10 November 2020: Clinical Research
Differential Levels of Endoplasmic Reticulum Stress in Peripheral Blood Mononuclear Cells from Patients with Sudden Sensorineural Hearing Loss
Zhibiao Liu12BCDEF, Bing Fei1BC, Xiaoping Du3CDF, Yanhong Dai145DG, Wandong She145AEFG*DOI: 10.12659/MSM.927328
Med Sci Monit 2020; 26:e927328
Abstract
BACKGROUND: Sudden sensorineural hearing loss (SSNHL) is currently treated with a combination of drugs, predominantly with glucocorticoids (GCs). However, the mechanisms of action of GCs in SSNHL are unknown. This study aimed to analyze the role of endoplasmic reticulum stress (ERS) in SSNHL pathogenesis and prognosis.
MATERIAL AND METHODS: In this study, we evaluated the expression and activation status of the protein kinase RNA-like endoplasmic reticulum kinase (PERK)-C/EBP homologous protein (CHOP) pathway in peripheral blood mononuclear cells (PBMCs) from patients with SSNHL and compared them with those in healthy controls. We also compared differences in expression of activating transcription factor 4 (ATF4) and CHOP before and after glucocorticoid treatment in patients with improved and unimproved SSNHL.
RESULTS: Treatment with GCs significantly improved hearing in 55% of patients with SSNHL. Levels of phosphorylated PERK (p-PERK) and phosphorylated eukaryotic initiation factor 2α were increased in PBMCs from patients with SSNHL compared with healthy controls. ATF4 and CHOP expression were also significantly elevated. After treatment, the amount of ATF4 and CHOP proteins in PBMCs in the patients whose SSNHL improved was significantly reduced compared with the levels measured before treatment in all patients with SSNHL. The expression of the ATF4 and CHOP proteins in PBMCs in the unimproved group, however, was not significantly changed relative to pretreatment levels.
CONCLUSIONS: ERS may play a significant role in the pathogenesis of SSNHL, and the responsiveness of the condition to GC-mediated mitigation of ERS may be one of the key factors that affect patient prognosis.
Keywords: Endoplasmic reticulum stress, Glucocorticoids, Hearing Loss, Sudden, Activating Transcription Factor 4, Down-Regulation, Eukaryotic Initiation Factor-2, Hearing Loss, Sensorineural, Leukocytes, Mononuclear, Transcription Factor CHOP, eIF-2 Kinase
Background
Sudden sensorineural hearing loss (SSNHL) refers to sensorineural hearing loss ≥30 dB over at least 3 contiguous audiometric frequencies that occurs over a period of 72 h due to unknown causes. It primarily presents as acute hearing loss, tinnitus, and dizziness requiring urgent care by an otorhinolaryngologist [1]. Some previous data suggested that SSNHL could be idiopathic, or originate from other diseases, such as vascular, infectious, or otologic conditions [1,2]. SSNHL is primarily treated with glucocorticoids (GCs) such as dexamethasone and methylprednisolone. However, in a significant number of patients, the condition does not respond to GC therapy [1]. Therefore, additional research is necessary into the pathogenesis of SSNHL and therapeutic response patterns to provide effective personalized treatment strategies for patients with SSNHL.
Endoplasmic reticulum stress (ERS) is defined as an accumulation of unfolded or misfolded proteins in the endoplasmic reticulum (ER) when cells are exposed to pathophysiological stressors. ERS is associated with various neurological and cardiovascular diseases [2,3]. During the early stages of ERS, cells can adapt to altered environmental conditions by reducing protein synthesis and enhancing protein folding and degradation of misfolded proteins. If the stress conditions persist, however, cells undergo apoptosis. ERS-induced apoptosis is achieved through 3 different signaling pathways that activate the C/EBP homologous protein (CHOP): inositol-requiring enzyme 1 (IRE1), activating transcription factor 6 (ATF6), and protein kinase RNA-like ER kinase (PERK) [3,4]. Under homeostatic conditions, CHOP is expressed at low levels in the cytoplasm. When cells are exposed to stress, CHOP expression increases considerably, leading to accumulation of the protein in the nucleus, and ultimately resulting in apoptosis [5]. Among the different signaling pathways that activate CHOP, PERK is the predominant one. As large quantities of unfolded proteins accumulate in the ER, PERK is activated by phosphorylation, thereby phosphorylating eukaryotic initiation factor 2α (eIF2α), which promotes the expression of activating transcription factor 4 (ATF4) and CHOP [3,6]. ERS is reportedly associated with inner ear injuries in several animal models of sensorineural hearing loss, including noise-induced hearing loss, cisplatin-induced ototoxicity, and in mice with Cdh23erl/erl mutations [7–9]. Because SSNHL is a form of acute sensorineural hearing loss, we hypothesized that ERS may play a role in its pathogenesis.
GCs regulate many complex signaling pathways, such as those for nuclear factor erythroid 2-related factor 2 and nuclear factor kappa β [10]. It has been reported that, under ERS conditions, there is crosstalk between CHOP and glucocorticoid receptor (GR) signaling, which is associated with a GR-CHOP heterocomplex formation [11]. Therefore, we hypothesized that GCs might protect cells and tissues of patients with SSNHL from ERS damage.
In the present study, we examined the PERK-CHOP pathway in the peripheral blood mononuclear cells (PBMCs) of patients with SSNHL who were treated with methylprednisolone and Ginkgo biloba and observed the effects of GCs on the expression of proteins associated with the PERK-CHOP pathway in PBMCs to validate a putative role of ERS in SSNHL and to determine patterns of ERS response to GC treatment.
Material and Methods
RECRUITMENT OF PATIENTS WITH SSNHL AND COLLECTION OF PBMCS:
This study was approved by the Ethics Committee of Nanjing Drum Tower Hospital Clinical College of Nanjing Medical University (Protocol No 2016-194-01). Forty-seven patients with SSNHL with pure-tone averages (PTA, 0.5–4 kHz) >60 dB were recruited from March 2017 to March 2018 in the Department of Otolaryngology-Head and Neck Surgery, Nanjing Drum Tower Hospital Clinical College of Nanjing Medical University. All patients met the criteria for SSNHL [1]. Briefly, SSNHL is defined as sensorineural hearing loss ≥30 dB over ≥3 contiguous audiometric frequencies, occurring over a period of 72 h due to unknown causes [1]. Patients with hereditary deafness, acoustic neuroma, diabetes, severe hypertension, and individuals who voluntarily withdrew from the study or were lost to follow-up were excluded. Twenty-five healthy volunteers matched by sex and age were recruited as a control group. All volunteers underwent a hearing test. Only volunteers with normal hearing were enrolled in the present study and the average PTA in the control group was 15.5±3.2 dB. All patients received a 10-day course of conventional treatment, which included intravenous (IV) injections of methylprednisolone (Pfizer Inc., U.S.A.) (80 mg/d for 4 days, 40 mg/d for 3 days, and 20 mg/d for 3 days) and of Ginkgo biloba extract (an antioxidant; Dr Willmar Schwabe, GmBH & Co KG, Essen, Germany) (105 mg/d for 10 days). GC was administrated simultaneously with the Ginkgo biloba extract.
Follow-up was conducted for more than 2 months after patients were discharged. All of the patients with SSNHL received hearing tests before treatment, 24 hours after completion of the 10-day treatment regimen, and once every 2 to 3 weeks thereafter. The hearing levels measured at 3 months after onset were considered as the final post-treatment levels in the study. The patients were assigned to 2 groups, according to PTA gain 3 months after onset: the improved group (PTA ≥15 dB gan) and the unimproved group (<15 dB PTA gain) [1]. PTA was calculated as an average of the thresholds measured at 0.5 to 4.0 kHz.
PBMCs were isolated by gradient centrifugation from 15- to 20-mL fasting blood samples collected from all patients with SSNHL 1 day before and immediately (within 24 hours) after the 10-day IV treatment and from healthy volunteers. The PBMCs were stored at −80 until the time of analysis.
REAL-TIME PCR OF PERK, EIF2α, ATF4, AND CHOP IN PBMCS:
To determine the expression status of the PERK-CHOP pathway in patients with SSNHL, we examined the messenger RNA (mRNA) levels of PERK, eIF2α, ATF4, and CHOP in PBMCs of patients with SSNHL before treatment. Total RNA was extracted from the PBMCs using TRIzol reagent. Complementary DNA was then obtained by reverse transcription. Real-time polymerase chain reaction (rt-PCR) was performed with the Applied Biosystems QuantStudio 6 Flex Real-Time PCR System (Applied Biosystems, Singapore). The thermal cycle conditions for rt-PCR included an initial denaturation at 95°C for 30 seconds, followed by 40 cycles of 5-s denaturation at 95°C and a 30-s extension at 60°C. The mRNA levels were normalized to the levels of the internal reference standard, β-actin, and determined by using the 2−ΔΔCt method [12]. Each experiment was repeated 3 times. Table 1 lists the primer sequences of PERK, eIF2α, ATF4, CHOP, and β-actin that were used in rt-PCR.
WESTERN BLOTTING FOR PERK, EIF2α, ATF4, AND CHOP IN PBMCS:
To determine the status of the PERK-CHOP pathway in patients with SSNHL, we examined the protein levels of PERK, eIF2α, ATF4, and CHOP in PBMCs of patients with SSNHL before treatment. Total protein was extracted from PBMCs by using a radioimmunoprecipitation assay buffer with protease and phosphatase inhibitors. Protein concentration was determined with a bicinchoninic acid assay. Thirty micrograms of protein were resolved by sodium dodecyl sulphate-polyacrylamide gel electrophoresis and then transferred onto a polyvinylidene difluoride membrane. The membrane was blocked with 5% bovine serum albumin for 1 h at room temperature and then incubated with 1 of the primary antibodies (PERK, eIF2α, p-eIF2α, or CHOP, Cell Signaling Tech, U.S.A.; p-PERK, Immunoway, U.S.A.; ATF4, Abcam, U.K., 1: 1,000/each antibody) at 4°C overnight. After washing with Tris-buffered saline, the membranes were incubated with the appropriate species-specific secondary antibody (anti-rabbit immunoglobulin G [IgG] or anti-mouse IgG, 1: 10 000, FCMACS, China) for 2 h at room temperature. Enhanced chemiluminescence substrate was used to visualize the bands, and the blots were developed with a Tanon 5200 Multi Automatic Fluorescence/Chemiluminescence Imaging Analysis System (Tanon Science & Technology Co, Ltd, Shanghai, China). Protein bands were analyzed for densitometry, using NIH Image J software. Each experiment was repeated 3 times.
QUANTITATIVE PROTEIN DETECTION OF ATF4 AND CHOP BY SIMPLE WESTERN BLOT ANALYSIS IN PBMCS:
To determine the relationship between the ERS-associated proteins (ATF4 and CHOP) and the prognosis of SSNHL, we examined the protein levels of ATF4 and CHOP in PBMCs in the patients in whom SSNHL improved and did not improve. Five micrograms of purified protein were mixed with dithiothreitol (DDT), fluorescent 5X Master Mix, and a biotinylated ladder in a special 384-well microplate according to the manufacturer’s instructions, and then automatically analyzed with the Protein Simple platform (ProteinSimple Inc., U.S.A.).
STATISTICAL ANALYSIS:
All data were expressed as means±standard deviation. SPSS Statistics 20.0 software (IBM Corp., Armonk, New York, U.S.A.) was used for statistical analyses. The normal distribution of data was verified using the Kolmogorov-Smirnov test. An independent samples
Results
GENERAL CLINICAL DATA FOR PATIENTS WITH SSNHL:
A total of 47 eligible patients with SSNHL were enrolled in this study and completed follow-up. All patients received a full 10 days of IV treatment and their hearing levels were measured at least 3 times over the course of the study. According to the recovery of PTA after final follow-up, patients were assigned into the improved group (PTA gain ≥15 dB, accounting for 55.3%) and the unimproved group (PTA gain <15 dB, accounting for 44.7%). There was no significant difference in sex or age among the improved, the unimproved, and the control groups. There was no significant difference between the improved and the unimproved groups in the course of disease, PTA before the treatment, or the number of patients with tinnitus and/or vertigo before treatment. The demographic data for the patients with SSNHL and the control group are summarized in Table 2.
UPREGULATION OF THE PERK/CHOP PATHWAY IN PBMCS OF PATIENTS WITH SSNHL:
The expression patterns of PERK, eIF2α, and their downstream genes, and of ATF4 and CHOP were examined with rt-PCR and western blot evaluations of the PBMCs from all patients with SSNHL before treatment (total 47 samples). Compared with the control group (total 25 samples), no significant differences were observed in the levels of either total protein or total mRNA for PERK or eIF2α in PBMCs from patients with SSNHL (0.77±0.05 vs. 0.76±0.07, t=0.088, P=0.930 for PERK protein; 1.16±0.14 vs. 1.09±0.12, t=1.896, P=0.062 for eIF2α protein; 1.00±0.11 vs. 0.96±0.15, t=1.042, P=0.301 for PERK mRNA; 1.00±0.11 vs. 1.07±0.14, t=1.834, P=0.071 for eIF2α mRNA. All ν=70) (Figure 1A–1D). However, significantly higher levels of phosphorylated, activated PERK, and eIF2α (p-PERK and p-eIF2α) were observed in PBMCs from patients with SSNHL (0.10±0.01 vs. 0.29±0.04, t=22.019, P=0.000 for p-PERK protein; 0.05±0.004 vs. 0.33±0.04, t=35.615, P=0.000 for p-eIF2α protein. All ν=70) (Figure 1A, 1B). Furthermore, ATF4 and CHOP protein and mRNA levels were also significantly upregulated compared with those in the control group (0.62±0.07 vs. 0.15±0.05, t=29.211, P=0.000 for ATF4 protein; 0.40±0.04 vs. 0.06±0.02, t=40.327, P=0.000 for CHOP protein; 1.36±0.29 vs. 1.00±0.20, t=5.454, P=0.000 for ATF4 mRNA; 1.62±0.35 vs. 1.00±0.22, t=7.934, P=0.000 for CHOP mRNA. All ν=70) (Figure 1A, 1B, 1E, 1F). These results suggest that ERS may be involved in the pathogenesis of SSNHL.
DOWNREGULATION ATF4 AND CHOP PROTEIN LEVELS IN PBMCS IN THE IMPROVED GROUP AFTER TREATMENT:
ATF4 and CHOP protein levels in PBMCs (52 samples from the improved group, 42 samples from the unimproved group, 25 samples from the control group) were quantitatively examined with simple western blot analysis. After treatment, the amount of ATF4 and CHOP proteins in PBMCs in the improved group was significantly decreased compared with the level measured before treatment in patients with SSNHL (0.15±0.02 vs. 0.34±0.06, t=18.329, P=0.000 for ATF; 0.14±0.02 vs. 0.21±0.03, t=8.566, P=0.000 for CHOP. All ν=50) (Figure 2A, 2C, 2D, 2F), while the expression in PBMCs of the unimproved group was not significantly changed relative to pretreatment levels (0.30±0.04 vs. 0.32±0.06, t=0.631, P=0.531 for ATF4; 0.21±0.02 vs. 0.22±0.02, t=1.025, P=0.311 for CHOP. All ν=40) (Figure 2B, 2C, 2E, 2F). The amounts of ATF4 and CHOP proteins in PBMCs in the control group were 0.09±0.03 and 0.03±0.01, respectively. These results suggest that responsiveness to GC-mediated mitigation of ERS may a key factor that affects the prognosis of SSNHL. In theory, automatic protein quantitative analysis system can generate higher-quality data than traditional western blot analysis, which is reproducible [13].
Discussion
GC-based therapy is still the criterion standard for the treatment of SSNHL. Dexamethasone and methylprednisolone are the 2 GCs commonly used, and some previous studies have compared their therapeutic effects. Tarkan et al. found that the therapeutic success rate was 62.5% for the methylprednisolone group and 54.6% for the dexamethasone group. Berjis et al. found that in patients with SSNHL, there was an 84% improvement in general hearing after treatment with methylprednisolone, which was significantly higher than in patients treated with dexamethasone (64%). Their results suggested that both dexamethasone and methylprednisolone have good therapeutic efficacy for treatment of SSNHL. However, while methylprednisolone is a GC with medium effect, dexamethasone has long-term effects [14,15]. Therefore, for the treatment of SSNHL, methylprednisolone is more commonly used because it is safer than dexamethasone for systemic administration [15]. Ginkgo biloba is the main active component extracted from the traditional Chinese medicine Ginkgo, and it has been used in clinics for the treatment of SSNHL [16]. Based on multicenter studies [17] and guidelines for the diagnosis and treatment of sudden hearing loss in China [18], Ginkgo biloba extract is recommended as a supplement to GCs for the treatment of SSNHL. In recent research, the expression of ERS proteins in PBMCs of patients with SSNHL has been investigated and their potential mechanism have been discussed.
SNHL has been associated with ERS in animal studies [6–8] and ERS may serve as an indicator of vascular or nerve damage [2]. To date, however, there are no reports in the literature about the role of ERS in SSNHL, probably because of the inability to obtain tissue samples from the inner ear of patients at the onset of SSNHL, and the lack of reliable SSNHL animal models. Recently, researchers have found that PBMCs are good surrogates in which to study inflammation, oxidative stress, and ERS [19–21]. PBMCs, which are easy to collect in the clinic, are ideal tissue samples with which to monitor systemic changes and study molecular signaling mechanisms in pathologic processes [22]. In our previous studies, we have used PBMCs to study SSNHL and achieved some meaningful results. For instance, GC receptor levels in PBMCs were positively correlated with those in the cochlea, and histone deacetylase 2 levels in PBMCs were correlated with prognosis of SSNHL [23–25]. In the present study, we have extended our prior SSNHL research by examining the PERK-CHOP pathway in PBMCs. We observed that the PERK-CHOP pathway was activated in patients with SSNHL, which suggests that ERS could be involved in SSNHL-related pathogenesis. The cause of ERS in patients with SSNHL is still unknown. However, in previous studies, many patients had been exposed to chronic stress (e.g., anxiety, mood swings, irregular life style, and sleep disorders) and/or had lipid metabolism disorders [18] before the onset of SSNHL, which suggests that their nervous systems were highly susceptible to ERS [26,27].
Spontaneous recovery of hearing has been reported in patients with SSNHL, especially those who have mild hearing loss [28]. However, the spontaneous recovery rate is much lower in patients with severe than mild hearing loss [28]. The rates of overall hearing recovery reported here in patients with severe hearing loss is similar to those in a previous report [29]. The therapeutic efficacy of GCs in the treatment of SSNHL remains controversial because of the lack of valid randomized controlled trials [28,30]. Some studies have shown that patients may not benefit from the drugs [28,30]. We believe that the benefits of GC use outweigh the potential adverse effects in patients with moderate or severe SSNHL [30]. A large retrospective study reported significant hearing improvement in patients with severe hearing loss who were treated with GCs, compared with those who declined the treatment [30].
Considering that the rate of spontaneous recovery is high in patients with SSNHL who have mild hearing loss, we included in the present study only individuals with severe to profound hearing loss (initial average hearing threshold shift >60 dB). We treated patients with SSNHL with GCs in accordance with Chinese guidelines for diagnosis and treatment of sudden deafness [18]. Furthermore, to reduce the influence of human factors [13], we used an automatic protein quantitative analysis system to compare the changes in ERS-associated protein expression between the patients with (PTA gain >15 dB) and without improvement (PTA gain <15 dB) before and after GC treatment. Interestingly, after GC treatment, significantly decreased ATF and CHOP protein levels were observed only in the patients with improvement, whereas the levels of either ATF and CHOP were not significantly changed in patients in the group without improvement. Therefore, the levels of ATF and CHOP in PBMCs of patients with SSNHL may serve as an indicator of their responses to GC treatment, and the decrease in ATF and CHOP levels may suggest a better prognosis for them. However, we still do not know why the response of ATF and CHOP to GC treatment was low or absent in the patients who did not have hearing improvement.
GCs have many different effects on metabolic, immunologic, and homeostatic functions. However, the relationship between GCs and ERS is immensely complex, and the effects of GCs on ERS can be completely different in different cells, from inhibiting to promoting ERS [31,32]. In the present study, we observed that levels of ERS-related proteins were elevated in PBMCs of all patients with SSNHL before they received GCs, and that the treatment downregulated the protein levels in patients who had hearing improvement compared with those did not have hearing improvement. The underlying mechanism of action of GCs in this context is still unknown. However, multiple mechanisms or pathways could be involved. For example, GCs can inhibit ERS by promoting the secretion of correctly-folded proteins and degradation of misfolded proteins [33]. They also may selectively antagonize ER stress-induced apoptosis by reducing the transcription of growth differentiation factor 15 (GDF15) [34]. Furthermore, GCs can alleviate ERS response by inducing leucine zipper protein and interacting with CHOP to attenuate ERS-induced apoptotic cell death [11,32].
The present study has limitations. First, we included a relatively small number of participants and the results should be verified with further analysis in a larger sample. Also, the data were based on experience in the Chinese Han population and should be verified in people of other races.
Conclusions
This study aimed to analyze the role of ERS in SSNHL pathogenesis and prognosis using rt-PCR, western blot, and quantitative analyses. The results suggest that ERS may contribute to SSNHL pathogenesis, and the effects on ERS of treatment with GCs may be a key factor that affects the prognosis of SSNHL.
Figures
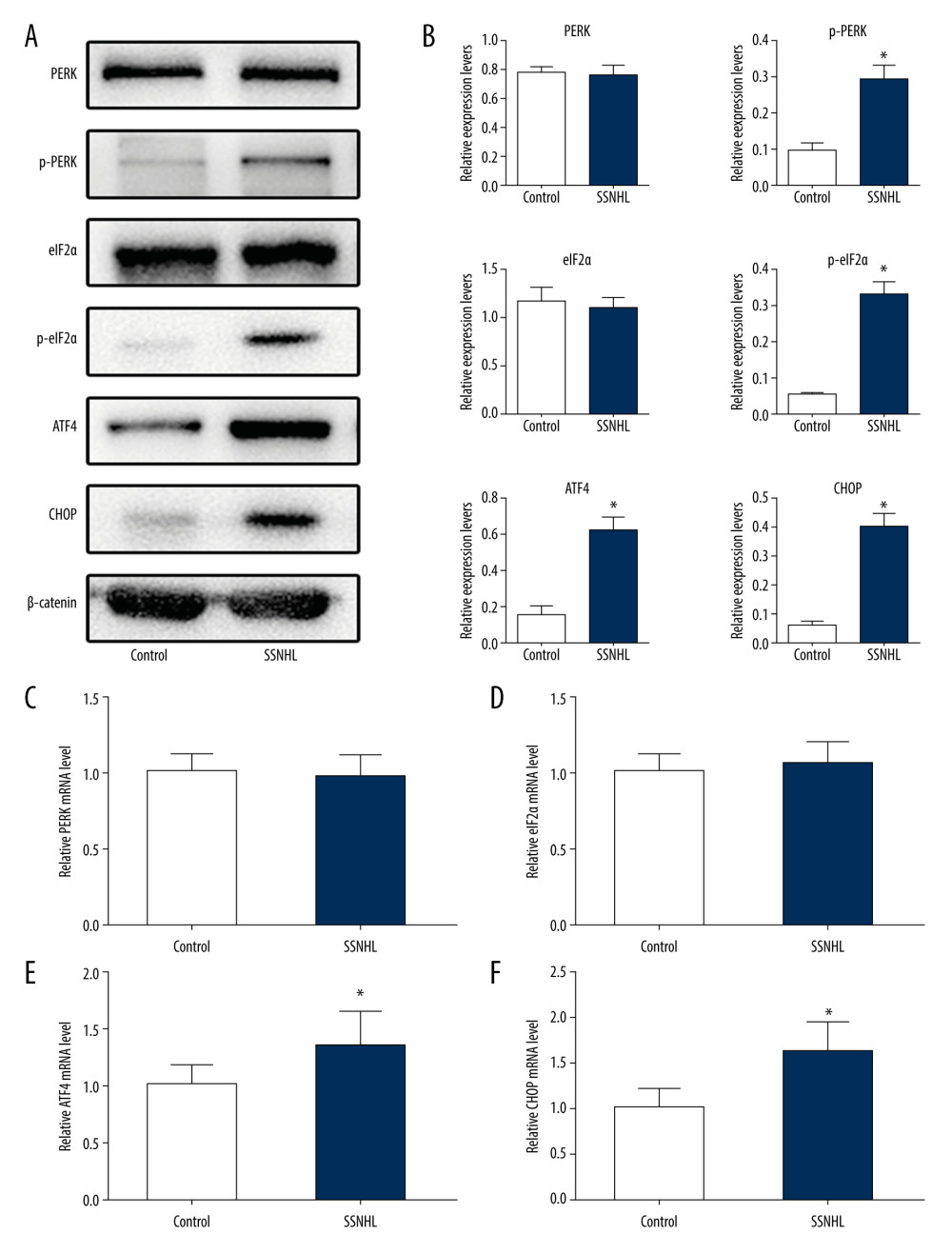 Figure 1. Patterns of expression of protein kinase RNA-like endoplasmic reticulum kinase (PERK), eukaryotic initiation factor 2α (eIF2α), activating transcription factor 4 (ATF4), and C/EBPO homologous protein (CHOP) in peripheral blood mononuclear cells of patients with sudden sensorineural hearing loss before treatment. (A) Examples of western blot of PERK, eIF2α, ATF4, and CHOP. (B) The western blots were quantitatively analyzed. (C–F) The messenger RNA levels of PERK, eIF2α, ATF4, and CHOP were also examined with by real-time polymerase chain reaction. * P<0.05 compared with the control group.
Figure 1. Patterns of expression of protein kinase RNA-like endoplasmic reticulum kinase (PERK), eukaryotic initiation factor 2α (eIF2α), activating transcription factor 4 (ATF4), and C/EBPO homologous protein (CHOP) in peripheral blood mononuclear cells of patients with sudden sensorineural hearing loss before treatment. (A) Examples of western blot of PERK, eIF2α, ATF4, and CHOP. (B) The western blots were quantitatively analyzed. (C–F) The messenger RNA levels of PERK, eIF2α, ATF4, and CHOP were also examined with by real-time polymerase chain reaction. * P<0.05 compared with the control group. 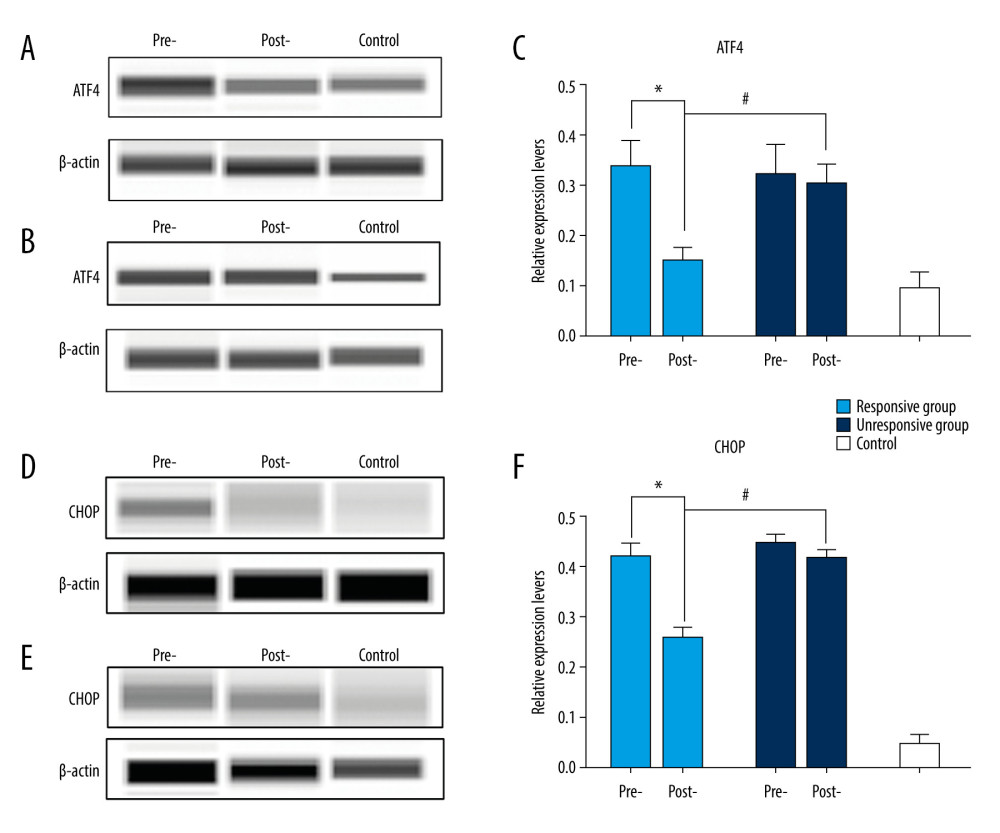 Figure 2. Comparison of activating transcription factor 4 (ATF4) and C/EBPO homologous protein (CHOP) protein levels in peripheral blood mononuclear cells (PBMCs) of patients with sudden sensorineural hearing loss (SSNHL) before and after treatment. Examples of quantitative protein detection of ATF4 in PBMCs from (A) patients with SSNHL in the improved group and (B) in the unimproved group before (pre-) and after (post-) treatment. (C) The protein levels of ATF4 were quantitatively analyzed. Examples of quantitative protein detection of CHOP in PBMCs in the (D) improved group and (E) in the unimproved group before (pre-) and after (post-) treatment. (F) The protein levels of CHOP were quantitatively analyzed. * and # indicate P<0.05.
Figure 2. Comparison of activating transcription factor 4 (ATF4) and C/EBPO homologous protein (CHOP) protein levels in peripheral blood mononuclear cells (PBMCs) of patients with sudden sensorineural hearing loss (SSNHL) before and after treatment. Examples of quantitative protein detection of ATF4 in PBMCs from (A) patients with SSNHL in the improved group and (B) in the unimproved group before (pre-) and after (post-) treatment. (C) The protein levels of ATF4 were quantitatively analyzed. Examples of quantitative protein detection of CHOP in PBMCs in the (D) improved group and (E) in the unimproved group before (pre-) and after (post-) treatment. (F) The protein levels of CHOP were quantitatively analyzed. * and # indicate P<0.05. References
1. Chandrasekhar SS, Tsai Do BS, Schwartz SR, Clinical practice guideline: Sudden hearing loss (update): Otolaryngol Head Neck Surg, 2019; 161(1 Suppl); S1-45
2. Ciodaro F, Freni F, Alberti G, Application of cervical vestibular-evoked myogenic potentials in adults with moderate to profound sensorineural hearing loss: A preliminary study: Int Arch Otorhinolaryngol, 2020; 24(1); e5-10
3. Louessard M, Bardou I, Lemarchand E, Activation of cell surface GRP78 decreases endoplasmic reticulum stress and neuronal death: Cell Death Differ, 2017; 24; 1518-29
4. Nougarède A, Tesnière C, Ylanko J, Improved IRE1 and PERK pathway sensors for multiplex endoplasmic reticulum stress assay reveal stress response to nuclear dyes used for image segmentation: Assay Drug Dev Technol, 2018; 16; 350-60
5. Yao Y, Lu Q, Hu Z, A non-canonical pathway regulates ER stress signaling and blocks ER stress-induced apoptosis and heart failure: Nat Commun, 2017; 8; 133
6. Wang C, Tan Z, Niu B, Inhibiting the integrated stress response pathway prevents aberrant chondrocyte differentiation thereby alleviating chondrodysplasia: Elife, 2018; 7; e37673
7. Xue Q, Li C, Chen J, The Protective effect of the endoplasmic reticulum stress-related factors BiP/GRP78 and CHOP/Gadd153 on noise-induced hearing loss in guinea pigs: Noise Health, 2016; 18; 247-55
8. Zong S, Liu T, Wan F, Endoplasmic reticulum stress is involved in cochlear cell apoptosis in a cisplatin-induced ototoxicity rat model: Audiol Neurootol, 2017; 22; 160-68
9. Hu J, Li B, Apisa L, ER stress inhibitor attenuates hearing loss and hair cell death in Cdh23erl/erl mutant mice: Cell Death Dis, 2016; 7; e2485
10. Alam MM, Okazaki K, Nguyen L, Glucocorticoid receptor signaling represses the antioxidant response by inhibiting histone acetylation mediated by the transcriptional activator NRF2: J Biol Chem, 2017; 292; 7519-30
11. Mihailidou C, Panagiotou C, Kiaris H, Crosstalk between C/EBP homologous protein (CHOP) and glucocorticoid receptor in lung cancer: Mol Cell Endocrinol, 2016; 436; 211-23
12. Livak KJ, Schmittgen TD, Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)), ethod: Methods, 2001; 25; 402-8
13. Nguyen U, Squaglia N, Boge A, The Simple Western™: A gel-free, blot-free, hands-free Western blotting reinvention: Nat Methods, 2011; 8; v-vi
14. Tarkan Ö, Dağkıran M, Sürmelioğlu Ö, Intratympanic methylprednisolone versus dexamethasone for the primary treatment of idiopathic sudden sensorineural hearing loss: J Int Adv Otol, 2018; 14(3); 451-55
15. Berjis N, Soheilipour S, Musavi A, Hashemi SM: Adv Biomed Res, 2016; 5; 111
16. Koo JW, Chang MY, Yun SC, The efficacy and safety of systemic injection of Ginkgo biloba extract, EGb761, in idiopathic sudden sensorineural hearing loss: A randomized placebo-controlled clinical trial: Eur Arch Otorhinolaryngol, 2016; 273(9); 2433-41
17. Zheng H, Dai QQ, Zhou LChinese sudden hearing loss multi-center clinical study group. (Multicenter study on the treatment of sudden total deafness): Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi, 2013; 48(5); 379-84 [in Chinese]
18. Guideline of diagnosis and treatment of sudden deafness (2015): Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi, 2015; 50(6); 443-47 [in Chinese]
19. Bagheri V, Khorramdelazad H, Hassanshahi G, CXCL12 and CXCR4 in the peripheral blood of patients with Parkinson’s disease: Neuroimmunomodulation, 2018; 25; 201-5
20. Al Dubayee MS, Alayed H, Almansour R, Differential expression of human peripheral mononuclear cells phenotype markers in type 2 diabetic patients and type 2 diabetic patients on metformin: Front Endocrinol (Lausanne), 2018; 9; 537
21. Lenna S, Assassi S, Farina GA, The HLA-B*35 allele modulates ER stress, inflammation and proliferation in PBMCs from limited cutaneous systemic sclerosis patients: Arthritis Res Ther, 2015; 17; 363
22. Hsiao CP, Hoppel C, Analyzing mitochondrial function in human peripheral blood mononuclear cells: Anal Biochem, 2018; 549; 12-20
23. Hou J, She W, Du X, Histone deacetylase 2 in sudden sensorineural hearing loss patients in response to intratympanic methylprednisolone perfusion: Otolaryngol Head Neck Surg, 2016; 154; 164-70
24. Lu L, Dai Y, Du X, Relationship of glucocorticoid receptor expression in peripheral blood mononuclear cells and the cochlea of guinea pigs and effects of dexamethasone administration: PLoS One, 2013; 8; e56323
25. Zhang X, Chen J, Gao Z, Response of glucocorticoid receptor alpha and histone deacetylase 2 to glucocorticoid treatment predicts the prognosis of sudden sensorineural hearing loss: Clin Exp Otorhinolaryngol, 2019; 12; 367-75
26. Dong L, Wang S, Li Y, ru486 reverses emotional disorders by influencing astrocytes and endoplasmic reticulum stress in chronic restraint stress challenged rats: Cell Physiol Biochem, 2017; 42; 1098-108
27. Jangra A, Sriram CS, Dwivedi S, Sodium phenylbutyrate and edaravone abrogate chronic restraint stress-induced behavioral deficits: Implication of oxido-nitrosative, endoplasmic reticulum stress cascade, and neuroinflammation: Cell Mol Neurobiol, 2017; 37; 65-81
28. Rauch SD, Clinical practice. Idiopathic sudden sensorineural hearing loss: N Engl J Med, 2008; 359; 833-40
29. Jo SY, Lee S, Eom TH, Outcomes of severe to profound idiopathic sudden sensorineural hearing loss: Clin Exp Otorhinolaryngol, 2015; 8; 206-10
30. Schreiber BE, Agrup C, Haskard DO, Sudden sensorineural hearing loss: Lancet, 2010; 375; 1203-11
31. Smith M, Wilkinson S, ER homeostasis and autophagy: Essays Biochem, 2017; 61; 625-35
32. André F, Corazao-Rozas P, Idziorek T, GILZ overexpression attenuates endoplasmic reticulum stress-mediated cell death via the activation of mitochondrial oxidative phosphorylation: Biochem Biophys Res Commun, 2016; 478; 513-20
33. Das I, Png CW, Oancea I, Glucocorticoids alleviate intestinal ER stress by enhancing protein folding and degradation of misfolded proteins: J Exp Med, 2013; 210; 1201-16
34. Varadarajan S, Breda C, Smalley JL, The transrepression arm of glucocorticoid receptor signaling is protective in mutant huntingtin-mediated neurodegeneration: Cell Death Differ, 2015; 22; 1388-96
Figures
 Figure 1. Patterns of expression of protein kinase RNA-like endoplasmic reticulum kinase (PERK), eukaryotic initiation factor 2α (eIF2α), activating transcription factor 4 (ATF4), and C/EBPO homologous protein (CHOP) in peripheral blood mononuclear cells of patients with sudden sensorineural hearing loss before treatment. (A) Examples of western blot of PERK, eIF2α, ATF4, and CHOP. (B) The western blots were quantitatively analyzed. (C–F) The messenger RNA levels of PERK, eIF2α, ATF4, and CHOP were also examined with by real-time polymerase chain reaction. * P<0.05 compared with the control group.
Figure 1. Patterns of expression of protein kinase RNA-like endoplasmic reticulum kinase (PERK), eukaryotic initiation factor 2α (eIF2α), activating transcription factor 4 (ATF4), and C/EBPO homologous protein (CHOP) in peripheral blood mononuclear cells of patients with sudden sensorineural hearing loss before treatment. (A) Examples of western blot of PERK, eIF2α, ATF4, and CHOP. (B) The western blots were quantitatively analyzed. (C–F) The messenger RNA levels of PERK, eIF2α, ATF4, and CHOP were also examined with by real-time polymerase chain reaction. * P<0.05 compared with the control group. Figure 2. Comparison of activating transcription factor 4 (ATF4) and C/EBPO homologous protein (CHOP) protein levels in peripheral blood mononuclear cells (PBMCs) of patients with sudden sensorineural hearing loss (SSNHL) before and after treatment. Examples of quantitative protein detection of ATF4 in PBMCs from (A) patients with SSNHL in the improved group and (B) in the unimproved group before (pre-) and after (post-) treatment. (C) The protein levels of ATF4 were quantitatively analyzed. Examples of quantitative protein detection of CHOP in PBMCs in the (D) improved group and (E) in the unimproved group before (pre-) and after (post-) treatment. (F) The protein levels of CHOP were quantitatively analyzed. * and # indicate P<0.05.
Figure 2. Comparison of activating transcription factor 4 (ATF4) and C/EBPO homologous protein (CHOP) protein levels in peripheral blood mononuclear cells (PBMCs) of patients with sudden sensorineural hearing loss (SSNHL) before and after treatment. Examples of quantitative protein detection of ATF4 in PBMCs from (A) patients with SSNHL in the improved group and (B) in the unimproved group before (pre-) and after (post-) treatment. (C) The protein levels of ATF4 were quantitatively analyzed. Examples of quantitative protein detection of CHOP in PBMCs in the (D) improved group and (E) in the unimproved group before (pre-) and after (post-) treatment. (F) The protein levels of CHOP were quantitatively analyzed. * and # indicate P<0.05. Tables
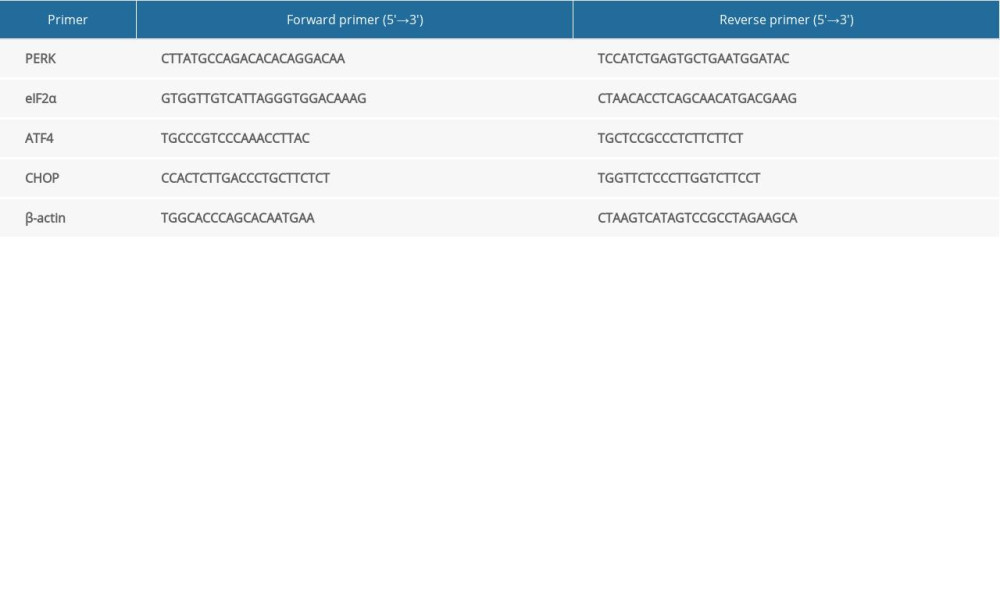 Table 1. Primer sequences for PERK, eIF2α, ATF4, CHOP, and β-actin used in rt-PCR.
Table 1. Primer sequences for PERK, eIF2α, ATF4, CHOP, and β-actin used in rt-PCR.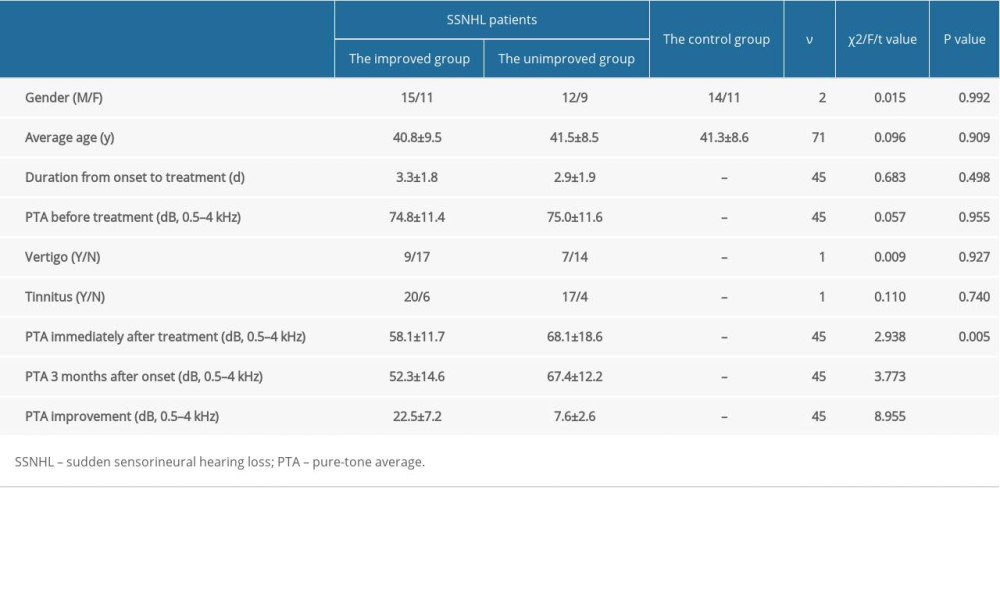 Table 2. Demographic data for patients with SSNHL patients and the control group. (n, χ̄±SD).
Table 2. Demographic data for patients with SSNHL patients and the control group. (n, χ̄±SD). Table 1. Primer sequences for PERK, eIF2α, ATF4, CHOP, and β-actin used in rt-PCR.
Table 1. Primer sequences for PERK, eIF2α, ATF4, CHOP, and β-actin used in rt-PCR. Table 2. Demographic data for patients with SSNHL patients and the control group. (n, χ̄±SD).
Table 2. Demographic data for patients with SSNHL patients and the control group. (n, χ̄±SD). In Press
05 Mar 2024 : Clinical Research
Muscular Function Recovery from General Anesthesia in 132 Patients Undergoing Surgery with Acceleromyograph...Med Sci Monit In Press; DOI: 10.12659/MSM.942780
05 Mar 2024 : Clinical Research
Effects of Thermal Insulation on Recovery and Comfort of Patients Undergoing Holmium Laser LithotripsyMed Sci Monit In Press; DOI: 10.12659/MSM.942836
05 Mar 2024 : Clinical Research
Role of Critical Shoulder Angle in Degenerative Type Rotator Cuff Tears: A Turkish Cohort StudyMed Sci Monit In Press; DOI: 10.12659/MSM.943703
06 Mar 2024 : Clinical Research
Comparison of Outcomes between Single-Level and Double-Level Corpectomy in Thoracolumbar Reconstruction: A ...Med Sci Monit In Press; DOI: 10.12659/MSM.943797
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








