15 January 2021: Clinical Research
Wide-Field Fluorescein Angiography in the Diagnosis and Management of Retinal Vein Occlusion: A Retrospective Single-Center Study
Monika J. Turczyńska1ABCDEF, Przemysław Krajewski1CEF, Joanna E. Brydak-Godowska1AEFG*DOI: 10.12659/MSM.927782
Med Sci Monit 2021; 27:e927782
Abstract
BACKGROUND: The aim of this retrospective study was to evaluate the role of wide-field fluorescein angiography (WF-FA) in the diagnosis and management of retinal vein occlusion (RVO) at a single center in Poland.
MATERIAL AND METHODS: This study included 106 patients (112 eyes) diagnosed with RVO (102 eyes) or impending RVO (10 eyes) (54% women and 46% men, aged 26 to 86 years). The medical records of the participants were reviewed in search of documentation on ocular and systemic diseases. Results of FA of central and peripheral retina and optical coherence tomography (OCT) scans, which had been used to establish treatment indications, were analyzed. WF-FA was performed with Spectralis HRA+OCT or Optos Tx200.
RESULTS: Actual RVO was found in 102 eyes. Of those cases, 46.1% were CRVO (central retinal vein occlusion), 40.2% branch retinal vein occlusion, 11.8% small tributary vein occlusion, and 1.9% hemispheric retinal vein occlusion. Neovascularization on an optic disc, neovascularization elsewhere, and veno-venous collateral vessels were observed in 32.3%, 17.4%, and 41.2% of the eyes, respectively. Peripheral ischemic zones were present in 59.8% of the eyes, in 20.6% of which, ischemia was not observed in the posterior pole. Dye leaks limited to peripheral vessels, peripheral vascular amputations, and central macular edema in OCT were observed in 17.6%, 43.1%, and 63.7% of the eyes, respectively. Retinal laser photocoagulation was conducted on 73.5% of the eyes.
CONCLUSIONS: Decision-making about management of patients with RVO should be done after physical examination and analysis of central and peripheral retina FA. In 20.6% of patients, assessment of the peripheral retina resulted in a change in treatment. The first changes suggestive of progression of thrombotic disease to the ischemic form appeared on the periphery in images from WF-FA.
Keywords: Fluorescein Angiography, Tomography, Optical Coherence, Retinal Vein Occlusion, Aged, 80 and over
Background
Systemic diseases that involve arteries and/or veins may be associated with autoimmune, infectious, atherosclerotic or age-related degenerative factors. They are termed diseases of civilization and, because they can afflict multiple organs and systems, management requires a multidisciplinary approach. Retinal vein occlusion (RVO), which is one of the most common vascular complications of systemic diseases, affects the eye and causes sudden, painless vision loss [1].
Based on localization, RVO is classified as central retinal vein occlusion (CRVO), hemispheric retinal vein occlusion (HRVO) affecting the lower or upper vertical hemisphere, or branch retinal vein occlusion (BRVO) that involves 1 or more large vein branches or their smaller local tributaries (venules).
In 2008, according to Rogers et al., 16.4 million people worldwide aged 30 years and older had RVO in at least 1 eye; 2.5 million were affected by CRVO and 13.9 million by BRVO [2]. Klein et al. estimated the incidence of RVO in developed countries at 5.2 per 1000 and of CRVO at 0.8 per 1000 [3]. In 2015, according to Song et al., the global prevalences of RVO, BRVO, and CRVO in people aged 30 to 89 years were 0.77%, 0.64%, and 0.13%, equivalent to an overall 28.06 million, 23.38 million, and 4.67 million affected people, respectively [4]. It is estimated that 0.7% of patients are aged 49 to 60 years and 4.6% are older than age 80 [2], which suggests that older age is a risk factor [5].
RVO, defined as retinal artery occlusion due to emboli and anterior ischemic optic neuropathy, is an ocular complication of arterial hypertension [6]. Apart from older age and arterial hypertension, the risk factors for it include atherosclerosis, hyperlipidemia, diabetes mellitus, congenital or acquired thrombophilia, malignancy, renal insufficiency, smoking, use of hormonal contraception, obesity, sarcoidosis, thyroid-associated orbitopathy, Wegener’s granulomatosis, Goodpasture syndrome, and homocysteinemia [5,7,8]. Ocular factors predisposing to RVO include optic disc drusen, elevated intraocular pressure, glaucoma, and short axial length [5,9].
RVO occurs as a result of vasculitis, which causes damage to vascular endothelium. Enhanced platelet aggregation and disseminated intravascular coagulation ultimately lead to venous occlusion, which prevents blood from draining out of the retina. In the early stage of RVO, ophthalmoscopy of the involved retina reveals signs of damage to the veins and their increased permeability, such as dilated tortuous veins associated with flame-shaped retinal hemorrhages and/or cotton-wool spots or macular and optic disc edema. The course, management, and prognosis of a patient’s ultimate visual acuity depend on the diameter of the area of occlusion and its location (with or without macular involvement) and type (ischemic
Fluorescein angiography (FA) and optical coherence tomography angiography (OCTA) are the imaging studies used to visualize areas of the retina deprived of capillary circulation (nonperfusion areas).
Current clinical guidelines recommend use of OCTA for assessment of retinal edema, ischemia, neovascularization, and hemorrhage [10]. Currently, OCTA is used in clinical practice to visualize the macular region and there is a need to target wider fields of view [11]. Several studies have shown the utility of OCTA for examining the periphery of the retina; however, only FA can reveal wide areas of ischemia in that location [12,13].
FA was first used in 1961 by Novotny and Alvis [14]. Since the 1980s, it has been commonly used in clinical practice for diagnosis of retinal vascular disorders including RVO. Standard FA scans are taken using a 30º or 50º lens, which enables visualization of the posterior pole (a fovea-centered circle equal to 5 optic disc diameters), but in retinal vascular disorders, the evaluation must include large areas of the periphery. At present, with advances in technology such as wide-field angiography (WFA), it is possible to image the retinal periphery up to the ora serrata. WFA systems available include Optos Tx200, a scanning laser ophthalmoscope (a single image captures up to 200° or 82% of the retina) and Spectralis HRA+OCT (Heidelberg Retina Angiograph+Optical Coherence Tomography), a laser scanning instrument with a 102° lens (a single image captures the posterior pole with the periphery up to the level of the vortex veins). Both systems can capture a view of the entire retina up to the ora serrata (4 images with the patient instructed to look up, down, sideways and toward the nose) when the patient cooperates with the examiner and the position of gaze is suitably adjusted.
Numerous studies have shown that wide-field FA (WF-FA) is valuable for diagnosis of retinal venous occlusion, given the range of the peripheral retina that it reveals [10,15–17]. Therefore, the aims of this retrospective study were to evaluate the role of WF-FA in the diagnosis and management of RVO at a single center in Poland.
Material and Methods
The retrospective study included 106 patients (112 eyes, as in 6 patients, both eyes were affected) who were admitted to the Department of Ophthalmology at the Infant Jesus Clinical Hospital in Warsaw from 2014 to 2020, in whom either actual or impending RVO was diagnosed by ophthalmoscopy and FA.
The study group included 58 women (54%) and 48 men (46%) aged 26 to 86 years (mean ages 65 and 63, respectively). Four patients were younger than age 40 and 10 were older than age 80 years.
Participants’ medical records were reviewed with a special focus on ocular and systemic comorbidities considered risk factors for ocular thrombosis, any exposure to anticoagulant therapy, history of FA studies, and optical coherence tomography (OCT) findings of presence or absence of cystoid macular edema (CME). Ophthalmic treatments were analyzed, including the types, and in the case of laser photocoagulation the target therapeutic area, and the recorded indications for particular therapy guided by FA and OCT findings.
Patients were included in the analysis if they had at least 1 FA study of the retinal periphery. The studies were performed with the Spectralis HRA+OCT system using 55º and 102° lenses. In some patients, studies with the Optos Tx200 also were performed. FA was performed using 5 mL of 10% sodium fluorescein solution. The following were assessed: the vessel involved (CRVO, HRVO, BRVO or a smaller tributary vein); the presence or absence of ischemia, new vessels on the optic disc (NVD) or new vessels elsewhere (NVE) and veno-venous (VV) anastomoses (collateral circulation); and especially the presence of avascular zones, amputated vessels, and vascular leakage in the far periphery.
All of the procedures included in the present analysis were performed were in accordance with the ethical standards of the Bioethics Committee of the Medical University of Warsaw.
Results
Impending RVO was found in 8.9% (10) of the 112 eyes studied, in 7 women and 3 men. Systemic treatment with low-molecular-weight heparin (LMWH) at a dose of 1 mg/kg was instituted, and during the observation period, the condition did not progress to full-blown thrombosis and eventually resolved. Patients with clinical outcome were not included in the subsequent analysis.
RVO developed in 102 of the 112 eyes, CRVO in 46.1%, BRVO in 40.2%, a small tributary vein (venule) occlusion in 11.8%, and HRVO in 1.9% (Table 1).
In 47 patients with CRVO, FA of the posterior pole detected a non-ischemic occlusion in 76.6% of the eyes and an ischemic occlusion in 21.3%. In 1 patient (2.1%), the initial FA did not reveal ischemia, but avascular zones representing ischemia were noted on a subsequent FA (Table 2).
BRVO was found in 40.2% of the eyes. The superior temporal branch was involved in 58.3% of all patients with BRVO and the inferior temporal branch was involved in 36.6%; in a single patient, the inferior nasal branch was occluded, and in another patient, 2 sites were affected: the inferior temporal branch and the large branch draining the area above the optic disc. Information on the location of occlusion, its type, and the percentage of patients affected is presented in Table 3.
In 11.8% of the eyes (12/102), a venule was occluded, a small tributary of either the superior (75%; 9/12) or inferior (25%; 3/12) temporal branch. In 66.7% of the eyes (8/12), the occlusion was ischemic and in 33.3% (4/12), it was non-ischemic.
HRVO was diagnosed in 2 patients (1.9%), another patient had a non-ischemic superior HRVO, and a third patient had an inferior HRVO (initially non-ischemic, but a subsequent FA confirmed the conversion to ischemia).
Neovascularization of the optic disc (NVD) was found in 32.3% of all the eyes (33/102), 42.5% of the eyes with CRVO (20/47), 29.3% of the eyes with BRVO (12/41), and 50% of the eyes (1/2) with HRVO, but in no case of a small tributary vein (venule) occlusion (Table 4).
Peripheral retinal neovascularization (NVE) was seen in 17.4% of all the eyes (15/102), 14.9% of the eyes with CRVO (7/47), and 19.5% of the eyes with BRVO (8/41) (Table 4).
VV anastomoses (collaterals) were seen in 41.2% of all the eyes (42/102) and in 79.6% of the patients with BRVO (32/41), 56.2% of superior temporal branch occlusions (18/32), 37.5% of inferior temporal branch occlusions (12/32), 3.1% of inferior nasal branch occlusions (1/32), and in the single case of occlusions involving the inferior temporal branch and the branch above the optic disc (3.1%; 1/32). Collaterals were also observed in 100% of the eyes with HRVO (2/2) and 66.7% of the eyes with a venule occlusion (8/12). No veno-venous anastomoses were found in any of the eyes with CRVO (Table 4).
Peripheral retinal ischemia was found in 59.8% of all the eyes (61/102), including 51.1% of the eyes with CRVO (24/47), 82.9% with BRVO (34/41), 50% with HRVO (1/2), and 16.7% of the eyes with a venule occlusion (2/12) (Table 4).
In a number of cases, among the 61 eyes with avascular areas identified by FA in the periphery (20.6% of all eyes, 21/102), there was no ischemia in the posterior pole. This finding was more frequent in CRVO (34.0% of eyes; 16/47) than in BRVO (12.2%; 5/41). In those patients, FA that visualized the posterior pole up to an angle of 30º to 50º initially revealed a non-ischemic occlusion. On further analysis, those cases were classified accordingly. However, the classification should be changed when alterations in the retinal periphery best visualized by WF-FA are taken into consideration. In 4.9% of the 102 eyes, the occlusion at first was non-ischemic, but further FA studies revealed avascular areas in the posterior pole (a single patient each with CRVO, HRVO, and BRVO). In all of those patients, areas of ischemia in the far periphery were observed on the first FA evaluation.
Amputated peripheral vessels associated with avascular zones in the periphery were visualized by WF-FA in 43.1% of all the eyes (44/102).
Increased permeability is evidence of damage to vein walls in the course of thrombosis and the amount of dye leakage is proportional to the degree of vascular injury. Dye diffusion from veins damaged by a thrombus is usually observed in the posterior pole. In our study, in 17.6% of the eyes (18/102), dye leakage was seen exclusively in the far peripheral retina, from the vessels close to the ora serrata, but there was no leakage in the posterior pole. In that group, dye leakage was observed in 90.6% of the eyes (16/18) with an ischemic venous occlusion, a single eye with a non-ischemic venous occlusion, and a single eye with an initially non-ischemic occlusion with a zone of ischemia subsequently identified in the posterior pole.
Optic coherence tomography revealed cystic macular edema in 63.7% of all the eyes, including 76.6% with CRVO, 58.5% with BRVO, 50% with HRVO, and 33.3% of the eyes with a venule occlusion (Table 4).
Choice of therapy was guided by the FA and OCT findings and the degree of visual impairment. Overall, laser retinal photocoagulation (LRP) was performed in 73.5% of cases (80.9% in the eyes with CRVO, 73.2% with BRVO, and 50% with HRVO and venule occlusion [Table 4]). In cases of ischemic occlusion, LRP was done to the whole area of capillary nonperfusion. Pan photocoagulation was performed in all the eyes with CRVO and in a single case, transcorneal cryotherapy also was used.
LRP was applied to the areas of thrombosis in all eyes with ischemic occlusion in the posterior pole (pan photocoagulation); all eyes with areas of ischemia in the retinal periphery but no macular ischemia (peripheral retinal photocoagulation); and in some patients with non-ischemic occlusion as an adjunct treatment for CME. The use of LRP was guided by visual acuity and an unsatisfactory response of the retina to other treatments (macular grid photocoagulation).
Patients with CME confirmed by OCT or FA received standard treatments approved for this indication. An anti-vascular endothelial growth factor (VEGF) agent was used in 36.9% of the eyes (24/65), micropulse laser therapy in 27.7% (18/65), and a dexamethasone intravitreal implant (Ozurdex) in 7.7% of the eyes (5/65). Most patients had combination therapy (anti-VEGF intravitreal injections and micropulse laser treatment), which was frequently repeated, depending on the condition of the retina and its response to treatment.
Of the patients, 43.4% (46/106) were on systemic anticoagulants prescribed by their primary care physicians or cardiologists after consultation with an ophthalmologist. Choice of treatment was guided by a patient’s health status and any comorbidities and included LMWH at therapeutic doses, acenocoumarin (vitamin K antagonist), acetylsalicylic acid, and sulodexide. The systemic diseases reported by patients, some of which were risk factors for thrombosis, were arterial hypertension in 70.8% (75/106); ischemic heart disease in 17.9% (19/106), including a history of myocardial infarction in 11 patients and arrhythmia in 10.4% (11/106); thyroid disorders and diabetes mellitus in 18.9% (20/106); a history of cerebrovascular accident in 10.4% (11/106); renal insufficiency in 8.5% (9/106); cancer in 15.1% (16/106); and venous thromboembolic disease outside the eye in 7.5% (8/106). Confirmed coagulopathies were relatively rare (2.8%; 3/106). Ocular comorbidities included glaucoma in 23.6% of patients (25/106) and single cases of age-related macular degeneration, cataract, high myopia, and retinal detachment. Two patients had optic disc drusen.
Discussion
Populations around the world are aging rapidly, with consequent increases in the prevalence of diseases of civilization and their complications affecting the eye. RVO is the second most common cause of visual impairment from vascular diseases after diabetic retinopathy [5,18]. Proper control of arterial blood pressure, lipid levels, and glycemia, as well as maintaining body weight within normal range and recommended levels of physical activity, may prevent many lifestyle diseases or at least reduce the risk of their development. Importantly, health care professionals caring for patients at risk of thrombosis should instruct them to check their vision regularly. In some cases of ocular fundus thrombosis, when the macular region is not involved, patients are not aware of any deterioration of visual acuity. Prompt diagnosis supported by FA and use of appropriate local and systemic treatments offer a chance to avoid vision-threatening complications.
Age is a risk factor for vascular diseases [5]. In the present series of patients, the mean age was 60 years and only 4 were younger than age 40 years. According to Kolar, RVO is more frequent in men than in women [5], but we did not confirm that in our study, which had a female-to-male ratio of 1.13: 1, similar to observations by Rogers et al. [2].
Apart from the modifiable and non-modifiable risk factors for venous thrombosis listed previously, some local ocular conditions, such as glaucoma, may increase the risk of RVO [5]. More than one-fourth (26.6%) of patients with RVO in the present study were undergoing treatment for glaucoma. Drusen of the optic disc are another ocular pathology that may increase the risk of RVO [19,20], as these deposits often mechanically compress the blood vessels, resulting in occlusion. We found drusen in only 2 patients: a 28-year-old woman and a 60-year-old man (Figure 1).
The ophthalmoscopic appearance of RVO is characteristic, but in diagnostically doubtful cases FA, could be helpful. Despite its drawbacks (the test is invasive and relatively expensive) and the advent of newer diagnostic tools, FA remains the criterion standard for the detection of retinal vascular diseases including RVO. With standard FA, which provides a 30° to 50° view of the retina, avascular zones and areas of ischemia and the presence and activity of pathological vascular proliferations and VV collaterals can be visualized. FA can be used to identify the site of vein occlusion, assess vascular perfusion (blood flow rate), and visualize dye stasis and its leakage through the vessel wall, reflecting the degree of damage to the involved vein. With advances in technology, new digital systems have been introduced, such as a scanning laser ophthalmoscope and wide-field retinal imaging devices like the Optos Tx200 or Spectralis HRA+OCT, which employ a 102° lens that facilitates evaluation of the far retinal periphery. Wide-field imaging of the retinal periphery has considerably enhanced the diagnosis of retinal disorders by enabling visualization of the blood supply in areas that are unreachable with OCT imaging.
In cases of RVO, FA provides crucial information about whether an occlusion is ischemic (Figure 2) or non-ischemic (Figure 3), guiding future decisions about choice of therapy and frequency of follow-up visits. Until recently, FA was the only test available for differentiating between ischemic and non-ischemic occlusion. OCTA, which was introduced in 2014, visualizes areas of retinal ischemia and is faster and less expensive than FA. More importantly, it is noninvasive, making OCTA safer for patients, but it can only be used to visualize the posterior pole [11].
From our observations, peripheral ischemia was found in as many as 59.8% of the eyes with RVO, but in a fifth of those (20.6%), there was no ischemia in the posterior pole. Avascular zones were found in the periphery alone and were best visualized by WF-FA. When peripheral ischemia was revealed, a change in therapy was required and when laser photocoagulation (LPR) was to be performed, another therapeutic target area for photocoagulation was selected. In 43.1% of the eyes, amputated peripheral vessels were seen coexisting with avascular zones, visualized solely on the retinal periphery angiograms (Figures 1C, 4). These findings confirm the importance of thoroughly evaluating the retinal periphery, which also has been emphasized by several authors [15,17].
In 4.9% of all the eyes, non-ischemic RVO was initially visualized in the posterior pole and it subsequently converted to the ischemic type. According to the Central Vein Occlusion Study Group, 15% of eyes with perfusion convert to ischemia in the first 4 months of follow-up and an additional 19% of eyes in the next 32 months (a total of 34% after 3 years [21]). These findings differ from our observations, which is likely to be due to the study design – a retrospective observational study in the present case
In the present study, nonperfusion of the retinal periphery had already been seen on initial WF-FA in all the patients with a non-ischemic occlusion that converted to ischemia. This finding could strongly suggest that the presence of peripheral avascular zones increases the risk of development of ischemia in the posterior pole. Accordingly, in eyes with areas of nonperfusion in the peripheral retina, follow-up should be more frequent and more aggressive treatment should be instituted.
FA also visualizes vascular proliferation. Pathological new vessels are localized mostly in the posterior pole, on the optic disc, and at large vascular arches (Figure 5). In the eyes with CRVO and HRVO, the incidence of new vessels on the disc (NVD) was equal, meaning that a large area of the retina was involved. The presence of neovascularization in the anterior chamber angle strongly predicts the development of secondary neovascular glaucoma, which we observed in 3 eyes with CRVO. According to the literature, neovascular glaucoma can develop in 23% to 60% of eyes with CRVO within 12 to 15 months [22]. FA is the only test that can be used to assess neovascular activity by visualizing dye leakage through the damaged vessel wall. A larger leak reflects enhanced vascular permeability, which in turn is an indicator of neovascular activity. In some patients in the present study who had NVD/NVE on ophthalmoscopy, FA did not reveal any dye leakage, that is, neovascular activity on the disc or elsewhere. In such cases, LRP was not performed, but those patients remained under observation.
FA has a very important role in localizing VV anastomoses (Figure 6). Collaterals were present in the eyes with BRVO, but not in the eyes with CRVO; the involved vessels were mostly in the upper and lower temporal branches (58.3% and 37.3%) and the nasal branches were rarely affected. The anatomical course of VV collaterals and dye leakage through their walls are evaluated on FA. In some patients with collaterals, FA does not reveal any dye diffusion through the collateral wall, that is, the vessel wall is normal, which indicates normal development of protective mechanisms that prevent retinal ischemia and is a favorable prognostic factor [23].
FA is a dynamic study. A continuous sequence of images is captured with it and particular phases of the study are assessed. When a vein trunk or branch is occluded, the rate of blood flow through the involved vein, or perfusion, is assessed and hence, the degree of the vein damage. Occlusion is also demonstrated by prolonged dye retention until the late phases of the angiogram, when leakage through the damaged wall is visible (Figure 7).
FA is the only study that can be used to visualize dye leakage and leaks that are solely seen in the retinal periphery can be demonstrated on WF-FA (Figure 8). In the patients in the present study, 17.6% of WF-FAs detected dye leaks in the far periphery alone, but not in the posterior pole. These changes were seen in 90.6% of the eyes with a non-ischemic occlusion, which we think suggests the beginning of conversion of a non-ischemic occlusion to an ischemic type.
CME is the most common complication of RVO, which results in deterioration of visual acuity. According to the literature, CME may develop in 73% of eyes with ischemic CRVO and in 30% of eyes with BRVO and non-ischemic CRVO [5,22]. It was diagnosed in 63.7% of the eyes in the present study. OCT is the imaging study of choice for assessing and monitoring CME (Figure 9) [10], but FA is considered a valuable adjunct.
Imaging findings, especially FA of the periphery and OCT, guide decisions on the choice of therapy. Currently, intravitreal anti-VEGF agents are first-line therapy for RVO, especially when CME is present [10]. Intravitreal corticosteroids and micropulse laser treatment are used less frequently [24]. However, LPR remains the most common treatment because it is effective, accessible, and affordable [25].
FA, especially the wide-field modality, is used to confirm whether LPR should be used. LPR is indicated mainly for ischemic occlusion, especially of the vein trunk, as there is a risk of neovascularization in the anterior chamber angle, which results in development of neovascular glaucoma. LPR is also performed in cases of non-ischemic occlusion, with neovascular activity on the disc and elsewhere. LPR can be used as an adjunct treatment for CME and visual acuity of 0.1 to 0.5, preferably in combination with an anti-VEGF agent [26]. WF-FA is performed to identify therapeutic target areas for photocoagulation. In patients in the present study, LPR was performed on 80.9% of the eyes with CRVO, 73.2% with BRVO, 50% with HRVO, and in a single case of venule occlusion. Figure 10 shows an ischemic branch occlusion before (Figure 10A) and after (Figure 10B) LPR treatment. Most occlusive lesions are found in the posterior pole, but before any decisions concerning their management are made, it is necessary to evaluate the periphery. The present authors, like Nagiel et al., believe that peripheral retinal angiography should be the standard-of-care imaging modality for retinal vascular disease. Visualization of the avascular peripheral areas and amputated vessels guides the choice of appropriate management and identifies therapeutic target areas for LPR [16].
Conclusions
In RVO, decisions concerning management, choice of treatment modality, and the extent of therapy should be made individually after an ophthalmic examination, including a visual acuity test and ophthalmic imaging. FA is the most important of these studies, especially its wide-field modality, which enables evaluation of the peripheral retina. We observed that after visualization and evaluation of peripheral vascular changes, another therapy was instituted in 20.6% of patients. Also, vascular changes suggestive of conversion of a non-ischemic occlusion to the ischemic type are first seen in the periphery and require more frequent follow-up visits and more aggressive treatment. Evaluation of the peripheral retina should be recognized as the standard of care for diagnosing and monitoring retinal vascular disease including RVO.
Figures
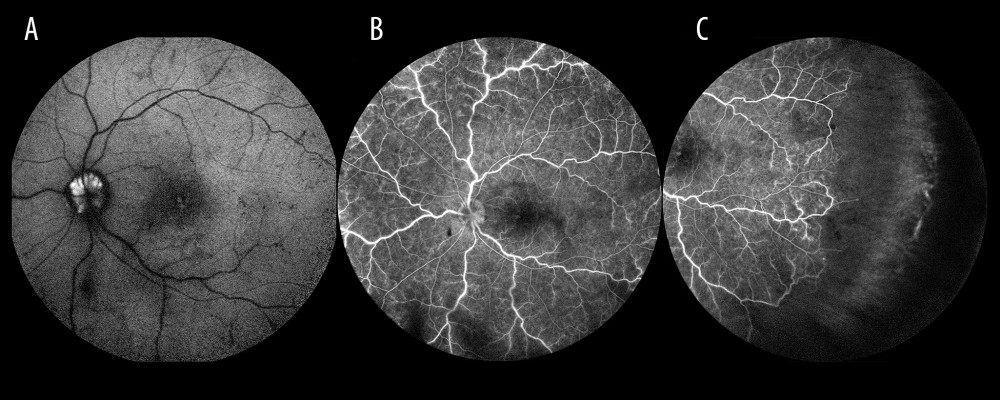 Figure 1. Optic disc drusen. (A) Autofluorescence from drusen on the optic disc. (B) Occlusion of the vein trunk without features of ischemia in the posterior pole (the same eye). (C) Extensive avascular zones in the peripheral temporal retina (the same eye).
Figure 1. Optic disc drusen. (A) Autofluorescence from drusen on the optic disc. (B) Occlusion of the vein trunk without features of ischemia in the posterior pole (the same eye). (C) Extensive avascular zones in the peripheral temporal retina (the same eye). 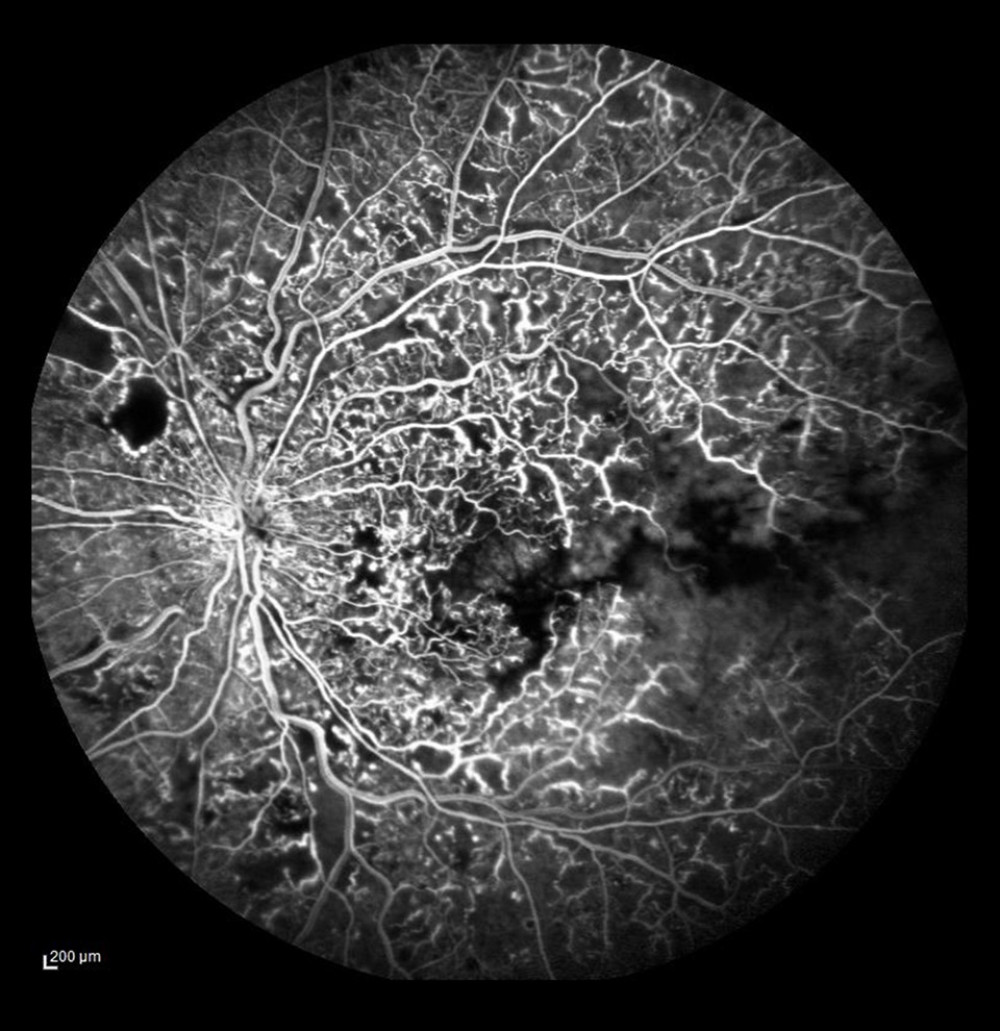 Figure 2. Ischemic occlusion with avascular zones in the posterior pole.
Figure 2. Ischemic occlusion with avascular zones in the posterior pole. 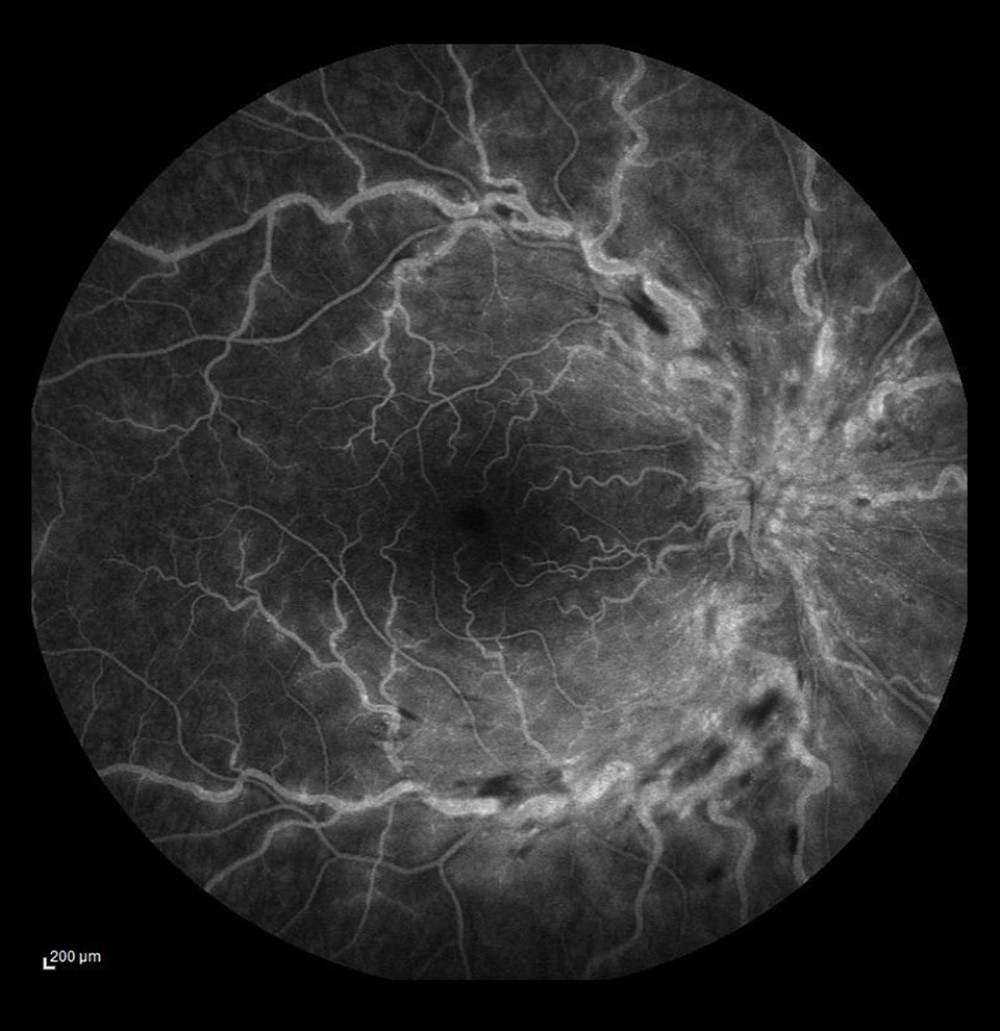 Figure 3. Non-ischemic occlusion. Dye leakage from the damaged veins and on the optic disc, but no avascular zones visualized in the posterior pole.
Figure 3. Non-ischemic occlusion. Dye leakage from the damaged veins and on the optic disc, but no avascular zones visualized in the posterior pole. 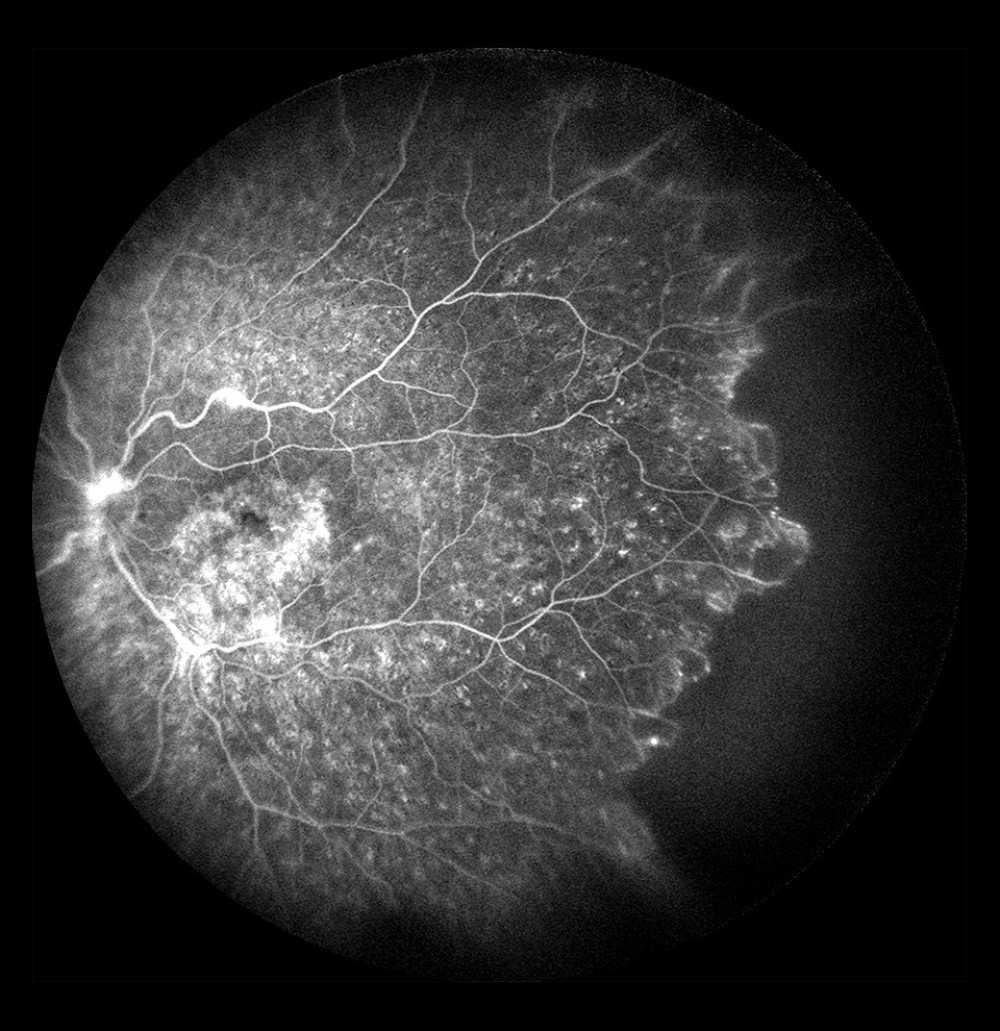 Figure 4. Non-ischemic occlusion in the posterior pole with extensive avascular zones and amputated vessels in the peripheral temporal retina. Dye leakage on the optic disc and cystoid macular edema (after laser photocoagulation).
Figure 4. Non-ischemic occlusion in the posterior pole with extensive avascular zones and amputated vessels in the peripheral temporal retina. Dye leakage on the optic disc and cystoid macular edema (after laser photocoagulation). 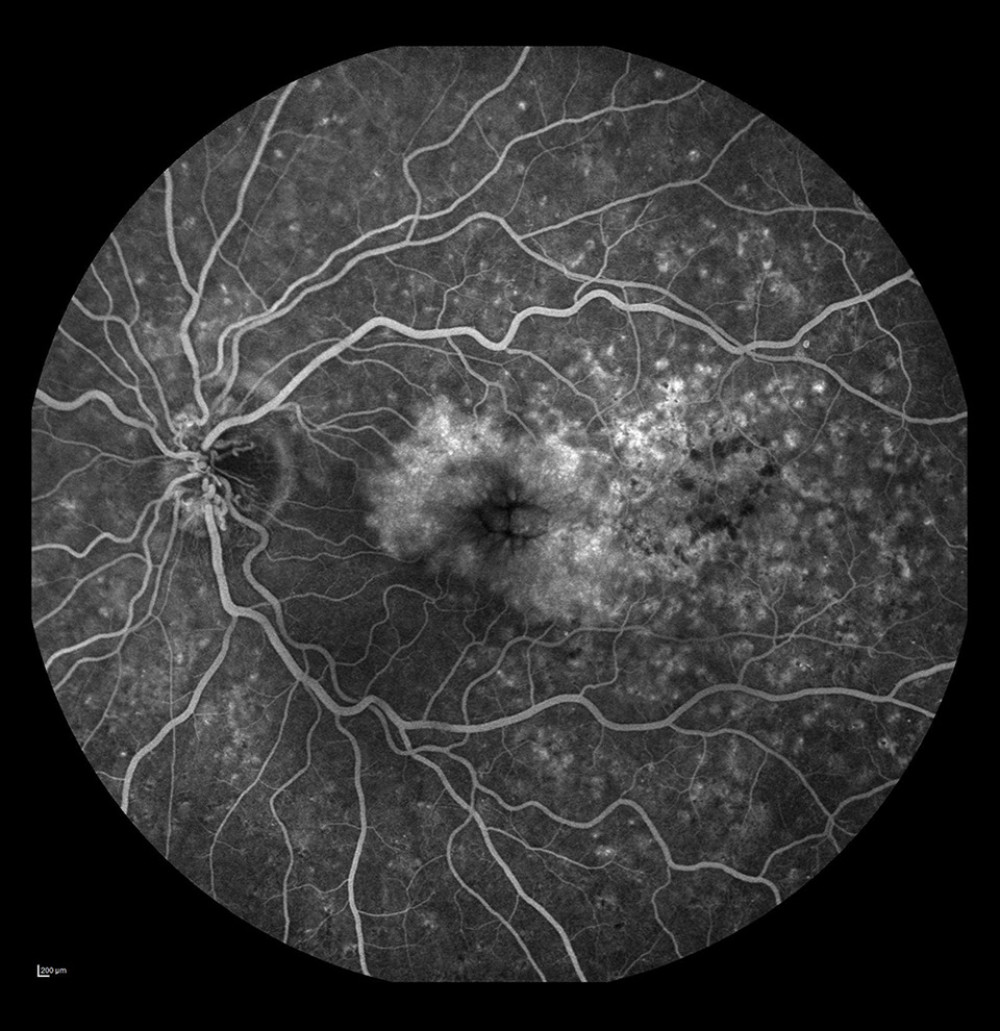 Figure 5. Occlusion of the vein trunk with new vessels on the disc and cystoid macular edema (after laser photocoagulation).
Figure 5. Occlusion of the vein trunk with new vessels on the disc and cystoid macular edema (after laser photocoagulation). 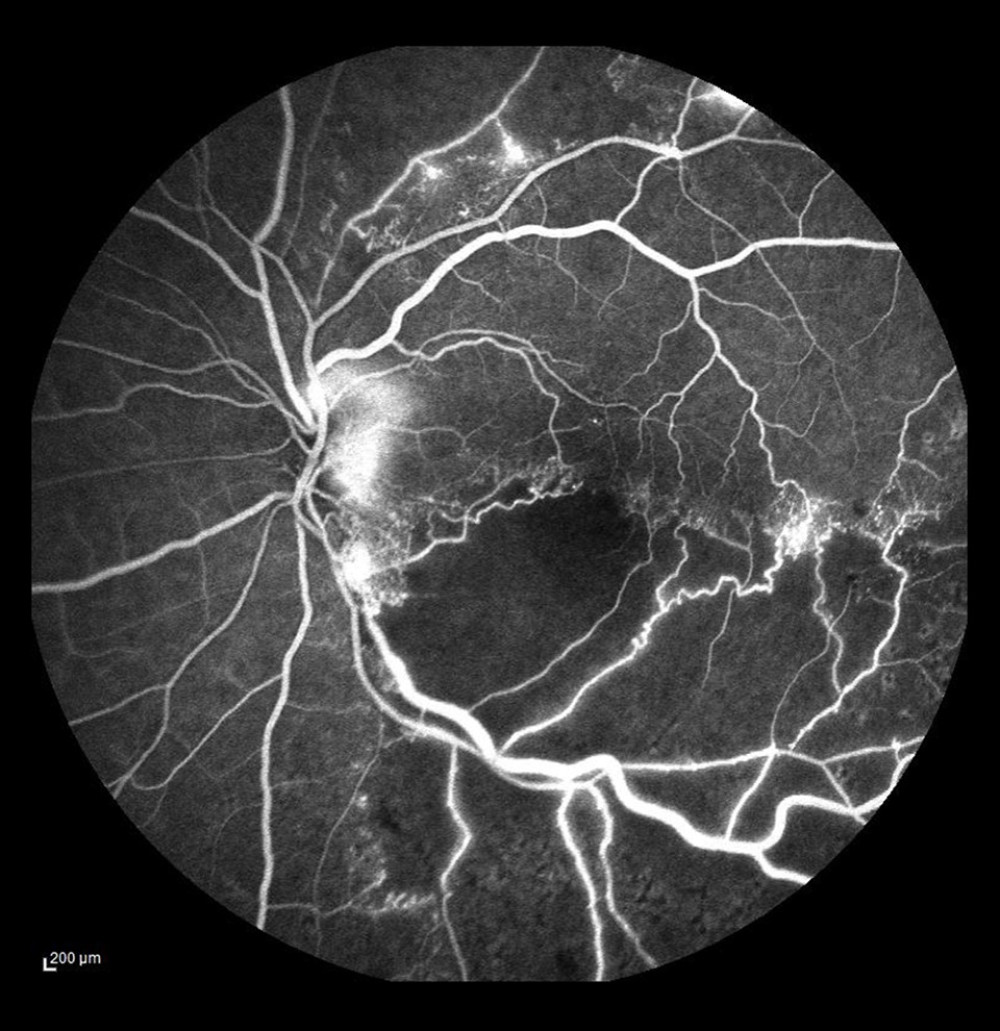 Figure 6. Ischemic occlusion of the inferior temporal bone with the development of collateral circulation. Veno-venous anastomoses are visible on the nasal and temporal sides of the macula.
Figure 6. Ischemic occlusion of the inferior temporal bone with the development of collateral circulation. Veno-venous anastomoses are visible on the nasal and temporal sides of the macula. 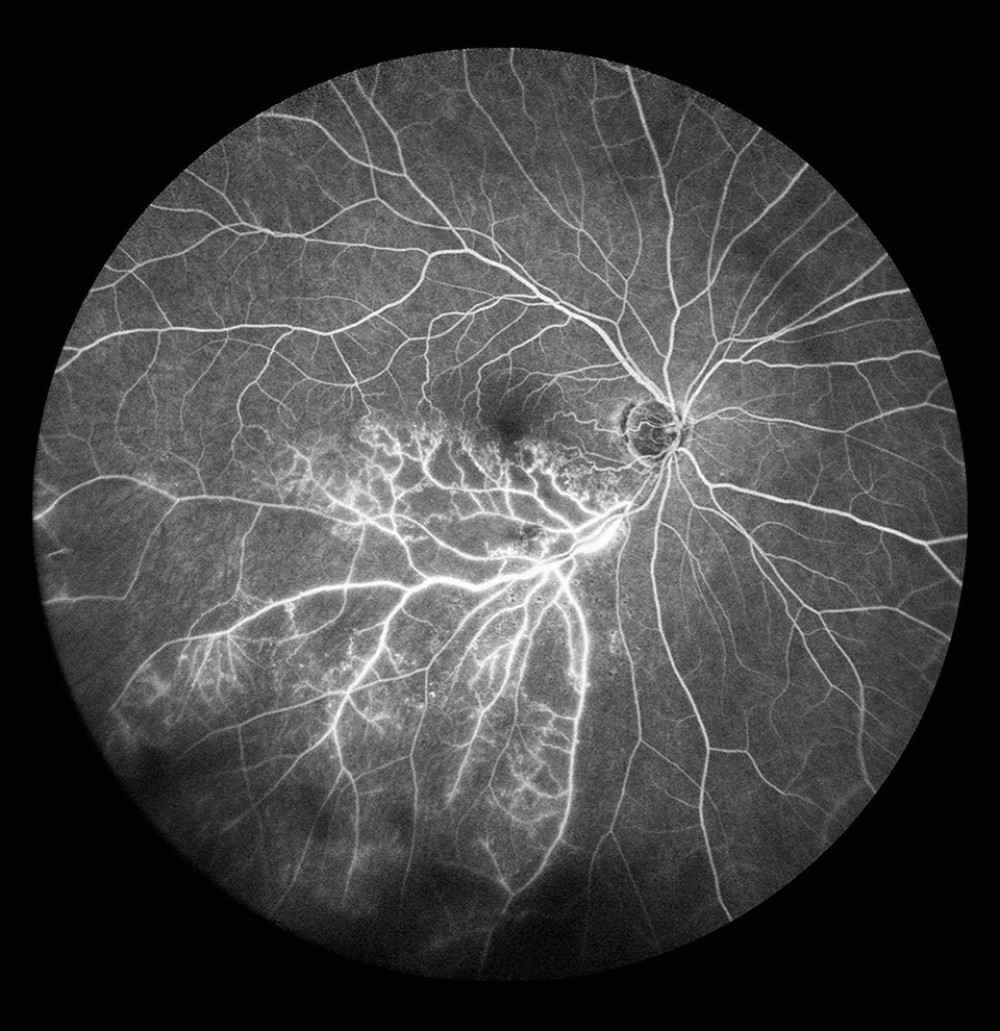 Figure 7. Occlusion of the inferior temporal branch with dye leakage in the tributaries of the entire occluded vein.
Figure 7. Occlusion of the inferior temporal branch with dye leakage in the tributaries of the entire occluded vein. 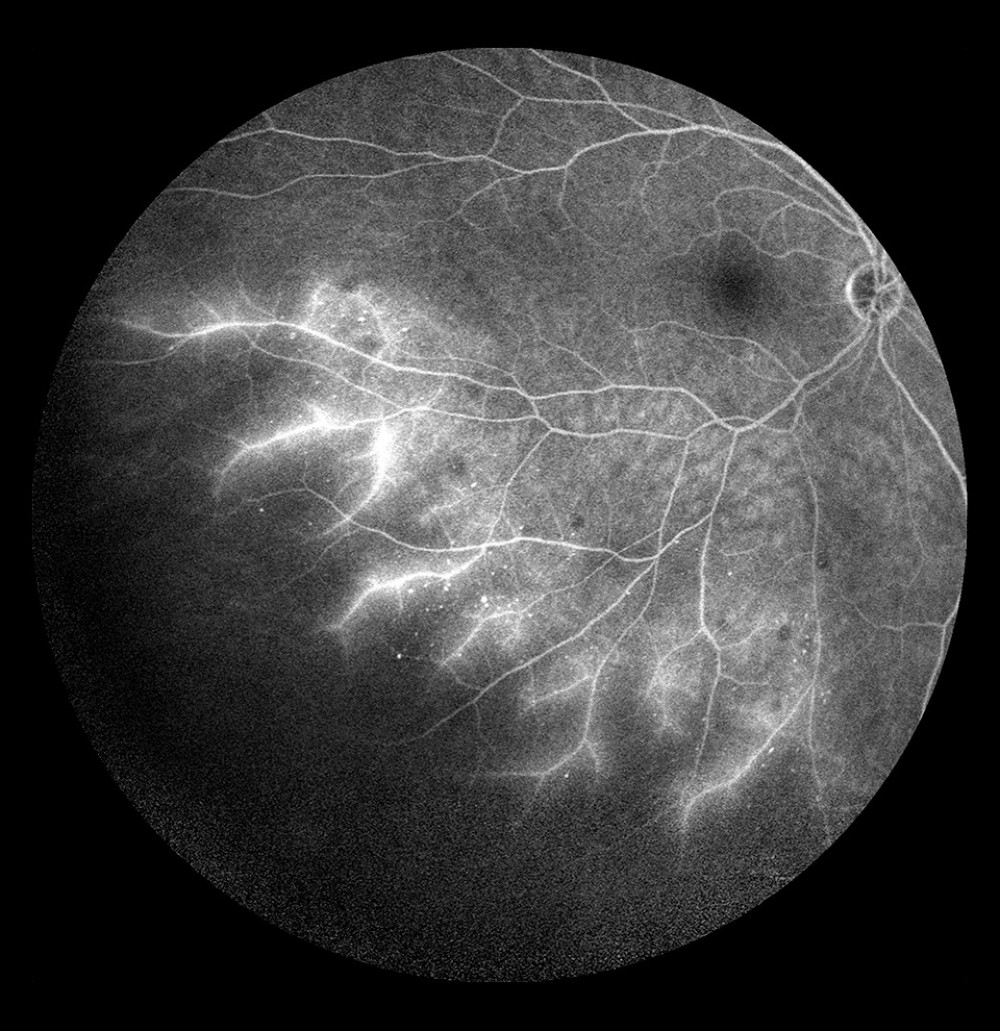 Figure 8. Occlusion of the inferior temporal branch with dye leakage in the peripheral retina.
Figure 8. Occlusion of the inferior temporal branch with dye leakage in the peripheral retina. 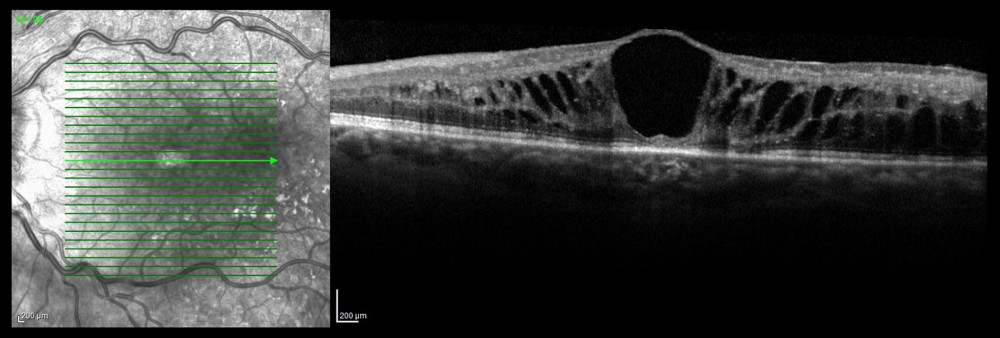 Figure 9. Optical coherence tomography scan of cystoid macular edema in the course of non-ischemic trunk occlusion.
Figure 9. Optical coherence tomography scan of cystoid macular edema in the course of non-ischemic trunk occlusion. 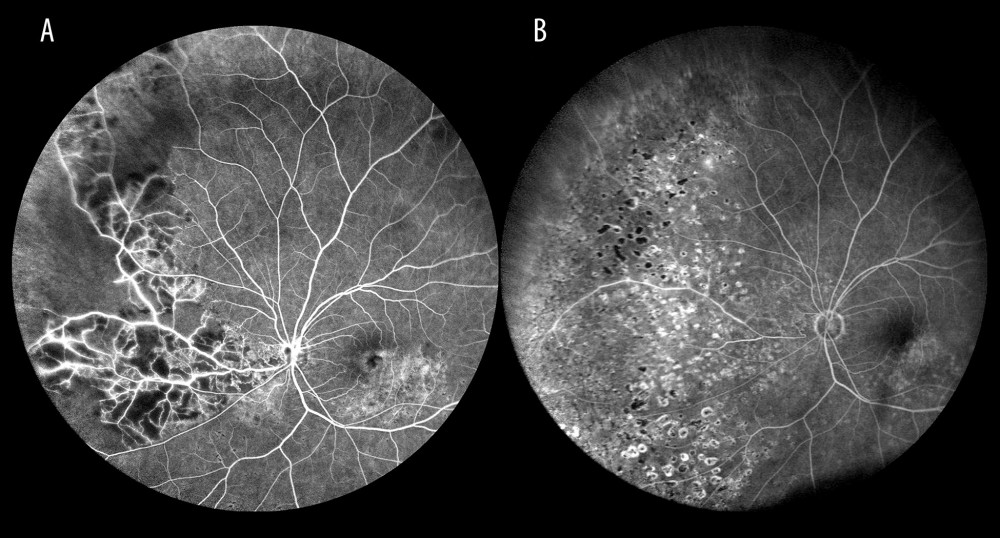 Figure 10. Ischemic occlusion of the inferior nasal branch. (A) Before laser photocoagulation. (B) After laser photocoagulation, the impact of photocoagulation is seen in the far retinal periphery.
Figure 10. Ischemic occlusion of the inferior nasal branch. (A) Before laser photocoagulation. (B) After laser photocoagulation, the impact of photocoagulation is seen in the far retinal periphery. Tables
Table 1. Number and percentage of patients with retinal vein occlusion in a specific location (vein trunk, vein branch, small tributary vein (venule), hemisphere).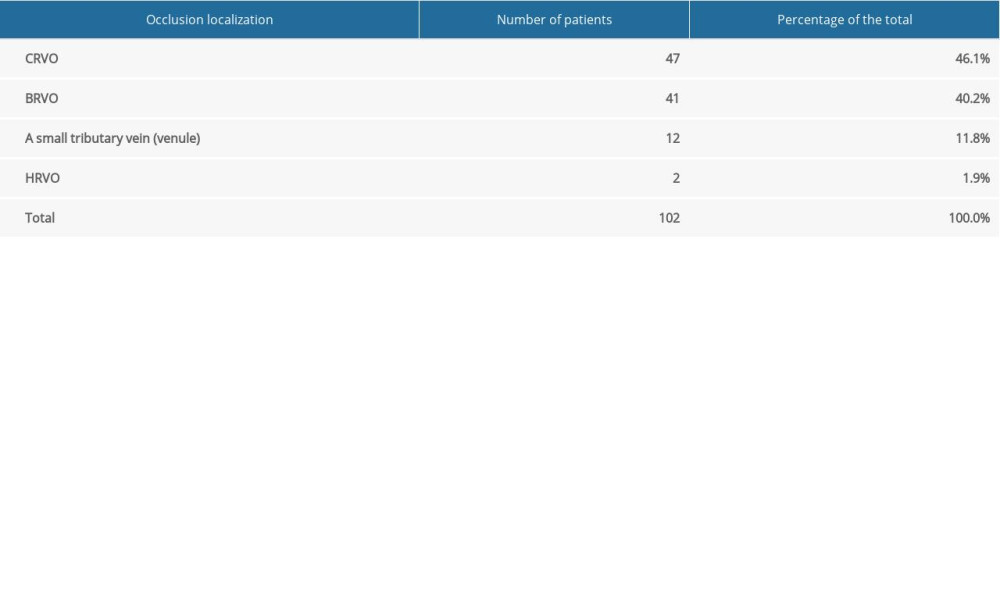 Table 2. Number and percentage of patients with ischemic and non-ischemic central retinal vein occlusion.
Table 2. Number and percentage of patients with ischemic and non-ischemic central retinal vein occlusion.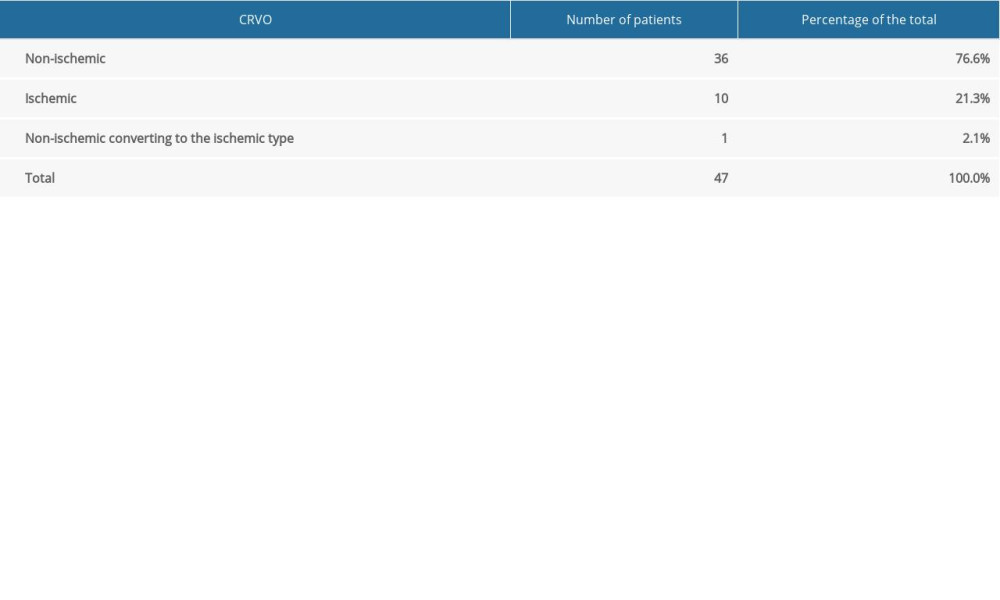 Table 3. Branch retinal vein occlusion. Involvement of specific retinal vein branches, and number and percentage of patients with ischemic and non-ischemic occlusions.
Table 3. Branch retinal vein occlusion. Involvement of specific retinal vein branches, and number and percentage of patients with ischemic and non-ischemic occlusions.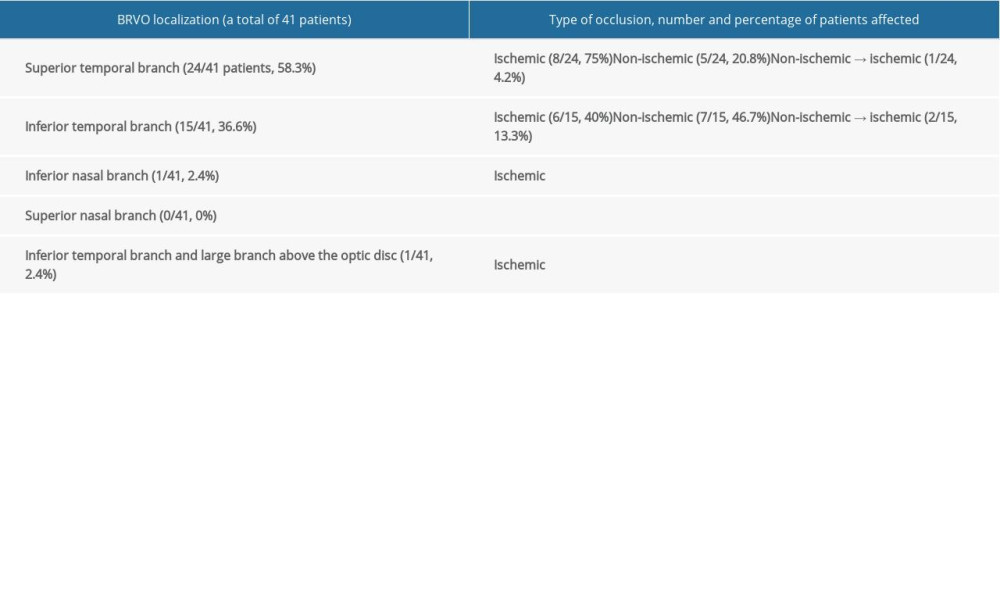 Table 4. Number and percentage of patients with specific fundal pathologies identified by fluorescein angiography and optical coherence tomography, and the use of laser retinal photocoagulation (LRP) in central retinal vein, branch retinal, hemispheric retinal vein, and venule occlusion.
Table 4. Number and percentage of patients with specific fundal pathologies identified by fluorescein angiography and optical coherence tomography, and the use of laser retinal photocoagulation (LRP) in central retinal vein, branch retinal, hemispheric retinal vein, and venule occlusion.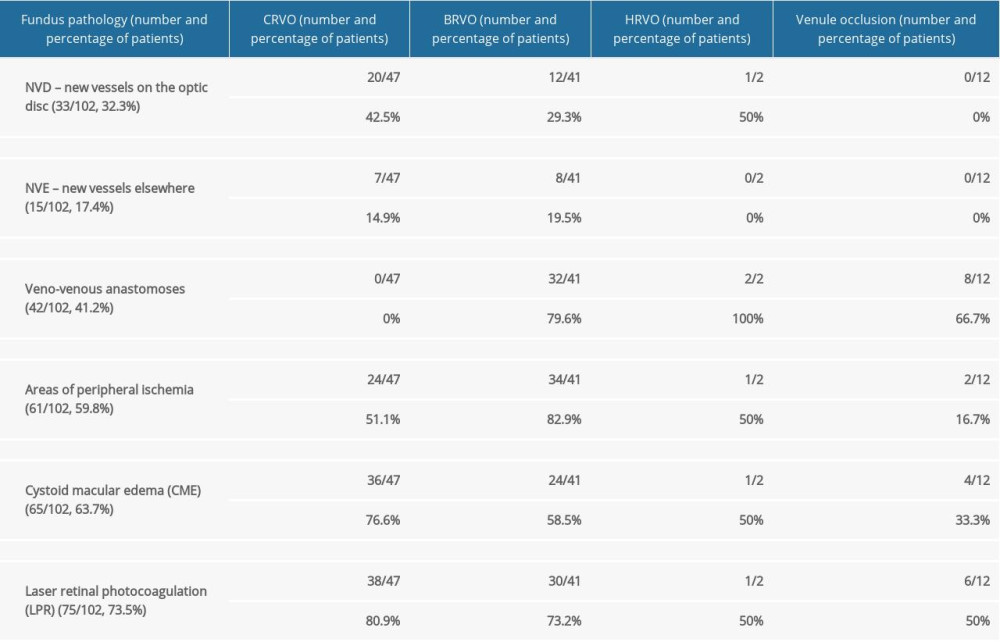
References
1. Pierru A, Girmens JF, Héron E, Retinal vein occlusion: J Fr Ophtalmol, 2017; 40(8); 696-705
2. Rogers S, McIntosh RL, Cheung NInternational Eye Disease Consortium, The prevalence of retinal vein occlusion: Pooled data from population studies from the United States, Europe, Asia, and Australia: Ophthalmology, 2010; 117; 313-19
3. Klein R, Moss SE, Meuer SM, The 15-year cumulative incidence of retinal vein occlusion: The Beaver Dam Eye Study: Arch Ophthalmol, 2008; 126(4); 513-18
4. Song P, Xu Y, Zha M, Global epidemiology of retinal vein occlusion: A systematic review and meta-analysis of prevalence, incidence, and risk factors: J Glob Health, 2019; 9(1); 010247
5. Kolar P, Risk factors for central and branch retinal vein occlusion: A meta-analysis of published clinical data: Ophthalmol, 2014; 2014 724780
6. Wong T, Mitchell P, The eye in hypertension: Lancet, 2007; 369(9559); 425-35
7. Wang Y, Wu S, Wen F, Diabetes mellitus as a risk factor for retinal vein occlusion: A meta-analysis: Medicine (Baltimore), 2020; 99(9); e19319
8. Cho BJ, Bae SH, Park SM, Comparison of systemic conditions at diagnosis between central retinal vein occlusion and branch retinal vein occlusion: PLoS One, 2019; 14(8); e0220880
9. Ghoghari H, Rizvi SF, Loya H, Axial length, a risk factor for retinal vein occlusion: A case control study: J Pak Med Assoc, 2019; 69(12); 1800-2
10. Schmidt-Erfurth U, Garcia-Arumi J, Gerendas BS, Guidelines for the Management of Retinal Vein Occlusion by the European Society of Retina Specialists (EURETINA): Ophthalmologica, 2019; 242(3); 123-62
11. Ang M, Tan ACS, Cheung CMG, Optical coherence tomography angiography: A review of current and future clinical applications: Graefes Arch Clin Exp Ophthalmol, 2018; 256(2); 237-45
12. Shiraki A, Sakimoto S, Tsuboi K, Evaluation of retinal nonperfusion in branch retinal vein occlusion using wide field optical coherence tomography angiography: Acta Ophthalmologica, 2019; 97(6); e913-18
13. Kimura M, Nozaki M, Yoshida M, Wide-field optical coherence tomography angiography using extended field imaging technique to evaluate the nonperfusion area in retinal vein occlusion: Clin Ophthalmol, 2016; 10; 1291-95
14. Novotny HR, Alvis DL, A method of photographing fluorescence in circulating blood of the human retina: Circulation, 1961; 24; 82-86
15. Spaide RF, Peripheral areas of nonperfusion in treated central retinal vein occlusion as imaged by wide-field fluorescein angiography: Retina, 2011; 31; 829-37
16. Nagiel A, Lalane RA, Sadda SR, Ultra-widefield fundus imaging. A review of clinical applications and future trends: Retina, 2016; 36(4); 660-78
17. Tan CS, Li KZ, Sadda SR, Wide-field angiography in retinal vein occlusions: Int J Retin Vitr, 2019; 5(Suppl 1); 18
18. La Spina C, De Benedetto U, Battaglia Parodi M, Practical management of retinal vein occlusions: Ophthalmol Ther, 2012; 1(1); 3
19. Padhy SK, Behera UC, Optic disc drusen precipitating central retinal vein occlusion in young: BMJ Case Rep, 2019; 12(7); e230677
20. Auw-Haedrich C, Staubach F, Witschel H, Optic disk drusen: Survey Ophthalmol, 2002; 47(6); 515-32
21. The Central Vein Occlusion Study Group, Natural history and clinical management of central retinal vein occlusion: Arch Ophthalmol, 1997; 115(4); 486-91
22. McIntosh RL, Rogers SL, Lim L, Natural history of central retinal vein occlusion: An evidence-based systematic review: Ophthalmology, 2010; 117(6); 1113-23.e15
23. Tsuboi K, Sasajima H, Kamei M, Collateral vessels in branch retinal vein occlusion: Anatomic and functional analyses by optical coherence tomography angiography: Ophthalmol Retina, 2019; 3; 767-76
24. Gawęcki M, Micropulse laser treatment of retinal diseases: J Clin Med, 2019; 8(2); 242
25. Zhao MW, Miao HWhole course management is the key to retinal vein occlusion: Zhonghua Yan Ke Za Zhi, 2020; 56(4); 246-49 [in Chinese]
26. Rehak M, Wiedemann P, Retinal vein thrombosis: Pathogenesis and management: J Thromb Haemost, 2010; 8(9); 1886-94
Figures
 Figure 1. Optic disc drusen. (A) Autofluorescence from drusen on the optic disc. (B) Occlusion of the vein trunk without features of ischemia in the posterior pole (the same eye). (C) Extensive avascular zones in the peripheral temporal retina (the same eye).
Figure 1. Optic disc drusen. (A) Autofluorescence from drusen on the optic disc. (B) Occlusion of the vein trunk without features of ischemia in the posterior pole (the same eye). (C) Extensive avascular zones in the peripheral temporal retina (the same eye). Figure 2. Ischemic occlusion with avascular zones in the posterior pole.
Figure 2. Ischemic occlusion with avascular zones in the posterior pole. Figure 3. Non-ischemic occlusion. Dye leakage from the damaged veins and on the optic disc, but no avascular zones visualized in the posterior pole.
Figure 3. Non-ischemic occlusion. Dye leakage from the damaged veins and on the optic disc, but no avascular zones visualized in the posterior pole. Figure 4. Non-ischemic occlusion in the posterior pole with extensive avascular zones and amputated vessels in the peripheral temporal retina. Dye leakage on the optic disc and cystoid macular edema (after laser photocoagulation).
Figure 4. Non-ischemic occlusion in the posterior pole with extensive avascular zones and amputated vessels in the peripheral temporal retina. Dye leakage on the optic disc and cystoid macular edema (after laser photocoagulation). Figure 5. Occlusion of the vein trunk with new vessels on the disc and cystoid macular edema (after laser photocoagulation).
Figure 5. Occlusion of the vein trunk with new vessels on the disc and cystoid macular edema (after laser photocoagulation). Figure 6. Ischemic occlusion of the inferior temporal bone with the development of collateral circulation. Veno-venous anastomoses are visible on the nasal and temporal sides of the macula.
Figure 6. Ischemic occlusion of the inferior temporal bone with the development of collateral circulation. Veno-venous anastomoses are visible on the nasal and temporal sides of the macula. Figure 7. Occlusion of the inferior temporal branch with dye leakage in the tributaries of the entire occluded vein.
Figure 7. Occlusion of the inferior temporal branch with dye leakage in the tributaries of the entire occluded vein. Figure 8. Occlusion of the inferior temporal branch with dye leakage in the peripheral retina.
Figure 8. Occlusion of the inferior temporal branch with dye leakage in the peripheral retina. Figure 9. Optical coherence tomography scan of cystoid macular edema in the course of non-ischemic trunk occlusion.
Figure 9. Optical coherence tomography scan of cystoid macular edema in the course of non-ischemic trunk occlusion. Figure 10. Ischemic occlusion of the inferior nasal branch. (A) Before laser photocoagulation. (B) After laser photocoagulation, the impact of photocoagulation is seen in the far retinal periphery.
Figure 10. Ischemic occlusion of the inferior nasal branch. (A) Before laser photocoagulation. (B) After laser photocoagulation, the impact of photocoagulation is seen in the far retinal periphery. Tables
 Table 1. Number and percentage of patients with retinal vein occlusion in a specific location (vein trunk, vein branch, small tributary vein (venule), hemisphere).
Table 1. Number and percentage of patients with retinal vein occlusion in a specific location (vein trunk, vein branch, small tributary vein (venule), hemisphere). Table 2. Number and percentage of patients with ischemic and non-ischemic central retinal vein occlusion.
Table 2. Number and percentage of patients with ischemic and non-ischemic central retinal vein occlusion. Table 3. Branch retinal vein occlusion. Involvement of specific retinal vein branches, and number and percentage of patients with ischemic and non-ischemic occlusions.
Table 3. Branch retinal vein occlusion. Involvement of specific retinal vein branches, and number and percentage of patients with ischemic and non-ischemic occlusions. Table 4. Number and percentage of patients with specific fundal pathologies identified by fluorescein angiography and optical coherence tomography, and the use of laser retinal photocoagulation (LRP) in central retinal vein, branch retinal, hemispheric retinal vein, and venule occlusion.
Table 4. Number and percentage of patients with specific fundal pathologies identified by fluorescein angiography and optical coherence tomography, and the use of laser retinal photocoagulation (LRP) in central retinal vein, branch retinal, hemispheric retinal vein, and venule occlusion. Table 1. Number and percentage of patients with retinal vein occlusion in a specific location (vein trunk, vein branch, small tributary vein (venule), hemisphere).
Table 1. Number and percentage of patients with retinal vein occlusion in a specific location (vein trunk, vein branch, small tributary vein (venule), hemisphere). Table 2. Number and percentage of patients with ischemic and non-ischemic central retinal vein occlusion.
Table 2. Number and percentage of patients with ischemic and non-ischemic central retinal vein occlusion. Table 3. Branch retinal vein occlusion. Involvement of specific retinal vein branches, and number and percentage of patients with ischemic and non-ischemic occlusions.
Table 3. Branch retinal vein occlusion. Involvement of specific retinal vein branches, and number and percentage of patients with ischemic and non-ischemic occlusions. Table 4. Number and percentage of patients with specific fundal pathologies identified by fluorescein angiography and optical coherence tomography, and the use of laser retinal photocoagulation (LRP) in central retinal vein, branch retinal, hemispheric retinal vein, and venule occlusion.
Table 4. Number and percentage of patients with specific fundal pathologies identified by fluorescein angiography and optical coherence tomography, and the use of laser retinal photocoagulation (LRP) in central retinal vein, branch retinal, hemispheric retinal vein, and venule occlusion. In Press
06 Mar 2024 : Clinical Research
Comparison of Outcomes between Single-Level and Double-Level Corpectomy in Thoracolumbar Reconstruction: A ...Med Sci Monit In Press; DOI: 10.12659/MSM.943797
21 Mar 2024 : Meta-Analysis
Economic Evaluation of COVID-19 Screening Tests and Surveillance Strategies in Low-Income, Middle-Income, a...Med Sci Monit In Press; DOI: 10.12659/MSM.943863
10 Apr 2024 : Clinical Research
Predicting Acute Cardiovascular Complications in COVID-19: Insights from a Specialized Cardiac Referral Dep...Med Sci Monit In Press; DOI: 10.12659/MSM.942612
06 Mar 2024 : Clinical Research
Enhanced Surgical Outcomes of Popliteal Cyst Excision: A Retrospective Study Comparing Arthroscopic Debride...Med Sci Monit In Press; DOI: 10.12659/MSM.941102
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








