23 August 2021: Clinical Research
Cost-Benefit Analysis of Using A Single Dose of Tranexamic Acid in Degenerative Lumbar Scoliosis Patients Undergoing Long-Segment Spinal Fusion Surgery: A Retrospective Study
Lei Yuan123BCE, Yu Jiang123BD, Yinhao Liu123BF, Yan Zeng123ADF*, Zhongqiang Chen123A, Weishi Li123BDOI: 10.12659/MSM.930352
Med Sci Monit 2021; 27:e930352
Abstract
BACKGROUND: Degenerative lumbar scoliosis (DLS) patients undergoing posterior long-segment spinal fusion surgery often require perioperative blood transfusions, and previous studies have reported that increased complications and additional costs accompany these transfusions. One method for decreasing transfusions is the administration of tranexamic acid (TXA). We sought to evaluate the costs and benefits of preoperative administration of 1 g of intravenous TXA, without maintenance, in DLS patients undergoing long-segment spinal fusion surgery.
MATERIAL AND METHODS: Patients who received TXA (TXA group) were compared with patients who did not receive TXA (NTXA group) with regard to blood loss, units of packed red blood cells (PRBC) transfused, hemostasis costs, and perioperative complications. The benefits and costs were estimated through analysis of the spending on NTXA and TXA patients, and were compared. The difference between the cost per patient in the 2 groups was designated as the net cost-benefit. Then, both groups were substratified into non-osteotomy and osteotomy subgroups for further analysis.
RESULTS: Of the 173 patients who met the inclusion criteria, 54 TXA patients had significantly reduced perioperative blood loss and total hemostasis costs compared with NTXA patients (n=119). In the group without osteotomy (n=72), TXA (n=13) reduced perioperative blood loss but did not significantly decrease PRBC units and hemostasis costs. However, in patients undergoing osteotomy (n=101), a remarkable net cost savings of ¥648.77 per patient was shown in the TXA group (n=41) (P<0.001). This was because patients undergoing osteotomy in the TXA group received fewer PRBC units (3.7 vs 5.7, P=0.001).
CONCLUSIONS: A single dose of TXA significantly decreased perioperative blood loss and total hemostasis costs for DLS patients undergoing osteotomy. Furthermore, TXA led to no additional net costs in patients without osteotomy.
Keywords: Blood Loss, Surgical, Cost-Benefit Analysis, Scoliosis, Tranexamic Acid, Antifibrinolytic Agents, Clinical Decision-Making, Combined Modality Therapy, Comorbidity, Disease Management, Spinal Fusion
Background
Degenerative lumbar scoliosis (DLS) is a degenerative spinal disease in which lumbar scoliosis develops in an adult with no previous scoliosis. The prevalence of DLS varies enormously among studies and ranges from 1.4 to 68% [1,2], which is about 13.3% of the Chinese Han population aged more than 40 years [3]. With the increased number of aged people and technical advances, more and more patients are requesting surgical treatment. Decompression for neurological compromise, long-segment fusion, and deformity correction are options for patients with severe sagittal and/or coronal imbalance [4]. Due to the complicated and extensive nature of the deformity, DLS patients undergoing posterior spinal fusion can experience massive intraoperative blood loss and often require perioperative allogeneic or autogenous blood transfusions, especially for patients undergoing osteotomy [5]. Excessive perioperative blood loss may lead to anemia, organ damage, coagulopathy, and the need for blood transfusion. Blood transfusion, in turn, increases the risk of transfusion-transmissible infections, immunological transfusion reactions, and mis-transfusion, as well as long-term mortality rates [6,7]. In addition, the use of allogeneic blood products results in significant cost increases.
Many preoperative and intraoperative strategies have been applied to minimize perioperative blood loss, including intraoperative blood salvage, coagulation factor substitution, antifibrinolytic drugs, hypotensive anesthesia, and measures to avoid hypothermia [8,9]. Aprotinin, tranexamic acid (TXA), and epsilon-aminocaproic acid are antifibrinolytics currently offered as prophylactic agents to reduce surgery-associated blood loss. A previous network meta-analysis found that high-dose TXA administration could be used as an optimal treatment to reduce blood loss and the need for transfusion in spinal surgeries [10]. TXA competitively blocks lysine-binding sites of plasminogen and reduces perioperative blood loss and the need for transfusion in major surgeries. Multiple studies have reported that TXA is widely used due to its proven benefits in reducing blood loss and blood transfusion requirements [11–14]. Furthermore, TXA has helped to decrease costs for total joint arthroplasty [15–17]. Evangelista et al [15] reported that perioperative TXA administration significantly reduced blood transfusion requirements and costs in total hip arthroplasty and total knee arthroplasty participants. McGoldrick et al [16] showed that intravenous TXA led to lower transfusion rates, shorter hospital stay, and an estimated financial savings of €114 586 per patient. Cost-benefit analysis can help decision-makers compare the costs and benefits of various options to manage population health, formulate reasonable, cost-effective prescriptions for treatment, and provide a decision-making basis for the clinical formulation of scientific treatment plans [18]. However, the optimal dose of TXA used in spinal surgery remains unclear, and, until now, there has been no study evaluating the benefit of TXA administration in DLS surgery.
In the present study, we performed a cost-benefit analysis of the use of a single dose of TXA, with no maintenance dose, for DLS patients undergoing correction surgery. We hypothesized that using a single dose of TXA would be cost-effective for DLS patients undergoing correction surgery, especially for those who are undergoing osteotomy.
Material and Methods
STUDY DESIGN AND PATIENT POPULATION:
After obtaining approval from the Ethics Committee (IRB00006761-M2018076) in our institution and registering the study in the Chinese Clinical Trial Registry (registration ID: ChiCTR2000030948.
ANESTHESIA AND INTRAOPERATIVE MONITORING:
Intravenous drugs, including midazolam, sufentanil, propofol, and atracurium, were used for general anesthesia induction. Remifentanil, propofol, and atracurium were used for anesthesia maintenance. Consecutive standard monitoring and arterial blood pressure monitoring were performed during the surgery. The mean arterial pressure (MAP) was maintained at 70 mmHg from the dissection phase until the spine was exposed. The MAP was then controlled at about 20% below the preoperative baseline pressure during the decompression, osteotomy, fusion, and instrumentation procedures, following which the MAP was returned to the baseline. MAP was raised higher if there were any changes in spinal cord evoked potentials. Indications for blood transfusion during the operation included a significant drop in systolic blood pressure (<50 mmHg), any perceived rapid loss of blood, decreased urine output, and alterations in the spinal cord monitoring responses decided by the surgeon and anesthetist. Packed red blood cells (PRBC) were administered when the postoperative hemoglobin (Hb) was less than 80 g/L, or 80 to 100 g/L, combined with symptoms such as tachycardia, fatigue, lethargy, pallor, and poor appetite.
DATA COLLECTION:
Demographic data collected included age, sex, body mass index (BMI), comorbidities, and coagulation status. Preoperative radiographic evaluation included the Cobb angle, the sagittal vertical axis (SVA), the coronal vertical axis from the central sacrum vertical line (CSVL), and overall lumbar lordosis. Operative details assessed included the adult spinal deformity surgery (ASD-S) score [19], the number of fused and fixation levels, the number of interbody fusion levels, and osteotomy grades as classified by Schwab et al [20], estimated intraoperative blood loss (EBL), postoperative drainage volume, intra-/postoperative packed red blood cells (PRBC) units, intraoperative autogenous transfusion volume by intraoperative cell salvage (ICS) system, and surgical duration. No preoperative blood donation was obtained from any of the patients. Cell salvage autologous blood recovery system was used in all cases. Intraoperative EBL was calculated by adding the blood volume collected by both suction and cell-saver systems and weighing the surgical sponges. The postoperative drainages were recorded daily at 6: 00 AM from the drainage bag until the drain tube was removed when the volume was less than 50 mL/day or the drainages were clear and colorless. The estimated blood volume (EBV) was calculated by multiplying 70 mL/kg by the patient’s weight, as previously reported [21]. The sum of the EBL and postoperative drainage was calculated as the total blood loss. The transfusion cost, perioperative hemoglobin, and hematocrit levels were assessed, as were possible thromboembolic complications related to TXA usage that required treatment, including stroke, symptomatic deep vein thrombosis (DVT), symptomatic pulmonary embolism, and myocardial infarction.
The present study’s primary outcome was the total cost of TXA and blood transfusions. The secondary outcomes were mean intraoperative blood loss, postoperative drainage, total blood loss, numbers per patient of intra-/post-operative PRBC units given, in-hospital thrombotic events, and length of hospital stay. To examine the influence of osteotomy in terms of cost-benefit, both groups were substratified into non-osteotomy and osteotomy groups for subanalysis. In calculating costs, we included the costs of drugs, the saline in which the drugs were dissolved, the consumables, and staff- and transfusion-related costs. Each vial of TXA (500 mg/vial) costs ¥4.8 (TXA cost per patient in the TXA group was ¥9.6), and the direct acquisition cost of PRBC transfusion is reported as ¥220 per unit (PRBC unit acquisition-only cost). The total transfusion cost per time includes PRBC cost and materials and staff costs, which is ¥25 per time. PRBC unit total cost refers to the total transfusion cost per patient. The difference between the cost for each patient given TXA vs each patient not using TXA was calculated as the net cost-benefit.
STATISTICAL METHODS:
The data were analyzed by SPSS software version 22 (SPSS, Inc., Chicago, IL). The descriptive results are expressed as mean and standard deviation (SD) for continuous variables with an approximately normal distribution or median and interquartile range. Categorical values are presented as the frequency and the percentage. A simple comparison of data between groups was performed by
Results
There were 173 patients (148 women and 25 men), with a mean age of 62.87±6.52 years, enrolled in either the TXA (N=54) or NTXA (n=119) group. Table 1 shows the preoperative, intraoperative, and postoperative variables for the TXA and NTXA groups. No significant difference was seen in demographic data, preoperative radiographic parameters, or preoperative or postoperative laboratory parameters. Similarly, the mean surgical invasiveness score was approximately 23 (22.92±4.88 vs 23.80±5.34,
Throughout the hospital stay, 93.3% of patients in the NTXA group and 87.04% in the TXA group needed at least 1 transfusion. Regarding total PRBC units used during the patients’ hospital stay, the NTXA group received an average of 4.91±3.39 units, and the TXA group received 3.50±2.34 units (
In the subanalysis, the without-osteotomy cohort consisted of 59 patients who had not received TXA and 13 patients who had received TXA. No significant differences were seen in demographic factors, preoperative radiographic values, preoperative and postoperative laboratory values, or operative factors between the 2 groups (Table 3). The patients who received TXA had significantly lower intraoperative blood loss (
The osteotomy cohort consisted of 41 patients without TXA and 60 patients receiving TXA. There were no significant differences between demographic factors, preoperative radiographic values, preoperative and postoperative laboratory values, or operative factors between the 2 groups (Table 5). The group that received TXA had significantly lower intraoperative blood loss (
Discussion
The present study found that a single dose of 1 g of TXA significantly decreased perioperative blood loss and total hemostasis costs for DLS patients undergoing osteotomy. For patients without osteotomy, TXA decreased perioperative blood loss and led to no additional net costs.
Our findings are consistent with those reported by some other authors investigating the use of TXA. Choi et al [22] reported a statistically significant reduction in intraoperative blood loss and postoperative transfusion relative to patients receiving placebo in 132 patients undergoing multilevel posterior spinal segmental instrumented fusion. A randomized controlled trial [13] of adult patients who underwent posterior instrumented spine surgery also found that administration of TXA could significantly reduce perioperative blood loss. Wong et al [23] similarly found that TXA significantly reduced intraoperative, postoperative, and total blood loss but did not influence transfusion outcomes. Regarding transfusion, previous studies have yielded varying results. In complex multilevel spine fusion with and without an osteotomy procedure, TXA was reported to reduce the total red blood cells transfused [24]. Raksakietisak and colleagues [13] observed that TXA reduced blood transfusion by 64.6% in a prospective randomized study. However, many other studies have concluded that TXA could not significantly reduce allogeneic transfusion requirements [13,23,25]. In the present study, the TXA group had a lower transfusion rate than the NTXA group but this difference was not statistically significant. Nevertheless, TXA reduced postoperative and total transfusion volume.
In the present study, the allogeneic transfusion rate was 87.04% in patients receiving TXA vs 93.28% in the control group. This rate seems much higher than in some previous studies [23,26]. The transfusion trigger in related studies may impact the transfusion rate, as may surgery characteristics, such as decompression levels, osteotomy grade, surgery duration, preoperative Hb level, and TXA dose. In our study, the postoperative transfusion trigger was Hb level <80 g/L or 80 to 100 g/L combined with exhibited symptomatic anemia, although some other studies set the transfusion trigger at Hb <7 g/dL [23]. More than 80% of our study patients underwent fusion of more than 5 segments and decompressed more than 3 levels, with 50.42% of the patients in the NTXA group and 75.93% of those in the TXA group undergoing osteotomy. The intraoperative allogeneic transfusion rate (73.95% in the NTXA group vs 66.67% in the TXA group) in our study was less than the rate reported by Choi et al [22] (88.4% in the NTXA group vs 74.2% in the TXA group), who reported results from spinal deformity surgery. Recent studies have concluded that osteotomy and a posterior approach in spine surgery are risk factors for blood transfusion requirement [8,27].
There has been no consensus on the optimal regimen for TXA delivery in spine surgery. A meta-analysis [28] demonstrated that high-dose TXA (>20 mg/kg) had a better effect than low-dose TXA (<20 mg/kg) in controlling blood loss during scoliosis surgery. Hui et al [29] also revealed that high-dose rather than low-dose TXA could reduce both operative duration and perioperative allogeneic transfusion rates. The blood half-life of TXA is 2 h, and the plasma concentration of TXA is maintained during the first 16 h after administration [30]. We used a single dose of 1000 mg TXA without a maintenance dose, which was lower than 20 mg/kg. That may be a contributory cause for the high blood transfusion rate in our study.
The present study’s main objective was to determine whether a single dose of 1000 mg TXA is cost-effective for DLS patients. A recent study showed that TXA administration significantly reduced intraoperative bleeding and total hemostasis costs for patients undergoing surgery of more than 4-level fusion [18]. However, this study included patients with degenerative pathology, and neither described osteotomy. In our analysis, we first performed a comparison of the TXA cost among osteotomy and non-osteotomy patients. In this subanalysis, patients who underwent osteotomy and received TXA had a net cost-benefit of ¥ 648.77, while patients without osteotomy who received TXA had a net cost-benefit of ¥ 290.08. The ASD-S score has been demonstrated to be accurate in explaining variation in estimated blood loss and operative time in ASD surgery [19], and a significantly higher mean ASD-S score was seen in the osteotomy group (24.99±5.10 vs 20.67±3.68,
Reducing blood transfusions and the volume of blood transfused has many health and economic benefits. First of all, it reduces the risk of transfusion-transmissible infections [6] such as hepatitis C virus, hepatitis B virus, and human immunodeficiency virus. Various studies have estimated cost savings in arthroplasty [15,17,31]. Although many factors can lead to discrepancies, most studies offer strong evidence that TXA use is cost-effective [32]. In our study, from the evaluation of costs related to blood transfusions, we can estimate that with the introduction of TXA administration, our hospital saved ¥416.01 per DLS patient undergoing posterior long-segment fixation and fusion surgery. The prevalence of DLS in the Chinese Han population aged older than 40 years was approximately 13.3% [3]. Although we do not know exactly how many people need surgery, it is a large number of surgeries, and this savings would add up to substantial cost savings. As a result of the large volume of surgery, elective surgery is sometimes delayed due to insufficient blood products in the hospital’s blood banks, which results in prolonging the length of hospitalization and increasing other costs. Our analysis showed that using a single dose of TXA decreased total allogeneic transfusion, which may alleviate the blood shortage.
A major concern about TXA management is the potential for increasing the risk of perioperative thromboembolic complications. In our study, only 1 patient in the NTXA group (a patient with DVT) had a thrombotic complication, and there was no statistically significant difference in the complication rate between the 2 groups. Many studies and meta-analyses have demonstrated that using TXA has no association with any increase in incidence of pulmonary embolism, deep venous thrombosis, or myocardial infarction [14,23,33,34].
Our study has some limitations. Firstly, it was a single-center, retrospective study. However, the demographic data and surgical techniques, and the dose of TXA, were comparable. The only difference was a higher osteotomy rate in the NTXA group, and thus a subanalysis was performed next. Additionally, after adjusting for potential bias and confounding variables, TXA still effectively reduced perioperative bleeding. Secondly, the number of patients in the TXA group was lower than that in the NTXA group, which was also due to the study’s retrospective nature. Our results suggested that administration of a single dose of 1 g TXA, without a maintenance dose, also shows high efficacy and safety in DLS patients undergoing posterior correction surgery, but further studies are needed to find the optimal TXA dose.
Conclusions
A single dose of TXA significantly decreased perioperative blood loss and total hemostasis costs for DLS patients undergoing osteotomy. Furthermore, TXA decreased perioperative blood loss and led to no additional net costs in patients without osteotomy.
Tables
Table 1. Data for patients with or without intraoperative TXA.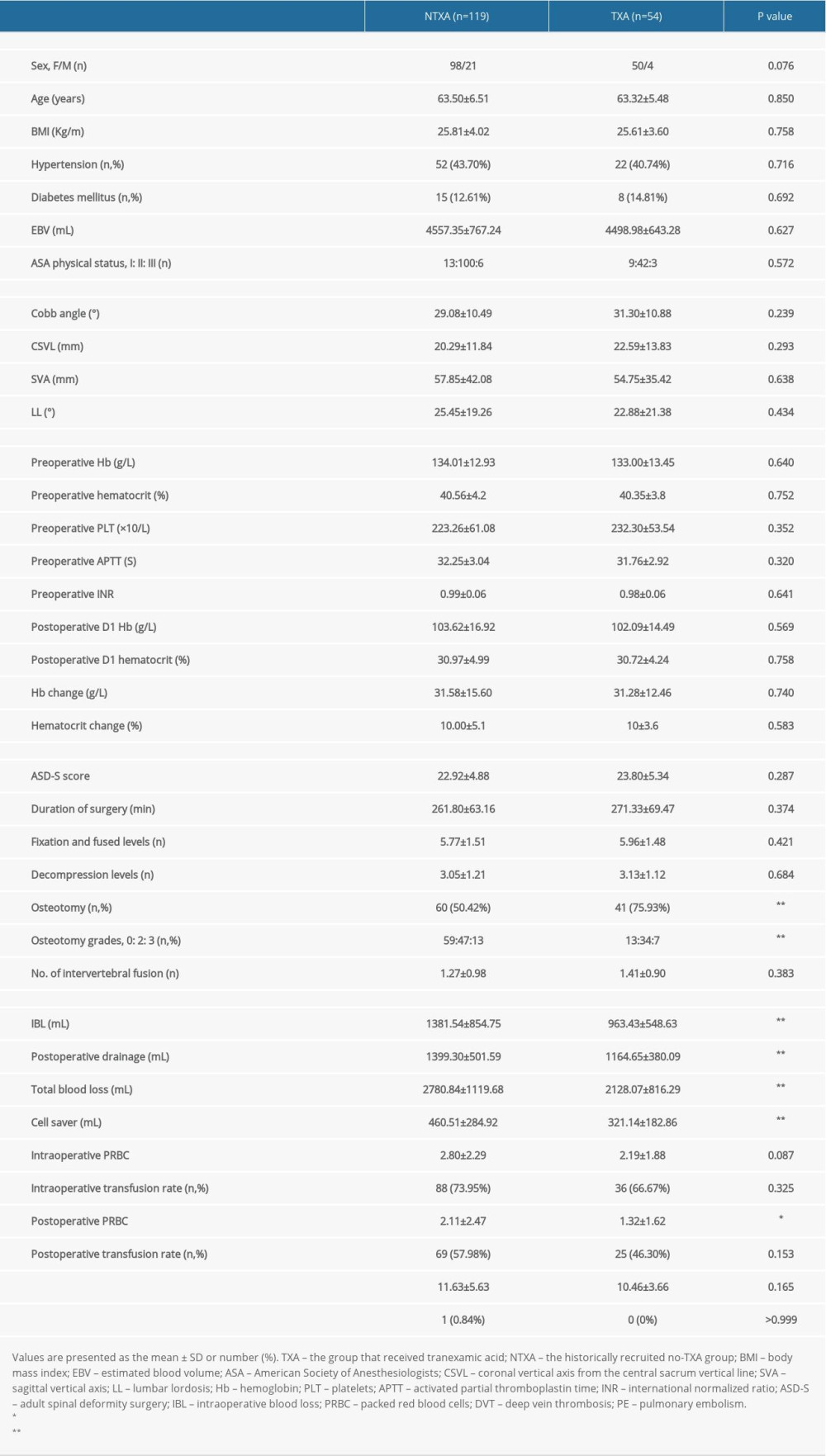 Table 2. Pricing of TXA and transfused PRBC units in patients with or without intraoperative TXA.
Table 2. Pricing of TXA and transfused PRBC units in patients with or without intraoperative TXA.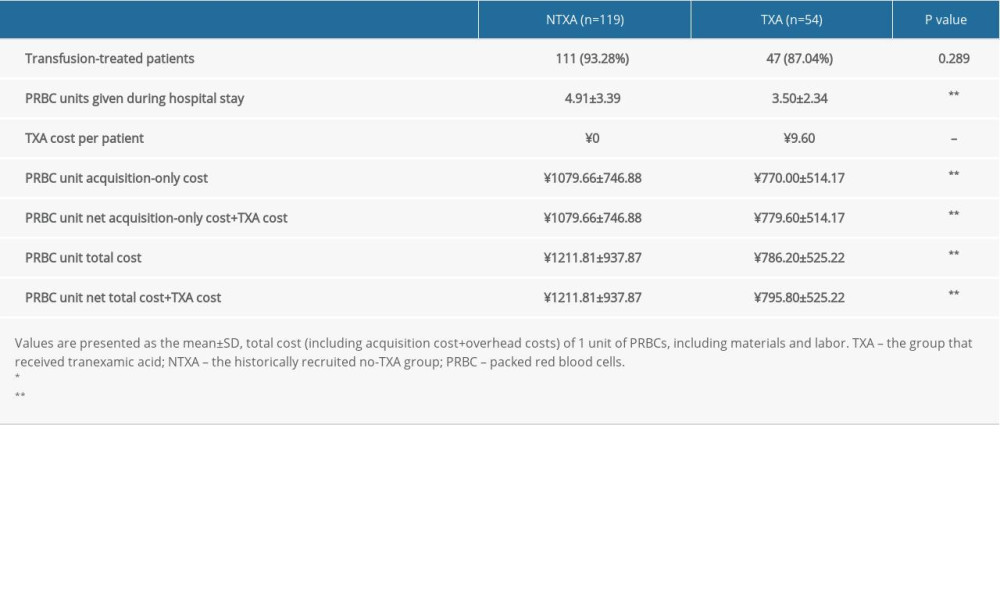 Table 3. Data from subanalyses of patients without osteotomy.
Table 3. Data from subanalyses of patients without osteotomy.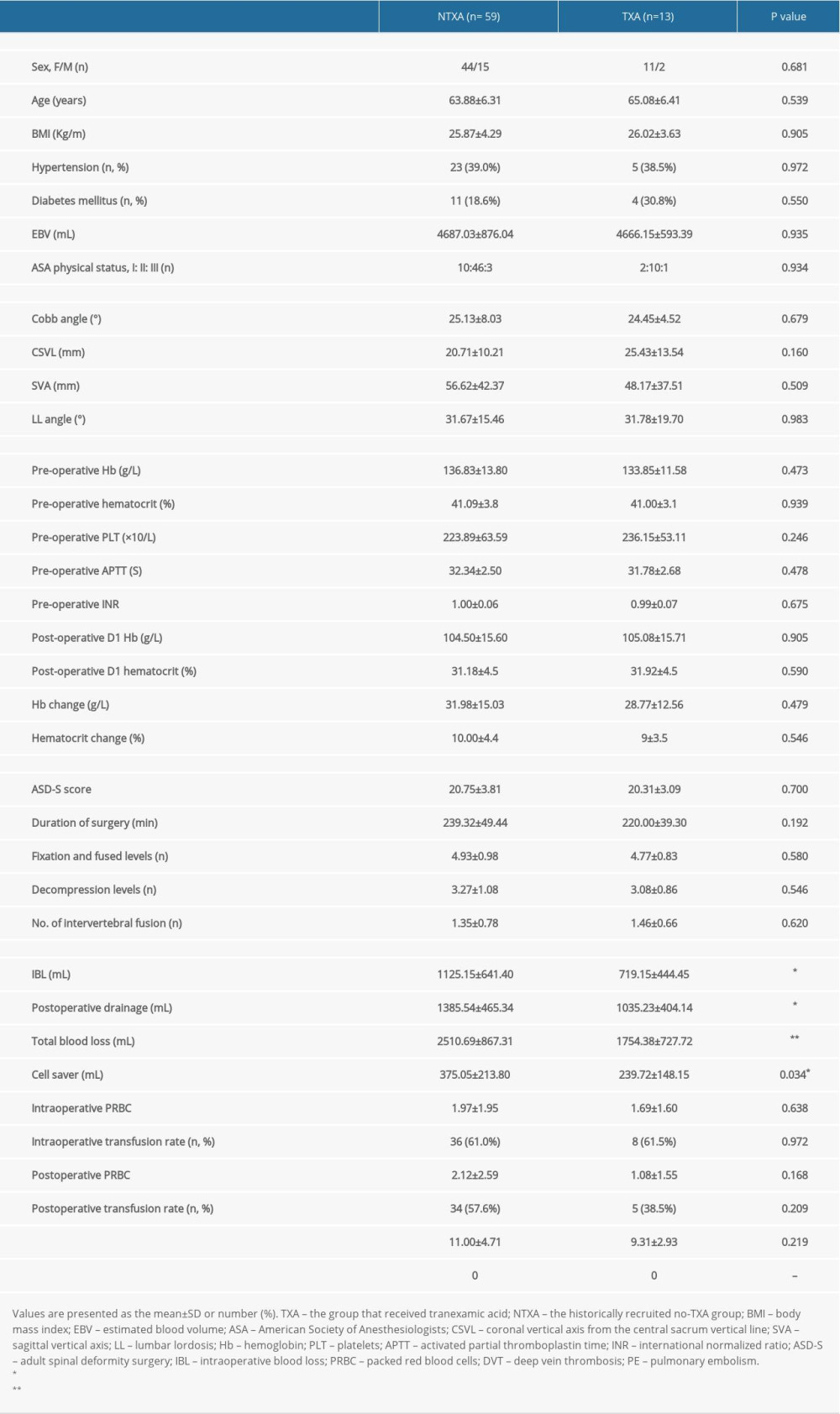 Table 4. Data from subanalyses of TXA pricing and units of transfused PRBCs for non-osteotomy and osteotomy constructs with or without intraoperative TXA.
Table 4. Data from subanalyses of TXA pricing and units of transfused PRBCs for non-osteotomy and osteotomy constructs with or without intraoperative TXA.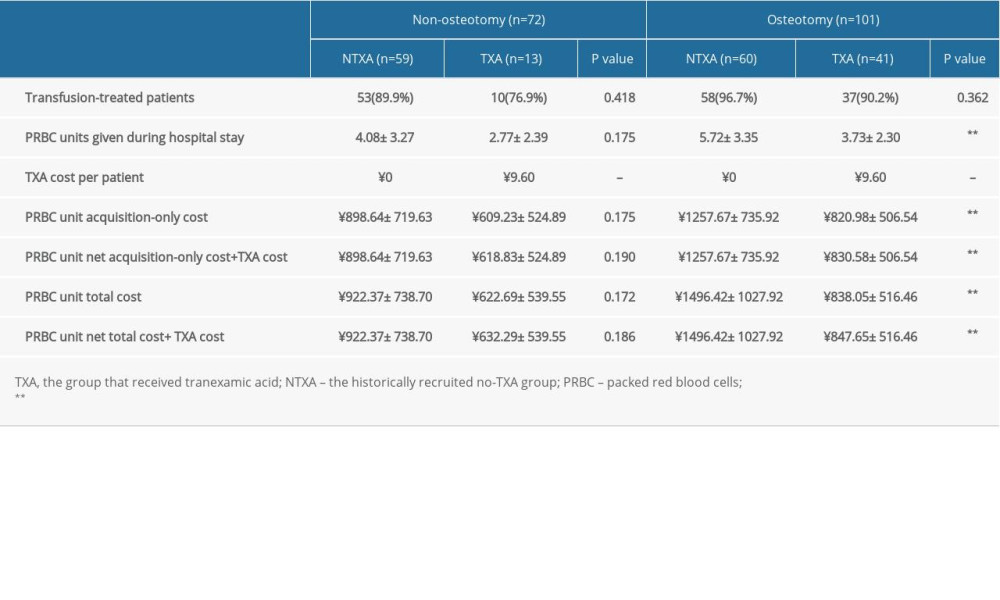 Table 5. Data from subanalyses of patients undergoing osteotomy surgery.
Table 5. Data from subanalyses of patients undergoing osteotomy surgery.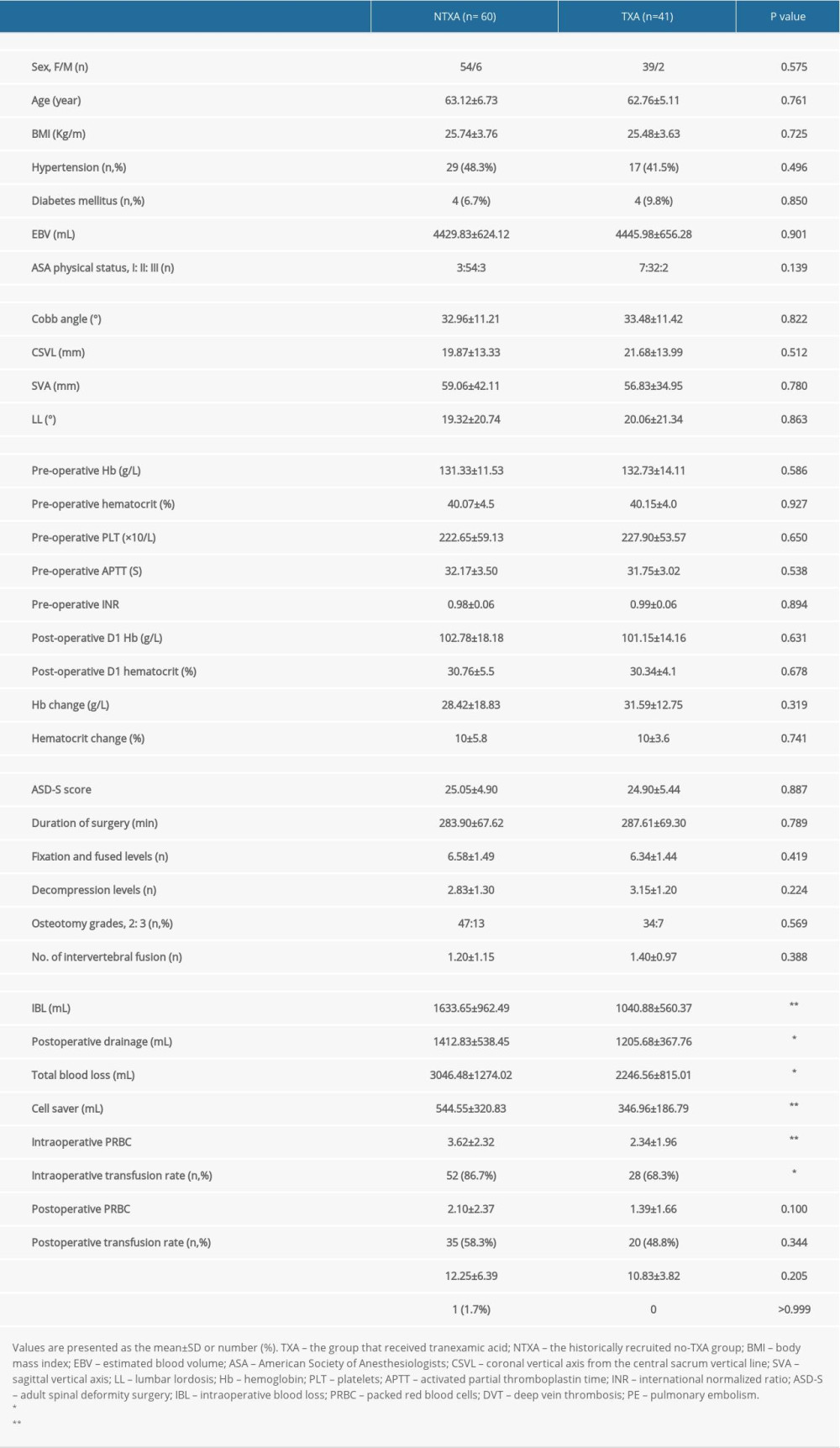
References
1. Schwab F, Dubey A, Gamez LM, Adult scoliosis: prevalence, SF-36, and nutritional parameters in an elderly volunteer population: Spine (Phila Pa 1976), 2005; 30(9); 1082-85
2. Hong JY, Suh SW, Modi HN, The prevalence and radiological findings in 1347 elderly patients with scoliosis: J Bone Joint Surg Br, 2010; 92(7); 980-83
3. Xu L, Sun X, Huang S, Degenerative lumbar scoliosis in Chinese Han population: Prevalence and relationship to age, gender, bone mineral density, and body mass index: Eur Spine J, 2013; 22(6); 1326-31
4. Ha KY, Jang WH, Kim YH, Park DC, Clinical relevance of the SRS-Schwab Classification for degenerative lumbar scoliosis: Spine (Phila Pa 1976), 2016; 41(5); E282-88
5. Wang X, Sun G, Sun R, Bipolar sealer device reduces blood loss and transfusion requirements in posterior spinal fusion for degenerative lumbar scoliosis: A randomized control trial: Clin Spine Surg, 2016; 29(2); E107-11
6. Janssen SJ, Braun Y, Wood KB, Cha TD, Schwab JH, Allogeneic blood transfusions and postoperative infections after lumbar spine surgery: Spine J, 2015; 15(5); 901-9
7. Madjdpour C, Spahn DR, Allogeneic red blood cell transfusions: Efficacy, risks, alternatives and indications: Br J Anaesth, 2005; 95(1); 33-42
8. White SJW, Cheung ZB, Ye I, Risk factors for perioperative blood transfusions in adult spinal deformity surgery: World Neurosurg, 2018; 115; e731-37
9. Theusinger OM, Spahn DR, Perioperative blood conservation strategies for major spine surgery: Best Pract Res Clin Anaesthesiol, 2016; 30(1); 41-52
10. Yuan L, Zeng Y, Chen Z-Q, Efficacy and safety of antifibrinolytic agents in spinal surgery: A network meta-analysis: Chin Med J (Engl), 2019; 132(5); 577-88
11. Nagabhushan RM, Shetty AP, Dumpa SR, Effectiveness and Safety of Batroxobin, Tranexamic Acid and a Combination in Reduction of Blood Loss in Lumbar Spinal Fusion Surgery: Spine (Phila Pa 1976), 2018; 43(5); E267-E273
12. Kim KT, Kim CK, Kim YC, The effectiveness of low-dose and high-dose tranexamic acid in posterior lumbar interbody fusion: a double-blinded, placebo-controlled randomized study: Eur Spine J, 2017; 26(11); 2851-57
13. Colomina MJ, Koo M, Basora M, Intraoperative tranexamic acid use in major spine surgery in adults: A multicentre, randomized, placebo-controlled trialdagger: Br J Anaesth, 2017; 118(3); 380-90
14. Raksakietisak M, Sathitkarnmanee B, Srisaen P, Two doses of tranexamic acid reduce blood transfusion in complex spine surgery: A prospective randomized study: Spine (Phila Pa 1976), 2015; 40(24); E1257-63
15. Evangelista PJ, Aversano MW, Koli E, Effect of tranexamic acid on transfusion rates following total joint arthroplasty: A cost and comparative effectiveness analysis: Orthop Clin North Am, 2017; 48(2); 109-15
16. McGoldrick NP, O’Connor EM, Davarinos N, Cost benefit analysis of the use of tranexamic acid in primary lower limb arthroplasty: A retrospective cohort study: World J Orthop, 2015; 6(11); 977-82
17. Vigna-Taglianti F, Basso L, Rolfo P, Tranexamic acid for reducing blood transfusions in arthroplasty interventions: A cost-effective practice: Eur J Orthop Surg Traumatol, 2014; 24(4); 545-51
18. Ehresman J, Pennington Z, Schilling A, Cost-benefit analysis of tranexamic acid and blood transfusion in elective lumbar spine surgery for degenerative pathologies: J Neurosurg Spine, 2020; 20; 1-9
19. Neuman BJ, Ailon T, Scheer JK, Development and validation of a novel adult spinal deformity surgical invasiveness score:Aanalysis of 464 patients: Neurosurg, 2018; 82(6); 847-53
20. Schwab F, Blondel B, Chay E, The comprehensive anatomical spinal osteotomy classification: Neurosurg, 2014; 74(1); 112-20
21. Jain A, Sponseller PD, Newton PO, Smaller body size increases the percentage of blood volume lost during posterior spinal arthrodesis: J Bone Joint Surg Am, 2015; 97(6); 507-11
22. Choi HY, Hyun SJ, Kim KJ, Jahng TA, Kim HJ, Effectiveness and safety of tranexamic acid in spinal deformity surgery: J Korean Neurosurg Soc, 2017; 60(1); 75-81
23. Wong J, El Beheiry H, Rampersaud YR, Tranexamic acid reduces perioperative blood loss in adult patients having spinal fusion surgery: Anesth Analg, 2008; 107(5); 1479-86
24. Carabini LM, Moreland NC, Vealey RJ, A randomized controlled trial of low-dose tranexamic acid versus placebo to reduce red blood cell transfusion during complex multilevel spine fusion surgery: World Neurosurg, 2018; 110; e572-79
25. Farrokhi MR, Kazemi AP, Eftekharian HR, Akbari K, Efficacy of prophylactic low dose of tranexamic acid in spinal fixation surgery: A randomized clinical trial: J Neurosurg Anesthesiol, 2011; 23(4); 290-96
26. Shi H, Ou Y, Jiang D, Tranexamic acid reduces perioperative blood loss of posterior lumbar surgery for stenosis or spondylolisthesis: A randomized trial: Medicine (Baltimore), 2017; 96(1); e5718
27. Lin JD, Lenke LG, Shillingford JN, Safety of a high-dose tranexamic acid protocol in complex adult spinal deformity: Analysis of 100 consecutive cases: Spine Deform, 2018; 6(2); 189-94
28. Yuan QM, Zhao ZH, Xu BS, Efficacy and safety of tranexamic acid in reducing blood loss in scoliosis surgery: A systematic review and meta-analysis: Eur Spine J, 2017; 26(1); 131-39
29. Hui S, Xu D, Ren Z, Can tranexamic acid conserve blood and save operative time in spinal surgeries? A meta-analysis: Spine J, 2018; 18(8); 1325-37
30. Kushioka J, Yamashita T, Okuda S, High-dose tranexamic acid reduces intraoperative and postoperative blood loss in posterior lumbar interbody fusion: J Neurosurg, 2017; 26(3); 363-67
31. Phan DL, Ani F, Schwarzkopf R, Cost analysis of tranexamic acid in anemic total joint arthroplasty patients: J Arthroplast, 2016; 31(3); 579-82
32. Lin ZX, Woolf SK, Safety, efficacy, and cost-effectiveness of tranexamic acid in orthopedic surgery: Orthopedics, 2016; 39(2); 119-30
33. Cheriyan T, Maier SP, Bianco K, Efficacy of tranexamic acid on surgical bleeding in spine surgery: A meta-analysis: Spine J, 2015; 15(4); 752-61
34. Yuan L, Zeng Y, Chen ZQ, Efficacy and safety of antifibrinolytic agents in spinal surgery: A network meta-analysis: Chin Med J, 2019; 132(5); 577-88
Tables
 Table 1. Data for patients with or without intraoperative TXA.
Table 1. Data for patients with or without intraoperative TXA. Table 2. Pricing of TXA and transfused PRBC units in patients with or without intraoperative TXA.
Table 2. Pricing of TXA and transfused PRBC units in patients with or without intraoperative TXA. Table 3. Data from subanalyses of patients without osteotomy.
Table 3. Data from subanalyses of patients without osteotomy. Table 4. Data from subanalyses of TXA pricing and units of transfused PRBCs for non-osteotomy and osteotomy constructs with or without intraoperative TXA.
Table 4. Data from subanalyses of TXA pricing and units of transfused PRBCs for non-osteotomy and osteotomy constructs with or without intraoperative TXA. Table 5. Data from subanalyses of patients undergoing osteotomy surgery.
Table 5. Data from subanalyses of patients undergoing osteotomy surgery. In Press
06 Mar 2024 : Clinical Research
Comparison of Outcomes between Single-Level and Double-Level Corpectomy in Thoracolumbar Reconstruction: A ...Med Sci Monit In Press; DOI: 10.12659/MSM.943797
21 Mar 2024 : Meta-Analysis
Economic Evaluation of COVID-19 Screening Tests and Surveillance Strategies in Low-Income, Middle-Income, a...Med Sci Monit In Press; DOI: 10.12659/MSM.943863
10 Apr 2024 : Clinical Research
Predicting Acute Cardiovascular Complications in COVID-19: Insights from a Specialized Cardiac Referral Dep...Med Sci Monit In Press; DOI: 10.12659/MSM.942612
06 Mar 2024 : Clinical Research
Enhanced Surgical Outcomes of Popliteal Cyst Excision: A Retrospective Study Comparing Arthroscopic Debride...Med Sci Monit In Press; DOI: 10.12659/MSM.941102
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








