24 October 2020: Clinical Research
Retrospective Comparison of the Safety and Effectiveness of Dexmedetomidine Versus Standard of Care Before and During Cesarean Delivery in a Maternity Unit in Zhengzhou, China
Wei Zhao1ABF, Ling Ma1ABDF, Jingjing Wang2EFG*, Xiaoyan Shi3ABCFDOI: 10.12659/MSM.925709
Med Sci Monit 2020; 26:e925709
Abstract
BACKGROUND: The objective of the present study was to test the hypothesis that intravenous dexmedetomidine is safe and effective when administered to women before and during cesarean section.
MATERIAL AND METHODS: The analysis included 392 women who received spinal anesthesia and no analgesia prior to undergoing elective cesarean delivery. Of them, 115 women received dexmedetomidine before anesthesia and during delivery (DX cohort), 109 received normal saline before anesthesia and during delivery and dexmedetomidine after delivery (SC cohort), and 168 received normal saline only before anesthesia and during delivery (CN cohort). Data about the women’s consumption of sufentanil and ondansetron during hospitalization, onset of lactation, and hospital stays were retrospectively collected and analyzed.
RESULTS: Most of the women in the study were primiparous (362/392). The women in the DX cohort received less sufentanil during their hospital stays than those in either of the other 2 cohorts (SC comparison: 151.45±11.15 μg vs. 175.12±25.15 μg, P<0.0001, q=8.776; CN comparison: 151.45±11.15 μg vs. 185.42±37.45 μg, P<0.0001, q=13.911). Also, the women in the DX cohort received less ondansetron before discharge and had shorter times to first lactation and hospital stays than those in the SC and CN cohorts.
CONCLUSIONS: Administering dexmedetomidine before spinal anesthesia appears to be safe and effective for women undergoing elective cesarean delivery.
Keywords: Anesthesia, Spinal, Cesarean Section, Dexmedetomidine, Hemodynamics, Lactation, Sufentanil, Administration, Intravenous, Analgesics, Non-Narcotic, Anesthesia, Obstetrical, Hypnotics and Sedatives, Intraoperative Care, Pregnancy, Preoperative Care, standard of care, young adult
Background
Cesarean deliveries are common [1] and post-delivery pain [2], anxiety, and depression [3] are seen more often in women who deliver via cesareans than vaginally. Effective post-delivery pain relief affects the recovery of women after cesarean delivery [4]. General anesthesia often is used for cesarean deliveries because many of the procedures are emergent, but also based on patient choice [5]. Sufentanil (a selective agonist of the μ-receptor) [6] combined with non-opioid analgesics commonly is used for pain management after cesarean delivery [7]. Maternal treatment with analgesics and sufentanil combined before delivery induces neonatal respiratory depression [5].
Dexmedetomidine is a highly selective α-2 receptor agonist that has anti-sympathetic, sedative, and analgesic effects [7]. It reduces release of damage-transmitting substances and glutamate, leading to suppression of pain [8]. Dexmedetomidine affects the locus coeruleus and induces sleep [9]. It also increases the frequency of contractions of smooth muscles in the uterus [7]. These effects can be of benefit to women who have cesarean deliveries [7,10], whereas postoperative infusion of dexmedetomidine combined with sufentanil is effective for analgesia and sedation [4]. However, when combined with sufentanil, dexmedetomidine results in excessive sedation [7], and adverse effects include hypotension [5] and bradycardia [7]. The maternal and fetal effects of administering dexmedetomidine after delivery have not been thoroughly evaluated [2]. Stronger evidence is required about the drug’s analgesic and sedative actions in obstetric settings. Retrospective analysis also should be performed to evaluate the effects of administration of different regimens of dexmedetomidine before and during cesarean delivery and for post-cesarean pain management.
The objectives of the present retrospective study were to evaluate the effectiveness and safety profile of preoperative and intraoperative use of dexmedetomidine in women undergoing cesarean deliveries.
Material and Methods
ETHICS APPROVAL AND CONSENT TO PARTICIPATE:
Protocol 2019-ZZJH-189, dated March 28, 2020, was approved by the Zhengzhou University Review Board and the Chinese Society of Anesthesiology. Written approval was received before the data were collected. All of the women who participated or their legally authorized representatives signed informed consent forms prior to hospital admission, which included information about anesthesia, delivery, treatment, and publication of the study.
STUDY POPULATION:
The population analyzed in the study was women who received spinal anesthesia but no predelivery analgesia and underwent elective cesarean delivery.
ANESTHESIA PROCEDURES:
All the women were given a 500-mL compound electrolyte solution (PLASMA-LYTE, Baxter Healthcare Corporation, Deerfield, Illinois, U.S.A.). Subarachnoid puncture into the L3/L4 space was performed on the parturients in the labor room using a 25G needle (Dr. Japan Co., Ltd., Tokyo, Japan). Bupivacaine 0.5% (Marcaine, Hospira, Inc., Lake Forest, Illinois, U.S.A.) then was administered at 0.1 mL/s for 1 to 2 min. to maintain sensory block plane at the T4/T6 level [2]. The anesthesia was administered by anesthesiologists with a minimum of 3 years of experience. Systolic and diastolic blood pressure, heart rate, and peripheral capillary oxygen saturation were monitored throughout delivery. If hypotension occurred, as defined as systolic blood pressure <90 mmHg or >20% drop in baseline systolic blood pressure, 50 μg of phenylephrine (Frenin, Wyeth Limited, New York, New York, U.S.A.) was administered. In the case of hypertension (systolic blood pressure >180 mmHg), 0.1 mg of nicardipine (Cardene IV, Baxter Healthcare Corporation, Deerfield, Illinois, U.S.A.) was administered. If a patient’s heart rate increased to 110 beats per minute, 10 mg of esmolol (Brevibloc, Baxter Healthcare Corporation, Deerfield, Illinois, U.S.A.) was administered [7]. All of the women received a 4-mg injection of ondansetron (Hospira Inc., Lake Forest, Illinois, U.S.A.) before their stitches were removed.
COHORT:
The 115 women in the DX cohort received 0.5 μg/kg of dexmedetomidine (Precedex™, Hospira Inc., Lake Forest, Illinois, U.S.A.) within 10 min before anesthesia and 0.5 μg/kg/h of dexmedetomidine in normal saline (Baxter Healthcare Corporation, Deerfield, Illinois, U.S.A.) during delivery. The 109 women in the SC cohort received 2 mL of normal saline within 10 min before anesthesia, 2 mL/h of normal saline during delivery, and 0.5 μg/kg of dexmedetomidine after delivery. The 168 women in the CN cohort received 2 mL of normal saline within 10 min before anesthesia and 2 mL/h of normal saline during delivery. For a maximum of 3 days after delivery, all of the women received no more than 3 infusions per day of 10 mg/mL, 100 mL paracetamol (Perfalgan, Bristol-Myers Squibb Pharmaceuticals Ltd., Fontana del Ceraso Anagni, Italy). They also received an injection of 1.5 μg/kg of sufentanil (Dsuvia™, Acelrx Pharmaceutical Inc., Redwood City, California, U.S.A.) after delivery. These regimens were approved by the institution and a shift in usual practice.
PAIN MEASUREMENT:
The women’s pain was assessed using a visual analog scale (VAS) with a range of 0 to 10, with 0 being no pain and 10 the maximum possible pain. Nursing staff with a minimum of 3 years of experience administered the VAS at 6 and 12 h and 1 day after delivery while the women were resting, having uterine contractions, and moving [7].
PAIN MANAGEMENT:
The institute’s nursing staff, in consultation with an obstetrician who had a minimum of 3 years of experience, administered 50-μg injections of sufentanil to women who had a VAS score >3 during rest.
MANAGEMENT OF NAUSEA AND VOMITING:
Working in consultation with an obstetrician, nursing staff from the institute who were blinded to the treatments administered 4-mg ondansetron injections to women who experienced nausea and vomiting.
FIRST LACTATION TIME:
The time from delivery to when at least 10 mL (2 tsp) of milk flowed from both breasts was defined as the first lactation time, based on self-reports by the women [7].
OTHER MATERNAL OUTCOMES AFTER DELIVERY:
After delivery, episodes of hypertension (systolic blood pressure >180 mmHg), hypotension (systolic blood pressure <90 mmHg), bradycardia (heart rate <50 beats per minutes), tachycardia (heart rate >110 beats per minutes), respiratory depression (defined as a decline in respiratory rate to 10 times/min for more than 10 min), and hypoxia (peripheral capillary oxygen saturation <95%) were recorded and analyzed [7].
HOSPITAL STAYS:
A hospital stay was defined as the period from admission for delivery to discharge.
STATISTICAL ANALYSIS:
SPSS v25.0 (IBM, Inc., New York, NY, U.S.A.) was used for statistical analysis. Data are shown as a frequency (percentage) and continuous and ordinal data as mean±SD. One-way analysis of variance (ANOVA) [2] was performed on continuous data and Fisher’s exact test [7] was performed on constant and ordinal data. Tukey’s test (with a critical value q>3.326) was performed for post hoc analysis. The sample size was calculated on the basis of 80% power and a 5% margin of error [2]. All results were considered significant at a 95% confidence level.
Results
STUDY POPULATION:
From January 1, 2019 to December 15, 2019, 1198 women underwent delivery at 30 to 42 weeks’ gestation in the Department of Obstetrics of the School of nursing and health, Zhengzhou University, Zhengzhou, Henan, China and the Second Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan, China. Of them, 7 had emergency cesareans, 585 gave birth vaginally, and 1 woman reported having an α-2 receptor agonist allergy. In 51 women, obstetricians used analgesia before delivery and 162 women did not receive spinal anesthesia, based on a decision by an anesthesiologist in consultation with an obstetrician. Therefore, data on the previously described women were excluded from the analysis.
Data from 392 women were included in the analysis who received spinal anesthesia, did not receive predelivery analgesia, and underwent elective cesarean delivery based on a decision made by an obstetrician (Figure 1). The women were ages 20 to 41 years and the average gestational age was 35.15±2.46 weeks. The majority of women in the study were primiparous (362/392). Table 1 lists the demographic characteristics, clinical conditions, and educational status of the women at the time of their admissions.
PAIN MEASUREMENT:
The same VAS score for pain was recorded in women in all of the cohorts during rest immediately after delivery (P=0.118). At 6 h after delivery, while resting, women in the DX cohort had significantly less pain than those in the SC cohort (P<0.0001, q=4.263) or the CN cohort (P<0.0001, q=7.064). While resting 6 h later, the women in the DX cohort (P<0.0001, q=8.437) and those in the SC cohort (P<0.0001, q=3.632) had significantly less pain than the women in the CN cohort. One day after delivery, while resting, women in the DX cohort (P<0.0001, q=10.804) and in the SC cohort (P<0.0001, q=6.715) had significantly less pain than those in the CN cohort. Figure 2 lists detailed data about pain while resting after delivery in the patients.
One day after delivery, the women in the DX cohort had significantly less pain during uterine contractions than those in the CN cohort (P<0.0001, q=9.569) or the SC cohort (P<0.0001, q=6.931). Table 2 lists detailed data about pain during uterine contractions after delivery in the patients.
The women in the DX cohort also had significantly less pain on movement 1 day after delivery than those in the CN cohort (P<0.0001, q=8.460) or the SC cohort (P<0.0001, q=5.106). Table 3 lists detailed data about pain on movement after delivery in the patients.
PAIN MANAGEMENT:
The women in the DX cohort received significantly less sufentanil during their hospitalizations than those in the SC cohort (151.45±11.15 μg vs. 175.12±25.15 μg, P<0.0001, q=8.776) or in the CN cohort (151.45±11.15 μg vs. 185.42±37.45 μg, P<0.0001, q=13.911). The women in the SC cohort received significantly less sufentanil before discharge than those in the CN cohort (P<0.0001, q=4.151, Figure 3).
MANAGEMENT OF NAUSEA AND VOMITING:
The women in the DX cohort received significantly less ondansetron before discharge than those in the SC cohort (6.15±1.14 mg/woman vs. 7.52±2.14 mg, P<0.0001, q=6.016) or in the CN cohort (6.15±1.14 mg vs. 9.12±3.11 mg, P<0.0001, q=14.404). The women in the SC cohort received significantly less ondansetron before discharge than those in the CN cohort (P<0.0001, q=7.636, Figure 4).
FIRST LACTATION TIME:
Time to first lactation was significantly shorter in the women in the DX cohort than in those in the SC cohort (25.12±3.12 min vs. 27.45±2.12 min, P<0.0001, q=10.592) or in the CN cohort (25.12±3.12 min vs. 28.08±1.75 min, P<0.0001, q=14.863, Figure. 5).
MATERNAL OUTCOME AFTER DELIVERY:
Incidences of adverse hemodynamic adverse events were the same in all of the study cohorts (P=0.998, Table 4).
HOSPITAL STAYS:
Women in the DX cohort had significantly shorter hospital stays than those in the SC cohort (3.01±0.15 days vs. 3.51±1.15 days, P<0.0001, q=3.964) or the CN cohort (3.01±0.15 days vs. 3.85±1.81 days, P<0.0001, q=7.356, Figure 6).
Discussion
Administration of dexmedetomidine before and during cesarean delivery resulted in less pain post-delivery in women during rest, uterine contractions, and while moving compared with post-delivery administration of normal saline combined with sufentanil. These outcomes parallel those reported in randomized trials [2,11]. Administration of dexmedetomidine before anesthesia has been shown to decrease post-delivery pain due to an opioid-sparing effect [7]. In the present study, post-delivery administration of dexmedetomidine combined with sufentanil resulted in less post-delivery pain at during rest in the SC cohort than was seen in the CH cohort with post-delivery administration of normal saline combined with sufentanil. These results also parallel those reported in randomized trials [4,7,12]. Post-delivery administration of dexmedetomidine with sufentanil has been shown to decrease pain associated with delivery [2]. The results of the present study suggest that dexmedetomidine may enhance the analgesic effect of sufentanil in the setting of cesarean delivery.
Women in the DX cohort received less sufentanil and ondansetron before discharge than those in the SC and CN cohorts. Those results parallel outcomes reported in some [2,5,7] but not all randomized trials [4]. The smaller sample size in the present study may be the reason for the contradictory results. Dexmedetomidine administration has been shown to decrease requirements for sufentanil [2,7]. The drug’s α-agonist action reduces nausea and vomiting [7] by reducing the minimum alveolar concentration of anesthetics [5]. Dexmedetomidine also reduces the need for administration of sufentanil, which can cause nausea and vomiting [6,7]. Dexmedetomidine administration may decrease nausea and vomiting after cesarean delivery.
Time to first lactation was shorter in women who received dexmedetomidine before and during delivery, but not in the same was not true in those who received the drug after delivery. Those results are consistent with reports from randomized trials [2,4,7]. Stress caused by delivery increases release of dynorphin and dopamine, which decreases release of prolactin and delays time to first lactation [2]. Therefore, time to first lactation is delayed in women who undergo cesarean delivery [13]. Dexmedetomidine improves time to first lactation by decreasing depression after delivery [14]. Because plasma dexmedetomidine levels were no higher in the women in the SC cohort than in those of the DX cohort, time to first lactation was not lengthened in the former cohort [4,7]. Difficulty in breastfeeding that is associated with cesarean delivery may be overcome by administering dexmedetomidine before and during delivery.
In the present study, there were no differences in hemodynamic parameters among the cohorts. These results parallel those reported in randomized trials [2,4,5,7] and in a prospective, observational study [15]. Administration of dexmedetomidine before delivery is safe for maternal health but further research is required to study its effects on neonates.
Hospital stays were shortest in the women who received dexmedetomidine before and during delivery. Long hospital stays are correlated with poor recovery in women who undergo cesarean delivery [16] and administration of dexmedetomidine before the procedure improves quality of life in these patients.
The present study has several limitations, the most important being that the analysis was retrospective and there was no randomization. The sample size was calculated on the basis of maternal outcomes rather than outcomes of hemodynamic parameters. Emergency cesarean deliveries were excluded and only data from elective cesarean deliveries were included. Data regarding milk volume during hospital stays were not evaluated. Dexmedetomidine is a highly selective α-2 receptor agonist and has anti-sympathetic, sedative, and analgesic effects. The reduction in the amount of sufentanil administered, from 175 μg to 150 μg, was not clinically significant because it did not amount to a difference equal to eliminating a full dose. The difference in time to lactation of 25 min versus 27 min also was statistically but not clinically significant. A number of confounders were introduced into the study, such as use of nicardipine, esmolol, and paracetamol, which were not addressed. The present study was conducted at a single maternity unit in China with established clinical protocols and methods of patient assessment, such as not using the Ramsay sedation score. In addition, the effects of dexmedetomidine on neonates were not evaluated. Most of the women in the study (362/392) were primiparous; therefore, they may have been more sensitive to pain than multiparas. Further studies of dexmedetomidine should be conducted with subgroup analysis.
Conclusions
Administration of dexmedetomidine combined with sufentanil can decrease post-cesarean pain, nausea, and vomiting, and help stimulate lactation, particularly if the drugs are given before the procedure. This treatment decreases lengths of hospital stays. Administering dexmedetomidine before spinal anesthesia is safe for women undergoing cesarean delivery.
Figures
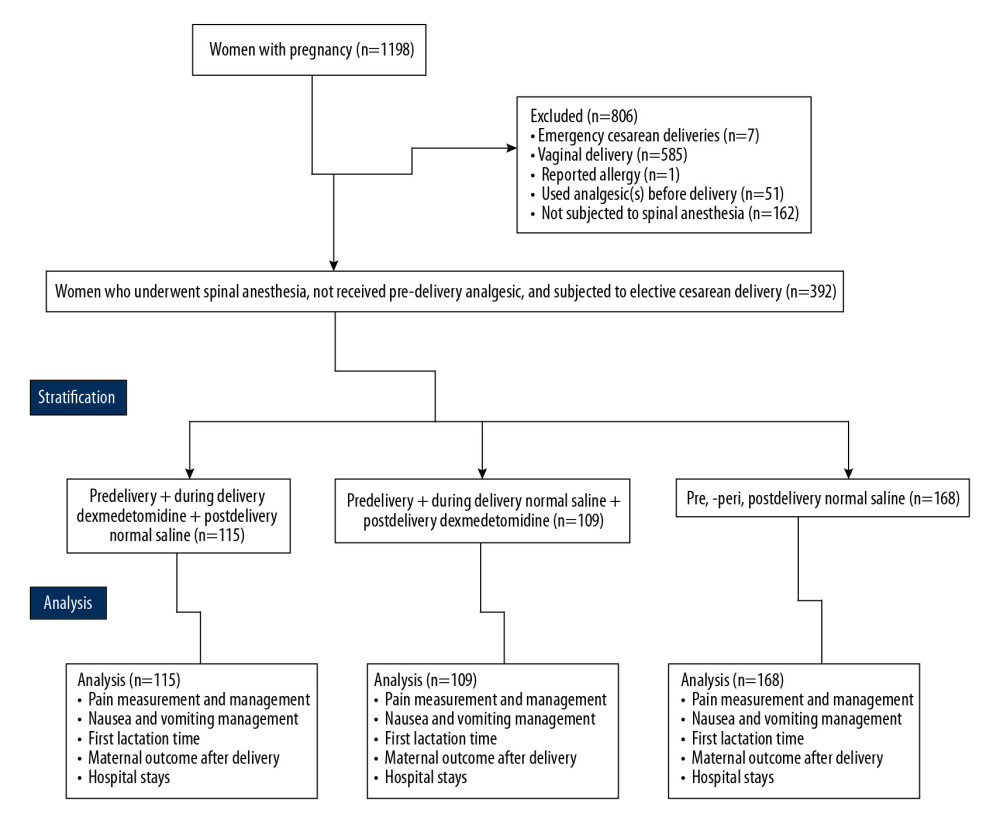 Figure 1. Flowchart of data collection.
Figure 1. Flowchart of data collection. 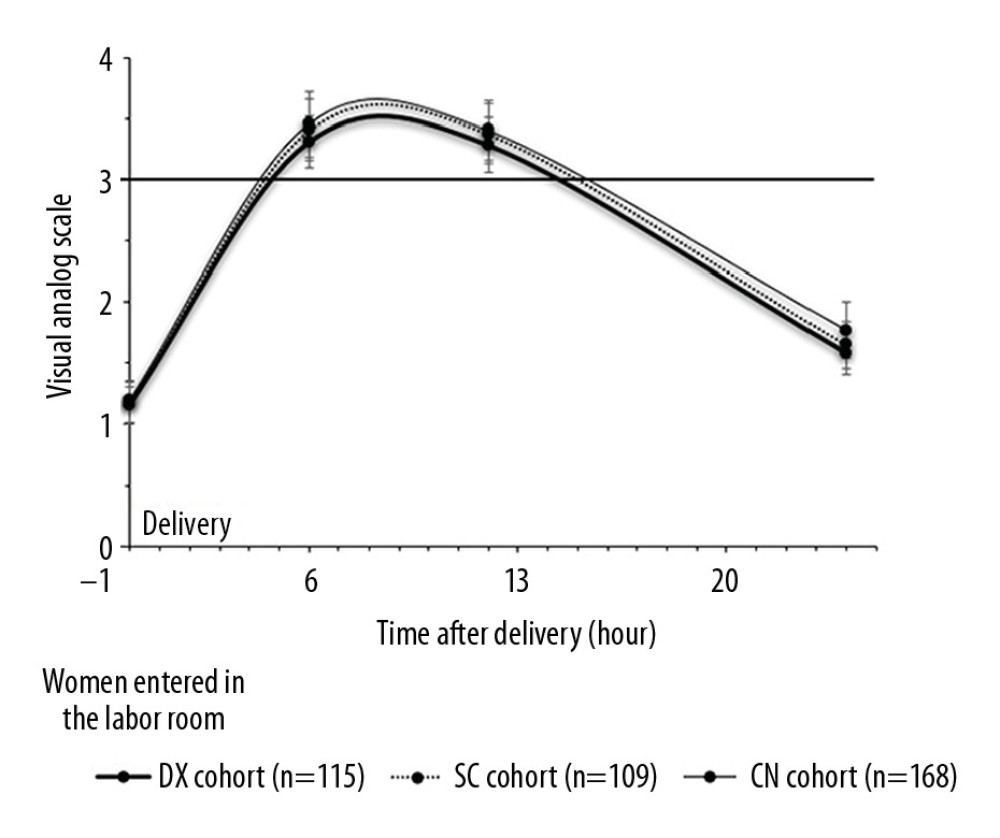 Figure 2. Measurement of pain during rest. Data are shown as mean±SD.
Figure 2. Measurement of pain during rest. Data are shown as mean±SD. 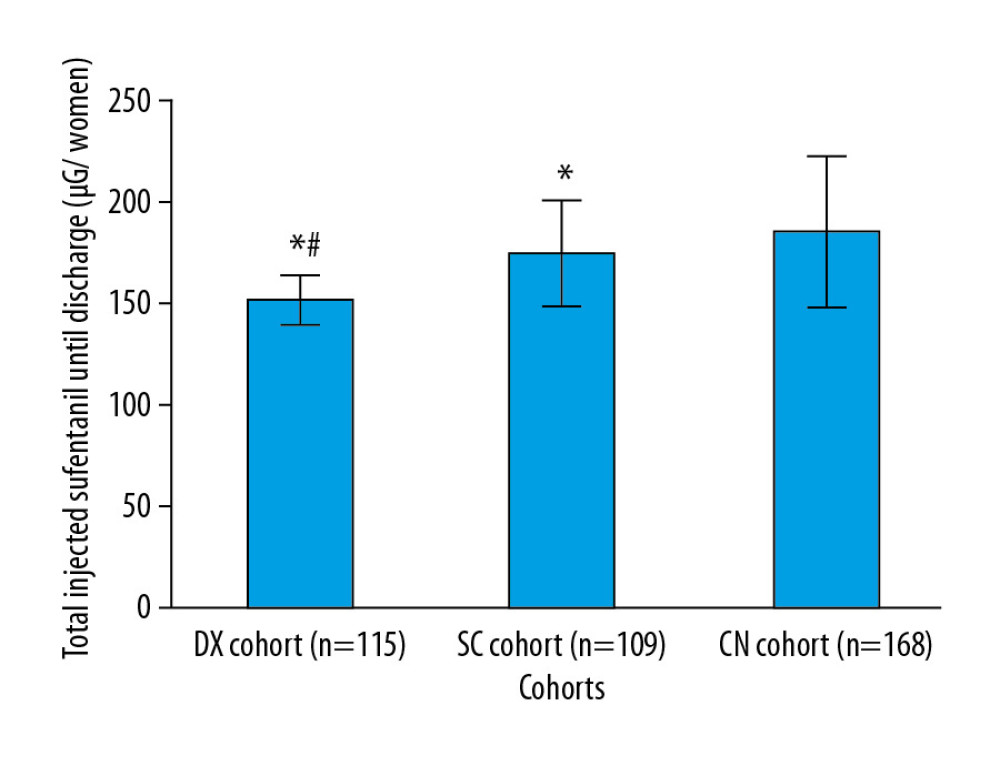 Figure 3. Management of pain. Data are shown as mean±SD. One-way analysis of variance was performed after Tukey’s post hoc test for statistical analysis. P<0.05 and q>3.326 were considered significant. * Significantly less than CN cohort. # Significantly less than in the SC cohort. When a patient’s VAS score was >3 during rest, an injection of 50 μg of sufentanil was administered.
Figure 3. Management of pain. Data are shown as mean±SD. One-way analysis of variance was performed after Tukey’s post hoc test for statistical analysis. P<0.05 and q>3.326 were considered significant. * Significantly less than CN cohort. # Significantly less than in the SC cohort. When a patient’s VAS score was >3 during rest, an injection of 50 μg of sufentanil was administered. 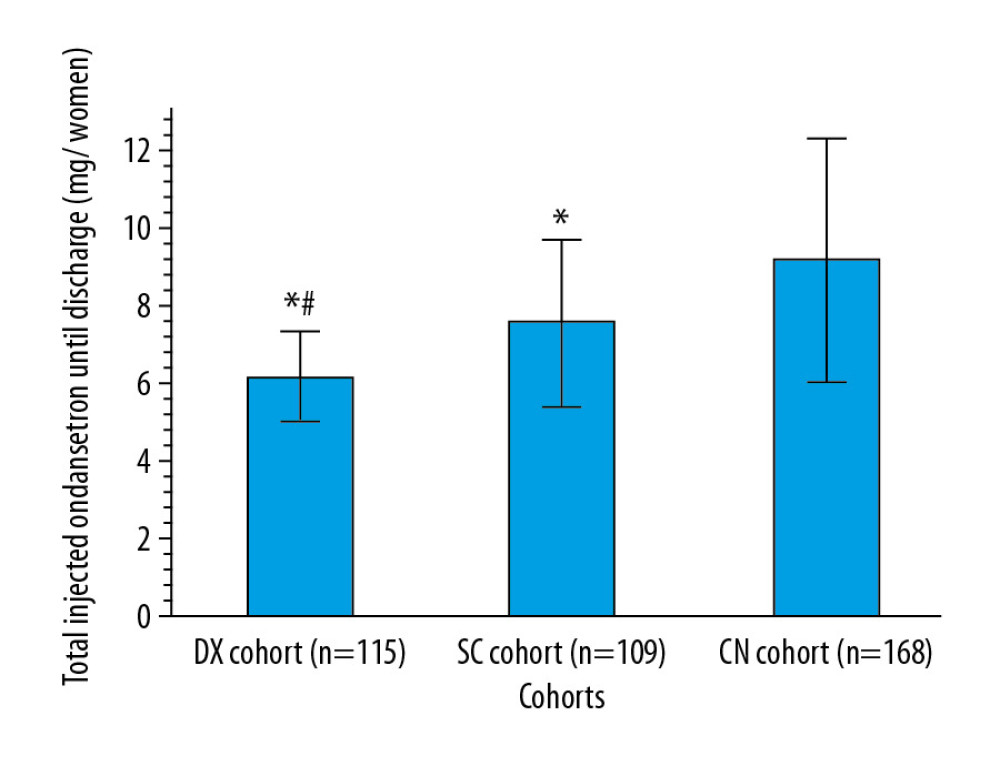 Figure 4. Management of nausea and vomiting. Data are shown as mean±SD. One-way analysis of variance was performed after Tukey’s post hoc test for statistical analysis. P<0.05 and q>3.326 were considered significant. * Significantly less than in the CN cohort. # Significantly less than in the SC cohort. An injection of 4 mg of ondansetron was administered for nausea and vomiting.
Figure 4. Management of nausea and vomiting. Data are shown as mean±SD. One-way analysis of variance was performed after Tukey’s post hoc test for statistical analysis. P<0.05 and q>3.326 were considered significant. * Significantly less than in the CN cohort. # Significantly less than in the SC cohort. An injection of 4 mg of ondansetron was administered for nausea and vomiting. 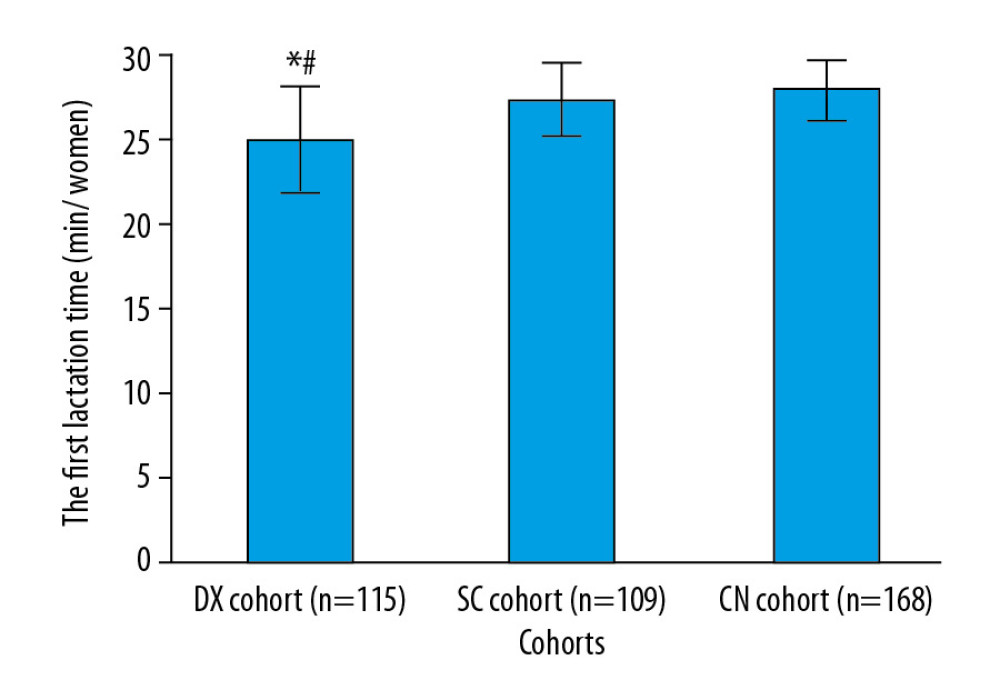 Figure 5. Time to first lactation. Data are shown as mean±SD. One-way analysis of variance was performed after Tukey’s post hoc test for statistical analysis. P<0.05 and q>3.326 were considered significant. * Significantly less than in the CN cohort. # Significantly shorter than in the SC cohort. Time to first lactation was defined as the period from delivery to when at least 10 mL of milk flowed from both breasts.
Figure 5. Time to first lactation. Data are shown as mean±SD. One-way analysis of variance was performed after Tukey’s post hoc test for statistical analysis. P<0.05 and q>3.326 were considered significant. * Significantly less than in the CN cohort. # Significantly shorter than in the SC cohort. Time to first lactation was defined as the period from delivery to when at least 10 mL of milk flowed from both breasts. 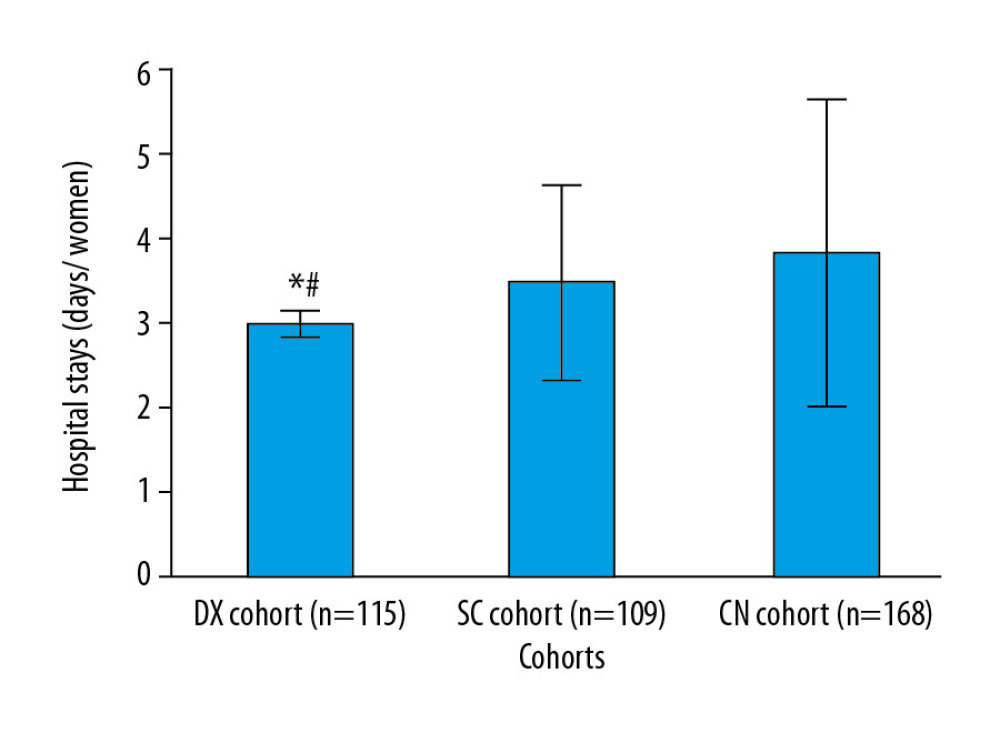 Figure 6. Hospital length of stay. Data are shown as mean±SD. One-way analysis of variance was performed after Tukey’s post hoc test for statistical analysis. P<0.05 and q>3.326 were considered significant. * Significantly less than in the CN cohort. # Significantly shorter than in the SC cohort. A hospital stay was defined as the time from admission for delivery to discharge.
Figure 6. Hospital length of stay. Data are shown as mean±SD. One-way analysis of variance was performed after Tukey’s post hoc test for statistical analysis. P<0.05 and q>3.326 were considered significant. * Significantly less than in the CN cohort. # Significantly shorter than in the SC cohort. A hospital stay was defined as the time from admission for delivery to discharge. Tables
Table 1. Demographic and clinical characteristics and educational status of pregnant women at the time of admission and neonatal parameters.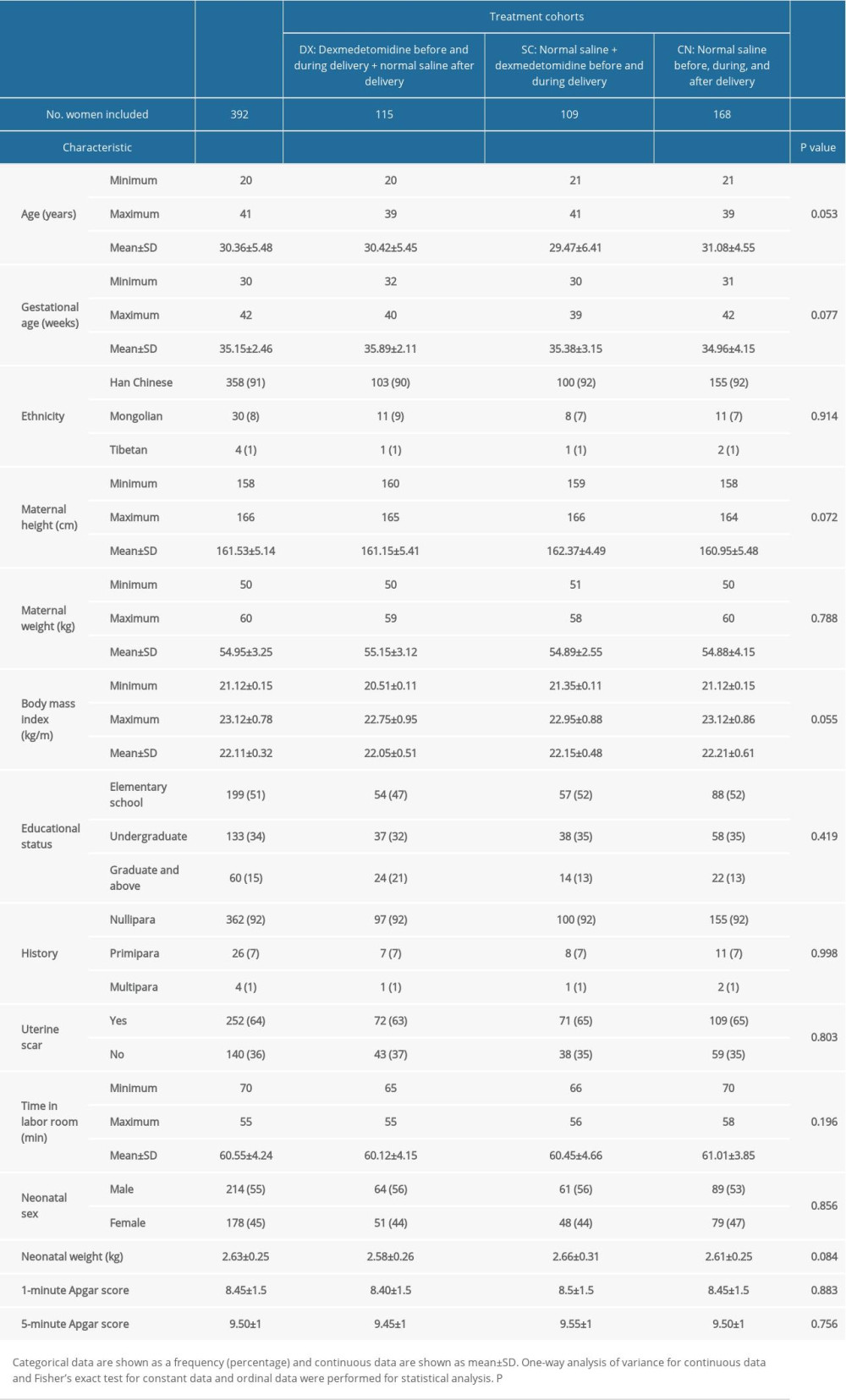 Table 2. Measurement of pain during uterine contractions.
Table 2. Measurement of pain during uterine contractions.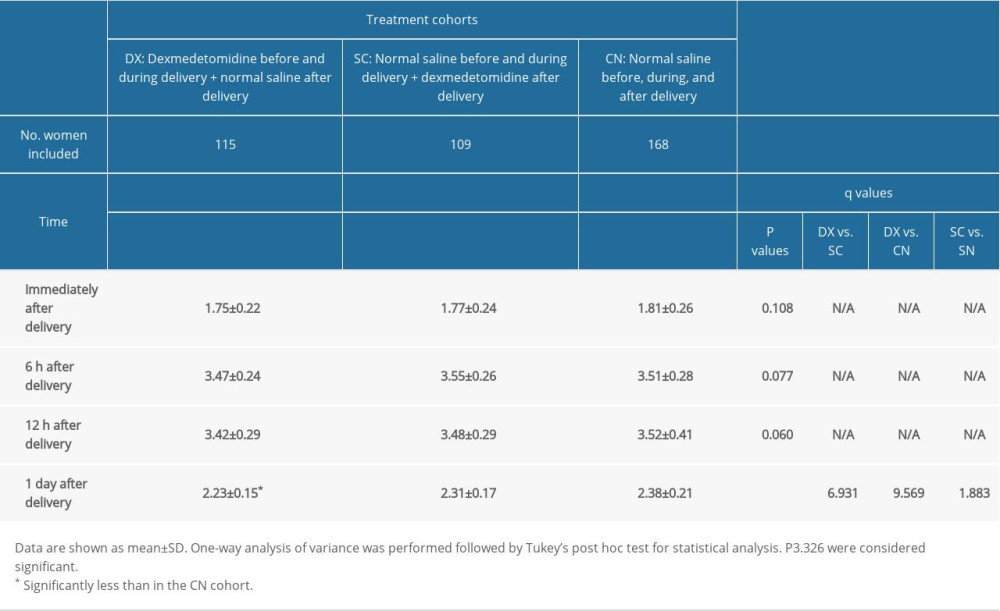 Table 3. Measurement of pain during movement.
Table 3. Measurement of pain during movement.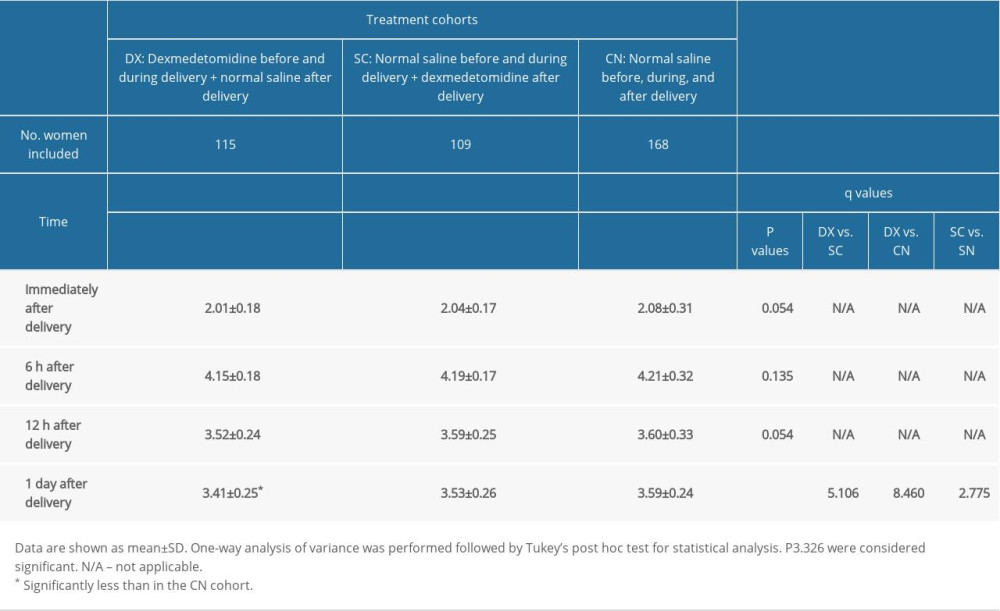 Table 4. Other maternal outcomes after delivery.
Table 4. Other maternal outcomes after delivery.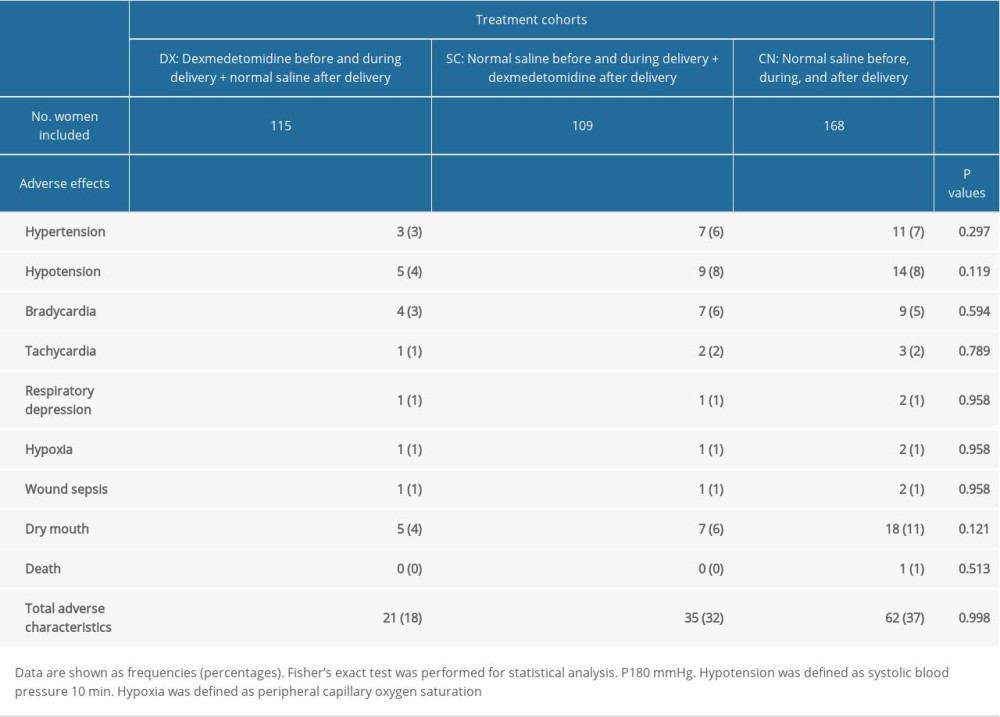
References
1. Desai E, Leuva H, Leuva B, Kanani M, A study of primary caesarean section in multipara: Int J Reprod Contracept Obstet Gynecol, 2013; 2; 320-24
2. Wang Y, Fang X, Liu C, Impact of intraoperative infusion and postoperative PCIA of dexmedetomidine on early breastfeeding after elective cesarean section: A randomized double-blind controlled trial: Drug Des Devel Ther, 2020; 14; 1083-93
3. Lau Y, Wang Y, Yin L, Validation of the mainland Chinese version of the Edinburgh postnatal depression scale in Chengdu mothers: Int J Nurs Stud, 2010; 47; 1139-51
4. Sedighinejad A, Haghighi M, Naderi Nabi B, Magnesium sulfate and sufentanil for patient-controlled analgesia in orthopedic surgery: Anesth Pain Med, 2014; 4; e11334
5. El-Tahan MR, Mowafi HA, Al Sheikh IH, Efficacy of dexmedetomidine in suppressing cardiovascular and hormonal responses to general anaesthesia for caesarean delivery: A dose-response study: Int J Obstet Anesth, 2012; 21; 222-29
6. Nie Y, Tu W, Shen X, Dexmedetomidine added to sufentanil patient-controlled intravenous analgesia relieves the postoperative pain after cesarean delivery: A prospective randomized controlled multicenter study: Sci Rep, 2018; 8; 9952
7. Nguyen V, Tiemann D, Park E, Salehi A, Alpha-2 agonists: Anesthesiol Clin, 2017; 35; 233-45
8. Funai Y, Pickering AE, Uta D: Pain, 2014; 155; 617-28
9. Feng M, Chen X, Liu T, Dexmedetomidine and sufentanil combination versus sufentanil alone for postoperative intravenous patient-controlled analgesia: A systematic review and meta-analysis of randomized controlled trials: BMC Anesthesiol, 2019; 19; 81
10. Nie Y, Liu Y, Luo Q, Huang S, Effect of dexmedetomidine combined with sufentanil for post-caesarean section intravenous analgesia: A randomised, placebo-controlled study: Eur J Anaesthesiol, 2014; 31; 197-203
11. Ren C, Xu H, Xu G, Effect of intraoperative infusion of dexmedetomidine on postoperative recovery in patients undergoing endovascular interventional therapies: A prospective, randomized, controlled trial: Brain Behav, 2019; 9; e01317
12. Lin TF, Yeh YC, Lin FS, Effect of combining dexmedetomidine and morphine for intravenous patient-controlled analgesia: Br J Anaesth, 2009; 102; 117-22
13. Hobbs AJ, Mannion CA, McDonald SW, The impact of caesarean section on breastfeeding initiation, duration and difficulties in the first four months postpartum: BMC Pregnancy Childbirth, 2016; 16; 1-9
14. Silva CS, Lima MC, Sequeira-de-Andrade LA, Association between postpartum depression and the practice of exclusive breastfeeding in the first three months of life: J Pediatr, 2017; 93; 356-64
15. Yoshimura M, Kunisawa T, Suno M, Intravenous dexmedetomidine for cesarean delivery and its concentration in colostrum: Int J Obstet Anesth, 2017; 32; 28-32
16. Ciechanowicz S, Setty T, Robson E, Development and evaluation of an obstetric quality-of-recovery score (ObsQoR-11) after elective Caesarean delivery: Br J Anaesth, 2019; 122; 69-78
Figures
 Figure 1. Flowchart of data collection.
Figure 1. Flowchart of data collection. Figure 2. Measurement of pain during rest. Data are shown as mean±SD.
Figure 2. Measurement of pain during rest. Data are shown as mean±SD. Figure 3. Management of pain. Data are shown as mean±SD. One-way analysis of variance was performed after Tukey’s post hoc test for statistical analysis. P<0.05 and q>3.326 were considered significant. * Significantly less than CN cohort. # Significantly less than in the SC cohort. When a patient’s VAS score was >3 during rest, an injection of 50 μg of sufentanil was administered.
Figure 3. Management of pain. Data are shown as mean±SD. One-way analysis of variance was performed after Tukey’s post hoc test for statistical analysis. P<0.05 and q>3.326 were considered significant. * Significantly less than CN cohort. # Significantly less than in the SC cohort. When a patient’s VAS score was >3 during rest, an injection of 50 μg of sufentanil was administered. Figure 4. Management of nausea and vomiting. Data are shown as mean±SD. One-way analysis of variance was performed after Tukey’s post hoc test for statistical analysis. P<0.05 and q>3.326 were considered significant. * Significantly less than in the CN cohort. # Significantly less than in the SC cohort. An injection of 4 mg of ondansetron was administered for nausea and vomiting.
Figure 4. Management of nausea and vomiting. Data are shown as mean±SD. One-way analysis of variance was performed after Tukey’s post hoc test for statistical analysis. P<0.05 and q>3.326 were considered significant. * Significantly less than in the CN cohort. # Significantly less than in the SC cohort. An injection of 4 mg of ondansetron was administered for nausea and vomiting. Figure 5. Time to first lactation. Data are shown as mean±SD. One-way analysis of variance was performed after Tukey’s post hoc test for statistical analysis. P<0.05 and q>3.326 were considered significant. * Significantly less than in the CN cohort. # Significantly shorter than in the SC cohort. Time to first lactation was defined as the period from delivery to when at least 10 mL of milk flowed from both breasts.
Figure 5. Time to first lactation. Data are shown as mean±SD. One-way analysis of variance was performed after Tukey’s post hoc test for statistical analysis. P<0.05 and q>3.326 were considered significant. * Significantly less than in the CN cohort. # Significantly shorter than in the SC cohort. Time to first lactation was defined as the period from delivery to when at least 10 mL of milk flowed from both breasts. Figure 6. Hospital length of stay. Data are shown as mean±SD. One-way analysis of variance was performed after Tukey’s post hoc test for statistical analysis. P<0.05 and q>3.326 were considered significant. * Significantly less than in the CN cohort. # Significantly shorter than in the SC cohort. A hospital stay was defined as the time from admission for delivery to discharge.
Figure 6. Hospital length of stay. Data are shown as mean±SD. One-way analysis of variance was performed after Tukey’s post hoc test for statistical analysis. P<0.05 and q>3.326 were considered significant. * Significantly less than in the CN cohort. # Significantly shorter than in the SC cohort. A hospital stay was defined as the time from admission for delivery to discharge. Tables
 Table 1. Demographic and clinical characteristics and educational status of pregnant women at the time of admission and neonatal parameters.
Table 1. Demographic and clinical characteristics and educational status of pregnant women at the time of admission and neonatal parameters. Table 2. Measurement of pain during uterine contractions.
Table 2. Measurement of pain during uterine contractions. Table 3. Measurement of pain during movement.
Table 3. Measurement of pain during movement. Table 4. Other maternal outcomes after delivery.
Table 4. Other maternal outcomes after delivery. Table 1. Demographic and clinical characteristics and educational status of pregnant women at the time of admission and neonatal parameters.
Table 1. Demographic and clinical characteristics and educational status of pregnant women at the time of admission and neonatal parameters. Table 2. Measurement of pain during uterine contractions.
Table 2. Measurement of pain during uterine contractions. Table 3. Measurement of pain during movement.
Table 3. Measurement of pain during movement. Table 4. Other maternal outcomes after delivery.
Table 4. Other maternal outcomes after delivery. In Press
06 Mar 2024 : Clinical Research
Prevalence and Variation of Medical Comorbidities in Oral Surgery Patients: A Retrospective Study at Jazan ...Med Sci Monit In Press; DOI: 10.12659/MSM.943884
08 Mar 2024 : Clinical Research
Evaluation of Foot Structure in Preschool Children Based on Body MassMed Sci Monit In Press; DOI: 10.12659/MSM.943765
15 Apr 2024 : Laboratory Research
The Role of Copper-Induced M2 Macrophage Polarization in Protecting Cartilage Matrix in OsteoarthritisMed Sci Monit In Press; DOI: 10.12659/MSM.943738
07 Mar 2024 : Clinical Research
Knowledge of and Attitudes Toward Clinical Trials: A Questionnaire-Based Study of 179 Male Third- and Fourt...Med Sci Monit In Press; DOI: 10.12659/MSM.943468
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








