27 January 2021: Clinical Research
Percentage of Natural Killer (NK) Cells in Peripheral Blood Is Associated with Prognosis in Patients with Gastric Cancer: A Retrospective Study from a Single Center
Ming-zhi XieBCE, Yan-ping TangBDE, Bang-li HuCE, Ke-zhi LiCDE, Ji-lin LiBCD, Xin-qiang LiangAEGDOI: 10.12659/MSM.927464
Med Sci Monit 2021; 27:e927464
Abstract
BACKGROUND: Natural killer (NK) cells are important for the prognosis of multiple cancers, but their prognostic value remains to be evaluated in patients with gastric cancer. Thus, this retrospective study was conducted at a single center to investigate the association between percentage of NK cells in the peripheral blood and prognosis in patients with gastric cancer.
MATERIAL AND METHODS: The data of 180 gastric cancer patients were collected. Univariate and multivariate Cox regression models were applied to screen candidate prognostic factors. A time-dependent receiver operating characteristic curve was employed to evaluate the ability of NK cells as a prognostic marker. Furthermore, we determined the correlation between the NK cells percentage and other parameters and their clinical significance.
RESULTS: Patients with a higher percentage of NK cells survived longer than those with a lower percentage of NK cells. Cox analysis revealed that NK cells could be used as an independent indicator for patients with gastric cancer. The percentage of NK cells was positively correlated with lymphocyte count and albumin, but was negatively correlated with CA125 and neutrophil-lymphocyte ratio. The area under the curve for NK cells in predicting the 5-year survival rate for gastric cancer was 0.792. This increased to 0.830 upon combining NK cells with neutrophil-lymphocyte ratio. Patients at early T, N, and clinical stages possessed a significantly higher percentage of NK cells compared to those at advanced T, N, and clinical stages of gastric cancer.
CONCLUSIONS: Our results suggest that a higher percentage of NK cells predicts is associated with longer survival of gastric cancer patients and could serve as an independent prognostic biomarker.
Keywords: Biological Markers, Natural Killer T-Cells, Biomarkers, Tumor, CA-125 Antigen, Lymphocyte Count, Lymphocytes, Membrane Proteins, Neutrophils, Proportional Hazards Models, ROC Curve, Serum Albumin, Survival Rate
Background
Gastric cancer remains one of the most common malignant tumors worldwide, especially at later stages, with a high mortality rate [1]. Despite the significant advancements in therapeutic approaches, the cure and prognosis of gastric cancer patients remain poor [2]. Currently, conventional tumor biomarkers, including CA153, CA199, and CA125, are used to assess the diagnosis and prognosis of gastric cancer; however, these biomarkers exhibit low sensitivity and specificity and are poor markers for the prognosis of cancer [3,4]. Therefore, efforts are being made to identify effective biomarkers for gastric cancer; studies have reported some biomarkers in the peripheral blood, such as neutrophil-lymphocyte ratio (NLR) [5] and platelet-lymphocyte ratio (PLR) [6].
Immune cells, including T lymphocytes, B lymphocytes, and natural killer (NK) cells, contribute to the host antitumor immune response. The percentage concentration of these immune cells is crucial for the pathogenesis of certain cancers [7–9]. NK cells are good markers for the prognosis of cancers. Tang et al. [10] showed that NK cells in the blood were an independent predictor of survival in CRC patients. A recent study reported that low NK cell counts in the circulation or tumor tissue before treatment were associated with poorer outcomes in patients with follicular lymphoma and diffuse large B cell lymphoma undergoing antibody-based therapy [11]. Similar results were observed in patients with mantle cell lymphoma [12] and acute lymphoblastic leukemia [13]. In addition, the above-mentioned biomarkers are nonspecific, and the clinical significance and utility of these biomarkers are still unclear at best, as many of them are statistically but not clinically significant [14].
Pervious research has reported that higher percentages of NK cells lead to better prognosis in gastric cancer patients [15,16], suggesting the potential of NK cells in prognosis. However, the sample size was small, which reduced the robustness of the results. Moreover, there is no evidence for the importance of NK cells in gastric cancer patients undergoing radical surgery and adjuvant chemotherapy. Therefore, this retrospective study was conducted at a single center to investigate the association between percentage of NK cells in the peripheral blood and prognosis in patients with gastric cancer.
Material and Methods
COLLECTION OF GASTRIC CANCER PATIENTS:
In this single-center study, we retrospectively assessed all patients who underwent surgery between January 2014 and December 2016 from the electronic medical record system in our hospital. The sample size was determined using the powerandsamplesize online tool (
DATA EXTRACTION:
The potential candidates for indicators for the prognosis of gastric cancers were extracted, including sex, age, preoperative blood counts, tumor biomarkers (including CA153, CA199, CA125, and CEA), immune cell counts (NK cells, T lymphocytes, and B lymphocytes), and inflammatory markers, such as C-reactive protein (CRP) and high-sensitivity CRP (hsCRP). NLR and PLR were calculated. Details of the tumor location, degree of differentiation, and TNM staging (American Joint Committee on Cancer criteria, 8th Edition) [10] were also collected. All the patients were followed up after treatment until December 2016 or death. Overall survival (OS) was defined as the date from treatment to the date of last follow-up or death.
COLLECTION OF BLOOD SAMPLES:
Fresh blood samples were collected prior to surgery. Venous blood (10 mL) was collected from each patient in the morning to determine fasting levels. Samples (5 mL) were placed in a water bath at 37°C for 30 min and centrifuged at 1400×g. Subsequently, the supernatants were collected and stored at −20°C until analysis. The remaining 5 mL of blood was transferred to collection tubes containing the general anti-coagulator EDTA-K3, and the samples were prepared for flow cytometry within 30 min. To ensure maximum viability, stained cells were analyzed promptly to detect the lymphocyte subsets.
FLOW CYTOMETRY FOR T AND B LYMPHOCYTES AND NK CELLS:
Lymphocytes were gated according to the CD45/side scatter dot plots. T cell subsets were defined as CD3+/CD4+, and CD3+/CD8+, B cells were defined as CD19+, and NK cells were CD3−/CD16+/CD56+. Flow cytometry (BD Biosciences, Franklin Lakes, NJ, USA) determination for the cells was performed as previously described [17]. Briefly, blood cells were lysed with the red blood cell lysis buffer, then the samples were centrifuged and supernatants were discarded and heavy precipitates were retained. Membrane-labeled monoclonal antibodies were added to the precipitates and incubated at 4°C for 30 min.
STATISTICAL ANALYSIS:
SPSS 19.0 and R language (version 3.6.1) were used to conduct the statistical analyses. The normal distribution of NK cell number was confirmed by histogram prior to analysis. Comparison between the NK cells within groups was the continuous parameter that was evaluated using the t test or Mann-Whitney U test, as needed. Comparison of the NK cells between 3–4 groups differing in tumor location and grade was performed using ANOVA. Correlations between NK cells and other variables were determined using Pearson analysis. Kaplan-Meier analysis and log-rank test were used to compare the OS in different groups. A Cox regression model was established to identify the risk factors associated with OS by univariate and multivariate analyses, respectively. The proportional hazards assumption was checked using statistical tests based on the scaled Schoenfeld residuals; P value >0.05 means that the Cox regression model meets the proportional hazards, meaning that the ratio of the hazards for any 2 individuals is constant over time. Because there were more male patients than female patients, sex-stratified models were created, and we also considered the unbalanced numbers between males and females. The optimal cut-off of NK cells was selected using the survminer package (version 0.4.6; http://cran.r-project.org/). The significance of NK cells for prognosis was determined by time-dependent receiver operating characteristic (time-ROC) curves and the area under the curve (AUC) using the R packages of “timeROC” [18], and the comparison of AUC values was also performed. P values of <0.05 were considered statistically significant.
Results
PATIENT CHARACTERISTICS:
After filtering out the incomplete records, the data from 180 gastric cancer patients were selected for this study. The cohort comprised 111 male and 69 female patients with the mean age of 57.56±12.34 years. The follow-up appointments were conducted at 12–58 months (median: 27 months); 81 patients died during the follow-up. The clinical pathological characteristics are listed in Table 1.
NK CELLS AS A RISK FACTOR FOR PROGNOSIS IN GASTRIC CANCER PATIENTS:
Based on the histogram, the distribution of NK cells was normal (Supplementary Figure 1). We included age, lymphocyte count, albumin, tumor biomarkers, NK cells, CRP, hsCRP, N stage, M stage, and NLR data from the pathological parameters in the univariate Cox regression models. These parameters were subsequently analyzed using a multivariate Cox regression model. The proportional hazards assumption showed that differences were not statistically significant (P=0.084), suggesting that this model met the proportional hazards assumption. We observed that the percentages of lymphocytes, NK cells, CA199, and hsCRP and M stages were correlated with the prognosis of patients with gastric cancer (Table 2). The C-index of the Cox regression models was 0.672. To determine whether sex is associated with the prognostic ability of the above indexes, we included interactions between NK cell percentages and sex in the model and assessed whether the interaction was significant. As Supplementary Table 1 shows, sex did not significantly affect the prognostic ability of the above indexes.
CORRELATION BETWEEN ABSOLUTE NK CELL NUMBERS AND OTHER MARKERS:
As listed in Table 3, analysis revealed that the percentage of NK cells was positively correlated with the levels of lymphocytes and albumin (P<0.05), but was negatively correlated with CA125 levels and NLR (P<0.001).
PROGNOSTIC VALUE OF NK CELLS IN PATIENTS WITH GASTRIC CANCER:
We calculated the optimal cut-off for the percentage of NK cells and found that at a cut-off of 5.07 the high and low percentages NK cells helped divide the patients into high-risk and low-risk groups based on the cut-off value (log-rank value <0.001; Figure 1). To determine the effect of NK cells on survival in gastric cancer patients, we calculated AUC using time-ROC curves; the results showed that the percentage of NK cells had a moderate prognostic association with the 1-, 3-, and 5-year OS, with AUC values of 0.732, 0.806, and 0.792, respectively (Figure 2A).
PROGNOSTIC VALUE OF NK CELLS COMBINED WITH NLR IN GASTRIC CANCER PATIENTS:
NLR is an independent predictor of gastric cancer, as previous study described [5]. Here, we confirmed that NLR was also related to the survival of gastric cancer patients. Therefore, we assessed whether the combination of the percentage of NK cells with NLR could improve the prognostic value. As shown in Figure 2B, this combination increased the prognosis of 1-, 3-, and 5-year OS, for which the AUC was 0.758, 0.823, 0.830, respectively, and the differences at all 3 timepoints were remarkable compared with NK cells alone (all P<0.01). These results indicated that this combination could improve the prediction of prognosis of gastric cancer patients.
CORRELATION BETWEEN NK CELLS AND CLINICAL FEATURES OF GASTRIC CANCER PATIENTS:
To understand the correlation between percentage of NK cells and clinical features of gastric cancer, we analyzed the percentage of NK cells in patients with different clinical features. As shown in Table 4, the percentage of NK cells was obviously higher in patients at early T stages (T1+T2), N stage (N0), and clinical stages (I+II) compared with those at advanced T stages (T3+T4), N stages (N1+N2+N3), and clinical stages (III+IV; P<0.05). There were no significant differences in patient age, sex, tumor location, histological differentiation, and M stage (P>0.05).
Discussion
NK cells are crucial in innate immunity; they can recognize and kill tumor cells and produce multiple cytokines to regulate adaptive immunity [19]. NK cells exert a robust protective response to reactivated pathogens [20,21]. The higher percentage of NK cells allowed treatment of gastric cancer patients to be effective. A huge decrease in the percentage of NK cells and secreted cytokines in gastric cancer patients damages the immune system [22,23]. Increasing the percentage of or activating NK cells are efficient approaches that are tolerated by the immune system to treat gastric cancer [24–26].
Previous studies have shown the prognostic value of NK cells in several cancers. Bar et al. [27] reported that lower percentages of NK cells resulted in a weaker immune response to residual leukemia and predicted a poor outcome in acute myeloid leukemia. The percentage of NK cells is an independent indicator for prognosis of colon cancer and is significantly correlated with the lymph nodes [28]. Yang et al. [29] reported that patients with advanced pancreatic cancer possess NK cells of a unique subtype with anergic features; a high percentage of NKs predicts poor survival of these patients. A recent study reported that baseline concentration of circulating tumor cells is positively correlated with concentration of peripheral NK cells; a combination of the circulating tumor cells and NK cells predicts progression-free survival in triple-negative breast cancer patients without any pre-existing conditions [30].
In the present study, the percentage of NK cells was correlated with the OS of gastric cancer patients, indicating that NK cells can act as an independent biomarker. This result was inconsistent with that reported by Rosso et al. [31]. We also identified an optimal cut-off for the percentage of NK cells that allowed a clear division of patients into low- and high-risk groups. Subsequently, we analyzed the prognostic value in patients’ survival. However, the prognostic value of NK cells for 1-, 3-, and 5-year survival was moderate. Therefore, we combined other biomarkers to improve the prognostic ability. As expected, combining the NK cells with NLR greatly increased the prognostic ability compared to using only NK cells, suggesting that this combination can better predict the prognosis of gastric cancer patients.
Although previous studies have reported the prognostic utility of the percentage of NK cells in patients with gastric cancer, the results obtained were inconsistent. Pernot et al. [32] showed that gastric cancer patients with a high percentage of circulating NK cells exhibit better OS than patients with a low percentage. Similarly, Rosso et al. [31] showed that the NK cell percentage in tissues acted as an independent prognostic predictor for gastric cancer patients. In contrast, Yu et al. [14] observed that the circulating NK cells were increased in gastric cancer patients compared to those in healthy controls; however, the percentage of NK cells did not correlate with the survival of patients. Akagi et al. [33] have shown similar results. Thus, the correlation between the percentage of NK cells and survival of patients with gastric cancer remains unclear. The present study provides evidence that the percentage of NK cells is correlated with the survival of gastric cancer patients.
The strength of our study was the relatively large cohort with data from extended follow-up appointments. However, there are some limitations to this study. First, the data originated from patients at a single-center setting, thereby decreasing the generalizability of our findings. Second, the design of this study was of a retrospective nature, which could have introduced bias and reduced the reliability of the results. Third, owing to the limited data, some well-known risk factors for gastric cancer, such as
Conclusions
This study demonstrated that the percentage of peripheral NK cells is correlated with the survival of gastric cancer patients, and could be a promising prognostic biomarker. However, these results need further validation owing to the study limitations.
Figures
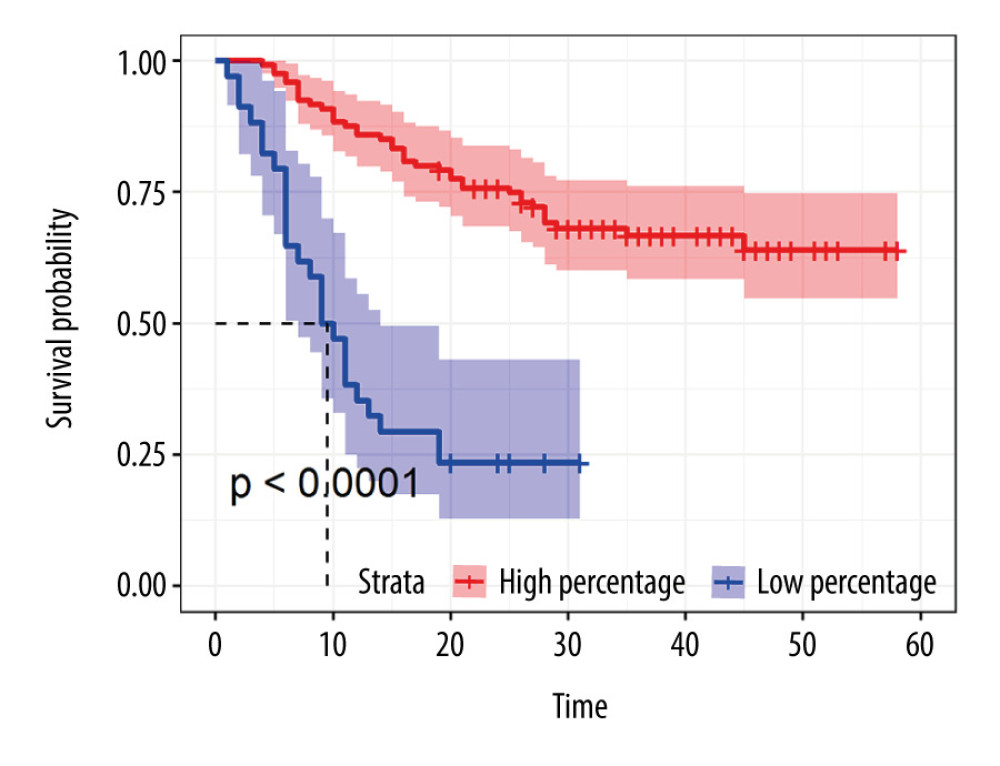 Figure 1. Kaplan-Meier curve of NK cells using the optimal cut-off value in patients with gastric cancer.
Figure 1. Kaplan-Meier curve of NK cells using the optimal cut-off value in patients with gastric cancer. 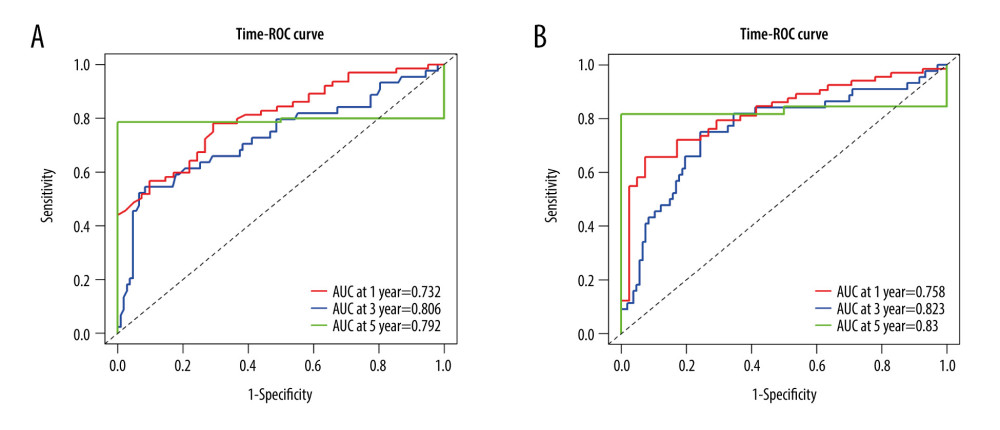 Figure 2. (A) Time-ROC curve of NK cells in 1-, 3-, 5- year survival in patients with gastric cancer. (B) Time-ROC curve of NK cells combined with NLR in 1-, 3-, 5- year survival in patients with gastric cancer.
Figure 2. (A) Time-ROC curve of NK cells in 1-, 3-, 5- year survival in patients with gastric cancer. (B) Time-ROC curve of NK cells combined with NLR in 1-, 3-, 5- year survival in patients with gastric cancer. Tables
Table 1. Clinical pathological characteristic of gastric cancer patients. Table 2. Risk factors for the prognosis in gastric cancer patients, with N0 and M0 as reference groups.
Table 2. Risk factors for the prognosis in gastric cancer patients, with N0 and M0 as reference groups.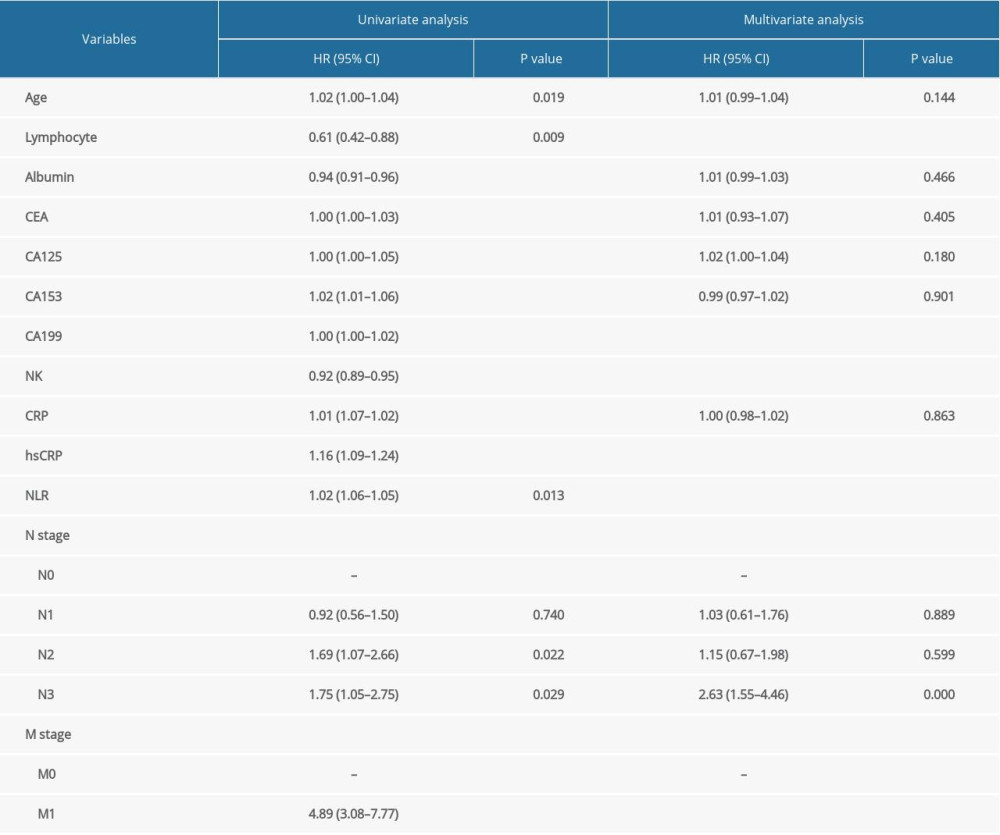 Table 3. Correlations between the percentage of NK cells and the other indicators.
Table 3. Correlations between the percentage of NK cells and the other indicators.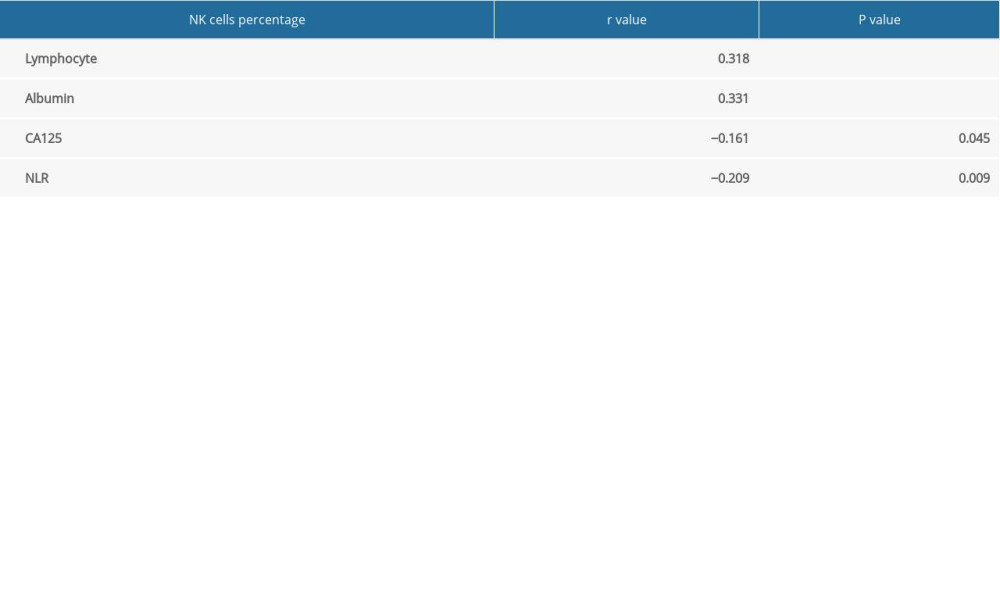 Table 4. Association of percentage of NK cells with the clinical features of gastric cancer patients.
Table 4. Association of percentage of NK cells with the clinical features of gastric cancer patients.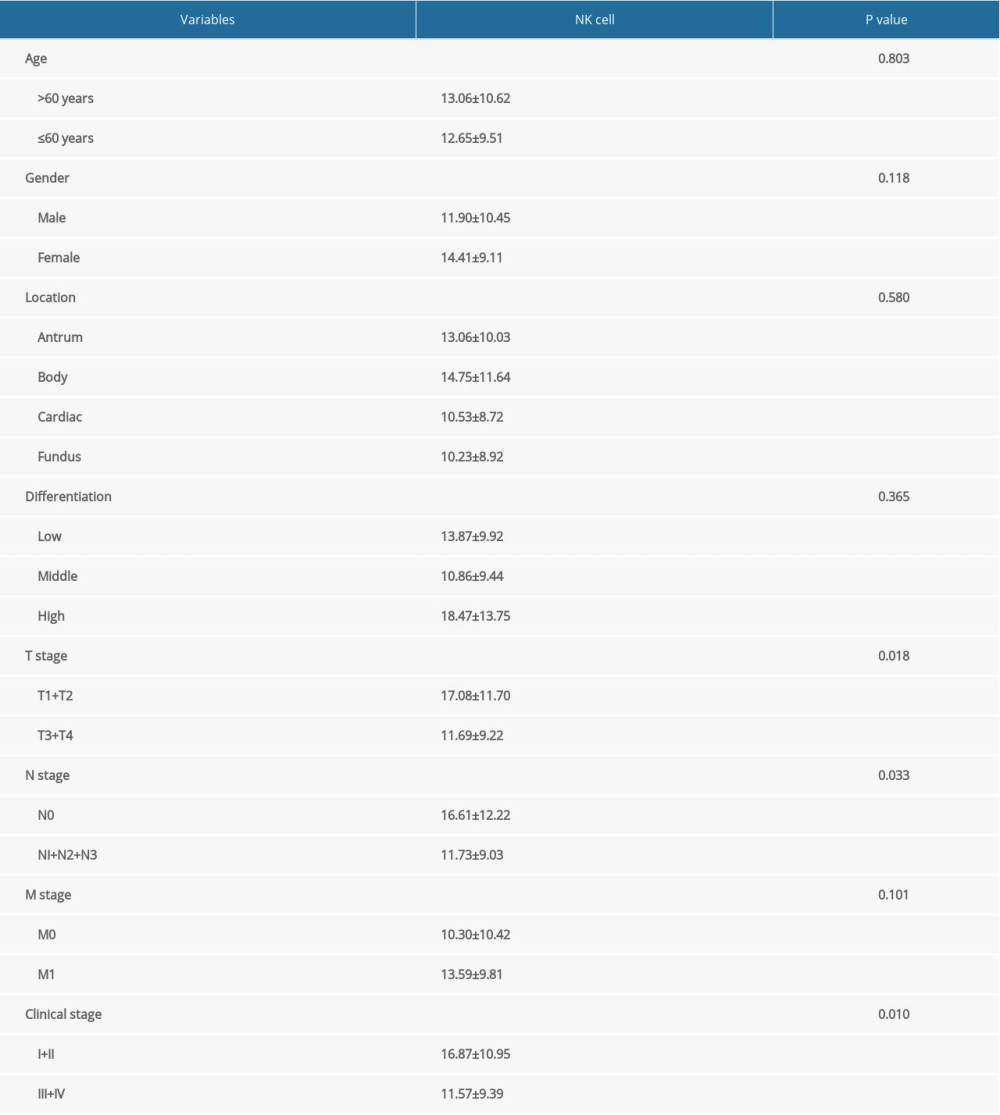 Supplementary Table 1. Multivariate Cox regression analysis of variables in prognosis of gastric cancer after stratification by sex.
Supplementary Table 1. Multivariate Cox regression analysis of variables in prognosis of gastric cancer after stratification by sex.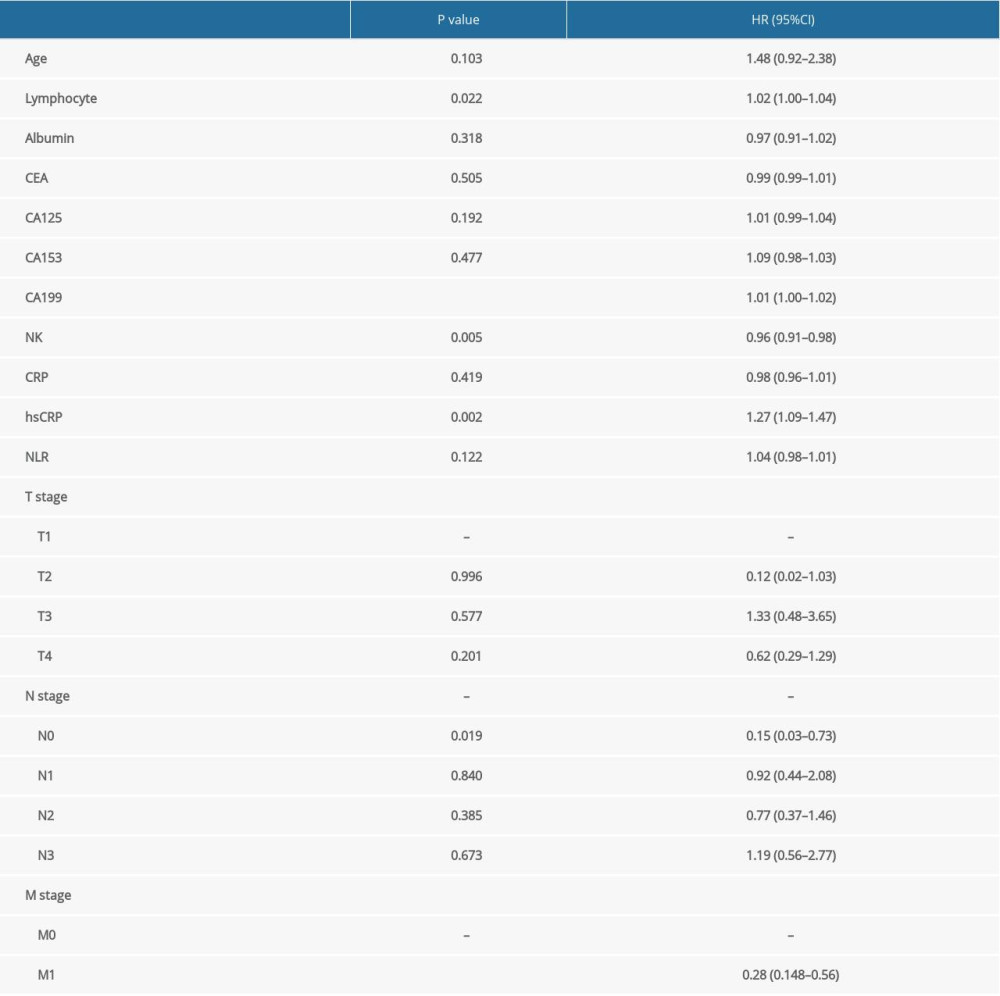
References
1. Ferlay J, Soerjomataram I, Dikshit R, Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012: Int J Cancer, 2015; 136; E359-86
2. Hundahl SA, Phillips JL, Menck HR, The National Cancer Data Base Report on poor survival of U.S. gastric carcinoma patients treated with gastrectomy: Fifth Edition American Joint Committee on Cancer staging, proximal disease, and the “different disease” hypothesis: Cancer, 2000; 88; 921-32
3. Tian SB, Yu JC, Kang WM, Combined detection of CEA, CA 19-9, CA 242 and CA 50 in the diagnosis and prognosis of resectable gastric cancer: Asian Pac J Cancer Prev, 2014; 15; 6295-300
4. Feng F, Tian Y, Xu G, Diagnostic and prognostic value of CEA, CA19-9, AFP and CA125 for early gastric cancer: BMC Cancer, 2017; 17; 737
5. Mellor KL, Powell A, Lewis WG, Systematic review and meta-analysis of the prognostic significance of neutrophil-lymphocyte ratio (NLR) after R0 gastrectomy for cancer: J Gastrointest Cancer, 2018; 49; 237-44
6. Xu Z, Xu W, Cheng H, The prognostic role of the platelet-lymphocytes ratio in gastric cancer: A meta-analysis: PLoS One, 2016; 11; e0163719
7. Gonzalez H, Hagerling C, Werb Z, Roles of the immune system in cancer: From tumor initiation to metastatic progression: Genes Dev, 2018; 32; 1267-84
8. Tse E, Kwong YL, Immunologic milieu of mature T-cell and NK-cell lymphomas-implications for therapy: Curr Hematol Malig Rep, 2018; 13; 37-43
9. Valipour B, Velaei K, Abedelahi A, NK cells: An attractive candidate for cancer therapy: J Cell Physiol, 2019; 234(11); 19352-65
10. Tang YP, Xie MZ, Li KZ, Prognostic value of peripheral blood natural killer cells in colorectal cancer: BMC Gastroenterol, 2020; 20; 31
11. Klanova M, Oestergaard MZ, Trněný M, Prognostic impact of natural killer cell count in follicular lymphoma and diffuse large B-cell lymphoma patients treated with immunochemotherapy: Clin Cancer Res, 2019; 25(15); 4634-43
12. Zhou XH, Zhang XY, Liang JH, Low absolute NK cell counts in peripheral blood are associated with inferior survival in patients with mantle cell lymphoma: Cancer Biomark, 2019; 24; 439-47
13. Mizia-Malarz A, Sobol-Milejska G, NK cells as possible prognostic factor in childhood acute lymphoblastic leukemia: Dis Markers, 2019; 2019; 3596983
14. Ng QX, Soh AYS, Yeo WS, Statistically but not clinically significant? Biomarkers in gastric cancer: Clin Nutr, 2018; 37; 2292-93
15. Chen J, Yang J, Jiang J, Function and subsets of dendritic cells and natural killer cells were decreased in gastric cancer: Int J Clin Exp Pathol, 2014; 7; 8304-11
16. Chen Y, Chen B, Yang T, Human fused NKG2D-IL-15 protein controls xenografted human gastric cancer through the recruitment and activation of NK cells: Cell Mol Immunol, 2017; 14; 293-307
17. Liu HZ, Deng W, Li JL, Peripheral blood lymphocyte subset levels differ in patients with hepatocellular carcinoma: Oncotarget, 2016; 7; 77558-64
18. Heagerty PJ, Lumley T, Pepe MS, Time-dependent ROC curves for censored survival data and a diagnostic marker: Biometrics, 2000; 56; 337-44
19. Stabile H, Fionda C, Gismondi A, Santoni A, Role of distinct natural killer cell subsets in anticancer response: Front Immunol, 2017; 8; 293
20. Sun JC, Beilke JN, Lanier LL, Adaptive immune features of natural killer cells: Nature, 2009; 457; 557-61
21. Ugolini S, Vivier E, Immunology: Natural killer cells remember: Nature, 2009; 457; 544-45
22. Du Y, Wei Y, Therapeutic potential of natural killer cells in gastric cancer: Front Immunol, 2018; 9; 3095
23. Xu Q, Li J, Zhang N, Utilization of invariant natural killer T cells for gastric cancer treatment: Future Oncol, 2018; 14; 2053-66
24. Wang W, Jin J, Dai F, Interleukin-15 suppresses gastric cancer liver metastases by enhancing natural killer cell activity in a murine model: Oncol Lett, 2018; 16; 4839-46
25. Li T, Zhang Q, Jiang Y, Yu J, Gastric cancer cells inhibit natural killer cell proliferation and induce apoptosis via prostaglandin E2: Oncoimmunology, 2016; 5; e1069936
26. Oberg HH, Kellner C, Gonnermann D, Tribody [(HER2)2xCD16] is more effective than trastuzumab in enhancing gammadelta T cell and natural killer cell cytotoxicity against HER2-expressing cancer cells: Front Immunol, 2018; 9; 814
27. Bar M, Othus M, Park HM, Elevated lymphocyte count at time of acute myeloid leukemia diagnosis is associated with shorter remission: Leuk Lymphoma, 2015; 56; 3109-15
28. Okada K, Sadahiro S, Chan LF, The Number of Natural Killer Cells in the Largest Diameter Lymph Nodes Is Associated with the Number of Retrieved Lymph Nodes and Lymph Node Size, and Is an Independent Prognostic Factor in Patients with Stage II colon cancer: Oncology, 2018; 95; 288-96
29. Yang C, Cheng H, Zhang Y, Anergic natural killer cells educated by tumor cells are associated with a poor prognosis in patients with advanced pancreatic ductal adenocarcinoma: Cancer Immunol Immunother, 2018; 67; 1815-23
30. Liu X, Ran R, Shao B, Combined peripheral natural killer cell and circulating tumor cell enumeration enhance prognostic efficiency in patients with metastatic triple-negative breast cancer: Chin J Cancer Res, 2018; 30; 315-26
31. Rosso D, Rigueiro MP, Kassab PCorrelation of natural killer cells with the prognosis of gastric adenocarcinoma: Arq Bras Cir Dig, 2012; 25; 114-17 [in Portuguese]
32. Pernot S, Terme M, Radosevic-Robin N, Infiltrating and peripheral immune cell analysis in advanced gastric cancer according to the Lauren classification and its prognostic significance: Gastric Cancer, 2020; 23(1); 73-81
33. Akagi J, Baba H, Prognostic value of CD57(+) T lymphocytes in the peripheral blood of patients with advanced gastric cancer: Int J Clin Oncol, 2008; 13; 528-35
Figures
 Figure 1. Kaplan-Meier curve of NK cells using the optimal cut-off value in patients with gastric cancer.
Figure 1. Kaplan-Meier curve of NK cells using the optimal cut-off value in patients with gastric cancer. Figure 2. (A) Time-ROC curve of NK cells in 1-, 3-, 5- year survival in patients with gastric cancer. (B) Time-ROC curve of NK cells combined with NLR in 1-, 3-, 5- year survival in patients with gastric cancer.
Figure 2. (A) Time-ROC curve of NK cells in 1-, 3-, 5- year survival in patients with gastric cancer. (B) Time-ROC curve of NK cells combined with NLR in 1-, 3-, 5- year survival in patients with gastric cancer. Tables
 Table 1. Clinical pathological characteristic of gastric cancer patients.
Table 1. Clinical pathological characteristic of gastric cancer patients. Table 2. Risk factors for the prognosis in gastric cancer patients, with N0 and M0 as reference groups.
Table 2. Risk factors for the prognosis in gastric cancer patients, with N0 and M0 as reference groups. Table 3. Correlations between the percentage of NK cells and the other indicators.
Table 3. Correlations between the percentage of NK cells and the other indicators. Table 4. Association of percentage of NK cells with the clinical features of gastric cancer patients.
Table 4. Association of percentage of NK cells with the clinical features of gastric cancer patients. Table 1. Clinical pathological characteristic of gastric cancer patients.
Table 1. Clinical pathological characteristic of gastric cancer patients. Table 2. Risk factors for the prognosis in gastric cancer patients, with N0 and M0 as reference groups.
Table 2. Risk factors for the prognosis in gastric cancer patients, with N0 and M0 as reference groups. Table 3. Correlations between the percentage of NK cells and the other indicators.
Table 3. Correlations between the percentage of NK cells and the other indicators. Table 4. Association of percentage of NK cells with the clinical features of gastric cancer patients.
Table 4. Association of percentage of NK cells with the clinical features of gastric cancer patients. Supplementary Table 1. Multivariate Cox regression analysis of variables in prognosis of gastric cancer after stratification by sex.
Supplementary Table 1. Multivariate Cox regression analysis of variables in prognosis of gastric cancer after stratification by sex. In Press
05 Mar 2024 : Clinical Research
Muscular Function Recovery from General Anesthesia in 132 Patients Undergoing Surgery with Acceleromyograph...Med Sci Monit In Press; DOI: 10.12659/MSM.942780
05 Mar 2024 : Clinical Research
Effects of Thermal Insulation on Recovery and Comfort of Patients Undergoing Holmium Laser LithotripsyMed Sci Monit In Press; DOI: 10.12659/MSM.942836
05 Mar 2024 : Clinical Research
Role of Critical Shoulder Angle in Degenerative Type Rotator Cuff Tears: A Turkish Cohort StudyMed Sci Monit In Press; DOI: 10.12659/MSM.943703
06 Mar 2024 : Clinical Research
Comparison of Outcomes between Single-Level and Double-Level Corpectomy in Thoracolumbar Reconstruction: A ...Med Sci Monit In Press; DOI: 10.12659/MSM.943797
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








