18 March 2021: Clinical Research
Atosiban Combined with Ritodrine for Late Threatened Abortion or Threatened Premature Labor Patients with No Response to Ritodrine: A Clinical Trial
Shuai Fu1ABC, Haitian Xie1DEF, Yilei Zhong12BCD, Qi Xu1D, Liqiong Zhu1B, Hanjie Mo1B, Jianping Zhang1AG, Yinglin Liu1CF, Hui Chen1AG, Yonghong Zhong2AD*, Jianping Tan1ADDOI: 10.12659/MSM.929743
Med Sci Monit 2021; 27:e929743
Abstract
BACKGROUND: Premature labor is an important cause of infant death and long-term disability. This study aimed to explore the safety and effectiveness of combining the tocolytic agents atosiban and ritodrine to extend gestation.
MATERIAL AND METHODS: The study included 52 patients with late threatened abortion and threatened premature labor between 20⁰⸍⁷ and 33⁶⸍⁷ weeks’ gestation who were administrated continuous tocolytic agents for 48 h. Patients were divided into a research group receiving ritodrine combined with atosiban, owing to having no response to ritodrine alone (n=30), and a control group receiving ritodrine alone (n=22). The mean infusion rate and duration of tocolytic administration, gestation extension, pregnancy outcomes, and adverse effects were recorded. Routine blood tests, including C-reactive protein, and cultures for leukorrhea, candida, and mycoplasma were performed before and 1 week after treatment.
RESULTS: Patients receiving ritodrine with atosiban had a mean gestation extension of 42.53±31.70 days. The extension of gestation of the research group was statistically shorter than that of the control group (P<0.05). The fetal loss rate, newborn birth weight, and Apgar score at 1 min were similar between the 2 groups (all, P>0.05). The research group had a lower incidence of palpitations than the control group (P<0.05).
CONCLUSIONS: For patients with late threatened abortion or threatened premature labor not controlled with ritodrine alone, ritodrine combined with atosiban extends gestation and improves pregnancy outcomes. For patients with abnormal uterine contractions, routine testing for reproductive tract infection should be performed. When infection is present, anti-infective therapy should be administered.
Keywords: Abortion, Threatened, Premature Birth, Tocolytic Agents, Drug Therapy, Combination, Gestational Age, Infant, Newborn, Obstetric Labor, Premature, Pregnancy, Pregnancy Outcome, Ritodrine, Vasotocin
Background
Premature labor is an important cause of infant death and long-term disability and its incidence ranges from 5% to 15% [1]. About 40% of premature labor cases are spontaneous [2]. Proper and effective application of tocolytic agents can postpone delivery, give time for promoting fetal lung maturation, and enable the intrauterine transport [3].
In 2012, the American College of Obstetricians and Gynecologists recommended ritodrine (a β2-receptor agonist) as the first-line medication for suppressing premature contractions [4]. However, owing to the wide distribution of β2 receptors in human tissues, long-term and high-dose use of this drug can cause adverse effects such as increased heart rate, chest pain, disorder of glucose metabolism, and hypokalemia. High concentrations of β2-adrenoceptor agonists have an anti-diuretic effect, which can result in sodium and water retention and even lead to pulmonary edema [5,6].
Atosiban is an oxytocin-receptor antagonist. It is specific for oxytocin receptors and has no effects on organs without these receptors. Extensive research has shown that atosiban and indomethacin are the only 2 drugs of this drug type not associated with severe adverse drug reactions [7]. Atosiban is the first-line drug to suppress contractions in Europe, but the cost of atosiban exceeds the cost of other tocolytic drugs, such as ritodrine [8].
Currently, a single drug is primarily used to suppress contractions. An ideal tocolytic should prolong gestational weeks at a low cost without maternal and fetal adverse effects [7]. However, none of the single tocolytics meet these criteria because either their effects are not adequate or the adverse effects are not tolerable; therefore, it is necessary to consider combining different drugs [9]. However, very little is known about the combined drug use. In view of this uncertainty, we aimed to compare the effectiveness and safety of the β2-adrenoceptor agonist ritodrine combined with the oxytocin antagonist atosiban in women with late threatened abortion and threatened preterm birth.
Material and Methods
THERAPEUTIC REGIMENS:
For treatment with ritodrine alone, 100 mg of ritodrine was added to 500 mL of 5% glucose solution to achieve a dilution of 0.2 mg/mL. The initial intravenous infusion rate was 0.05 mg/min (5 drops/min), and then the dosage was gradually increased according to the effect on contractions, with a maximum of 0.35 mg/min (35 drops/min).
For the treatment with atosiban combined with ritodrine
The same procedure described above was followed in patients with a maternal heart rate of >130 beats per min without the control of contractions on ritodrine alone.
MATERNAL-FETAL MONITORING DURING DRUG TREATMENT:
Maternal vital signs were monitored daily. Routine blood tests including WBC, red blood cell, platelets, hemoglobin, percentage of neutrophils, C-reactive protein (CRP), and high-sensitivity CRP were done with the original matching reagents and were performed every week to monitor for indications of infection. Maternal biochemical and blood glucose examinations were performed weekly. Routine prenatal examinations including blood pressure and fetal monitoring were performed weekly. Cervical ultrasounds were performed every 2 to 4 weeks to examine cervical morphology and length. Every 4 weeks, fetal ultrasound was performed to examine fetal size. Every 4 weeks, ECG and cardiac color Doppler ultrasound were performed to determine if there were changes of cardiac function and morphology due to the drugs.
INDICATIONS FOR WITHDRAWAL OF DRUG:
There were 4 indications for the withdrawal of drug treatment: (1) At the 34th week of gestation, the tocolytic agents were stopped. If the mother and fetus were in good condition, the mother was expected to give birth naturally; (2) atosiban combined with ritodrine were at the maximum dosages and the contractions were not controlled; (3) there was any evidence of maternal infection or cardiac dysfunction; and (4) there was fetal distress or evidence of intrauterine infection.
STATISTICAL METHODS:
Data were analyzed using SPSS 22.0 statistical software. The
Results
CLINICAL DATA:
The patients in the research group ranged in age from 24 to 40 years, with a mean age of 32.63 years, and the patients in the control group ranged in age from 18 to 40 years, with a mean age of 30.68 years. There was no statistically significant difference in age between the 2 groups. The average gestational age of the research group was 25.90 weeks and the average gestational age of the control group was 22.00 weeks; the difference was statistically significant (P=0.002). The length of drug treatment in the control group was statistically longer than that in the research groups (Table 1).
INDICATORS OF INFECTION:
There were no statistically significant differences in indicators of infection including WBC count, the presence of leukorrhea, and positive mycoplasma culture before treatment and 1 week after treatment between the 2 groups. The research group had a significantly higher CRP level and neutrophilic granulocyte percentage than the control group (both, P<0.05). Patients with the presence of leukorrhea suggesting a monilial infection were administrated clotrimazole vaginal tablets 2 times every 3 days (Canesten, 500 mg/tablet; Bayer, Germany). If the vaginal secretion test suggested mycoplasma infection, the patients were administered 4 azithromycin tablets (Zithromax, 250 mg/tablet, Pfizer PGM, France) (Tables 2, 3). The incidence of monilial infection (odds ratio 1.27; 95% confidence interval [CI] 0.27–5.97; P=0.765) was higher in the research group than in the control group, but the difference was not statistically significant. The incidence of mycoplasma infection (odds ratio 0.78; 95% CI 0.23–2.61; P=0.686) was lower in the research group than in the control group, but the difference was not statistically significant. After 1 week of treatment, the incidence of monilial infection (odds ratio 1.50; 95% CI 0.13–17.67; P=0.747) was higher in the research group than in the control group, but the difference was not statistically significant. The incidence of mycoplasma infection (odds ratio 0.97; 95% CI 0.20–4.87; P=0.975) was lower in the research group than in the control group, but the difference was not statistically significant.
DOSAGE OF RITODRINE AND PREGNANCY OUTCOMES:
The mean infusion rate of ritodrine was similar between the 2 groups (P>0.05). The extension of gestation of the control group was significantly longer than that of the research group (P<0.05). The fetal loss rate, newborn birth weight, and Apgar score at 1 min were similar between the 2 groups (all, P>0.05) (Table 4). The incidence of fetal loss (odds ratio 2.30; 95% CI 0.53–9.94; P=0.263), was higher in the research group than in the control group, but the difference was not statistically significant.
ADVERSE DRUG REACTIONS:
The control group had a significantly higher rate of palpitations than did the research group (P>0.05) (Table 5).
Discussion
Premature labor is a leading cause of newborn disease and death. Newborn deaths due to premature labor account for two-thirds of all newborn deaths, including those due to congenital malformations [11]. About one-fourth of surviving newborns develop mental impairment or neurological sequelae, resulting in huge social and economic burdens. At present, the intervention for premature labor is to extend the pregnancy to 34 weeks, providing the fetal membranes are intact and the mother is in good health [12].
The proper and effective application of tocolytic agents extends gestation, allowing for fetal lung maturation [13]. Ritodrine can effectively suppress contractions but can have serious adverse effects. Atosiban is the only tocolytic agent approved by the European Medicine Agency for treatment premature labor [14]. Many studies have reported no difference in the effectiveness of atosiban and ritodrine for suppressing contractions, but atosiban has fewer adverse effects [15–17]. However, due to its high cost, atosiban is not widely used in China. It is mainly used in patients who have no response to ritodrine or have intolerable adverse effects from ritodrine.
This study showed that atosiban combined with ritodrine has certain effects in patients with late threatened abortion and threatened premature labor who have no response to ritodrine alone. The extension of gestation in the combined drug group was slightly shorter than that of the ritodrine-only group (control group). However, the fetal loss rate, newborn birth weight, and Apgar score at 1 min were similar between the 2 groups. The research group had a lower incidence of palpitations and adverse drug reactions than the control group. These results suggest that ritodrine combined with atosiban can effectively treat premature labor and suppress contractions with no adverse effects.
At present, ritodrine combined with atosiban is not routinely used, and there is little experience using them in combination. However, their mechanisms of suppressing contractions are different, providing a theoretical basis for using them in combination. While there were no obvious adverse reactions when using ritodrine combined with atosiban in our hospital, intensive monitoring is required because of the potential of an adverse reaction [18].
It has been shown that about 40% of late abortions before the 28th week of pregnancy and 30% of premature labors before the 30th week of pregnancy are associated with infections [19]. Animal experiments and in vitro and in vivo studies have confirmed the mechanism of premature labor caused by infections is bacterial invasion in the gap between the chorion and decidua. The endotoxins and exotoxins released activate decidual cells to produce various cytokines, which activate the synthesis of prostaglandins, resulting in uterine contractions [20,21]. Interestingly, a recent study found a marked increase of CB1, anandamide, and inflammatory cytokine downregulation in human placental samples of a term delivery group, compared with those in preterm groups [22]. In addition, the proteolytic enzymes produced reduce the tensile strength of fetal membranes, decreasing collagenous fibers and increasing membrane brittleness, thus resulting in the rupture of the fetal membranes [23]. Infections can also lead to impaired function of multiple organs such as the heart, liver, and kidney, threatening the newborn’s health [24].
In this study, none of the patients had a fever, and their WBC counts were in the normal range. The patients had a strong desire to continue pregnancy, and they received treatment to extend gestation and achieved good outcomes. During treatment, some patients in both groups developed candida and mycoplasma genital infections. The infection rates were obviously reduced after anti-infection therapy for only 1 week. Patients with abnormal contractions should receive examinations for reproductive tract infections, and any infections should be actively treated. The research group in this study had a significantly higher CRP level and neutrophil granulocyte percentage than the control group. This may have been due to inflammatory responses or infections at sites other than the uterus or reproductive tract. Further research examining premature labor and infection should investigate inflammatory factors such as Th1 and perform amniocentesis to obtain amniotic fluid samples to perform testing, such as an amniotic fluid smear for bacteria, amniotic liquid glucose quantification, amniotic fluid lactate dehydrogenase, and amniotic fluid IL-6 determination [25].
Conclusions
For patients with late threatened abortion or threatened premature labor that are not controlled with ritodrine alone, ritodrine combined with atosiban extends the gestation period and improves pregnancy outcomes. For patients with abnormal uterine contractions, routine testing for reproductive tract infections should be performed. If an infection is present, anti-infective therapy should be administered.
Figures
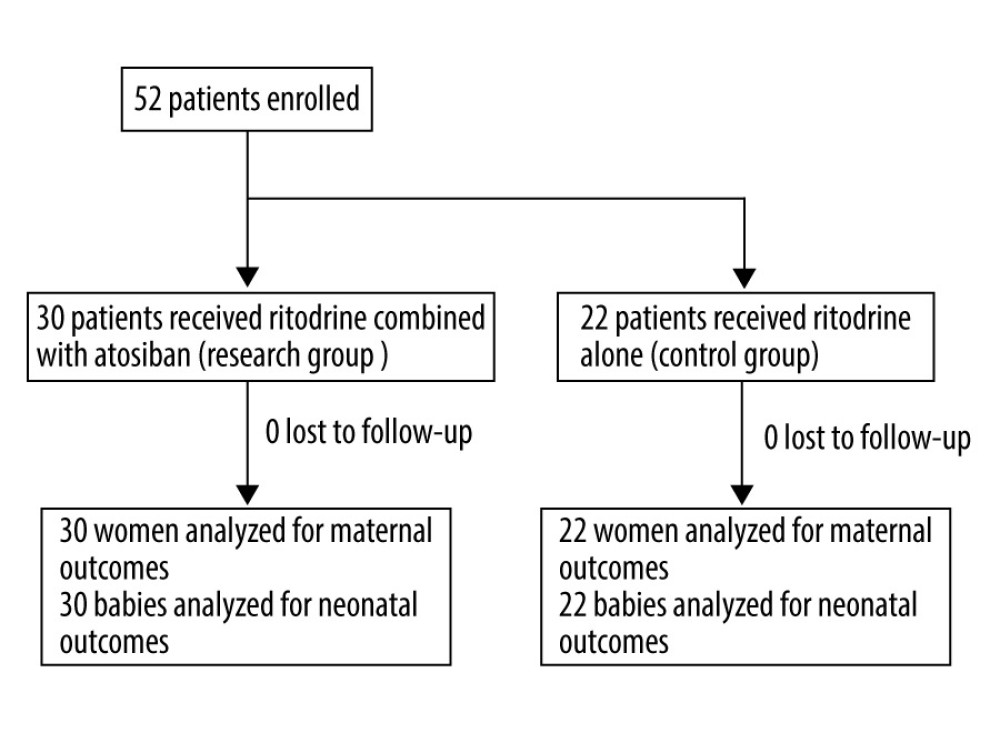 Figure 1. Study profile.
Figure 1. Study profile. Tables
Table 1. Characteristics of pregnant women at the time of admission. Continuous data are shown as mean±SD. The t test was used for analyzing quantitative data. P<0.05 was considered statistically significant.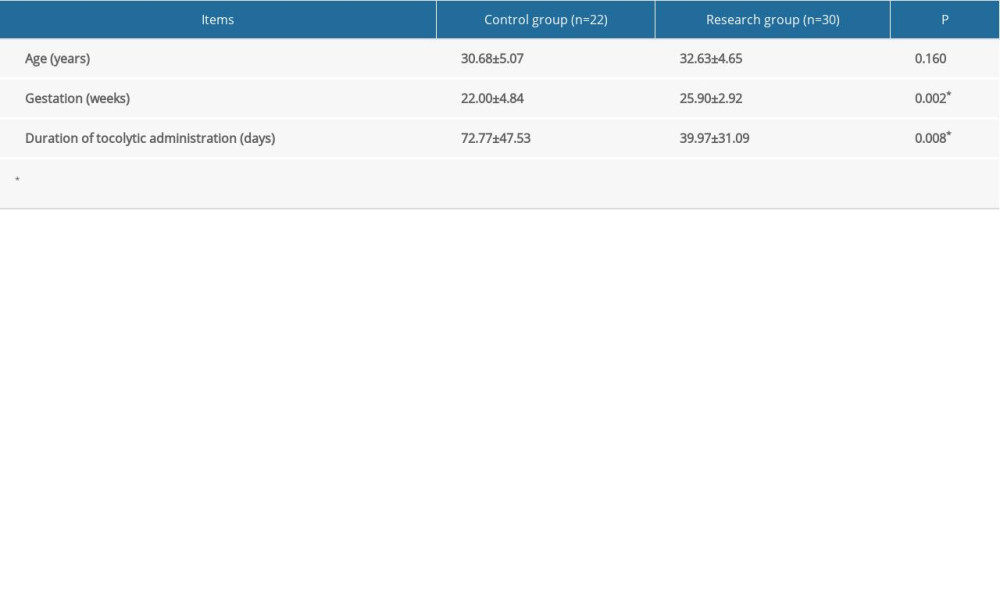 Table 2. Indicators of infection before treatment. Categorical data are shown as a frequency (percentage) and continuous data are shown as mean±SD. The t test was used for analyzing quantitative data, and the χ2 test was used for analyzing categorical data. P<0.05 was considered statistically significant.
Table 2. Indicators of infection before treatment. Categorical data are shown as a frequency (percentage) and continuous data are shown as mean±SD. The t test was used for analyzing quantitative data, and the χ2 test was used for analyzing categorical data. P<0.05 was considered statistically significant.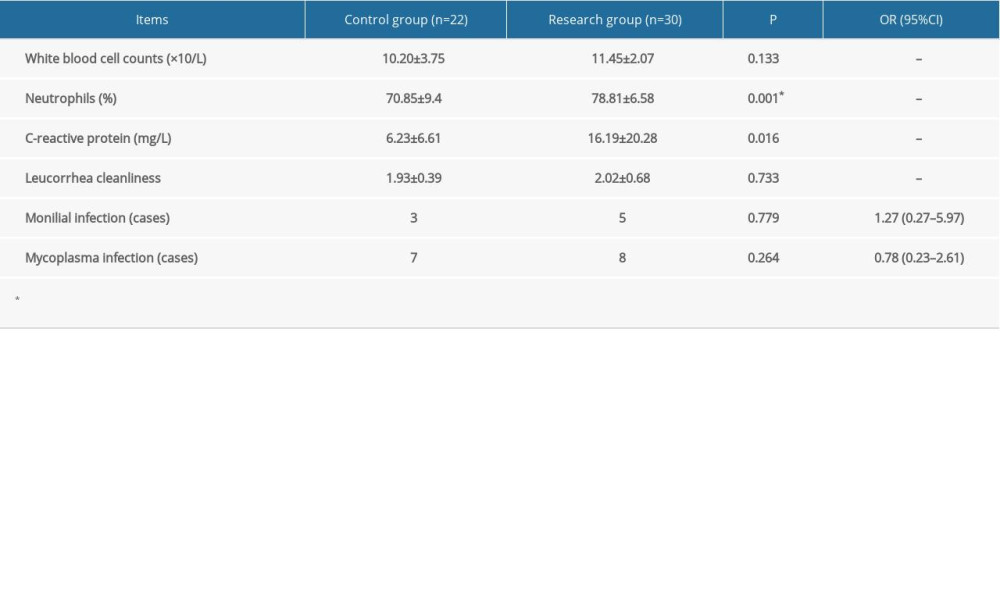 Table 3. Indicators of infection 1 week after treatment. Categorical data are shown as a frequency (percentage) and continuous data are shown as mean±SD. The t test was used for analyzing quantitative data, and the χ2 test was used for analyzing categorical data. P<0.05 was considered statistically significant.
Table 3. Indicators of infection 1 week after treatment. Categorical data are shown as a frequency (percentage) and continuous data are shown as mean±SD. The t test was used for analyzing quantitative data, and the χ2 test was used for analyzing categorical data. P<0.05 was considered statistically significant.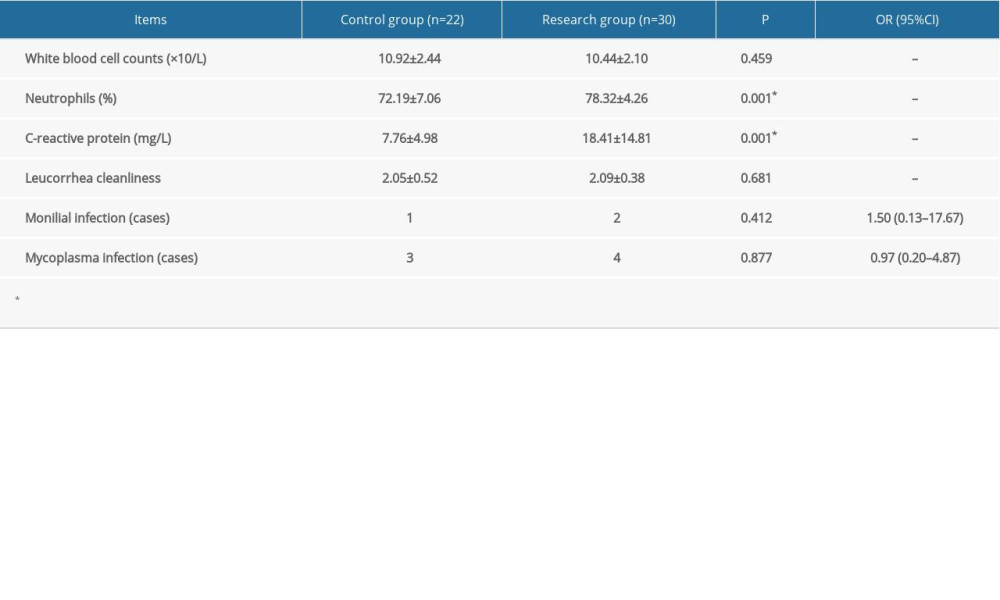 Table 4. Dosage and pregnancy outcomes. Categorical data are shown as a frequency (percentage) and continuous data are shown as mean±SD. The t test was used for analyzing quantitative data, while the χ2 test was used for analyzing categorical data. P<0.05 was considered statistically significant.
Table 4. Dosage and pregnancy outcomes. Categorical data are shown as a frequency (percentage) and continuous data are shown as mean±SD. The t test was used for analyzing quantitative data, while the χ2 test was used for analyzing categorical data. P<0.05 was considered statistically significant.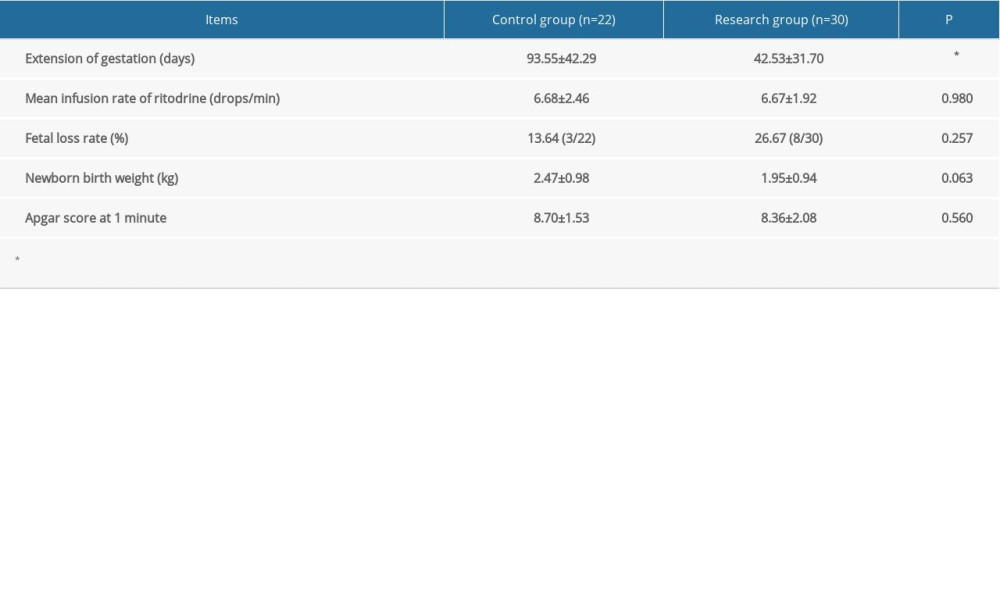 Table 5. Adverse drug reactions. Categorical data are shown as a frequency (percentage) and continuous data are shown as mean±SD. The t test was used for analyzing quantitative data, and the χ2 test was used for analyzing categorical data. P<0.05 was considered statistically significant.
Table 5. Adverse drug reactions. Categorical data are shown as a frequency (percentage) and continuous data are shown as mean±SD. The t test was used for analyzing quantitative data, and the χ2 test was used for analyzing categorical data. P<0.05 was considered statistically significant.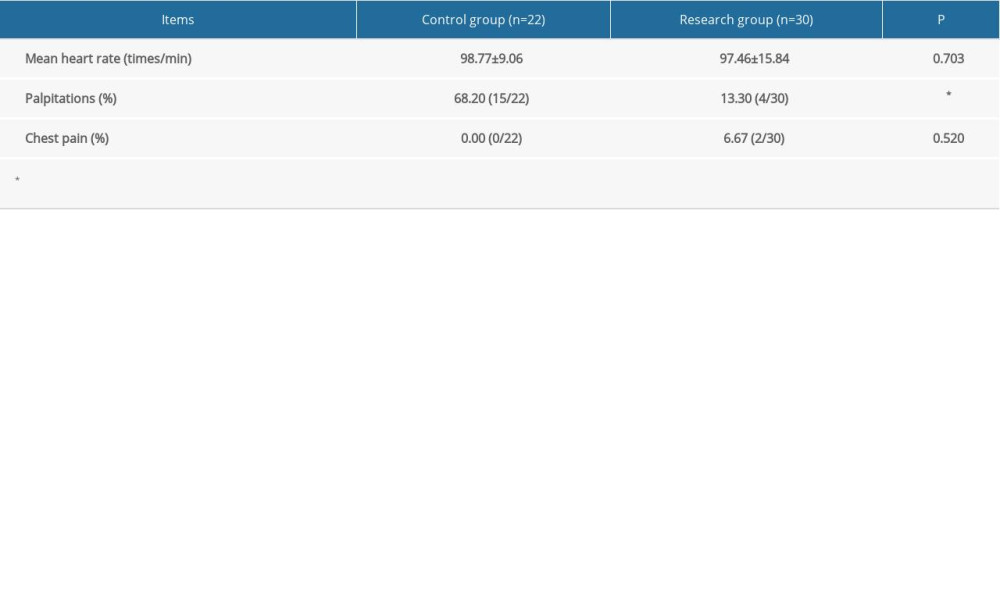
References
1. Goldenberg RL, Culhane JF, Iams JD, Epidemiology and causes of preterm birth: Lancet, 2008; 371; 75-84
2. Shaw GM, Wise PH, Mayo J, Maternal prepregnancy body mass index and risk of spontaneous preterm birth: Paediatr Perinat Epidemiol, 2014; 28; 302-11
3. Crowley P, Prophylactic corticosteroids for preterm birth: Cochrane Database Syst Rev, 2000; 2; CD000065
4. , American College of Obstetricians and Gynecologists (ACOG) Practice Bulletin No. 127: Management of preterm labor: Obstet Gynecol, 2012; 119; 1308
5. Yang GQ, Long SY, Yang P, Oxytocin receptor inhibitors and β-receptor agonist in the treatment of premature delivery: A meta-analysis: J Int Obstet Gynecol, 2014; 41; 686-91
6. Kimura M, Kato T, Nohara R, Pulmonary edema complicating ritodrine infusion in a patient with premature labor: Int Med, 2013; 52; 155
7. de Heus R, Mol BW, Erwich JJ, Adverse drug reactions to tocolytic treatment for preterm labour: Prospective cohort study: Br Med J, 2009; 338; b744
8. Lyndrup J, Lamont RF, The choice of a tocolytic for the treatment of preterm labor: A critical evaluation of nifedipine versus atosiban: Expert Opin Investig Drugs, 2007; 16; 843-53
9. Kuć P, Laudański P, Pierzyński P, The effect of combined tocolysis on in vitro uterine contractility in preterm labour: Adv in Med Sci, 2011; 56; 88-94
10. von Elm E, Altman DG, Egger M, The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies: Lancet, 2007; 370; 1453-57
11. Mercer BM, Merlino AA, Magnesium sulfate for preterm labor and preterm birth: Obstet Gynecol, 2009; 114; 650-68
12. Tan MY, To M, Recent advances in the prevention and management of preterm birth: F1000Prime Rep, 2015; 7; 40
13. Boots AB, Sanchezramos L, Bowers DM, The short-term prediction of preterm birth: A systematic review and diagnostic meta-analysis: Am J Obstet Gynecol, 2014; 210; 51-54
14. Salim R, Garmi G, Nachum Z, Nifedipine compared with atosiban for treating preterm labor: A randomized controlled trial: Obstet Gynecol, 2012; 120; 1323-31
15. Driul L, Londero AP, Adorati-Menegato A, Therapy side-effects and predictive factors for preterm delivery in patients undergoing tocolysis with atosiban or ritodrine for threatened preterm labour: J Obstet Gynaecol Res, 2014; 34; 684-89
16. Shim JY, Park YW, Yoon BH, Multicentre, parallel group, randomised, single-blind study of the safety and efficacy of atosiban versus ritodrine in the treatment of acute preterm labour in Korean women: BJOG, 2006; 113; 1228
17. Zhu LQ, Tan JP, Chen H, The significance of atosiban combined with ritodrine in the treatment of late threatened abortion and threatened preterm birth: J Trop Med, 2014; 14; 1148-50
18. Zhong YL, Zhu LQ, Liu YL, A case of cervix cerclage for pregnant women with twin pregnancies: Chin J Obstet Emerg (Electronic Edition), 2017; 6; 59-61
19. Chen C, Song X, Wei W, The microbiota continuum along the female reproductive tract and its relation to uterine-related diseases: Nat Commun, 2017; 8; 875
20. Kemp MW, Preterm birth, intrauterine infection, and fetal inflammation: Front Immun, 2014; 5; 574
21. Romero R, Miranda J, Chaiworapongsa T, Prevalence and clinical significance of sterile intra-amniotic inflammation in patients with preterm labor and intact membranes: Am J Rep Immun, 2015; 72; 458-74
22. Torella M, Bellini G, Punzo F, TNF-α effect on human delivery onset by CB1/TRPV1 crosstalk: New insights into endocannabinoid molecular signaling in preterm vs term labor. Analysis of the EC/EV pathway and predictive biomarkers for early diagnosis of preterm delivery: Minerva Ginecol, 2019; 71; 359-64
23. Rinaldi SF, Catalano RD, Wade J, Decidual neutrophil infiltration is not required for preterm birth in a mouse model of infection-induced preterm labor: J Immunol, 2014; 192; 2315-25
24. Shi CY, Dong Y, Infection and preterm birth: J Prac Obstet Gynecol, 2005; 21; 648-50
25. Lorenzen E, Follmann F, Secher JO: Micro Infect, 2017; 19; 334-42
Figures
Tables
 Table 1. Characteristics of pregnant women at the time of admission. Continuous data are shown as mean±SD. The t test was used for analyzing quantitative data. P<0.05 was considered statistically significant.
Table 1. Characteristics of pregnant women at the time of admission. Continuous data are shown as mean±SD. The t test was used for analyzing quantitative data. P<0.05 was considered statistically significant. Table 2. Indicators of infection before treatment. Categorical data are shown as a frequency (percentage) and continuous data are shown as mean±SD. The t test was used for analyzing quantitative data, and the χ2 test was used for analyzing categorical data. P<0.05 was considered statistically significant.
Table 2. Indicators of infection before treatment. Categorical data are shown as a frequency (percentage) and continuous data are shown as mean±SD. The t test was used for analyzing quantitative data, and the χ2 test was used for analyzing categorical data. P<0.05 was considered statistically significant. Table 3. Indicators of infection 1 week after treatment. Categorical data are shown as a frequency (percentage) and continuous data are shown as mean±SD. The t test was used for analyzing quantitative data, and the χ2 test was used for analyzing categorical data. P<0.05 was considered statistically significant.
Table 3. Indicators of infection 1 week after treatment. Categorical data are shown as a frequency (percentage) and continuous data are shown as mean±SD. The t test was used for analyzing quantitative data, and the χ2 test was used for analyzing categorical data. P<0.05 was considered statistically significant. Table 4. Dosage and pregnancy outcomes. Categorical data are shown as a frequency (percentage) and continuous data are shown as mean±SD. The t test was used for analyzing quantitative data, while the χ2 test was used for analyzing categorical data. P<0.05 was considered statistically significant.
Table 4. Dosage and pregnancy outcomes. Categorical data are shown as a frequency (percentage) and continuous data are shown as mean±SD. The t test was used for analyzing quantitative data, while the χ2 test was used for analyzing categorical data. P<0.05 was considered statistically significant. Table 5. Adverse drug reactions. Categorical data are shown as a frequency (percentage) and continuous data are shown as mean±SD. The t test was used for analyzing quantitative data, and the χ2 test was used for analyzing categorical data. P<0.05 was considered statistically significant.
Table 5. Adverse drug reactions. Categorical data are shown as a frequency (percentage) and continuous data are shown as mean±SD. The t test was used for analyzing quantitative data, and the χ2 test was used for analyzing categorical data. P<0.05 was considered statistically significant. Table 1. Characteristics of pregnant women at the time of admission. Continuous data are shown as mean±SD. The t test was used for analyzing quantitative data. P<0.05 was considered statistically significant.
Table 1. Characteristics of pregnant women at the time of admission. Continuous data are shown as mean±SD. The t test was used for analyzing quantitative data. P<0.05 was considered statistically significant. Table 2. Indicators of infection before treatment. Categorical data are shown as a frequency (percentage) and continuous data are shown as mean±SD. The t test was used for analyzing quantitative data, and the χ2 test was used for analyzing categorical data. P<0.05 was considered statistically significant.
Table 2. Indicators of infection before treatment. Categorical data are shown as a frequency (percentage) and continuous data are shown as mean±SD. The t test was used for analyzing quantitative data, and the χ2 test was used for analyzing categorical data. P<0.05 was considered statistically significant. Table 3. Indicators of infection 1 week after treatment. Categorical data are shown as a frequency (percentage) and continuous data are shown as mean±SD. The t test was used for analyzing quantitative data, and the χ2 test was used for analyzing categorical data. P<0.05 was considered statistically significant.
Table 3. Indicators of infection 1 week after treatment. Categorical data are shown as a frequency (percentage) and continuous data are shown as mean±SD. The t test was used for analyzing quantitative data, and the χ2 test was used for analyzing categorical data. P<0.05 was considered statistically significant. Table 4. Dosage and pregnancy outcomes. Categorical data are shown as a frequency (percentage) and continuous data are shown as mean±SD. The t test was used for analyzing quantitative data, while the χ2 test was used for analyzing categorical data. P<0.05 was considered statistically significant.
Table 4. Dosage and pregnancy outcomes. Categorical data are shown as a frequency (percentage) and continuous data are shown as mean±SD. The t test was used for analyzing quantitative data, while the χ2 test was used for analyzing categorical data. P<0.05 was considered statistically significant. Table 5. Adverse drug reactions. Categorical data are shown as a frequency (percentage) and continuous data are shown as mean±SD. The t test was used for analyzing quantitative data, and the χ2 test was used for analyzing categorical data. P<0.05 was considered statistically significant.
Table 5. Adverse drug reactions. Categorical data are shown as a frequency (percentage) and continuous data are shown as mean±SD. The t test was used for analyzing quantitative data, and the χ2 test was used for analyzing categorical data. P<0.05 was considered statistically significant. In Press
06 Mar 2024 : Clinical Research
Comparison of Outcomes between Single-Level and Double-Level Corpectomy in Thoracolumbar Reconstruction: A ...Med Sci Monit In Press; DOI: 10.12659/MSM.943797
21 Mar 2024 : Meta-Analysis
Economic Evaluation of COVID-19 Screening Tests and Surveillance Strategies in Low-Income, Middle-Income, a...Med Sci Monit In Press; DOI: 10.12659/MSM.943863
10 Apr 2024 : Clinical Research
Predicting Acute Cardiovascular Complications in COVID-19: Insights from a Specialized Cardiac Referral Dep...Med Sci Monit In Press; DOI: 10.12659/MSM.942612
06 Mar 2024 : Clinical Research
Enhanced Surgical Outcomes of Popliteal Cyst Excision: A Retrospective Study Comparing Arthroscopic Debride...Med Sci Monit In Press; DOI: 10.12659/MSM.941102
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








