04 November 2021: Lab/In Vitro Research
Association of Single-Nucleotide Polymorphisms of Gab1 Gene with Susceptibility to Meningioma in a Northern Chinese Han Population
Weifeng Chen12ABCEF, Jiahui Zhao3ABC, Qianlan Wu4BC, Hongshan Yan1DF, Xiaoyan WangDOI: 10.12659/MSM.933444
Med Sci Monit 2021; 27:e933444
Abstract
BACKGROUND: The Gab1 gene has an important role in cell proliferation in meningioma via various signaling pathways. However, the relationship between polymorphisms of the Gab1 gene and meningioma remains unknown. In this study, we aimed to investigate the plausible association of single-nucleotide polymorphisms (SNPs) of the Gab1 gene and meningioma risk in a northern Chinese Han population.
MATERIAL AND METHODS: This case-control study included 205 patients with meningioma and 297 healthy controls. Four loci of the Gab1 gene were genotyped using the multiplex snapshot technique. The odds ratio (OR) and 95% confidence interval (CI) were calculated by chi-squared and logistic regression analysis. The distributions of Gab1 SNP genotypes and allele frequencies were compared between patients with meningioma and healthy controls and among patients stratified by clinical phenotypes.
RESULTS: The allelic frequency distributions of G at rs3805236 and C at rs1397529 were significantly higher in patients with meningioma than in healthy controls. The frequency of the rs3805236-GG and rs1397529-AC genotypes were significantly higher in patients with meningioma than in controls. Furthermore, there was a statistically significant difference between the genotypes of patients versus healthy individuals at rs1397529, according to stratification by dural invasion. The allelic frequency distributions of alleles or genotypes at rs3805246 and rs3828512 were not different in patients with meningioma and healthy controls.
CONCLUSIONS: The Gab1 gene rs3805236A>G and rs1397529A>C SNPs increased the risk of meningioma in the northern Chinese Han population. Furthermore, rs1397529A>C may be related to enhanced dural invasion in patients with meningioma.
Keywords: GAB1 Protein, Human, Meningioma, Polymorphism, Single Nucleotide, Adaptor Proteins, Signal Transducing, Female, Humans, Male, Meningeal Neoplasms
Background
Meningiomas are the most common non-glial primary tumor of the central nervous system (CNS), arising from the arachnoidal “cap” cells in the meninges and connected to the dura [1,2]. Meningiomas account for 37.6% of all primary CNS tumors and 53.2% of nonmalignant primary CNS tumors [3]. The incidence of meningioma is 8.83 per 100 000 according to the most recent Central Brain Tumor Registry of the United States and has been increasing in recent years [3–5]. According to the 2016 revision of the World Health Organization (WHO) classification, meningiomas are further categorized into grades I to III: 80% to 90% of cases are WHO I (benign), 20% to 25% are WHO II (atypical), and 1% to 3% are WHO III (anaplastic) [6]. The location and size of a meningioma determine the clinical symptoms, such as headaches, seizures, changes in vision, smell, and hearing, and physical disorders, which severely reduce the life quality of patients. However, there is a lack of an effective biomarker for metastasis prediction and drug target for therapeutic intervention.
Although the etiology and pathogenesis of meningioma remain largely unclear, many studies have verified that the activation of mitogen-activated protein kinases (MAPK) or phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt) signaling pathways are implicated in the pathogenesis of meningioma [7–9]. Presently, the underlying mechanisms of activation of MAPK or PI3K/Akt cascades in meningioma remain unclear.
The growth factor receptor-bound (Grb2)-associated binder (Gab) family of proteins include Gab1, Gab2, and Gab3. Gab1 is the most abundant and widely distributed Gab protein, and Gab3 is mainly expressed in the blood and lymphatic tissues [10–12]. Previous studies have shown that Gab1 prompts cell growth, differentiation, proliferation, migration, and other biological functions mainly through the 2 classical pathways of PI3K/Akt and Ras/MAPK [13–15]. To date, there has been little research on Gab2 or Gab3 in meningioma. However, most studies have confirmed that Gab1 was highly expressed in meningioma [16–18], and the expression might be involved in the invasion of meningioma through the P13K/AKT and Ras/MAPK pathways.
The Gab1 gene is located on chromosome 4, and its single-nucleotide polymorphisms (SNPs) are reported to be closely related to the susceptibility of several human diseases, such as biliary tract cancer and lung cancer [19,20]. The Gab1 SNPs may be involved in the pathogenesis of human diseases by regulating the expression of key genes of disease progression. Whether its genetic SNPs are associated with the risk of meningioma has not yet been reported.
In this study, we genotyped 4 loci of the Gab1 gene and found that Gab1 rs3805236A>G and rs1397529A>C SNPs increased the risk of meningioma in a northern Chinese Han population and the rs1397529A>C SNP may be related to enhanced dural invasion in patients with meningioma. These findings provide a new gene biomarker of meningioma and the basis for our next mechanism research.
Material and Methods
STUDY PARTICIPANTS:
A total of 205 patients with meningioma and 297 healthy controls who were enrolled at the Second Affiliated Hospital of Hebei Medical University from January 2019 to May 2021 were included in this study. No patients with meningioma received radiotherapy or chemotherapy before surgery. Every patient was diagnosed by the Departments of Pathology and Molecular Diagnostics in our hospital according to the 2016 World Health Organization (WHO) classification of CNS tumors [4]. Patients were excluded if they had a history of other tumors, chronic diseases, autoimmune diseases, or endocrine disorders. The study was approved by the Ethics Committee and Institutional Review Board of our hospital. Written informed consent was obtained from each participant.
The study procedure was approved by the Ethics Committee of Hebei Medical University and was in accordance with the principles of the Declaration of Helsinki.
SAMPLE COLLECTION:
For each patient and healthy control participant, 5 mL of peripheral venous blood was collected in an EDTA anticoagulant tube. DNA was obtained from the peripheral blood by the standard salting-out procedure and stored at −20°C until use.
GENOMIC DNA ISOLATION AND SNP GENOTYPING:
The SNPs of Gab1 were selected from the Single-Nucleotide Polymorphism Database of the National Center for Biotechnology Information and the International HapMap Project database with the criteria of linkage disequilibrium value (r2 >0.8) and minor allele frequency (MAF) >0.1 in the Chinese Han population. Thus, 4 selected sites of Gab1 were tested (rs3805246, rs3828512, rs3805236, and rs1397529). The following were included for further study: rs3805246 G/A (located in chr4: 143382955, intron, G>A), rs3828512 A/G (located in chr4: 143395607, intron, A>G), rs3805236 A/G (located in chr4: 143436584, intron, A>G), rs1397529 A/C (located in chr4: 143471009, 3′UTR, A>C).
Primers were designed using Primer5 and were synthesized by the Sangon Company (Shanghai, China). Following the manufacturer’s instructions, genotyping was done using the snapshot multiplex assay and analyzed using an ABI 3730XL genetic analyzer. Quality control analysis was performed on the SNPs, and samples that passed the 95% quality threshold were subjected to further statistical analysis.
STATISTICAL ANALYSES:
The distribution of age, sex, smoking status, and drinking status was analyzed by the
Results
GENOTYPING AND HARDY-WEINBERG EQUILIBRIUM:
Table 1 summarizes the demographic and clinical characteristics of all participants. In total, 205 patients with meningioma and 297 healthy controls were recruited in this study. The mean age was 55.84±11.01 years for the patients and 53.85±13.30 years for the healthy controls. No significant differences were observed in age, sex, smoking history, and alcohol drinking history between the patients and the healthy controls (P>0.05).
The goodness-of-fit chi-squared test was used to calculate the Hardy-Weinberg equilibrium (HWE) between healthy controls and patients. The observed genotype and allelic frequency distributions in healthy controls for all SNPs conformed to the Hardy-Weinberg equilibrium (
GAB1 GENE SNPS WERE CORRELATED WITH MENINGIOMA RISK:
Logistic regression analysis was used to determine the genotype and allele frequencies of the Gab1 SNPs in patients with meningioma and healthy controls under dominant, recessive, and additive models (Table 2). Among the 4 SNPs, 2 SNPs (rs3805236 and rs1397529) were significantly associated with a predisposition for meningioma. For Gab1 rs3805236, the frequency of the G allele was higher in the patient group than in the healthy control group (P=0.001, OR 1.675, 95% CI 1.252–2.240). The frequencies of the GG homozygote and AG+GG genotype in the patient group were significantly higher than those in the control group (GG: P=0.002, OR 3.369, 95% CI 1.704–6.661; AG+GG: P=0.016, OR 1.561, 95% CI 1.085–2.246).
For Gab1 rs1397529, patients with meningioma showed significantly higher frequencies of the rs1397529 C allele (11.2%) than did healthy controls (7.1%) (OR 0.602, 95% CI 0.388–0.934, P=0.023, Pcorr=0.028). Under the codominant model, the heterozygous AC genotype of rs1397529 was associated with a high risk of meningioma, compared with the wild AA genotype (OR 1.737, 95% CI, 1.090–2.768, Pcorr=0.020). With respect to Gab1 rs3805246 and rs3828512, there were no differences in the genotype distribution and allele frequencies between patients with meningioma and healthy controls (P>0.05, Table 2).
LINKAGE DISEQUILIBRIUM AND HAPLOTYPE ANALYSES:
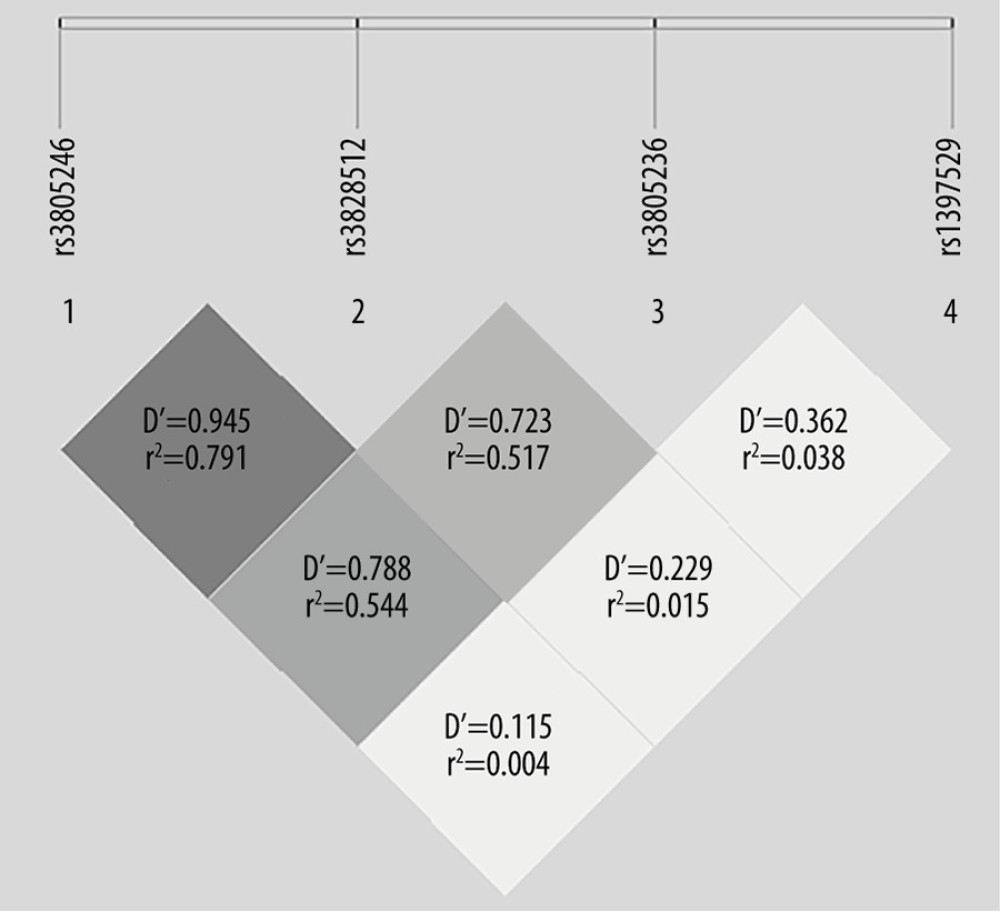
Linkage disequilibrium and haplotype analyses of the SNPs in the patient and healthy control samples were further studied. Linkage disequilibrium relationships between SNPs evaluated using the r2 measure. In Figure 1, the graphical representation generated by SHEsis of the r2 linkage disequilibrium relationship between each SNP is expressed in different colors, where deep red represents high linkage disequilibrium (r2 >0.80) and light red represents low linkage disequilibrium (r2 <0.80). However, the linkage disequilibrium was not strong enough to create any haplotype blocks; therefore, we were not able to perform a haplotype analysis.
STRATIFICATION ANALYSES WERE PERFORMED ACCORDING TO THE CLINICAL FEATURES:
Patients were stratified according to tumor size, status, location, WHO grade, peritumoral edema, and dural invasion status. Tumor size, status, location, WHO grade, and peritumoral edema were not associated with the sites. However, the rs1397529 AC frequencies were determined to be higher in patients with meningioma with dural invasion (P=0.027, Table 3). This may indicate that the SNP of rs1397529 on Gab1 increased the risk of meningioma development and might facilitate dural invasion in patients with meningioma.
Discussion
It has been shown that Gab1 is highly expressed in meningioma, and its downstream signaling pathways have recently been implicated in human meningioma pathogenesis [16–18]. However, the association of the Gab1 SNPs and meningioma risk has not been reported. In our study, we found 2 loci (rs1397529 and rs3805236) were significantly associated with meningioma risk. The distributions of genotypes and alleles of Gab1 rs1397529 and rs3805236 were significantly different between patients with meningioma and healthy controls. For rs1397529, logistic regression analysis showed that the MAF of rs1397529 on the Gab1 gene was significantly higher in patients with meningioma than in healthy controls, suggesting that the C allele elevated the risk of meningioma and the higher heterozygous AC genotype on rs1397529 increased the risk of meningioma in the Chinese Han population. For rs3805236, the G allele of rs3805236 was also higher in meningioma and might be another risk allele of meningioma. Also, the GG homozygote on rs3805236 increased the risk of meningioma in our study. However, no strong linkage disequilibrium relationship between rs1397529 and rs3805236 was observed. Furthermore, there was a statistically significant relationship between the genotypes at rs1397529, as revealed by stratification analysis of dural invasion, suggesting that the AC genotype was significantly more aggressive than the AA genotype in patients with meningioma. The stratification analysis by dural invasion indicated that the SNP of rs1397529 on Gab1 increased the risk of meningioma development, and might have also facilitated dural invasion in patients with meningioma.
Gab1 SNPs have been found to increase the risk of a number of human diseases [14,19–21]. A previous study showed that the Gab1 rs3805246 AA genotype was associated with a low risk of
We found that Gab1 rs3805236 was linked to an increased risk of meningioma; in other words, rs3805236 G was a risk factor for meningioma. Meng reported that the Gab1 rs3805236 gene SNP was not associated with biliary tract cancer susceptibility in the Liaoning Han population [19]. However, our study has some similarities to Meng’s study. The GG genotype frequencies in the control groups of both studies were similar (5.1% vs 6.0%). Also, the genotypes of rs3805236 in the control groups conformed to Hardy-Weinberg equilibrium in both studies (P>0.01). The results that differ could be attributed to the fact that SNPs can generate diverse effects in individuals for different diseases [26].
In the present study, we found that rs1397529 C alleles were risk factors for meningioma. Li et al reported that the MAF of rs1397529 was significantly lower in the case group (5.8%) than in the control group (8.2%); the C allele was a protective factor, and a dominant genetic model of rs1397529 was associated with a reduced risk of lung cancer, compared with the AA genotype in a previous study [20]. We found 2 genotypes (AA and AC) at this locus, while 3 genotypes (AA, AC, and CC) were found in the study by Li et al. The CC genotype in the latter was only 0.8%. The variation may be attributed to a relatively small sample size in our study; however, we achieved a statistical power of 85%. Few studies have investigated the relationship of the Gab1 gene SNPs and meningioma risk in other populations. Therefore, further studies of these SNPs in a larger sample size should be conducted.
According to the stratified dural invasion analysis, there was a significant difference in the genotypes of patients with meningioma at rs1397529. This may indicate that rs1397529 plays a role in meningioma. The rs1397529 site is located in the 3′UTR of Gab1. It has been shown that the main functions of SNPs in 3′UTR are related to the regulation of DNA transcription and the subsequent gene translation and tissue-specific expression. The same gene expression may differently affect the separate cell behaviors through different signaling pathways. 3′UTR is an essential regulatory region for the expression of many genes that regulate the translation, degradation, and subcellular localization of mRNAs by influencing RNA-binding proteins or non-coding RNAs [27,28]. 3′UTR SNPs can significantly impact gene expression by abolishing, weakening, or creating miRNA binding sites [29,30]. The results of the present study indicated that the rs1397529 C allele may facilitate dural invasion in patients with meningioma through regulating 3′UTR of the Gab1 gene and may ultimately influence Gab1 transcription or translation.
Several studies have documented that Gab1 can promote the growth, migration, and invasion of tumor cells through MAPK or PI3K/Akt signaling pathways [31–34]. It seems that decreased MAPK activity was related to the recurrence of meningioma and PI3K activation was related to poor preclinical conditions and brain invasion of malignant meningioma [7]. The expression of phosphorylated Raf and MAPK is reduced in aggressive meningioma; however, inhibition of MAPK leads to reduced tumor cell apoptosis and proliferation [9]. Our results indicated that the rs1397529 C allele may facilitate dural invasion in patients with meningioma through affecting the regulation of the 3′UTR of the Gab1 gene, ultimately influencing Gab1 transcription or translation. In view of findings from published studies and our experimental data, we further speculated that the genetic SNPs in Gab1 may functionally regulate protein expression through the P13K/AKT or MAPK pathway to affect the growth, proliferation, and invasion characteristics of meningioma.
There are limitations to this study. Although we identified the association between the Gab1 SNPs and meningioma risk, further investigations are needed in a larger and more ethnically diverse population of patients with meningioma to confirm our conclusions. Also, patients with meningioma with certain diseases were excluded from our study to maintain the homogeneity of samples; however, the Gab1 genotypes that are inversely associated with these diseases may have been excluded.
Conclusions
In the present study, we were the first researchers to find that the Gab1 gene rs3805236A>G and rs1397529A>C SNPs were associated with meningioma susceptibility in the northern Chinese Han population and that rs1397529A>C may be related to enhanced dural invasion in patients with meningioma. These findings provide a new gene biomarker of meningioma and the basis for our future mechanism research.
Tables
Table 1. Demographic data of the participants for Gab1 single-nucleotide polymorphisms.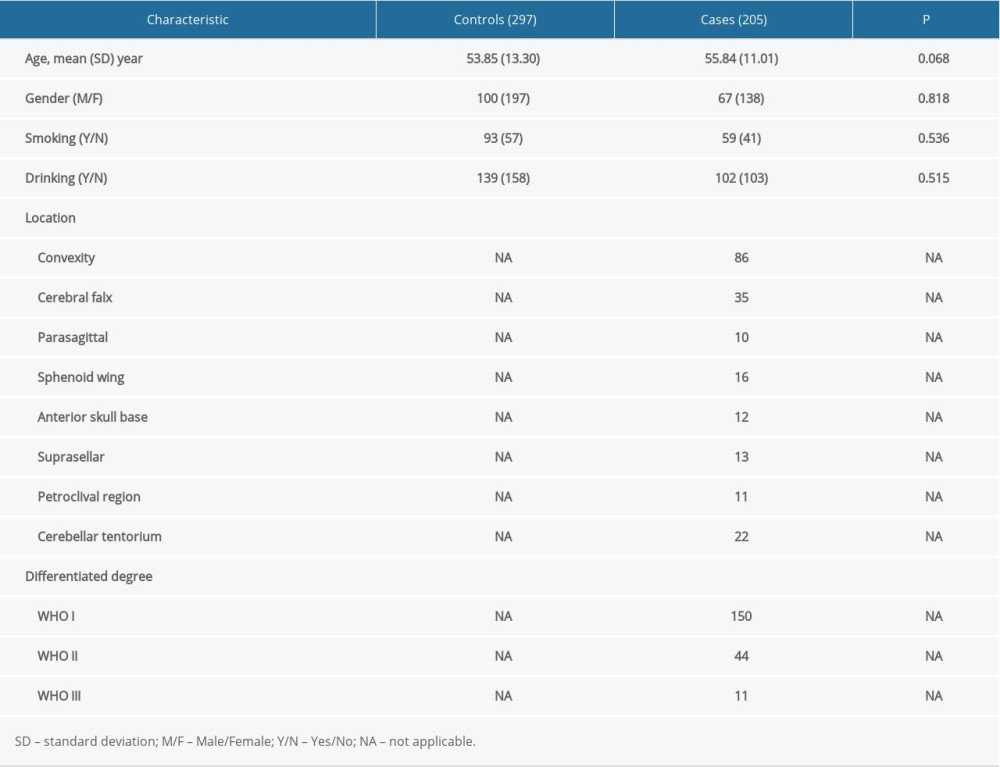 Table 2. Relationship of allele frequencies and genotype distribution of Gab1 single-nucleotide polymorphisms and the risk of meningioma.
Table 2. Relationship of allele frequencies and genotype distribution of Gab1 single-nucleotide polymorphisms and the risk of meningioma.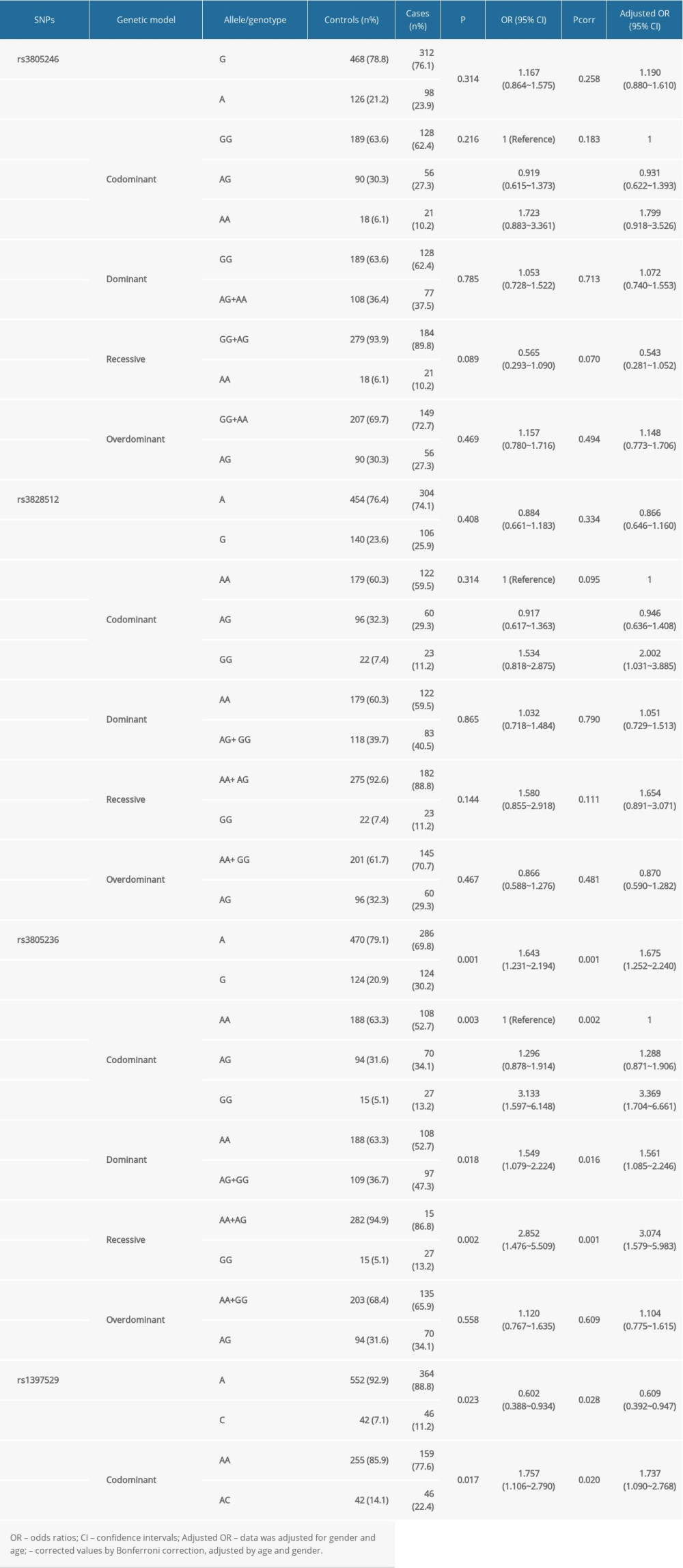 Table 3. Stratification analysis between Gab1 single-nucleotide polymorphisms and the clinical characteristics of meningioma.
Table 3. Stratification analysis between Gab1 single-nucleotide polymorphisms and the clinical characteristics of meningioma.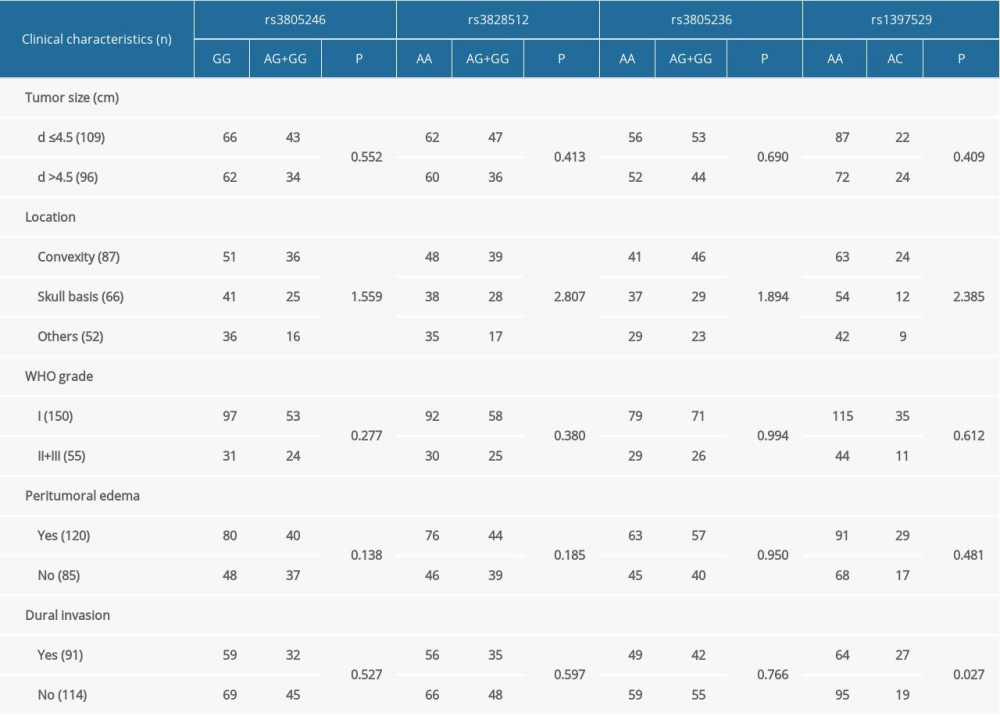
References
1. Buerki RA, Horbinski CM, Kruser T, An overview of meningiomas: Future Oncol (London, England), 2018; 14(21); 2161-77
2. Huntoon K, Toland AMS, Dahiya S, Meningioma: A Review of clinicopathological and molecular aspects: Front Oncol, 2020; 10; 579599
3. Ostrom QT, Cioffi G, Gittleman H, CBTRUS Statistical Report: Primary brain and other central nervous system tumors diagnosed in the United States in 2012–2016: Neuro Oncol, 2019; 21(Suppl 5); v1-v100
4. Louis DN, Perry A, Reifenberger G, The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary: Acta Neuropathol, 2016; 131(6); 803-20
5. Yin B, Liu L, Zhang BY, Correlating apparent diffusion coefficients with histopathologic findings on meningiomas: Eur J Radiol, 2012; 81(12); 4050-56
6. Wang N, Osswald M, Meningiomas: Overview and new directions in therapy: Semin Neurol, 2018; 38(1); 112-20
7. Mawrin C, Sasse T, Kirches E, Different activation of mitogen-activated protein kinase and Akt signaling is associated with aggressive phenotype of human meningiomas: Clin Cancer Res, 2005; 11(11); 4074-82
8. El-Habr EA, Levidou G, Trigka EA, Complex interactions between the components of the PI3K/AKT/mTOR pathway, and with components of MAPK, JAK/STAT and Notch-1 pathways, indicate their involvement in meningioma development: Virchows Arch, 2014; 465(4); 473-85
9. Johnson MD, Okedli E, Woodard A, Toms SA, Allen GS, Evidence for phosphatidylinositol 3-kinase-Akt-p7S6K pathway activation and transduction of mitogenic signals by platelet-derived growth factor in meningioma cells: J Neurosurg, 2002; 97(3); 668-75
10. Gu H, Pratt JC, Burakoff SJ, Neel BG, Cloning of p97/Gab2, the major SHP2-binding protein in hematopoietic cells, reveals a novel pathway for cytokine-induced gene activation: Mol Cell, 1998; 2(6); 729-40
11. Burdon T, Stracey C, Chambers I, Suppression of SHP-2 and ERK signalling promotes self-renewal of mouse embryonic stem cells: Dev Biol, 1999; 210(1); 30-43
12. Nishida K, Yoshida Y, Itoh M, Gab-family adapter proteins act downstream of cytokine and growth factor receptors and T- and B-cell antigen receptors: Blood, 1999; 93(6); 1809-16
13. Eulenfeld R, Schaper F, A new mechanism for the regulation of Gab1 recruitment to the plasma membrane: J Cell Sci, 2009; 122(Pt 1); 55-64
14. Gao L, Weck MN, Nieters A, Brenner H: Mol Carcinog, 2010; 49(10); 869-73
15. Nishida K, Hirano T, The role of Gab family scaffolding adapter proteins in the signal transduction of cytokine and growth factor receptors: Cancer Sci, 2003; 94(12); 1029-33
16. Kakkar A, Kumar A, Das A, 1p/14q Co-deletion: A determinant of recurrence in histologically benign meningiomas: Indian J Pathol Microbiol, 2015; 58(4); 433-38
17. Battu S, Kumar A, Pathak P, Clinicopathological and molecular characteristics of pediatric meningiomas: Neuropathology, 2018; 38(1); 22-33
18. Boetto J, Lerond J, Peyre M, GAB1 overexpression identifies hedgehog-activated anterior skull base meningiomas: Neuropathol Appl Neurobiol, 2021 [Online ahead of print]
19. Meng LQ, Essential role of polymorphism of Gab1, EGFR, and EGF for the susceptibility of biliary tract cancer: Tumour Biol, 2014; 35(12); 12497-508
20. Li X, Li X, Ren Y, Polymorphisms of rs1347093 and rs1397529 are associated with lung cancer risk in northeast Chinese population: Oncotarget, 2017; 8(55); 94862-71
21. Goto Y, Ando T, Nishio K, Grb2-associated binder 1 polymorphism was associated with the risk of Helicobactor pylori infection and gastric atrophy: Int J Med Sci, 2006; 4(1); 1-6
22. Wang H, Pang C, Zeng N, Association between the IL-6 gene polymorphism and tuberculosis risk: A meta-analysis: Infect Drug Resist, 2017; 10; 445-54
23. Zhang Y, Chen L, Chen H, Associations between polymorphisms in IL-10 gene and the risk of viral hepatitis: A meta-analysis: Gut Pathog, 2020; 12; 36
24. Norris JM, Rich SS, Genetics of glucose homeostasis: implications for insulin resistance and metabolic syndrome: Arterioscler Thromb Vasc Biol, 2012; 32(9); 2091-96
25. Bullich G, Ballarín J, Oliver A, HLA-DQA1 and PLA2R1 polymorphisms and risk of idiopathic membranous nephropathy: Clin J Am Soc Nephrol, 2014; 9(2); 335-43
26. Rosen HR, Golden-Mason L, Daly AK, Variants in the LGALS9 gene are associated with development of liver disease in heavy consumers of alcohol: Clin Gastroenterol Hepatol, 2016; 14(5); 762-768.e1
27. Conklin D, Jonassen I, Aasland R, Taylor WR, Association of nucleotide patterns with gene function classes: Application to human 3′ untranslated sequences: Bioinformatics (Oxford, England), 2002; 18(1); 182-89
28. Adyshev DM, Elangovan VR, Moldobaeva N, Mechanical stress induces pre-B-cell colony-enhancing factor/NAMPT expression via epigenetic regulation by miR-374a and miR-568 in human lung endothelium: Am J Respir Cell Mol Biol, 2014; 50(2); 409-18
29. Karthi S, Rajeshwari M, Francis A, 3′-UTR SNP rs2229611 in G6PC1 affects mRNA stability, expression and glycogen storage disease type-Ia risk: Clin Chim Acta, 2017; 471; 46-54
30. Kim JO, Park HS, Ryu CS, Interplay between 3′-UTR polymorphisms in the methylenetetrahydrofolate reductase (MTHFR) gene and the risk of ischemic stroke: Sci Rep, 2017; 7(1); 12464
31. Wang X, Peng J, Yang Z, Elevated expression of Gab1 promotes breast cancer metastasis by dissociating the PAR complex: J Exp Clin Cancer Res, 2019; 38(1); 27
32. Xu L, Li J, Kuang Z, Kuang Y, Wu H, Knockdown of Gab1 inhibits cellular proliferation, migration, and invasion in human oral squamous carcinoma cells: Oncol Res, 2018; 26(4); 617-24
33. Hu L, Liu R, Expression of Gab1 is associated with poor prognosis of patients with epithelial ovarian cancer: Tohoku J Exp Med, 2016; 239(3); 177-84
34. Sang H, Li T, Li H, Liu J, Down-regulation of Gab1 inhibits cell proliferation and migration in hilar cholangiocarcinoma: PLoS One, 2013; 8(11); e81347
Tables
 Table 1. Demographic data of the participants for Gab1 single-nucleotide polymorphisms.
Table 1. Demographic data of the participants for Gab1 single-nucleotide polymorphisms. Table 2. Relationship of allele frequencies and genotype distribution of Gab1 single-nucleotide polymorphisms and the risk of meningioma.
Table 2. Relationship of allele frequencies and genotype distribution of Gab1 single-nucleotide polymorphisms and the risk of meningioma. Table 3. Stratification analysis between Gab1 single-nucleotide polymorphisms and the clinical characteristics of meningioma.
Table 3. Stratification analysis between Gab1 single-nucleotide polymorphisms and the clinical characteristics of meningioma. Table 1. Demographic data of the participants for Gab1 single-nucleotide polymorphisms.
Table 1. Demographic data of the participants for Gab1 single-nucleotide polymorphisms. Table 2. Relationship of allele frequencies and genotype distribution of Gab1 single-nucleotide polymorphisms and the risk of meningioma.
Table 2. Relationship of allele frequencies and genotype distribution of Gab1 single-nucleotide polymorphisms and the risk of meningioma. Table 3. Stratification analysis between Gab1 single-nucleotide polymorphisms and the clinical characteristics of meningioma.
Table 3. Stratification analysis between Gab1 single-nucleotide polymorphisms and the clinical characteristics of meningioma. In Press
05 Mar 2024 : Clinical Research
Muscular Function Recovery from General Anesthesia in 132 Patients Undergoing Surgery with Acceleromyograph...Med Sci Monit In Press; DOI: 10.12659/MSM.942780
05 Mar 2024 : Clinical Research
Effects of Thermal Insulation on Recovery and Comfort of Patients Undergoing Holmium Laser LithotripsyMed Sci Monit In Press; DOI: 10.12659/MSM.942836
05 Mar 2024 : Clinical Research
Role of Critical Shoulder Angle in Degenerative Type Rotator Cuff Tears: A Turkish Cohort StudyMed Sci Monit In Press; DOI: 10.12659/MSM.943703
06 Mar 2024 : Clinical Research
Comparison of Outcomes between Single-Level and Double-Level Corpectomy in Thoracolumbar Reconstruction: A ...Med Sci Monit In Press; DOI: 10.12659/MSM.943797
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952