20 September 2020: Meta-Analysis
Efficacy and Toxicity of Adjuvant Therapies for High-Risk Endometrial Cancer in Stage I–III: A Systematic Review and Network Meta-Analysis
Mengyin Ao12ABCDEF, Ting Ding12BF, Dan Tang12E, Mingrong Xi12E*DOI: 10.12659/MSM.925595
Med Sci Monit 2020; 26:e925595
Abstract
BACKGROUND: The use of adjuvant therapy for high-risk endometrial cancer patients (HREC) in International Federation of Gynecology and Obstetrics (FIGO) stage I-III remains debatable. This network meta-analysis was conducted to compare and rank adjuvant therapies based on efficacies and toxicities to facilitate clinical decision-making and further research.
MATERIAL AND METHODS: We searched 3 databases – PubMed, EMBASE, and the Cochrane Central Register of Controlled Trials – from inception to December 9, 2019. Only randomized controlled trials that compared any of these adjuvant therapies (pelvic radiotherapy, vaginal brachytherapy, chemotherapy, and chemoradiotherapy) with each other or surgery alone were included. The network meta-analysis was performed in a frequentist framework using Stata software 15.0.
RESULTS: Fourteen RCTs with 5872 participants were eligible. No significant difference between treatments was observed in 5-year overall survival (OS) or distant metastasis. Compared with surgery alone, adjuvant pelvic radiotherapy plus chemotherapy (pelvic RT-CT) prolonged 5-year progression-free survival (PFS) and pelvic radiotherapy (pelvic RT) (RR=0.61, 95% CI 0.39–0.96; RR=0.779, 95% CI 0.63–0.95). Compared with surgery alone, pelvic RT, the combination of pelvic RT and vaginal brachytherapy (pelvic RT-VBT), chemotherapy (CT), and pelvic RT-CT led to fewer local recurrences (RR=0.33, 95% CI 0.21–0.50; RR=0.15, 95% CI 0.03–0.74; RR=0.39, 95% CI 0.21–0.73; RR=0.17, 95% CI 0.06–0.46). Adjuvant CT was found to result in more grade III/IV late toxicities than surgery alone (RR=11.8, 95% CI 1.02–137.14). Pelvic RT-CT ranked first for OS, PFS, distant metastasis, and local recurrence.
CONCLUSIONS: Pelvic RT-CT is superior to other treatments for PFS and local recurrence rate, and associated related toxicities are tolerable, suggesting it may be an ideal adjuvant therapy for HREC patients.
Keywords: Chemoradiotherapy, Adjuvant, Chemotherapy, Adjuvant, Radiotherapy, Adjuvant, Endometrial Neoplasms
Background
Endometrial cancer is a common cancer in women and its prevalence is increasing [1]. In 2018, around 382 069 new cases of endometrial cancer were diagnosed worldwide and there were 89 929 related deaths [2,3]. Most patients are diagnosed at early stages and cured through surgery alone. However, patients in International Federation of Gynecology and Obstetrics (FIGO) stages I–III with risk factors (non-endometrioid disease (serous or clear cell adenocarcinoma and other types of carcinoma), grade II or III histology, positive lympho-vascular space invasion (LVSI), pelvic or para-aortic nodes metastasis, myometrial invasion >50%, cervical stroma involvement, and invasion of adnexa) had higher recurrence rates [4,5]. Consequently, adjuvant radiotherapy, chemotherapy, and chemoradiotherapy have been applied to reduce the recurrence rate when risk factors exist.
Pelvic radiotherapy has been the standard adjuvant treatment for high-risk endometrial cancer (HREC) for many years. The adjuvant radiotherapy (RT) for HREC patients has been evaluated in randomized controlled trials (RCTs), all of which demonstrated that the use of RT decreased the rate of loco-regional recurrence but did not improve the 5-year overall survival rate (OS) or reduce distant metastasis [6–9]. Distant metastases remain a significant cause of death in these patients; therefore, adjuvant chemotherapy is proposed to prevent distant recurrences. The therapeutic benefit of chemotherapy for patients with endometrial cancer was first confirmed in GOG-122, which suggested that chemotherapy with doxorubicin-cisplatin improved clinical outcomes compared with whole abdominal irradiation [10]. Nevertheless, several RCTs in which CT alone and RT alone were compared showed that, although chemotherapy delayed distant relapses and the RT delayed local recurrences, OS and PFS were similar between groups [11,12]. Therefore, some trials evaluated the efficacy of chemoradiotherapy in the treatment of HREC patients after surgical management [13–17]. The results are controversial, showing either that chemoradiotherapy prolonged the PFS or had no survival benefit. According to the ESMO-ESGO-ESTRO consensus guidelines for endometrial cancer and the NCCN clinical practice guidelines for uterine neoplasms stage I–II, endometrioid HREC is recommended as adjuvant pelvic RT with or without CT, while there is more evidence supporting use of the combination of pelvic RT and CT for stage III endometrioid HREC [5,18]. Additionally, CT with or without pelvic RT is recommended for non-endometrioid cancer [5,18]. However, this leaves clinicians with a dilemma of how to choose the optimal treatment strategy for patients: pelvic RT and CT in combination or alone. Therefore, there is still no widely accepted adjuvant therapy for HREC patients.
Based on direct head-to-head comparisons of 2 adjuvant therapies, several systematic reviews have compared different adjuvant therapies for HREC patients [19–21]. In the absence of RCTs comparing all available adjuvant therapies, it is still uncertain which is the most effective and safest option. Network meta-analyses have compared numerous treatments simultaneously by combining direct and indirect evidence and provide a hierarchy of these treatments [22,23]. Therefore, the present network meta-analysis was conducted to analyze the effectiveness and toxicity of adjuvant therapies for HREC to identify the most effective treatment with the least toxicity, which could potentially better inform clinical decision-making.
Material and Methods
DATA SOURCES AND SEARCHES:
PubMed, EMBASE, the Cochrane Central Register of Controlled Trials (CENTRAL) were searched for eligible studies up to December 9, 2019. We also hand-searched the citation lists of relevant trials, systematic reviews, and the reports of conferences. The Medical Subject Headings (MeSH) used in the search strategy included “Endometrial Neoplasms”, “Radiotherapy”, “Chemotherapy, Adjuvant,” and “Chemoradiotherapy”. Searches were limited to literature in English or Chinese.
STUDY SELECTION:
The inclusion criteria were as follows: 1. RCTs comparing at least 2 of the following intervention after surgery: no further treatment, adjuvant pelvic RT, adjuvant vaginal brachytherapy (VBT), adjuvant CT, and chemoradiotherapy; 2. Surgically-treated endometrial cancer diagnosed with histology; 3. stage I to stage III disease according to FIGO staging classification involved with at least 1 risk factors including non-endometrioid disease (serous or clear cell adenocarcinoma, other types of carcinoma), grade II or III histology, positive LVSI, pelvic or para-aortic nodal metastasis, myometrial invasion >50%, cervical stroma involvement, invasion of adnexa. The exclusion criteria were as follows: 1. chemotherapy or radiotherapy before surgery; 2. treatment with targeted therapy or hormone therapy. 3. quasi-randomized trials. All search results were imported into EndNote X9 reference management software. Titles, keywords, and abstracts were screened independently by 2 authors (MYA and TD). Then, the inclusion and exclusion criteria were applied to full texts for further evaluation. Any disagreements were resolved via discussion between 2 reviewers or via consulting the third reviewer, if necessary.
DATA EXTRACTION:
The data from included studies were extracted independently by 2 reviewers (MYA and TD) using the same pre-populated form. The primary outcomes evaluated in the network meta-analysis were 5-year OS and 5-year PFS. The secondary outcomes included the distant metastasis rate, the local recurrence rate, and grade III/IV acute and late toxicities. The details collected also contained authors, year of publication, study design, study location, inclusion and exclusion criteria, intervention details, total number enrolled, age, and FIGO stage of participants. The methodological quality of included studies were evaluated independently by 2 reviewers (MYA and TD) using the Revised Cochrane risk of bias tool for randomized trials (RoB 2) [25]. Any disagreements on data extraction or quality assessment were resolved by discussion or consulting the third reviewer.
DATA SYNTHESIS AND STATISTICAL ANALYSIS:
Traditional pair-wise meta-analyses were performed to directly compare different adjuvant therapies. The risk ratios (RR) and 95% confidence intervals (CI) were calculated for all outcomes using a fixed-effects model or random-effects model. The heterogeneity was estimated using the I2 test. If the I2 value was greater than 50%, a random-effects model was performed for each variable; otherwise, a fixed-effects model was used for meta-analysis.
The network meta-analysis was conducted in a frequentist framework using Stata software 15.0. As all outcomes involved in this study were dichotomous variables, we used a random-effects model to assess RR and 95% CI for all outcomes as conservative estimates. We analyzed the data on an intention-to-treat basis as far as possible. In network diagrams, interventions were presented by nodes, and head-to-head studies between interventions were presented by edges. Network diagrams were produced with node size corresponding to the number of participants assigned to receive each intervention and the line width corresponding to the number of studies comparing the interventions. Use of the inconsistency test was waived because of the absence of a closed-loop in the network meta-analysis. To assess the plausibility of assumption of transitivity, we summarized and compared the clinical and methodological characteristics of studies, then the transitivity assumption was considered valid. We estimated the cumulative probabilities for each adjuvant therapy being at each possible rank and obtained a treatment hierarchy using the surface under the cumulative ranking curve (SUCRA). The larger the value of SUCRA, the higher its rank among all available adjuvant therapies. Comparison-adjusted funnel plots were used to explore the possibility of small study effects.
Results
STUDY SELECTION:
The results of the research were summarized in the PRISMA flow diagram (Figure 1). The electronic database searches yielded 2834 potentially relevant studies, from which we excluded 600 studies as duplicates. A total of 2182 studies were ruled out after screening the titles and abstracts. We further reviewed 52 full-text articles for eligibility. Finally, 14 RCTS involving 5872 participants were included in analysis [7–9,11–17,26–28].
CHARACTERISTICS AND QUALITY ASSESSMENT OF INCLUDED STUDIES:
The characteristics of included studies were summarized in Table 1. Fourteen RCTs were 2-arm trials involving a total of 5872 patients. The characteristics of patients were not identical across included studies, but the vast majority of patients were at high risk of recurrence and the study groups were well-balanced. A total of 2628 patients among 12 RCTs underwent pelvic RT and the doses were in the range of 44–56 Gy. In 5 RCTs, VBT was added for patients with cervical involvement [8,13,14,17]. Five RCTs involving 773 patients experienced pelvic RT-CT [14–17]. Adjuvant CT was given following completion of RT in 3 RCTs [14,15]. In Kuoppala et al. [16], CT and RT were given in a “sandwich” fashion CT used during the pause in RT. In another RCT, 2 cycles of cisplatin were administered in the first and fourth week of RT, then followed by 4 cycles of carboplatin and paclitaxel at 21-day intervals [17]. In 3 RCTs [14,16], patients in the pelvic RT-CT arm received anthracycline-based CT. However, only doxorubicin was used in 1 RCT [15]. Patients in the other RCT received CT comprising cisplatin, carboplatin, and paclitaxel [17]. In 2 RCTs, 366 patients in the CT group received cyclophosphamide, doxorubicin, and cisplatin [11,12]. All 289 patients in 2 RCTs were given VBT after the completion of pelvic RT [26,29]. Only 1 study contained the combination of VBT and CT, and VBT was given first, then paclitaxel was given 3 weeks later [13].
The results of methodological quality assessment are presented in Figure 2. All of the enrolled RCTs were evaluated according to the following items: random sequence generation, allocation concealment, blinding of participants and personnel, detection bias, incomplete outcome data, selective reporting, and other bias.
PAIR-WISE META-ANALYSIS OF THE EFFICACY AND TOXICITY OF DIFFERENT THERAPIES:
The results of individual studies and pair-wise meta-analysis were presented in forest plots. There was no significant difference in OS and distant metastasis rate among direct comparisons (Supplementary Figures 1 and 2). As presented in Supplementary Figure 3, the PFS of pelvic RT was relatively lower than that of pelvic RT-CT (RR=1.31, 95% CI 1.09–1.58). Compared to no further treatment, pelvic RT significantly reduced the local recurrence rate (RR=0.32, 95% CI 0.21–0.48) (Supplementary Figure 4). Direct comparison showed that pelvic RT caused more grade III/IV late toxicities than no further treatment (RR=3.09, 95% CI 1.79–5.33) (Supplementary Figure 5).
NETWORK META-ANALYSIS OF THE EFFICACY AND TOXICITY OF DIFFERENT THERAPIES:
The network plots for OS, PFS, distant metastasis, local recurrence, and grade III/IV late toxicities are summarized in Figure 3, in which node size corresponds to the number of participants assigned to receive each intervention, and the line width corresponds to the number of studies comparing the interventions. Given the scarcity of data on grade III/IV acute toxicities, it was impossible to quantify them in different adjuvant therapies.
There were 14 RCTs involving 5872 patients that reported data on OS [7–9,11–17,26–28]. As shown in Table 2, there was no significant difference in network comparisons. The SUCRA values for each therapy for OS are shown in Table 3. The largest value of SCURA was 76.1 for pelvic RT-CT, indicating that pelvic RT-CT was more likely to improve OS.
Twelve RCTs reported PFS in 4977 patients [8,9,11–14,16,17, 26–28]. Pelvic RT-CT significantly prolonged 5-year PFS compared to no further treatment and pelvic RT (RR=0.61, 95% CI 0.39–0.96; RR=0.77, 95% CI 0.63–0.95), while there was no significant difference among the remaining network comparisons (Table 2). The SUCRA values for PFS are shown in Table 3, which suggests that pelvic RT-CT with the largest SUCRA value was most likely to prolong PFS among these adjuvant therapies.
Data on the number of distant metastases were available from 11 RCTs involving 4737 patients [7–9,11,12,15–17,26–28], but no significant difference was observed (Table 2). When treatments were ranked by SUCRA values, pelvic RT-CT still ranked first, followed by pelvic RT-VBT, CT, no further treatment, and pelvic RT (Table 3).
Eleven RCTs reported the total number of local recurrence events in 4430 patients [7–9,11,12,14,16,26–28]. The local recurrence rate in pelvic RT-VBT was lower than that of VBT (RR=0.23, 95% CI 0.07–0.71). Compared with no further treatment, pelvic RT-CT, pelvic RT, CT, and pelvic RT-VBT led to less local recurrence (RR=0.17, 95% CI 0.06–0.46; RR=0.33, 95% CI 0.21–0.50; RR=0.39, 95% CI 0.21–0.73; RR=0.15, 95% CI 0.03–0.74) (Table 2). As shown in Table 3, pelvic RT-CT still ranked highest and pelvic RT-VBT ranked second.
For grade III/IV late toxicities, network comparison was based on data extracted from 8 RCTs involving 4611 patients [7–9,11,13,17,26,28]. CT was found to result in more grade III/IV late toxicities than in groups with no further treatment (RR=11.8, 95% CI 1.02–137.14). Groups with no further treatment clearly ranked highest, while chemotherapy ranked lowest (Table 3).
PUBLICATION BIAS:
Comparison-adjusted funnel plots for all the above-mentioned outcomes are presented in Supplementary Figure 1. No evidence of publication bias or other small study effects were observed.
Discussion
To the best of our knowledge, this is the first systematic review and network meta-analysis of various adjuvant therapies for HREC. For OS and distant metastasis, no significant differences between treatments were found. Adjuvant pelvic RT-CT prolonged PFS compared to pelvic RT and surgery alone, and pelvic RT-CT reduced local recurrence after surgery. As for late toxicities, there was no significant difference among pelvic RT-CT, pelvic RT, and surgery alone. The ranks indicated that pelvic RT-CT was most likely to improve OS and PFS among all adjuvant therapies. Additionally, pelvic RT-CT ranked first for distant metastasis and local recurrence. In summary, adjuvant pelvic RT-CT may be an ideal strategy in treatment of HREC, which is consistent with results from previous meta-analyses [19,30]. Yi et al. performed a meta-analysis in 2018 enrolled 6 RCTs and suggested that adjuvant chemoradiotherapy is well tolerated and can significantly improve PFS and cancer-specific survival compared with radiotherapy [19].
For many years, adjuvant radiotherapy has been the cornerstone of adjuvant therapy for HREC. It seems clear that adjuvant radiotherapy can effectively inhibit its recurrence inside the field that is treated. Three RCTs comparing adjuvant pelvic RT to surgery alone all demonstrated that pelvic RT led to a highly significant reduction of loco-regional recurrence, but there was no clear tendency toward prevention of distant metastases or improvement of OS [6, 7, 9]. The potential OS benefits by reduced loco-regional recurrence were probably offset by a high incidence of distant metastases, thus generating the idea of adding CT to or replacing RT for HREC. In several studies, adjuvant CT combined with RT was reported to have an advantage over RT or CT alone for PFS and CSS, even for OS [14, 16, 31–33]. The retrospective study by Marchetti et al. assessed stage III endometrial cancer patients in which PFS at 3 years was 86.5%, 65.8%, and 44.1% with pelvic RT-CT, CT, and RT alone, respectively [33]. Another large retrospective study, by Alvarez et al., found a significant difference between the adjuvant therapies groups for OS and PFS (p<0.001), with those accepting combination therapy having better 3-year OS (79%) and PFS (62%) compared with either RT (70% and 59%) or CT (33% and 19%) [32]. In the NSGO-EC-9501/EORTC-55991 trial, additional adjuvant CT to pelvic RT was associated with a 36% reduction in the risk of recurrence or death (HR 0.64, 95% CI 0.41–0.99; P=0.04), but there was no significant difference in OS. In combined analysis with the Mango ILIADE-III trial, OS approached statistical significance (HR 0.69 95% CI 0.46–1.03; P=0.07) and CSS was significant (HR 0.55 95% CI 0.44–0.89; P=0.01) [14]. In the present study, although 27.7% of patients in pelvic RT-CT groups had stage III disease, which was relatively higher than that of groups with other adjuvant therapies, pelvic RT-CT still ranked first for efficacies, without increased late toxicities. This reaffirms the role of pelvic RT-CT for HREC. Based on the above-mentioned trials and our results, the addition of CT to pelvic RT appears to improve outcomes for HREC patients whose survival was severely limited by recurrence.
The beneficial effects of CT in treatment of endometrial seem to be restricted to cisplatin, doxorubicin, and paclitaxel-based regimens. The GOG 122 study was the first to recommend doxorubicin plus cisplatin as the standard treatment regimen of adjuvant chemotherapy [10]. Recent studies reported that taxanes combined with platinum showed good efficacy and tolerability [34–36]. In the NSGO/EORTC and Mango study, in which HREC were randomly allocated to adjuvant RT with or without sequential CT consisting of cisplatin and doxorubicin, the addition of CT improved PFS, but OS did not differ significantly [14]. However, there is no widely accepted RCT comparing different scheduling of CT and RT (sequential, sandwich, and synchronization). Consequently, the optimal scheduling is still unknown. In the present study, all included studies used sequential chemoradiotherapy except for Kuopalla et al. Several studies have been conducted to evaluate chemoradiotherapy given in sandwich fashion, most of which supported that sandwich therapy was feasible, efficacious, and well tolerated for endometrial cancer patients [37–42]. All trials included in our study used pathological features to stratify endometrial cancer patients into intermediate, high-intermediate, and high risk. With the advent of newly discovered molecular markers of endometrial cancers, scholars have proposed risk stratification criteria based on analysis from the Cancer Genome Atlas (TCGA) and verified it by data from some prospective and retrospective studies [43,44]. In general, more attention should be paid to the application of new risk stratification criteria and optimization of scheduling for pelvic RT and CT.
It may be confusing that pelvic RT ranked behind no further treatment for OS, distant metastasis, and local recurrence. The possible reason for this result is that all patients with no further treatment had FIGO stage I–II diseases, while 14.1% of patients treated with pelvic RT had FIGO stage III diseases. Most studies indicated that adjuvant therapy has no impact on OS, including the present study. There are several possible explanations for this. Firstly, the most likely explanation is that not all enrolled patients were at high risk of recurrence, especially risk of distant metastases. A few patients at intermediate risk were mixed with high-risk patients, hiding the potential benefits for survival. The PORTEC-3 trial enrolled 660 patients, of whom 47% had stage III disease. In subgroup analysis for FIGO stage, patients with stage III disease had relatively lower OS and PFS than those with stage I–II disease. Also, patients with serous cancers had worse survival outcomes than those with other histological subtypes [17]. In a meta-analysis conducted by Park et al., subgroup analysis for FIGO stage suggested that, for advanced endometrial cancer, chemoradiotherapy had a larger survival benefit compared to radiotherapy (OS HR 0.53, 95% CI 0.36–0.80; PFS HR 0.54, 95% CI 0.37–0.77) [21]. In view of above results, a subgroup analysis for FIGO stage or histological subtypes should be performed to evaluate those effects on survival, but the available data was insufficient. Secondly, although all patients were treated with hysterectomy and bilateral salpingo-oophorectomy, some patients did not undergo comprehensive surgical staging because para-aortic and pelvic lymphadenectomy was optional in most included RCTs. Thirdly, differences in chemotherapy regimens, sequence of CT and RT, and VBT boost in case of cervical involvement also have influences on survival outcomes.
As adjuvant therapy can cause toxicities that affect quality of life and tolerance, the therapeutic benefits must overweigh the disadvantages. It is impossible to quantify acute toxicities of adjuvant therapies due to the lack of adequate data, but there are some available data. The ASTEC/EN.5 trial reported that acute toxicities were greater in the pelvic RT group than surgery alone (any toxicities: 57%
There are some limitations of our study. (a) Given the scarcity of head-to-head trials of treatments, only 14 RCTs were eligible for inclusion criteria, so the results of this study are mainly derived from indirect comparisons of adjuvant therapies. (b) The discrepancies in patient characteristics between groups, such as FIGO stage, the number of risk factors and different chemotherapy regimens, patterns of radiotherapy, and target volume, are sources of heterogeneity, which may lead inherent differences. (c) Subgroup analyses were impracticable due to the absence of detailed data. Therefore, we still cannot determine which patients are more likely to benefit from pelvic RT-CT. (d) The results on grade III/IV late toxicities are not accurate enough and we could not accurately quantify toxicities of adjuvant therapies due to the lack of data. (e) The literature search was limited to English and Chinese, and we might have missed related studies published in other languages. Despite these limitations, most of the studies we included were of high quality. Network meta-analysis can develop credible ranking systems of the likely efficacy and safety of different treatments, even in the absence of head-to-head trials [48]. Therefore, the results of our study can be still useful for clinical decision-making and further research.
Conclusions
In conclusion, pelvic RT-CT is superior to other treatments for PFS and local recurrence rate, and the related toxicities are tolerable. Therefore, the combination of pelvic RT and CT may be an ideal adjuvant therapy for HREC with FIGO stage I–III. However, none of these adjuvant treatments confers a significant advantage in OS and distant metastases. Further studies should be conducted to identify subgroups of HREC patients based on FIGO stages and histology types. To optimize the use of adjuvant therapy, attention also should be paid to the sequence of CT and pelvic RT, as well as to the implementation of new molecular-based risk classification. In clinical practice, the option of adjuvant treatment for individuals should consider the risk of local and distant relapse to balance benefits and toxicities.
Figures
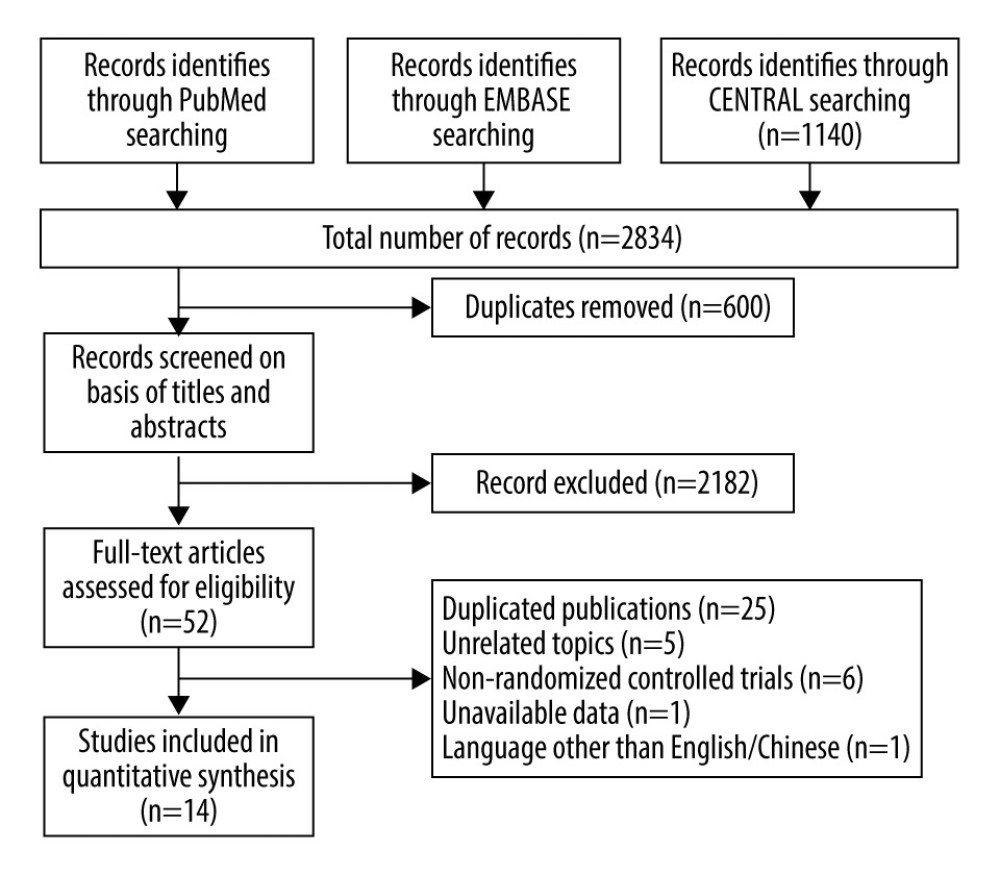 Figure 1. Flow diagram of the study selection procedure.
Figure 1. Flow diagram of the study selection procedure. 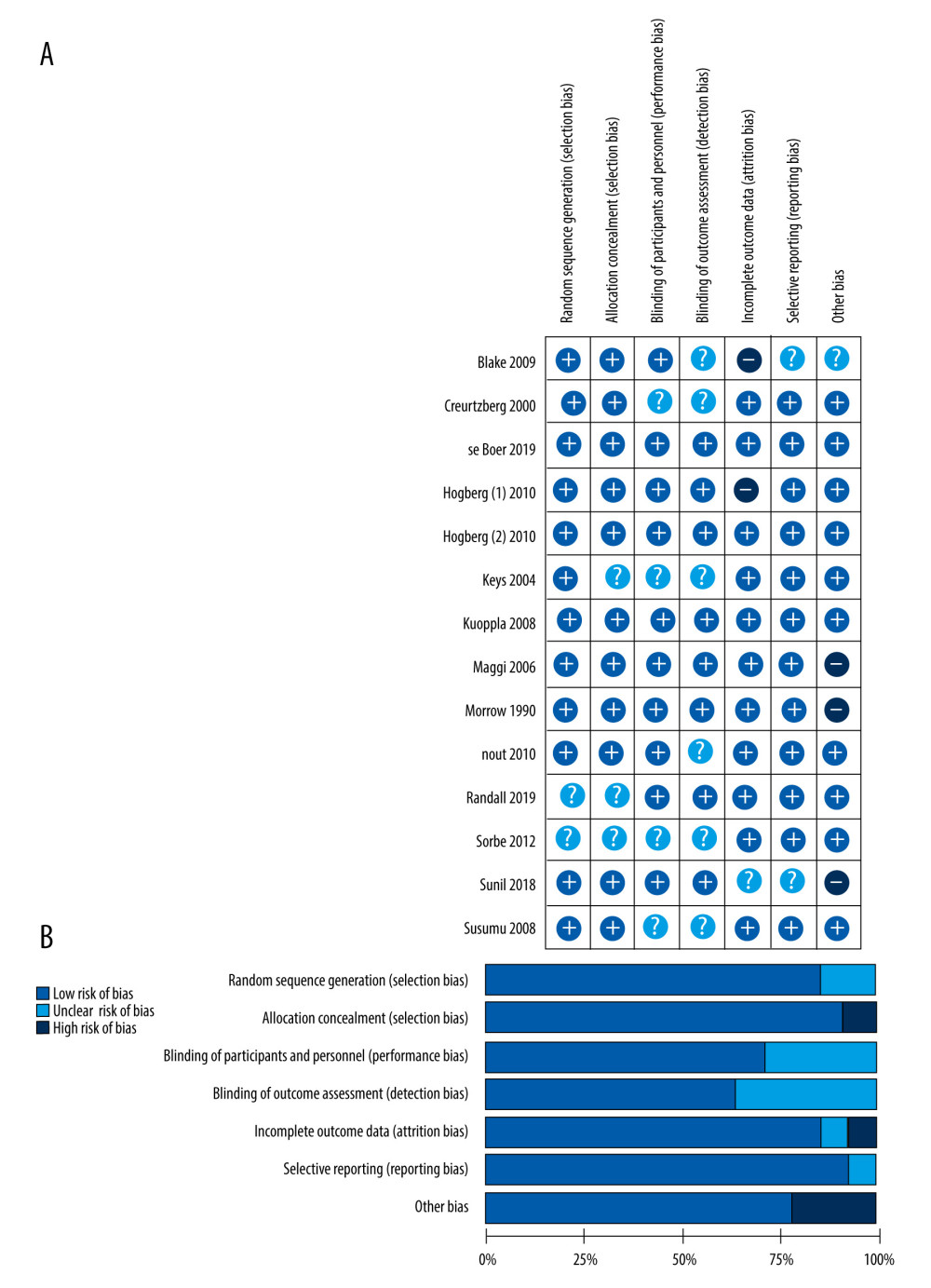 Figure 2. (A) The risk of bias graph showing the reviewers’ assessment of the risk of bias for each included study; (B) risk of bias summary showing the reviewers’ assessment of the risk of bias presented as percentages for all the included studies.
Figure 2. (A) The risk of bias graph showing the reviewers’ assessment of the risk of bias for each included study; (B) risk of bias summary showing the reviewers’ assessment of the risk of bias presented as percentages for all the included studies. 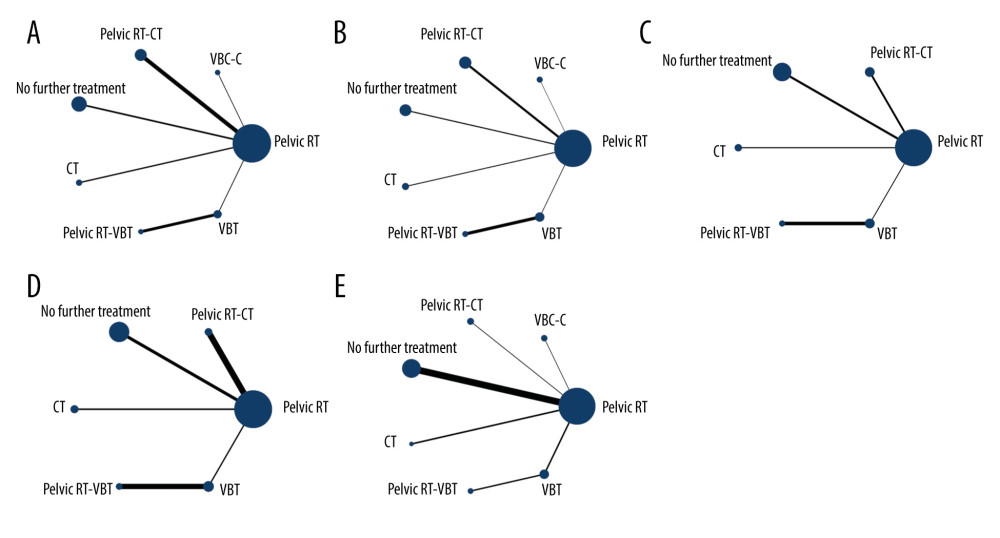 Figure 3. Network diagrams for (A) overall survival, (B) progression-free survival, (C) distant metastasis rate, (D) local recurrence rate and (E) grade III/IV late toxicities. Pelvic RT – pelvic radiotherapy; VCB-C – vaginal cuff brachytherapy and chemotherapy; pelvic RT-CT – pelvic radiotherapy plus chemotherapy; CT – chemotherapy; pelvic RT-VBT – pelvic radiotherapy and vaginal brachytherapy. Interventions with direct comparisons are linked with a line; the thickness of connecting lines corresponds to the number of trials evaluating the comparison. Node size corresponds to the number of participants assigned to receive each intervention.
Figure 3. Network diagrams for (A) overall survival, (B) progression-free survival, (C) distant metastasis rate, (D) local recurrence rate and (E) grade III/IV late toxicities. Pelvic RT – pelvic radiotherapy; VCB-C – vaginal cuff brachytherapy and chemotherapy; pelvic RT-CT – pelvic radiotherapy plus chemotherapy; CT – chemotherapy; pelvic RT-VBT – pelvic radiotherapy and vaginal brachytherapy. Interventions with direct comparisons are linked with a line; the thickness of connecting lines corresponds to the number of trials evaluating the comparison. Node size corresponds to the number of participants assigned to receive each intervention. Tables
Table 1. Characteristics of included randomized clinical trials.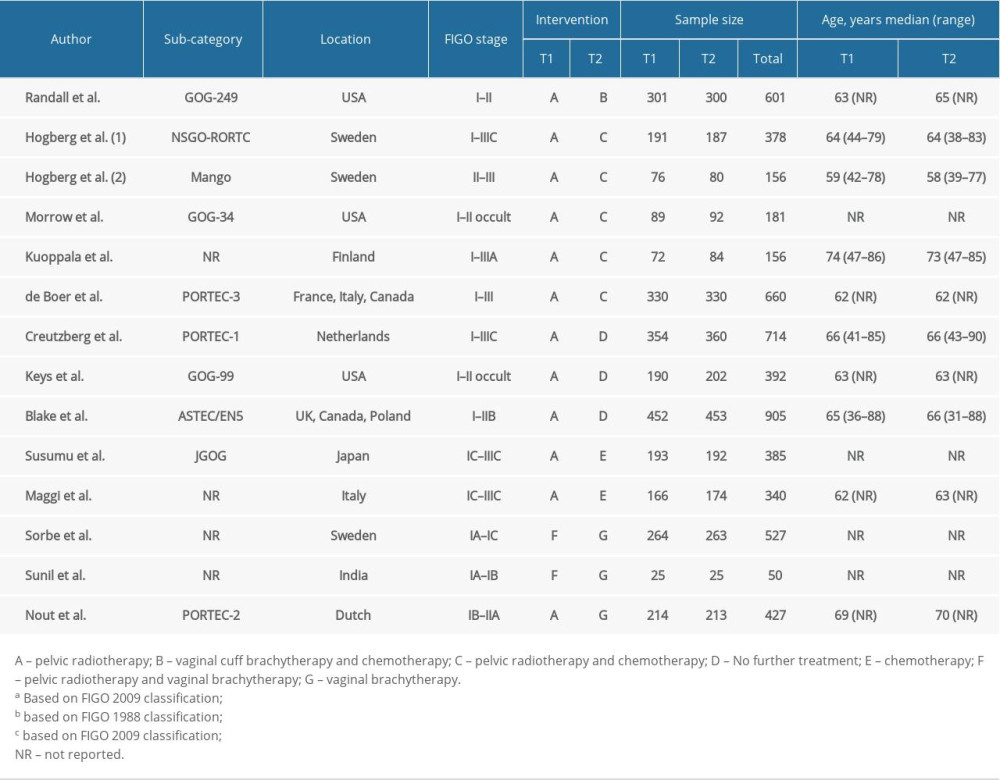 Table 2. League Table of pair-wise comparisons in the network meta-analysis for the relative risks (RR) of overall survival (OS), progression-free survival (PFS), distant metastasis rate, local recurrence rate, and grade III/IV late toxicities.
Table 2. League Table of pair-wise comparisons in the network meta-analysis for the relative risks (RR) of overall survival (OS), progression-free survival (PFS), distant metastasis rate, local recurrence rate, and grade III/IV late toxicities.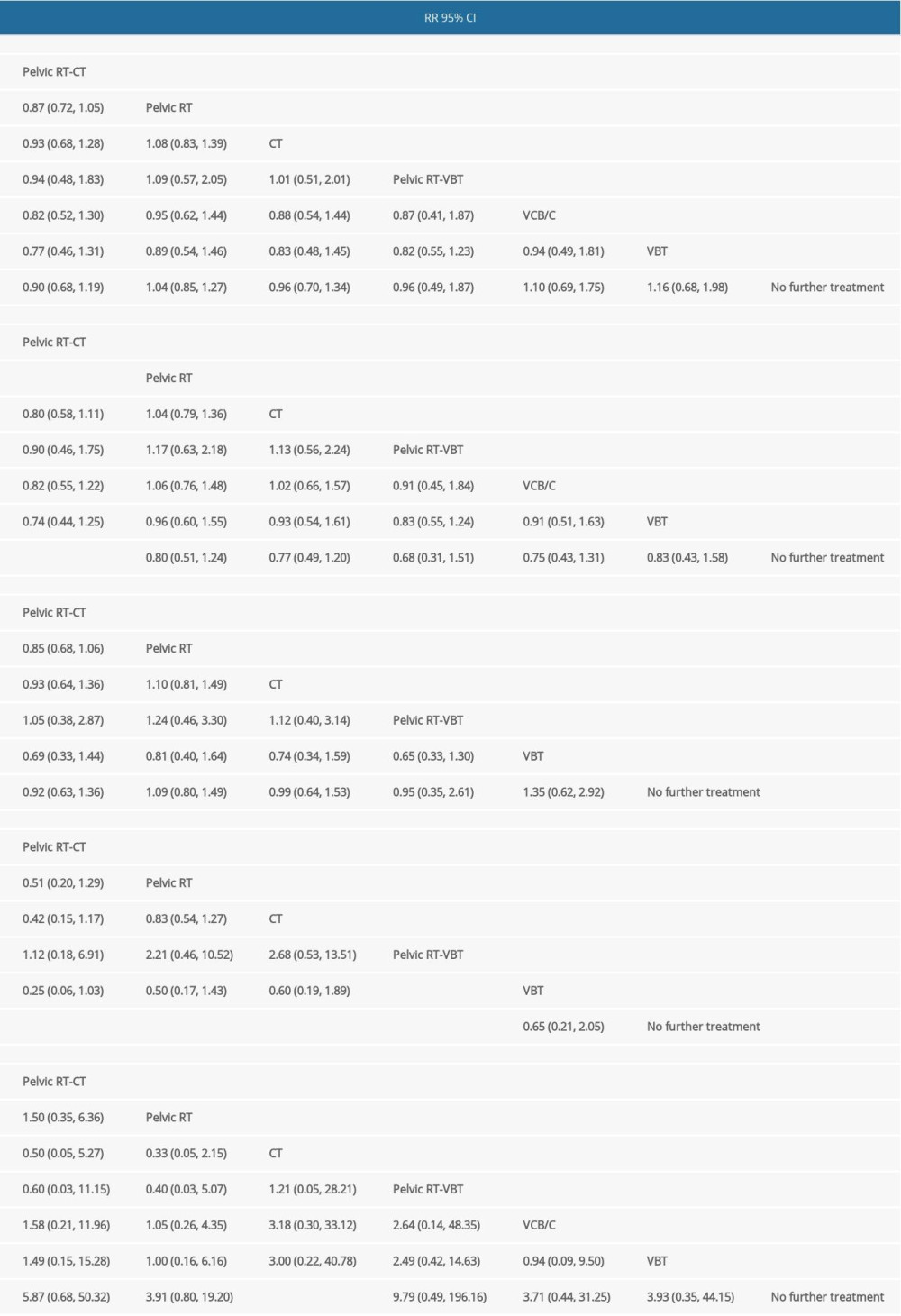 Table 3. SUCRA values of 7 adjuvant therapies for high-risk endometrial cancer patients under 5 outcomes.
Table 3. SUCRA values of 7 adjuvant therapies for high-risk endometrial cancer patients under 5 outcomes.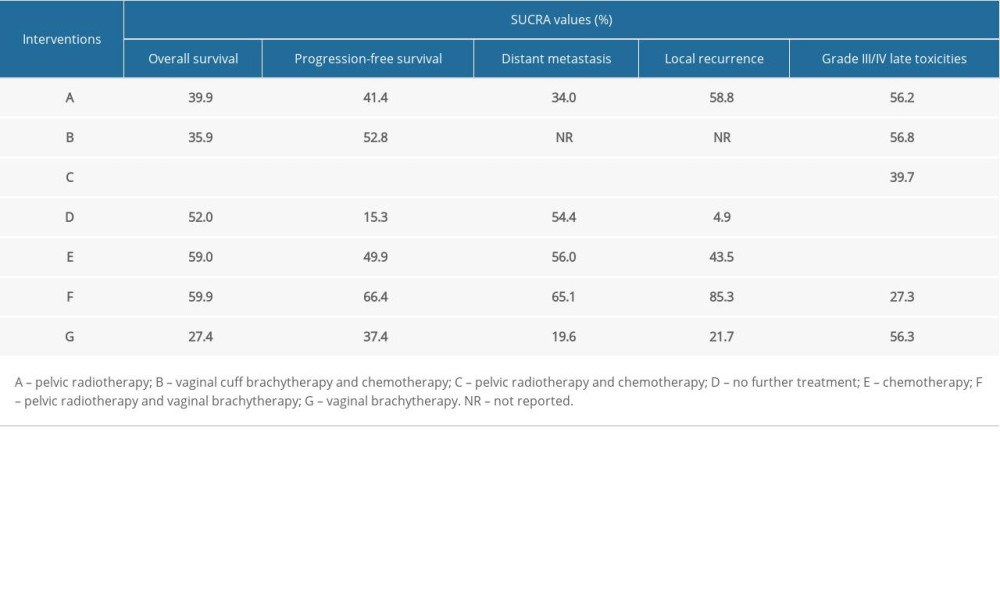
References
1. Siegel RL, Miller KD, Jemal A, Cancer statistics, 2019: Cancer J Clin, 2019; 69; 7-34
2. Bray F, Ferlay J, Soerjomataram I, Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries: Cancer J Clin, 2018; 68; 394-424
3. Morice P, Leary A, Creutzberg C, Endometrial cancer: Lancet, 2016; 387; 1094-108
4. Creutzberg CL, Van Putten WLJ, Wárláin-Rodenhtiis CC, Outcome of high-risk stage IC, grade 3, compared with stage I endometrial carcinoma patients: The postoperative radiation therapy in endometrial carcinoma trial: J Clin Oncol, 2004; 22; 1234-41
5. Colombo N, Creutzberg C, Amant FESMO-ESGO-ESTRO Consensus Conference Working Gropu, ESMO-ESGO-ESTRO Consensus Conference on Endometrial Cancer: Diagnosis, treatment and follow-up: Ann Oncol, 2016; 27; 16-41
6. Aalders J, Abeler V, Kolstad P, Onsrud M, Postoperative external irradiation and prognostic parameters in stage I endometrial carcinoma: Clinical and histopathologic study of 540 patients: Obstet Gynecol, 1980; 56; 419-27
7. Creutzberg CL, Van Putten WLJ, Koper PCM, Surgery and postoperative radiotherapy versus surgery alone for patients with stage-1 endometrial carcinoma: Multicentre randomised trial: Lancet, 2000; 355; 1404-11
8. Blake P, Swart AM, Orton J, Adjuvant external beam radiotherapy in the treatment of endometrial cancer (MRC ASTEC and NCIC CTG EN.5 randomised trials): Pooled trial results, systematic review, and meta-analysis: Lancet, 2009; 373; 137-46
9. Keys HM, Roberts JA, Brunetto VL, A phase III trial of surgery with or without adjunctive external pelvic radiation therapy in intermediate risk endometrial adenocarcinoma: A Gynecologic Oncology Group study: Gynecol Oncol, 2004; 92; 744-51
10. Randall ME, Filiaci VL, Muss H, Randomized phase III trial of whole-abdominal irradiation versus doxorubicin and cisplatin chemotherapy in advanced endometrial carcinoma: A Gynecologic Oncology Group Study: J Clin Oncol, 2006; 24; 36-44
11. Susumu N, Sagae S, Udagawa Y, Randomized phase III trial of pelvic radiotherapy versus cisplatin-based combined chemotherapy in patients with intermediate- and high-risk endometrial cancer: A Japanese Gynecologic Oncology Group study: Gynecol Oncol, 2008; 108; 226-33
12. Maggi R, Lissoni A, Spina F: Br J Cancer, 2006; 95; 266-71
13. Randall ME, Filiaci V, McMeekin DS, Phase III Trial: Adjuvant pelvic radiation therapy versus vaginal brachytherapy plus paclitaxel/carboplatin in high-intermediate and high-risk early stage endometrial cancer: J Clin Oncol, 2019; 37; 1810-18
14. Hogberg T, Signorelli M, de Oliveira CF, Sequential adjuvant chemotherapy and radiotherapy in endometrial cancer – results from two randomised studies: Eur J Cancer (Oxford, England: 1990), 2010; 46; 2422-31
15. Morrow CP, Bundy BM, Homesley HD, Doxorubicin as an adjuvant following surgery and radiation therapy in patients with high-risk endometrial carcinoma, stage I and occult stage II: A Gynecologic Oncology Group Study: Gynecol Oncol, 1990; 36; 166-71
16. Kuoppala T, Mäenpää J, Tomas E: Gynecol Oncol, 2008; 110; 190-95
17. de Boer SM, Powell ME, Mileshkin L, Adjuvant chemoradiotherapy versus radiotherapy alone for women with high-risk endometrial cancer (PORTEC-3): Final results of an international, open-label, multicentre, randomised, phase 3 trial: Lancet Oncol, 2018; 19; 295-309
18. Koh WJ, Abu-Rustum NR, Bean S, Uterine Neoplasms, Version 1.2018, NCCN Clinical Practice Guidelines in Oncology: J Natl Compr Cancer Netw, 2018; 16; 170-99
19. Yi L, Zhang H, Zou J, Adjuvant chemoradiotherapy versus radiotherapy alone in high-risk endometrial cancer: A systematic review and meta-analysis: Gynecol Oncol, 2018; 149; 612-19
20. Kong A, Johnson N, Kitchener HC, Lawrie TA, Adjuvant radiotherapy for stage I endometrial cancer: Cochrane Database Syst Rev, 2012; 2012; CD003916
21. Park HJ, Nam EJ, Kim S, The benefit of adjuvant chemotherapy combined with postoperative radiotherapy for endometrial cancer: A meta-analysis: Eur J Obstet Gynecol Reprod Biol, 2013; 170; 39-44
22. Caldvell DM, Ades AE, Higgins JPT, Simultaneous comparison of multiple treatments: combining direct and indirect evidence: BMJ, 2005; 331; 897-900
23. Lumley T, Network meta-analysis for indirect treatment comparisons: Stat Med, 2002; 21; 2313-24
24. Hutton B, Salanti G, Caldwell DM, The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: Checklist and explanations: Ann Intern Med, 2015; 162; 777-84
25. Sterne JAC, Savović J, Page MJ, RoB 2: A revised tool for assessing risk of bias in randomised trials: BMJ, 2019; 366; l4898
26. Sorbe B, Horvath G, Andersson H, External pelvic and vaginal irradiation versus vaginal irradiation alone as postoperative therapy in medium-risk endometrial carcinoma – a prospective randomized study: Int J Radiat Oncol Biol Phys, 2012; 82; 1249-55
27. Sunil RA, Bhavsar D, Shruthi MN, Combined external beam radiotherapy and vaginal brachytherapy versus vaginal brachytherapy in stage I, intermediate- and high-risk cases of endometrium carcinoma: J Contemp Brachytherapy, 2018; 10; 105-14
28. Nout RA, Smit VT, Putter H, Vaginal brachytherapy versus pelvic external beam radiotherapy for patients with endometrial cancer of high-intermediate risk (PORTEC-2): An open-label, non-inferiority, randomised trial: Lancet (London, England), 2010; 375; 816-23
29. Sunil RA, Bhavsar D, Combined external beam radiotherapy and vaginal brachytherapy versus vaginal brachytherapy in stage I, intermediate-, and high-risk cases of endometrium carcinoma: J Contemp Brachytherapy, 2018; 10; 389-90
30. Johnson N, Bryant A, Miles T, Adjuvant chemotherapy for endometrial cancer after hysterectomy: Cochrane Database Syst Rev, 2011; 2011; CD003175
31. El-Hadaad HA, Wahba HA, Gamal AM, Dawod T: Cancer Biol Med, 2012; 9; 168-71
32. Alvarez Secord A, Havrilesky LJ, Bae-Jump V, The role of multi-modality adjuvant chemotherapy and radiation in women with advanced stage endometrial cancer: Gynecol Oncol, 2007; 107; 285-91
33. Marchetti C, Pisano C, Mangili G, Use of adjuvant therapy in patients with FIGO stage III endometrial carcinoma: A multicenter retrospective study: Oncology, 2011; 81; 104-12
34. Nomura H, Aoki D, Michimae H: JAMA Oncol, 2019; 5; 833-40
35. Ball HG, Blessing JA, Lentz SS, Mutch DG, A phase II trial of paclitaxel in patients with advanced or recurrent adenocarcinoma of the endometrium: A Gynecologic Oncology Group Study: Gynecol Oncol, 1996; 62; 278-81
36. Katsumata N, Noda K, Nozawa S, Phase II trial of docetaxel in advanced or metastatic endometrial cancer: A Japanese Cooperative Study: Br J Cancer, 2005; 93; 999-1004
37. Geller MA, Ivy JJ, Ghebre R, A phase II trial of carboplatin and docetaxel followed by radiotherapy given in a “Sandwich” method for stage III, IV, and recurrent endometrial cancer: Gynecol Oncol, 2011; 121; 112-17
38. Einstein MH, Frimer M, Kuo DYS, Phase II trial of adjuvant pelvic radiation “sandwiched” between combination paclitaxel and carboplatin in women with uterine papillary serous carcinoma: Gynecol Oncol, 2012; 124; 21-25
39. Secord AA, Harvilesky LJ, O’Malley DM, A multicenter evaluation of sequential multimodality therapy and clinical outcome for the treatment of advanced endometrial cancer: Gynecol Oncol, 2009; 114; 442-47
40. Fields AL, Einstein MH, Nocetsky AP, Pilot phase II trial of radiation “sandwiched” between combination paclitaxel/platinum chemotherapy in patients with uterine papillary serous carcinoma (UPSC): Gynecol Oncol, 2008; 108; 201-6
41. Lan C, Huang X, Cao X, Adjuvant docetaxel and carboplatin chemotherapy administered alone or with radiotherapy in a “sandwich” protocol in patients with advanced endometrial cancer: a single-institution experience: Expert Opin Pharmacother, 2013; 14; 535-42
42. Abaid LN, Rettenmaier MA, Brown JV, Sequential chemotherapy and radiotherapy as sandwich therapy for the treatment of high risk endometrial cancer: J Gynecol Oncol, 2012; 23; 22-27
43. Stelloo E, Nout RA, Osse EM, Jurgenliemk-Schule IJ, Improved risk assessment by integrating molecular and clinicopathological factors in early-stage endometrial cancer-combined analysis of the PORTEC Cohorts: Clin Cancer Res, 2016; 22; 4215-24
44. Kommoss S, McConechy MK, Kommoss F, Final validation of the ProMisE molecular classifier for endometrial carcinoma in a large population-based case series: Ann Oncol, 2018; 29; 1180-88
45. Gadducci A, Greco C, The evolving role of adjuvant therapy in endometrial cancer: Crit Rev Oncol Hematol, 2011; 78; 79-91
46. Bakkum-Gamez JN, Gonzalez-Bosquet , Laack NN, Current issues in the management of endometrial cancer: Mayo Clinic Proc, 2008; 83; 97-112
47. de Boer SM, Powell ME, Mileshkin L, Toxicity and quality of life after adjuvant chemoradiotherapy versus radiotherapy alone for women with high-risk endometrial cancer (PORTEC-3): An open-label, multicentre, randomised, phase 3 trial: Lancet Oncol, 2016; 17; 1114-26
48. Caldwell DM, Simultaneous comparison of multiple treatments: combining direct and indirect evidence: BMJ, 2005; 331; 897-900
Errate
Figures
 Figure 1. Flow diagram of the study selection procedure.
Figure 1. Flow diagram of the study selection procedure. Figure 2. (A) The risk of bias graph showing the reviewers’ assessment of the risk of bias for each included study; (B) risk of bias summary showing the reviewers’ assessment of the risk of bias presented as percentages for all the included studies.
Figure 2. (A) The risk of bias graph showing the reviewers’ assessment of the risk of bias for each included study; (B) risk of bias summary showing the reviewers’ assessment of the risk of bias presented as percentages for all the included studies. Figure 3. Network diagrams for (A) overall survival, (B) progression-free survival, (C) distant metastasis rate, (D) local recurrence rate and (E) grade III/IV late toxicities. Pelvic RT – pelvic radiotherapy; VCB-C – vaginal cuff brachytherapy and chemotherapy; pelvic RT-CT – pelvic radiotherapy plus chemotherapy; CT – chemotherapy; pelvic RT-VBT – pelvic radiotherapy and vaginal brachytherapy. Interventions with direct comparisons are linked with a line; the thickness of connecting lines corresponds to the number of trials evaluating the comparison. Node size corresponds to the number of participants assigned to receive each intervention.
Figure 3. Network diagrams for (A) overall survival, (B) progression-free survival, (C) distant metastasis rate, (D) local recurrence rate and (E) grade III/IV late toxicities. Pelvic RT – pelvic radiotherapy; VCB-C – vaginal cuff brachytherapy and chemotherapy; pelvic RT-CT – pelvic radiotherapy plus chemotherapy; CT – chemotherapy; pelvic RT-VBT – pelvic radiotherapy and vaginal brachytherapy. Interventions with direct comparisons are linked with a line; the thickness of connecting lines corresponds to the number of trials evaluating the comparison. Node size corresponds to the number of participants assigned to receive each intervention. Tables
 Table 1. Characteristics of included randomized clinical trials.
Table 1. Characteristics of included randomized clinical trials. Table 2. League Table of pair-wise comparisons in the network meta-analysis for the relative risks (RR) of overall survival (OS), progression-free survival (PFS), distant metastasis rate, local recurrence rate, and grade III/IV late toxicities.
Table 2. League Table of pair-wise comparisons in the network meta-analysis for the relative risks (RR) of overall survival (OS), progression-free survival (PFS), distant metastasis rate, local recurrence rate, and grade III/IV late toxicities. Table 3. SUCRA values of 7 adjuvant therapies for high-risk endometrial cancer patients under 5 outcomes.
Table 3. SUCRA values of 7 adjuvant therapies for high-risk endometrial cancer patients under 5 outcomes. Table 1. Characteristics of included randomized clinical trials.
Table 1. Characteristics of included randomized clinical trials. Table 2. League Table of pair-wise comparisons in the network meta-analysis for the relative risks (RR) of overall survival (OS), progression-free survival (PFS), distant metastasis rate, local recurrence rate, and grade III/IV late toxicities.
Table 2. League Table of pair-wise comparisons in the network meta-analysis for the relative risks (RR) of overall survival (OS), progression-free survival (PFS), distant metastasis rate, local recurrence rate, and grade III/IV late toxicities. Table 3. SUCRA values of 7 adjuvant therapies for high-risk endometrial cancer patients under 5 outcomes.
Table 3. SUCRA values of 7 adjuvant therapies for high-risk endometrial cancer patients under 5 outcomes. In Press
07 Mar 2024 : Clinical Research
Knowledge of and Attitudes Toward Clinical Trials: A Questionnaire-Based Study of 179 Male Third- and Fourt...Med Sci Monit In Press; DOI: 10.12659/MSM.943468
08 Mar 2024 : Animal Research
Modification of Experimental Model of Necrotizing Enterocolitis (NEC) in Rat Pups by Single Exposure to Hyp...Med Sci Monit In Press; DOI: 10.12659/MSM.943443
18 Apr 2024 : Clinical Research
Comparative Analysis of Open and Closed Sphincterotomy for the Treatment of Chronic Anal Fissure: Safety an...Med Sci Monit In Press; DOI: 10.12659/MSM.944127
08 Mar 2024 : Laboratory Research
Evaluation of Retentive Strength of 50 Endodontically-Treated Single-Rooted Mandibular Second Premolars Res...Med Sci Monit In Press; DOI: 10.12659/MSM.944110
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








