSI treatments, as compared to oocytes with normal zona pellucida (non-HZP).
Material and Methods
PATIENTS:
This study retrospectively examined the IVF, and ICSI treatment cycles carried out at The Reproductive Medicine Center of The First Affiliated Hospital of Wenzhou Medical University, Wenzhou, Zhejiang, China, between June 2006 and March 2016. Only the cycles completed under controlled ovarian hyperstimulation with the protocol using gonadotropin-releasing hormone (GnRH) agonist were included. Twenty-nine IVF and 46 ICSI cycles with HZP oocytes were included (HZP group). Another 521 IVF and 206 ICSI cycles with oocytes retrieved on the same day were included as controls (non-HZP group). The detailed baseline information from the women is shown in Table 1.
The exclusion criteria were: 1) known previous poor ovarian response to ovarian stimulation; and 2) endometriosis, polycystic ovary syndrome (PCOS), hydrosalpinx, or uterine pathology.
This study was reviewed and approved by the Ethics Committee of The First Affiliated Hospital of Wenzhou Medical University (No. 2019-099), and the requirement for informed consent was waived due to the retrospective nature of the study.
STUDY DESIGN:
The patients were grouped according to the presence or absence of HZP in the oocytes used during IVF/ICSI. When all retrieved oocytes showed HZP and no normal oocytes could be found, the HZP oocytes were used for the procedure and assigned to the HZP group. The control (non-HZP group) group consisted of cycles with oocytes retrieved on the same day and considered to be morphologically normal.
STIMULATION REGIMENS:
During the study period, the luteal long downregulation protocol (LP) or the short flare-up protocol (SP) were routinely used, as previously described [19]. Specifically, in the LP, a 0.5–0.9 mg depot of a GnRH agonist (diphereline, 3.75 mg; IpsenPharma Biotech) was administered in the mid-luteal phase. Stimulation using gonadotropin (Gonal-F; EMD Serono) was initiated at 14 days, the injection of diphereline (usually on cycle day 3–9) or when pituitary downregulation was achieved, as previously described [19]. Downregulation was confirmed by an endometrial lining of up to 5 mm, serum estradiol <50 pg/L, and serum luteinizing hormone (LH) <5 U/L. For SP, the GnRH agonist (decapetyl, 0.1 mg; Ferring GmbH) was given as a daily dose of 0.1 mg starting on cycle day 2, followed by gonadotropin (Gonal-F; EMD Serono) starting on day 3, as previously described [19]. For both protocols, Gonal-F (150 or 225 U/day) was given for 5–6 days and adjusted based on follicle growth and serum levels of estradiol. Human chorionic gonadotropin (HCG; Livzon, China) was administered at 5000–10 000 IU when at least 3 follicles were observed to have reached 17 mm in diameter as assessed by B-mode ultrasound; oocyte retrieval was carried out 34–36 h later, as previously described [20].
OOCYTE INSEMINATION AND EMBRYO CULTURE:
IVF and ICSI were carried out according to standard protocols, as previously described [17,19]. Specifically, ICSI was used when sperm concentration was below 5×106/mL or when sperm motility was <10%. Insemination was performed by IVF or ICSI 3–6 h after oocyte retrieval. Sixteen to eighteen h later, fertilization was confirmed based on the observation of 2 pronuclei and 2 polar bodies. Fertilized oocytes were cultured in fresh 20 μl of G1 plus medium (Vitrolife AB, Sweden) in individual microdroplets covered with mineral oil at 37ºC in an atmosphere of 6% CO2. In the morning of day 3, embryos were graded according to morphology, blastomere number, blastomere size, and fragmentation, as described by the Istanbul Consensus [21]. The embryos with 7, 8, or 9 blastomeres, and those with less than 20 fragments were considered to be of high quality.
EMBRYO TRANSFER AND PREGNANCY DETECTION:
On day 3, embryo transfer was performed under abdominal ultrasound guidance, as previously described [22]. Specifically, 2 or 3 transferred embryos were selected according to embryo quality. If there were only poor-quality embryos or no embryos from the IVF/ICSI cycle, embryo transfer was canceled.
Pregnancy was confirmed as previously described [19]. Specifically, 2 weeks later, an HCG test was performed. In the case of positive signals, clinical pregnancy was defined as the presence of fetal heartbeat 4 to 5 weeks after embryo transfer, confirmed by ultrasound. Early abortion was defined as spontaneous abortion before 20 weeks of gestation.
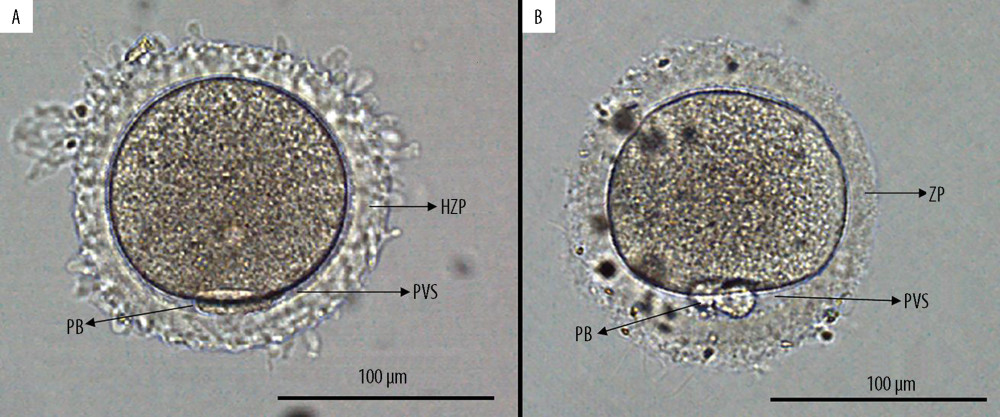
OBSERVATION OF THE ZP: The ZP was evaluated under an OLYMPUS IX73 microscope (OLYMPUS, Japan) equipped with a digital camera at 200× magnification. We defined HZP as a ZP with the bright and vitreous appearance and an irregular outer edge, as previously described [18]; an oocyte with HZP had overtly absent or extremely narrow perivitelline space (Figure 1).
DEFINITIONS AND OUTCOMES:
The numbers of oocytes retrieved, MII oocytes, fertilized oocytes, cleaved embryos, high-quality embryos, and available embryos for transfer were recorded. The corresponding rates were calculated as previously described [19], including MII oocyte rate (MII oocyte number by that of all retrieved oocytes×100), normal fertilization rate (number of fertilized oocytes by that of all retrieved oocytes [for IVF] or MII oocytes [for ICSI]×100), cleavage rate (number of cleaved embryos by that of all fertilized oocytes×100), high-quality embryo rate (the number of high-quality embryos [7, 8, or 9 blastomeres with less than 20 fragments] divided by that of cleaved embryos×100) and abandoned transfer rate (number of cycles of abandoned transfer divided by that of all cycles×100). For successfully transferred embryos, the number of transferred embryos, positive or negative HCG, implantation, pregnancy, gestational period, and miscarriage status were recorded. The positive HCG rate was defined as the total cycles of clinical, biochemical, intrauterine, and ectopic pregnancies divided by all transfer cycles×100. The implantation rate was defined as the number of fetal heartbeats divided by that of transferred embryos×100. The pregnancy rate was defined as the number of patients with 1 or more gestational sac(s) visualized by ultrasound at 6–7 weeks’ gestational age by that of transferred cycles×100. The miscarriage rate was defined as the number of miscarriage cycles divided by that of cycles with clinical pregnancy×100. The live birth rate was defined as the number of cycles with premature or mature delivery divided by that of transferred cycles×100.
STATISTICAL ANALYSIS:
Statistical analyses were carried out with SPSS version 16.0 (SPSS, Inc., USA). Continuous variables are presented as means±standard deviations (SD); comparisons between groups were performed using the
Results
BASELINE FEATURES OF THE INCLUDED PATIENTS:
When the ICSI cycles of both groups were compared, age and BMI were similar, but the duration of infertility was longer in the HZP group (p=0.002), and the diagnosis of infertility was more likely to be primary infertility (p<0.001) vs. the non-HZP group. When IVF cycles were compared between the 2 groups, the same factors were significant: the duration of infertility was longer in the HZP group (p=0.003), and the diagnosis of infertility was more likely to be primary infertility (p<0.001) compared with the non-HZP group, but age and BMI were similar. Other baseline features are shown in Table 1.
EMBRYO CHARACTERISTICS:
Embryological outcomes are shown in Table 2. For ICSI cycles, no differences were observed between the HZP and non-HZP groups in the number of oocytes retrieved, normal fertilization rate, and cleavage rate. The rates of MII oocytes and high-quality embryos were lower in the HZP group vs. the non-HZP group (p<0.05). In IVF cycles, no differences were detected between the HZP and non-HZP groups in the number of oocytes retrieved or high-quality embryo rate. MII oocyte rate, normal fertilization rate, and cleavage rate were lower in the HZP group vs. the non-HZP group (all p<0.001).
CLINICAL OUTCOMES:
Clinical outcomes are shown in Table 2. In ICSI cycles, 36 embryo transfers were performed in the HZP group and 161 in the non-HZP group. There were no significant differences in the abandoned transfer rate per retrieval cycle, positive HCG, implantation, pregnancy, and miscarriage rates between the 2 groups.
In IVF cycles, 12 embryo transfers were performed in the HZP group and 442 in the non-HZP group. The rate of abandoned transfer per retrieval cycle was higher in the HZP group
UNIVARIATE AND MULTIVARIATE ANALYSES:
Univariate analyses of factors related to pregnancy after ICSI are shown in Table 3. The results revealed that the woman’s age (OR=0.894, 95% CI 0.835–0.959; p=0.002), duration of infertility (OR=0.921, 95% CI 0.851–0.998; p=0.044), number of retrieved oocytes (OR=1.077, 95% CI 1.025–1.131; p=0.003), number of MII oocytes (OR=1.097, 95% CI 1.037–1.160; p=0.001), and number of D3 high-quality embryos (OR=1.286, 95% CI 1.109–1.491; p=0.001) were significant factors. The multivariate analysis is shown in Table 3, and no factors were independently related to pregnancy after ICSI.
Table 4 shows the univariate analysis of factors related to pregnancy after IVF. The results demonstrated that the woman’s age (OR=0.895, 95% CI 0.855–0.936; p<0.001), duration of infertility (OR=0.918, 95% CI 0.855–0.985; p=0.017), number of retrieved oocytes (OR=1.061, 95% CI 1.026–1.097; p=0.001), number of MII oocytes (OR=1.074, 95% CI 1.035–1.114; p<0.001), and number of D3 high-quality embryos (OR=1.205, 95% CI 1.098–1.323; p<0.001) were significant factors. As also shown in Table 4, the multivariate analysis found that the woman’s age (OR=0.916, 95% CI 0.873–0.962; p<0.001) and the number of D3 high-quality embryos (OR=1.120, 95% CI 1.004–1.249; p=0.043) were significantly associated with pregnancy. Whether the oocyte had HZP or not was not found to be related to pregnancy in case embryos were available for transfer in either ICSI or IVF cycle.
Discussion
LIMITATIONS:
Firstly, the definition of HZP has no established guidelines and could vary by study. Secondly, patients had different degrees of HZP, which were not graded or distinguished in this study. Thirdly, the sample size was relatively small. A larger study, including patients from multiple centers, might provide more evidence for these findings. Finally, this was a retrospective study, with possible selection bias.
Conclusions
Overall, women with oocytes showing HZP can become pregnant after either IVF or ICSI treatment. However, these women should avoid low fertilization and high abandoned transfer rates by choosing ICSI.
Tables
Table 1. Baseline patient information.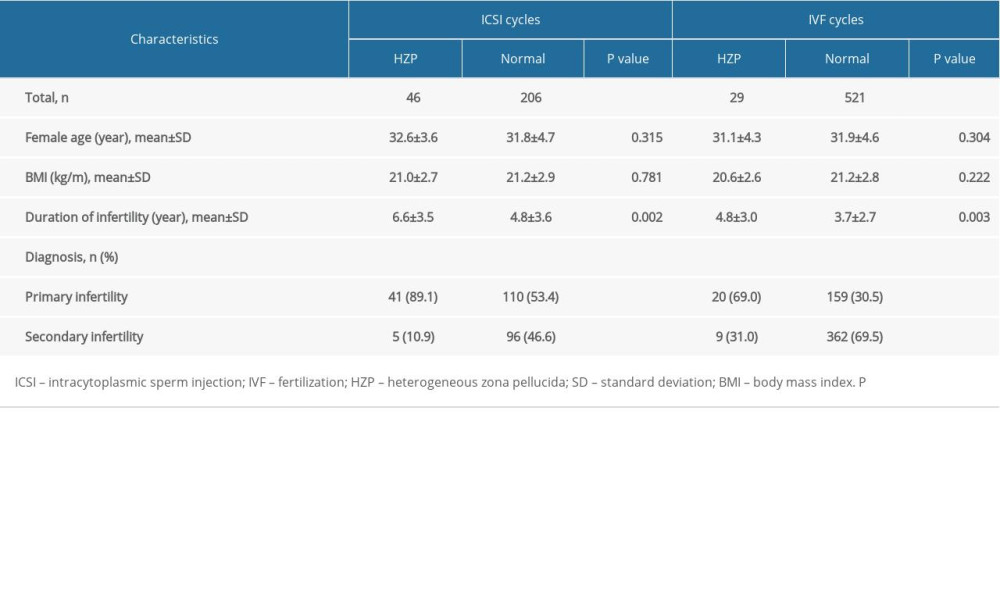 Table 2. Embryological and clinical outcomes for patients in both ICSI and IVF cycles.
Table 2. Embryological and clinical outcomes for patients in both ICSI and IVF cycles.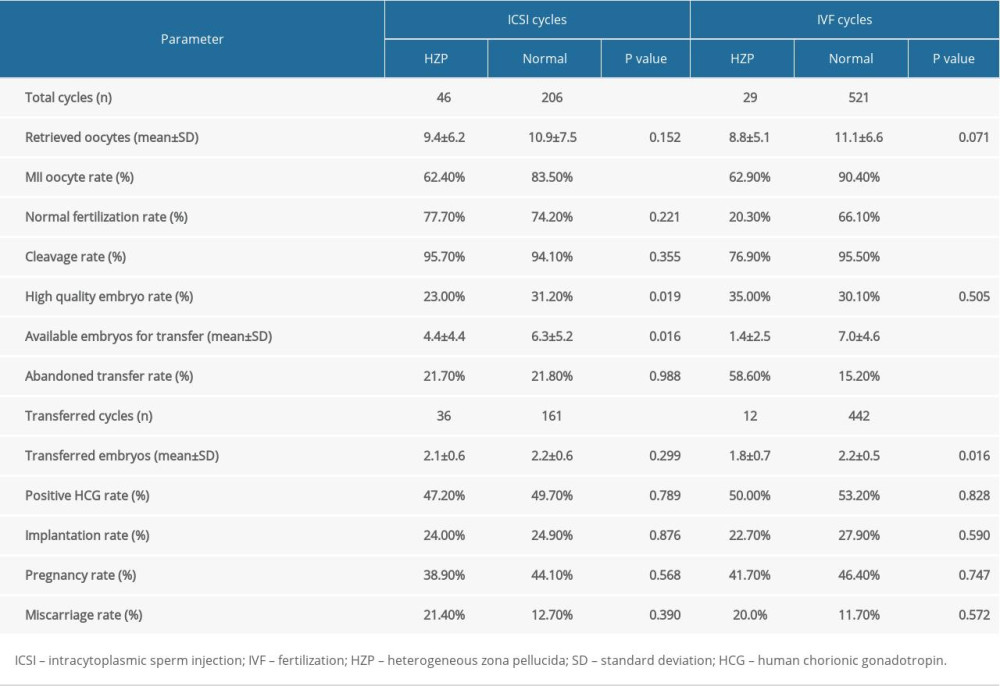 Table 3. Multivariate analysis of the main outcome (pregnancy or not) in ICSI cycles.
Table 3. Multivariate analysis of the main outcome (pregnancy or not) in ICSI cycles.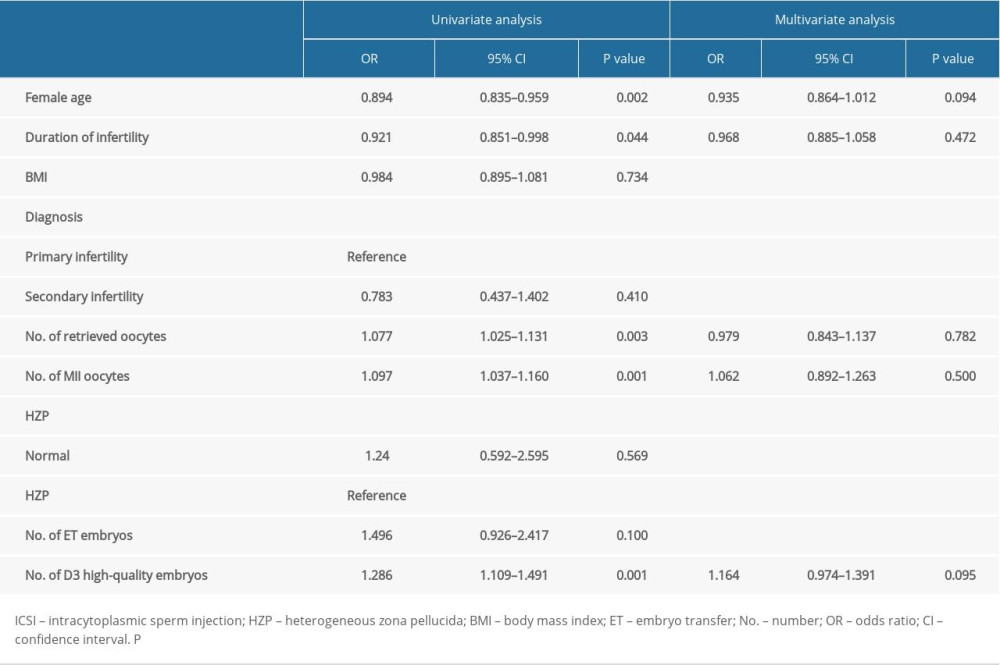 Table 4. Multivariate analysis of the main outcome (pregnancy or not) in IVF cycles.
Table 4. Multivariate analysis of the main outcome (pregnancy or not) in IVF cycles.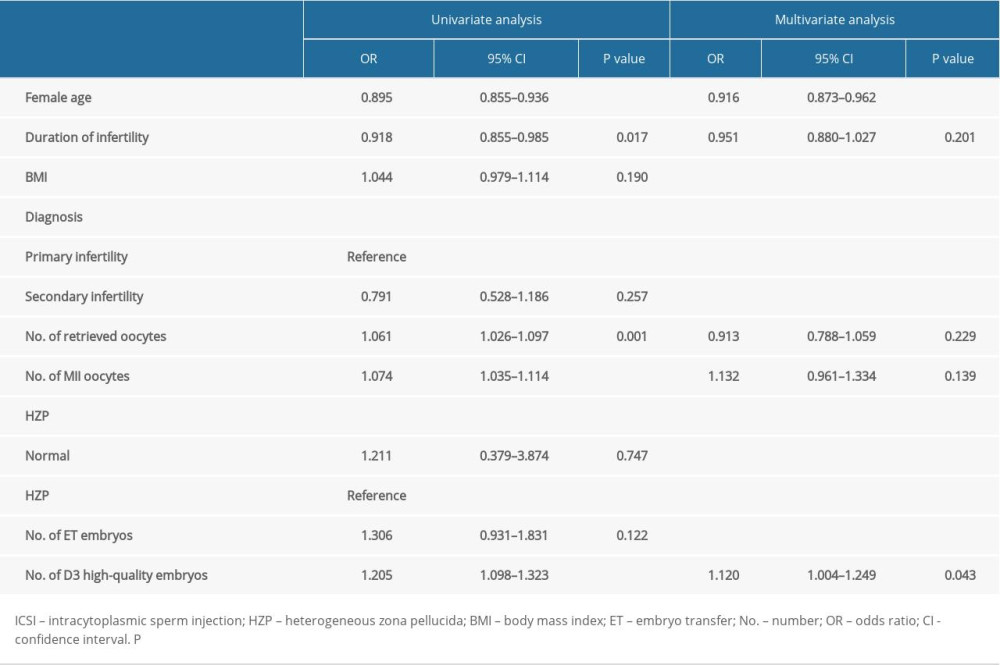
References
1. Davies MJ, Rumbold AR, Moore VM, Assisted reproductive technologies: A hierarchy of risks for conception, pregnancy outcomes and treatment decisions: J Dev Orig Health Dis, 2017; 8; 443-47
2. Uyar A, Torrealday S, Seli E, Cumulus and granulosa cell markers of oocyte and embryo quality: Fertil Steril, 2013; 99; 979-97
3. Rosenwaks Z, Introduction: Biomarkers of embryo viability: The search for the “holy grail” of embryo selection: Fertil Steril, 2017; 108; 719-21
4. Lefievre L, Conner SJ, Salpekar A, Four zona pellucida glycoproteins are expressed in the human: Hum Reprod, 2004; 19; 1580-86
5. Saldivar-Hernandez A, Gonzalez-Gonzalez ME, Sanchez-Tusie A, Human sperm degradation of zona pellucida proteins contributes to fertilization: Reprod Biol Endocrinol, 2015; 13; 99
6. Gupta SK, Role of zona pellucida glycoproteins during fertilization in humans: J Reprod Immunol, 2015; 108; 90-97
7. Choi JK, Yue T, Huang H, The crucial role of zona pellucida in cryopreservation of oocytes by vitrification: Cryobiology, 2015; 71; 350-55
8. Vajta G, Rienzi L, Bavister BD, Zona-free embryo culture: Is it a viable option to improve pregnancy rates?: Reprod Biomed Online, 2010; 21; 17-25
9. Rienzi L, Vajta G, Ubaldi F, Predictive value of oocyte morphology in human IVF: A systematic review of the literature: Hum Reprod Update, 2011; 17; 34-45
10. Ebner T, Shebl O, Moser M, Developmental fate of ovoid oocytes: Hum Reprod, 2008; 23; 62-66
11. Sauerbrun-Cutler MT, Vega M, Breborowicz A: J Ovarian Res, 2015; 8; 5
12. Gabrielsen A, Lindenberg S, Petersen K, The impact of the zona pellucida thickness variation of human embryos on pregnancy outcome in relation to suboptimal embryo development. A prospective randomized controlled study: Hum Reprod, 2001; 16; 2166-70
13. Marco-Jimenez F, Naturil-Alfonso C, Jimenez-Trigos E, Influence of zona pellucida thickness on fertilization, embryo implantation and birth: Anim Reprod Sci, 2012; 132; 96-100
14. Balaban B, Ata B, Isiklar A, Severe cytoplasmic abnormalities of the oocyte decrease cryosurvival and subsequent embryonic development of cryopreserved embryos: Hum Reprod, 2008; 23; 1778-85
15. Esfandiari N, Burjaq H, Gotlieb L, Casper RF, Brown oocytes: Implications for assisted reproductive technology: Fertil Steril, 2006; 86; 1522-25
16. Ten J, Mendiola J, Vioque J, de Juan J, Bernabeu R, Donor oocyte dysmorphisms and their influence on fertilization and embryo quality: Reprod Biomed Online, 2007; 14; 40-48
17. Shi W, Xu B, Wu LM, Oocytes with a dark zona pellucida demonstrate lower fertilization, implantation and clinical pregnancy rates in IVF/ICSI cycles: PLoS One, 2014; 9; e89409
18. Li M, Ma SY, Yang HJ, Pregnancy with oocytes characterized by narrow perivitelline space and heterogeneous zona pellucida: Is intracytoplasmic sperm injection necessary?: J Assist Reprod Genet, 2014; 31; 285-94
19. Brinsden PR, 2005, London, CRC Press
20. Jin J, Pan C, Fei Q: Fertil Steril, 2015; 103; 910-16
21. Alpha Scientists in Reproductive Medicine, ESHRE Special Interest Group of Embryology, The Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting: Hum Reprod, 2011; 26; 1270-83
22. Penzias A, Bendikson K, Butts S, ASRM standard embryo transfer protocol template: A committee opinion: Fertil Steril, 2017; 107; 897-900
23. Rankin TL, O’Brien M, Lee E, Defective zonae pellucidae in Zp2-null mice disrupt folliculogenesis, fertility and development: Development, 2001; 128; 1119-26
24. Rankin T, Talbot P, Lee E, Dean J, Abnormal zonae pellucidae in mice lacking ZP1 result in early embryonic loss: Development, 1999; 126; 3847-55
25. Rankin T, Familari M, Lee E, Mice homozygous for an insertional mutation in the Zp3 gene lack a zona pellucida and are infertile: Development, 1996; 122; 2903-10
26. Pokkyla RM, Lakkakorpi JT, Nuojua-Huttunen SH, Tapanainen JS, Sequence variations in human ZP genes as potential modifiers of zona pellucida architecture: Fertil Steril, 2011; 95; 2669-72
27. Sousa M, Teixeira da Silva J, Silva J, Embryological, clinical and ultrastructural study of human oocytes presenting indented zona pellucida: Zygote, 2015; 23; 145-57
28. Mrazek M, Fulka J, Failure of oocyte maturation: Possible mechanisms for oocyte maturation arrest: Hum Reprod, 2003; 18; 2249-52
29. Swain JE, Pool TB, ART failure: Oocyte contributions to unsuccessful fertilization: Hum Reprod Update, 2008; 14; 431-46
Tables
 Table 1. Baseline patient information.
Table 1. Baseline patient information. Table 2. Embryological and clinical outcomes for patients in both ICSI and IVF cycles.
Table 2. Embryological and clinical outcomes for patients in both ICSI and IVF cycles. Table 3. Multivariate analysis of the main outcome (pregnancy or not) in ICSI cycles.
Table 3. Multivariate analysis of the main outcome (pregnancy or not) in ICSI cycles. Table 4. Multivariate analysis of the main outcome (pregnancy or not) in IVF cycles.
Table 4. Multivariate analysis of the main outcome (pregnancy or not) in IVF cycles. Table 1. Baseline patient information.
Table 1. Baseline patient information. Table 2. Embryological and clinical outcomes for patients in both ICSI and IVF cycles.
Table 2. Embryological and clinical outcomes for patients in both ICSI and IVF cycles. Table 3. Multivariate analysis of the main outcome (pregnancy or not) in ICSI cycles.
Table 3. Multivariate analysis of the main outcome (pregnancy or not) in ICSI cycles. Table 4. Multivariate analysis of the main outcome (pregnancy or not) in IVF cycles.
Table 4. Multivariate analysis of the main outcome (pregnancy or not) in IVF cycles. In Press
05 Mar 2024 : Clinical Research
Role of Critical Shoulder Angle in Degenerative Type Rotator Cuff Tears: A Turkish Cohort StudyMed Sci Monit In Press; DOI: 10.12659/MSM.943703
06 Mar 2024 : Clinical Research
Comparison of Outcomes between Single-Level and Double-Level Corpectomy in Thoracolumbar Reconstruction: A ...Med Sci Monit In Press; DOI: 10.12659/MSM.943797
21 Mar 2024 : Meta-Analysis
Economic Evaluation of COVID-19 Screening Tests and Surveillance Strategies in Low-Income, Middle-Income, a...Med Sci Monit In Press; DOI: 10.12659/MSM.943863
10 Apr 2024 : Clinical Research
Predicting Acute Cardiovascular Complications in COVID-19: Insights from a Specialized Cardiac Referral Dep...Med Sci Monit In Press; DOI: 10.12659/MSM.942612
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952