12 January 2021: Clinical Research
Lumbar Sympathetic Nerve Modulation Using Absolute Ethanol for the Treatment of Primary Lower-Extremity Hyperhidrosis: A Dose-Effect Pilot Study
Mingjuan Liu1BCE, Huadong Ni1BCE, Jiachun Tao1BC, Keyue Xie1AE*DOI: 10.12659/MSM.928209
Med Sci Monit 2021; 27:e928209
Abstract
BACKGROUND: Primary lower-extremity hyperhidrosis (PLEH) can be treated by CT-guided lumbar sympathetic nerve modulation using absolute ethanol. However, doses of ethanol that are too high can cause nerve injury, and doses that are too low have suboptimal results. The present study aimed to investigate the dose-effect relationship of CT-guided lumbar sympathetic nerve modulation with absolute ethanol for PLEH.
MATERIAL AND METHODS: The study was conducted at the First Affiliated Hospital of Jiaxing University between 07/2014 and 02/2017. Twenty participants were enrolled in each group. The doses of absolute ethanol were 2.0 ml in the R₁ group, 2.5 ml in the R₂ group, 3.0 ml in the R₃ group, 3.5 ml in the R₄ group, and 4.0 ml in the R₅ group. Treatment effectiveness was assessed according to the time to complete hyperhidrosis relief: <10 min, effective; ≥10 min, non-effective.
RESULTS: The patient characteristics among the 5 groups were not statistically different (P>0.05). The onset time and time to complete hyperhidrosis relief decreased significantly with increasing dose of absolute ethanol (P<0.05). The effective rates in the 5 groups were 15.0%, 35.0%, 60.0%, 90.0%, and 100.0%, respectively. The ED₅₀ and ED₉₅ were 2.306 ml (95% CI: 2.003–2.512 ml) and 3.343 ml (95% CI: 3.051–3.962 ml), respectively.
CONCLUSIONS: This was the first dose-effect pilot study of consecutive PLEH patients treated by CT-guided lumbar sympathetic nerve modulation. CT-guided lumbar sympathetic nerve modulation with 2.306 ml (ED₅₀) and 3.343 ml (ED₉₅) of absolute ethanol showed treatment efficacy for PLEH. No complications were seen.
Keywords: Autonomic Nerve Block, Ethanol, hyperhidrosis, Dose-Response Relationship, Drug, Lower Extremity, Lumbosacral Plexus, Tomography, X-Ray Computed
Background
Hyperhidrosis is a benign skin condition of overproduction of sweat beyond the required thermoregulatory response. It may be focal or generalized and could be idiopathic (primary) or secondary to a variety of causes [1–4]. Hyperhidrosis affects 1–3% of individuals in the United States, but only about 50% of these individuals consult a physician [3,5]. Generalized hyperhidrosis is often caused by systemic diseases (e.g., autonomic dysreflexia, cerebrovascular accident, Parkinson’s disease, lymphoma, carcinoid, pheochromocytoma, hyperthyroidism, acromegaly, tuberculosis, or heart failure), medications (e.g., antidepressants or opiates) or toxins (alcohol) [1–4]. Focal hyperhidrosis affects discrete sites such as the palms, soles, axillae, and head, and is caused by overactivity of the sudomotor system (cholinergic innervation of the parasympathetic nervous system prominent in eccrine sweat glands) [1–4]. It is often idiopathic and triggered by emotion. Hyperhidrosis severely influences emotions, socialization, and quality of life [6,7]. Primary lower-extremity hyperhidrosis (PLEH) refers to spontaneous sweating of the lower extremities.
Beyond lifestyle counseling [4], treatments for PLEH include topical aluminum chloride hexahydrate [1,3,4], topical anticholinergics [3], botulinum toxin type A injection [8], tap water iontophoresis, oral anticholinergics [3,4], and lumbar sympathetic nerve modulation [3,4].
In recent years, the innovation team of the Pain Management Department of our hospital developed computed tomography (CT)-guided lumbar sympathetic nerve modulation with absolute ethanol to treat PLEH, which achieved good efficacy and improved the satisfaction degree of the patients [9–11]. Using high-dose absolute ethanol for lumbar sympathetic nerve modulation could lead to permanent injuries of the sciatic nerve, as well as severe complications such as spinal injuries if ethanol enters the spinal canal. On the other hand, if the dose is too low, the treatment efficacy could be suboptimal.
Therefore, this study aimed to investigate the dose-effect relationship of CT-guided lumbar sympathetic nerve modulation with absolute ethanol in treating PLEH, and to provide evidence for the appropriate selection of absolute ethanol dose in such patients.
Material and Methods
STUDY DESIGN AND PATIENTS:
This was a dose-effect pilot study of the most appropriate dose of absolute ethanol for CT-guided lumbar sympathetic nerve modulation in treating PLEH. Consecutive patients with PLEH treated with CT-guided lumbar sympathetic nerve modulation for the first time at the Pain Management Department of the First Affiliated Hospital of Jiaxing University between July 2014 and February 2017 were included. The study was approved by the Ethics Committee of the First Affiliated Hospital of Jiaxing University (No. 2011-037). All participants provided signed informed consent.
The inclusion criteria were: 1) diagnosis of PLEH [12]; 2) hyperhidrosis severity score (HDSS) grade 3–4 [13]; and 3) PLEH refractory to drug therapy. The exclusion criteria were: 1) severe cardiac, pulmonary, liver, or renal dysfunction; 2) coagulation disorders or infection of the puncture site; 3) allergy to lidocaine hydrochloride, iohexol, or absolute ethanol; 4) secondary hyperhidrosis; or 5) history of lumbar sympathectomy.
GROUPING:
The patients were divided into 5 groups (Group R1, Group R2, Group R3, Group R4, and Group R5) using a random number table. The patients were blinded to grouping.
TREATMENT METHOD:
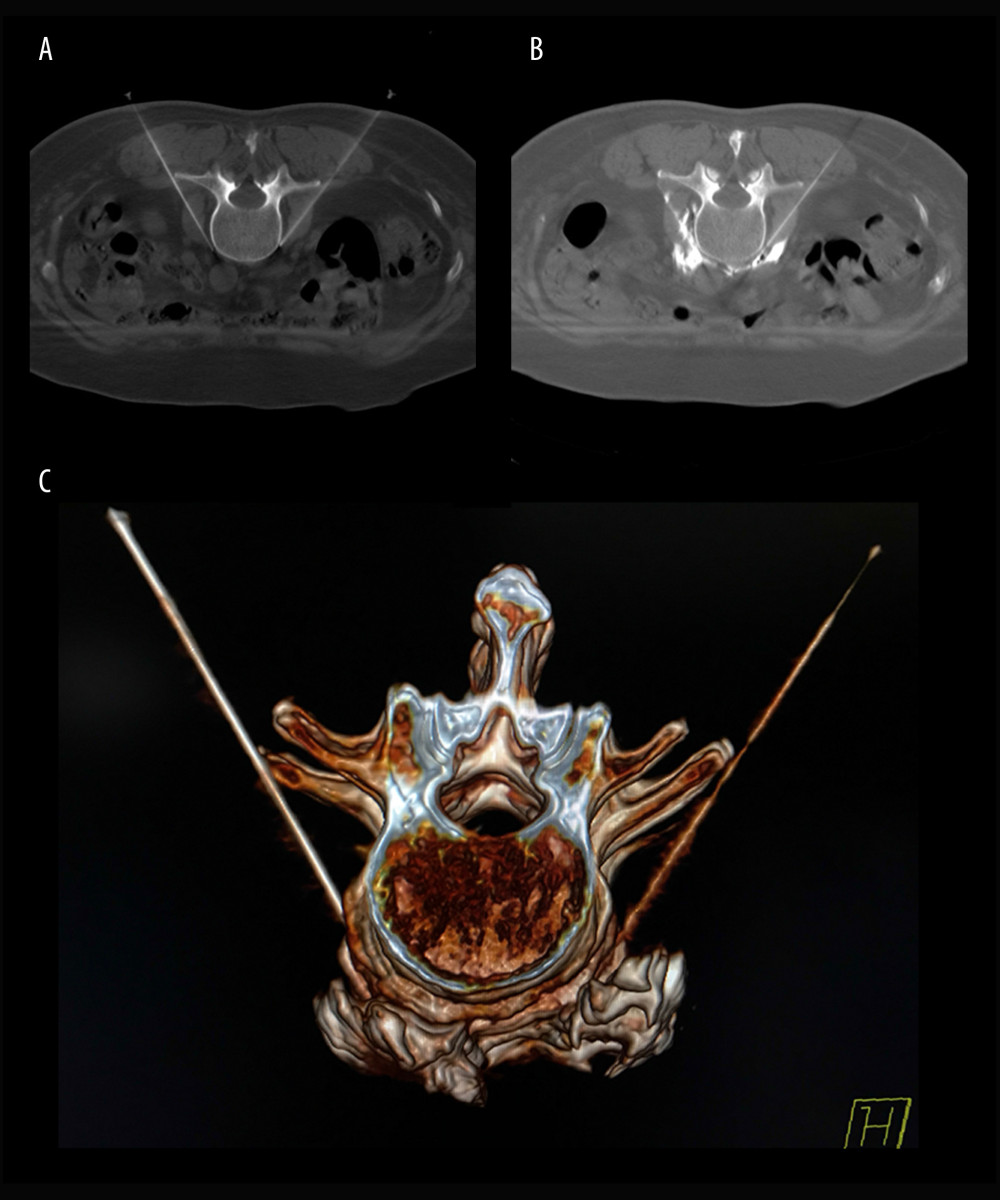
After the patients were transferred to the operating room, they were placed on the CT scanning table in the prone position. A multi-functional vital signs monitor (Radical-7 monitor, Masimo, Irvine, CA, USA) was used to monitor the non-invasive blood pressure, heart rate, pulse blood oxygen saturation, temperature of bilateral feet, and pulse perfusion index of the patients. CT (Siemens Somatom Emotion system, Siemens Healthcare, Malvern, PA, USA) was used for the positioning of the intervertebral space of L2–L3. The puncture needle was inserted until reaching the anterolateral margin of the L3 vertebral body (Figure 1A). When no blood, liquid, or gas was found during withdrawal, 1 ml of 30% iohexol was injected, and plain CT scanning was conducted for the anterolateral L3 vertebral body covered by iohexol. Then, a 1: 10 mixture of iohexol (batch number: 13385619, General Electric Pharmaceutical Shanghai Co., Ltd, Shanghai, China) and absolute ethanol (batch number: 20160911, Qingshan Chemical Reagent Factory, Lin’an, China) was injected into the bilateral sides (Figure 1B). The dose of absolute ethanol in previous studies was about 3 ml [9–11,14–17], so the median dose of absolute ethanol in this study was chosen accordingly, and increased or decreased in the different groups by increments of 0.5 ml across the various groups (2.0, 2.5, 3.0, 3.5, and 4.0 ml for the R1, R2, R3, R4, and R5 groups, respectively). Three-dimensional reconstruction of CT images was performed to assess the distribution of the fluid to the anterolateral L3 vertebral body (Figure 1C). Treatments in all patients were conducted by the same chief physician, who has 20 years of experience in clinical practice.
DATA COLLECTION:
The same investigator (an attending physician with 10 years of experience in clinical practice) assessed the onset time for drug effects, time to complete hyperhidrosis relief, and adverse effects during the treatment period. An increase in skin temperature by 1°C after injection of absolute ethanol was considered to indicate that ethanol had started to act. The onset time of ethanol effects referred to the time from injection of absolute ethanol to an increase in temperature of the skin of the bilateral foot soles by 1°C. Time to complete hyperhidrosis relief referred to the time from injection of absolute ethanol to the achievement of dryness of the skin of the bilateral foot soles. Treatment effectiveness was assessed according to time to complete hyperhidrosis relief, with <10 min being considered effective and ≥10 min being considered non-effective.
STATISTICAL ANALYSIS:
SPSS 19.0 (IBM, Armonk, NY, USA) was used for statistical analysis. Continuous data with a normal distribution were presented as mean and standard deviation (SD), and analyzed by one-way analysis of variance (ANOVA) with post hoc q test. Categorical data were presented as numbers and percentages, and were analyzed by the chi-square test.
Results
CHARACTERISTICS OF THE PARTICIPANTS:
A total of 112 patients were recruited, and 12 were subsequently excluded. Therefore, 100 patients (including 51 males and 49 females) were divided into 5 groups (n=20/group). The participants were 18 to 55 years old, with disease courses of 10 to 20 years. The characteristics of the participants were not statistically different among the 5 groups (P>0.05) (Table 1).
COMPARISON OF ONSET TIME AND TIME TO COMPLETE HYPERHIDROSIS RELIEF:
The onset time and time to complete hyperhidrosis relief were both reduced significantly with increasing dose of absolute ethanol (P<0.05) (Table 2).
TREATMENT EFFICACY AND COMPLICATIONS:
The effective rates in the 5 groups were 15.0%, 35.0%, 60.0%, 90.0%, and 100.0%, respectively. None of the patients showed complications, including alcohol intoxication, hypotension, numbness of lower limbs, movement disorders, dyspnea, or spinal cord injury.
:
The ED50 and ED95 were 2.306 ml (95% CI: 2.003–2.512 ml) and 3.343 ml (95% CI: 3.051–3.962 ml), respectively.
Discussion
PLEH can be treated by CT-guided lumbar sympathetic nerve modulation using absolute ethanol [9–11], but too high a dose of ethanol can cause nerve injury, and too low a dose can have suboptimal effects. Therefore, the aim of this pilot study was to investigate the dose-effect relationship of CT-guided lumbar sympathetic nerve modulation with absolute ethanol for PLEH. The results suggested that CT-guided lumbar sympathetic nerve modulation with absolute ethanol has clear treatment efficacy in PLEH, with no evident complications. The ED50 and ED95 were 2.306 ml and 3.343 ml, respectively.
CT can display the sites of puncture according to the anatomic positions of the lumbar sympathetic nerve. Thus, CT-guided insertion was conducted to insert the puncture needle at the anterolateral margin of the L3 vertebral body. The contrast agent was able to effectively display the spread of ethanol. The current study showed that the effective rates of the treatment in the 5 groups were 15.0%, 35.0%, 60.0%, 90.0%, and 100.0%. No patient had complications, including alcohol intoxication, numbness of lower limbs, dyspnea, or spinal cord injury induced by the entry of drugs into the spinal canal. Therefore, CT-guided lumbar sympathetic nerve modulation with absolute ethanol had clear treatment efficacy in PLEH, while no complications were found in the current patients. These results are similar to those seen in the literature. Huang et al. [9] and Guo et al. [10] treated patients with palmar hyperhidrosis using CT-guided thoracic sympathetic blockade, achieving success rates of 84% and 97%. CT-guided sympatholysis using ethanol was successfully applied for axillary and palmar hyperhidrosis in 8 out of 9 patients, with a primary efficacy of 74% at 1 year [11]. Brock et al. [18] reported a technical success rate of 100% and an overall efficacy of 60%; they used 2.0 ml of ethanol. Therefore, this method seems to achieve appropriate access to the nerve bundle and efficacy, but the dose of ethanol is probably an important factor affecting efficacy.
The use of high-dose absolute ethanol can possibly induce severe complications such as permanent alcohol intoxication and spinal cord injury due to ethanol entering the spinal canal. Previous studies have reported paraplegia, hypotension, and sensory and/or motor disorders in the lower limbs in some patients [14–16].
There were several limitations in this study. For instance, ethanol is liquid and thus uncontrollable to some degree; therefore, the drug might not distribute as anticipated. The use of iohexol, mixed with absolute ethanol, can indicate exactly where the ethanol is distributed but also could dilute ethanol and influence the study findings. Furthermore, the lasting time of the surgical efficacy could be influenced by ethanol dose, but no follow-up was conducted in this study. Moreover, although cost-effectiveness analysis is important in similar studies, this was a preliminary (pilot) trial with a very small sample for such assessment. Therefore, further studies are required to examine the efficacy and safety, including in the long-term, of this method. Nevertheless, this pilot study provides useful information for future trials, including the ED50 and ED95 values of absolute ethanol in this context.
Conclusions
CT-guided lumbar sympathetic nerve modulation with absolute ethanol has clear treatment efficacy in PLEH, with no evident complications. The ED50 and ED95 of this method are 2.306 ml and 3.343 ml, respectively.
References
1. Eisenach JH, Atkinson JL, Fealey RD, Hyperhidrosis: Evolving therapies for a well-established phenomenon: Mayo Clin Proc, 2005; 80; 657-66
2. Solish N, Bertucci V, Dansereau A, A comprehensive approach to the recognition, diagnosis, and severity-based treatment of focal hyperhidrosis: Recommendations of the Canadian Hyperhidrosis Advisory Committee: Dermatol Surg, 2007; 33; 908-23
3. McConaghy JR, Fosselman D, Hyperhidrosis: Management options: Am Fam Physician, 2018; 97; 729-34
4. Benson RA, Palin R, Holt PJ, Loftus IM, Diagnosis and management of hyperhidrosis: BMJ, 2013; 347; f6800
5. Strutton DR, Kowalski JW, Glaser DA, Stang PE, US prevalence of hyperhidrosis and impact on individuals with axillary hyperhidrosis: Results from a national survey: J Am Acad Dermatol, 2004; 51; 241-48
6. Lima SO, Aragao JF, Machado Neto J, Research of primary hyperhidrosis in students of medicine of the State of Sergipe, Brazil: An Bras Dermatol, 2015; 90; 661-65
7. Braganca GM, Lima SO, Pinto Neto AF, Evaluation of anxiety and depression prevalence in patients with primary severe hyperhidrosis: An Bras Dermatol, 2014; 89; 230-35
8. Lueangarun S, Sermsilp C, Tempark T, Topical botulinum toxin type A liposomal cream for primary axillary hyperhidrosis: A double-blind, randomized, split-site, vehicle-controlled study: Dermatol Surg, 2018; 44; 1094-101
9. Huang B, Sun K, Zhu Z, Oximetry-derived perfusion index as an early indicator of CT-guided thoracic sympathetic blockade in palmar hyperhidrosis: Clin Radiol, 2013; 68; 1227-32
10. Guo JG, Fei Y, Huang B, Yao M, CT-guided thoracic sympathetic blockade for palmar hyperhidrosis: Immediate results and postoperative quality of life: J Clin Neurosci, 2016; 34; 89-93
11. Tsitskari M, Friehs G, Zerris V, Georgiades C, CT-guided, ethanol sympatholysis for primary axillary-palmar hyperhidrosis: Cardiovasc Intervent Radiol, 2016; 39; 1722-27
12. Thorlacius L, Gyldenlove M, Zachariae C, Carlsen BC, Distinguishing hyperhidrosis and normal physiological sweat production: New data and review of hyperhidrosis data for 1980–2013: Int J Dermatol, 2015; 54; e409-15
13. Sener S, Karakoc Y, Effects of direct current administration on hyperhidrosis disease severity scale in patients with axillary hyperhidrosis: Biomed Res Int, 2019; 2019 3232015
14. Apiliogullari B, Esme H, Yoldas B, Early and midterm results of single-port video-assisted thoracoscopic sympathectomy: Thorac Cardiovasc Surg, 2012; 60; 285-89
15. Vicente P, Canelles E, Diaz A, Fons AIrreversible Horner’s syndrome after bilateral thoracoscopic sympathectomy: Arch Soc Esp Oftalmol, 2014; 89; 79-81 [in Spanish]
16. Joo S, Lee GD, Haam S, Lee S, Comparisons of the clinical outcomes of thoracoscopic sympathetic surgery for palmar hyperhidrosis: R4 sympathicotomy versus R4 sympathetic clipping versus R3 sympathetic clipping: J Thorac Dis, 2016; 8; 934-41
17. Brock M, Chung TH, Gaddam SR, Resolution of postural orthostatic tachycardia syndrome after CT-guided, percutaneous T2 ethanol ablation for hyperhidrosis: Cardiovasc Intervent Radiol, 2016; 39; 1785-88
18. Brock M, Frangakis C, Georgiades CS, CT-guided, percutaneous ethanol sympatholysis for primary hyperhidrosis: Cardiovasc Intervent Radiol, 2018; 41; 477-82
19. Huang SS, Chen CL, Huang FW, Ethanol enhances TGF-beta activity by recruiting TGF-beta receptors from intracellular vesicles/lipid rafts/caveolae to non-lipid raft microdomains: J Cell Biochem, 2016; 117; 860-71
20. Ayers-Ringler JR, Oliveros A, Qiu Y, Label-free proteomic analysis of protein changes in the striatum during chronic ethanol use and early withdrawal: Front Behav Neurosci, 2016; 10; 46
Tables
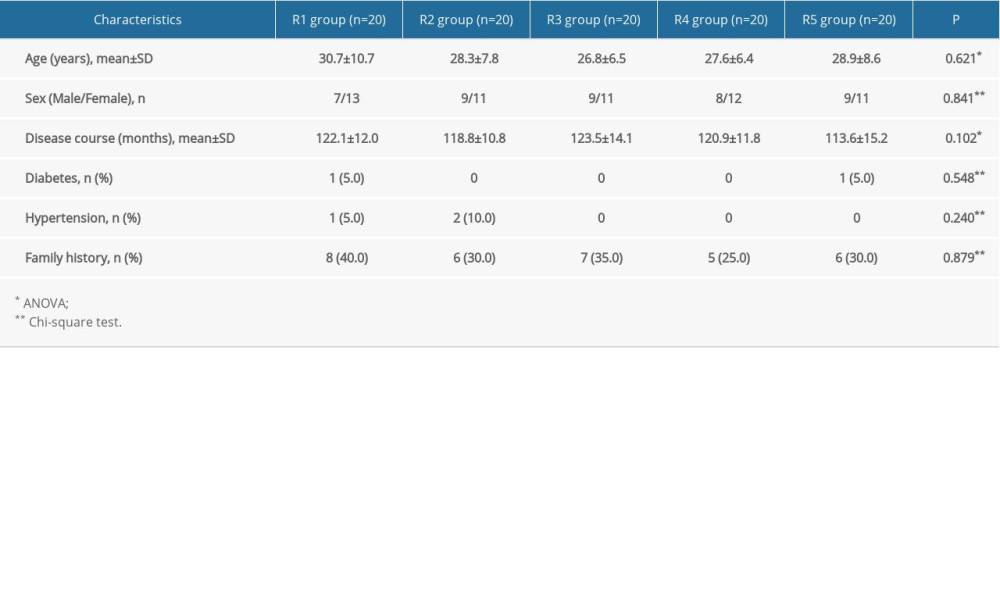 Table 1. Comparison of the general characteristics of the participants.
Table 1. Comparison of the general characteristics of the participants.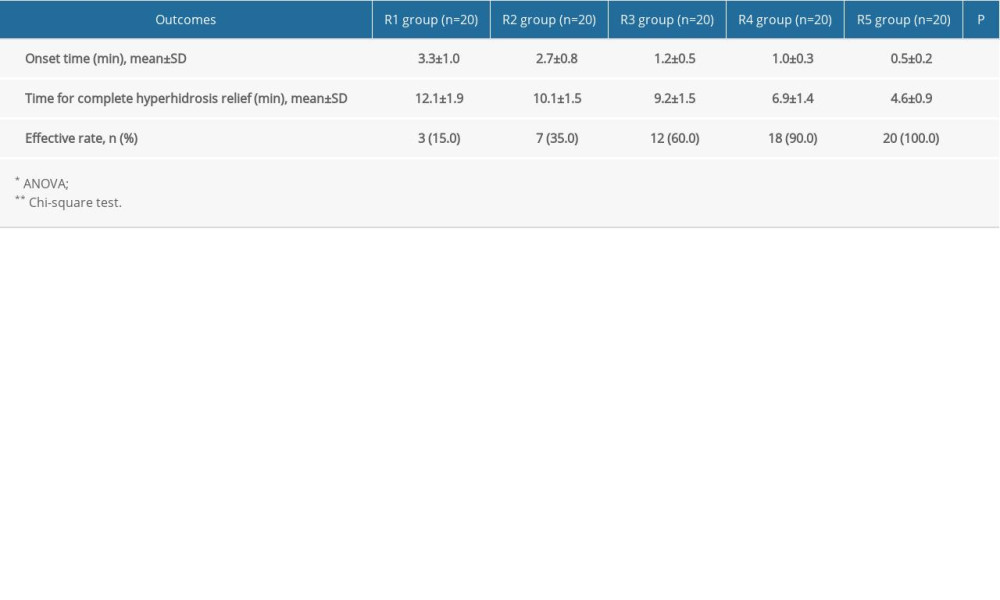 Table 2. Comparison of onset time and time for complete hyperhidrosis relief in the participants.
Table 2. Comparison of onset time and time for complete hyperhidrosis relief in the participants. Table 1. Comparison of the general characteristics of the participants.
Table 1. Comparison of the general characteristics of the participants. Table 2. Comparison of onset time and time for complete hyperhidrosis relief in the participants.
Table 2. Comparison of onset time and time for complete hyperhidrosis relief in the participants. In Press
21 Mar 2024 : Meta-Analysis
Economic Evaluation of COVID-19 Screening Tests and Surveillance Strategies in Low-Income, Middle-Income, a...Med Sci Monit In Press; DOI: 10.12659/MSM.943863
10 Apr 2024 : Clinical Research
Predicting Acute Cardiovascular Complications in COVID-19: Insights from a Specialized Cardiac Referral Dep...Med Sci Monit In Press; DOI: 10.12659/MSM.942612
06 Mar 2024 : Clinical Research
Enhanced Surgical Outcomes of Popliteal Cyst Excision: A Retrospective Study Comparing Arthroscopic Debride...Med Sci Monit In Press; DOI: 10.12659/MSM.941102
06 Mar 2024 : Clinical Research
Prevalence and Variation of Medical Comorbidities in Oral Surgery Patients: A Retrospective Study at Jazan ...Med Sci Monit In Press; DOI: 10.12659/MSM.943884
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952