24 August 2020: Human Study
Reference Ranges and Determinant Factors for Fractional Exhaled Nitric Oxide in a Healthy Saudi Adult Population
Syed Shahid Habib1ABCDEFG*, Mohammad A. Alzoghaibi1ABDFG, Syed Hamid Habib2ACDEF, Khalid A. Al-Regaiey1ACDFGDOI: 10.12659/MSMBR.926382
Med Sci Monit Basic Res 2020; 26:e926382
Abstract
BACKGROUND: BACKGROUND Fractional exhaled nitric oxide (FENO) has emerged as a promising marker in respiratory research. The aim of this study was to determine the reference range values of FENO for healthy Saudi adults and the factors associated with FENO levels. MATERIAL AND METHODS This cross-sectional study was conducted at the Department of Physiology, King Saud University, Riyadh, Saudi Arabia, from January 2016 to August 2017. A total of 429 healthy Saudi adults were initially recruited. The final selection included 412 participants, consisting of 307 men and 105 women. FENO measurements were performed according to the current recommendations of the American Thoracic Society. RESULTS We observed that the FENO levels of women were significantly lower than those of men (18.6 vs. 21.3, P=0.009). In women, the measured FENO ranged from 5.7 ppb to 42 ppb, and in men from 5.0 ppb to 55.0 ppb. The mean FENO level in the entire study population was 20.6, with a range of 5.0 ppb to 55.0 ppb. The difference became non-significant when we calculated the FENO after adjusting for body surface area by different percentile distributions. Multiple linear regression analysis showed that body surface area and weight were significant predictors of FENO levels. CONCLUSIONS In this study, FENO levels were significantly affected by demographic variables. Therefore, it is important to consider the factors influencing FENO values to make a valid clinical interpretation.
MATERIAL AND METHODS:
RESULTS:
CONCLUSIONS:
Keywords: Body Mass Index, Body Surface Area, Gender Identity, Nitric Oxide, Reference Values, Adolescent, Exhalation, Health, Linear Models, Saudi Arabia, young adult
Background
Fractional exhaled nitric oxide (FENO) is a molecule involved in the regulation of bronchial and vascular tone and inflammation of the bronchial mucosa [1,2]. FENO is a non-invasive marker of inflammation driven by helper T cells in airways, which are mediated by interleukin-4 and interleukin-13 [3,4]. It is currently one of the diagnostic tests used in clinical practice to determine the diagnosis and prognosis of many airway diseases [5,6]. The role of FENO is also described in the process of neurotransmission. Many diseases of the respiratory tract, such as bronchial asthma [7] and chronic obstructive pulmonary disease [8], are related to an increased concentration of FENO [9]. The use of anti-inflammatory drugs has been shown to reduce FENO levels [10,11]. In addition, FENO values are affected by age, race, sex, anthropometric measurements, atopy, and smoking habits [12]. These factors make the interpretation of diagnostic tests difficult. The diagnostic test can be interpreted in many ways, and the American Thoracic Society has suggested that using an absolute value for FENO is not recommended, which is also the case for lung function tests. Furthermore, fixed cutoff values for FENO are not specified in the society’s guidelines [13]. Similarly, reports in the literature differ regarding the normal FENO value among different populations, and there are different results reported for FENO values in various populations and in relation to confounding factors like smoking, which lowers FENO values [14,15].
FENO values have been very useful in assessing the diagnosis and prognosis of asthma [16]. However, its use seems to be limited in other chronic respiratory diseases, like chronic obstructive pulmonary disease and bronchiectasis [17]. We previously published the reference range values for the healthy male adult Saudi population [18]. However, differences in FENO levels due to sex and reference ranges for the healthy adult female population and their relation to age, height, weight, and BMI have not been evaluated in detail. Therefore, this study aimed to determine the reference range values of FENO for healthy Saudi adults and to determine the factors associated with FENO levels.
Material and Methods
MEASUREMENT OF FRACTIONAL EXHALED NITRIC OXIDE:
FENO measurements were performed and calculated as per the current American Thoracic Society guidelines (6) using an EVA Nox 4000 chemiluminescence analyzer (Seres, France), which has the sensitivity to measure FENO in 1 part per billion (ppb) of exhaled air. All participants were asked to rest and avoid eating, drinking, and any strenuous exercise for about 3 h before the measurement of FENO levels. All measurements were performed between 9–11 a.m., to minimize possible effects of the circadian rhythm. In addition, diet history was obtained to avoid any alterations in FENO levels due to nitrate-containing foods. To calculate the slow vital capacity, participants were asked to fill their lungs from the residual lung volume to total lung capacity and then perform slow expiration. They were asked to keep a constant expiratory flow rate of about 0.05 L/s, and to maintain it for at least 15 s, exhaling into a Teflon cylinder connected to Teflon tubing. An expiratory resistance of 10 cm to 20 cm was applied to exclude any nasal contamination. The resistance was detected by a Samba sensor 3200 (Samba Sensors AB, Vastera Frolunda, Sweden), a special sensor for making flow rate recordings. The expiratory flow rate for each participant was measured by a computerized data acquiring software system (BIOPAC MP-100, BIOPAC Systems Inc, Goleta, GA, USA). The plateau levels of FENO against time were determined, and FENO levels were calculated as ppb. FENO concentrations were graphed and averaged from 5 to 15 s after the start of expiration. The average values of 3 successive samples taken with at least a 1-min interval in between were calculated. For standardization, the EVA Nox machine was calibrated daily before each experiment, and the variation between tests was kept within the range of 10%.
VENTILATORY FUNCTIONS:
All spirometric recordings including FEV1, peak expiratory flow (PEF), forced vital capacity (FVC), and FEV1/FVC ratio were performed using an electronic spirometer (Vitalograph, Ireland) after FENO recordings. All measurements were taken 3 times and with participants in a seated position.
STATISTICAL ASNALYSIS:
Data were entered in Microsoft excel and data analyses were performed using SPSS for windows version 20.0. Prior to the final analysis, data were screened for normality assumption, homogeneity of variance, and presence of extreme scores. The homogeneity of the variance test and test of normality were done using the Shapiro-Wilk test. If these results were significant, defined as a
Results
In this study, we determined the reference range values of FENO and their distribution in adult female Saudi participants. All participants were healthy and nonsmokers. Table 1 shows the clinical characteristics and FENO levels of all participants. The mean FENO level of the entire study population was 20.6±9.1 ppb, ranging from 5.0 ppb to 55.0 ppb. Table 2 shows the comparison of clinical characteristics and FENO levels between male and female participants. We observed that women had significantly lower FENO levels compared to those of men (18.6
Figure 6 shows the comparison of FENO levels between male and female participants according to different percentiles of body surface area, with mean values and 95% confidence intervals. It was observed that at <25%, 25–50%, 50–75%, and >75% of body surface area the difference was non-significant with
Discussion
The primary objectives of this study were to determine differences in FENO levels according to sex, and to develop reference ranges of FENO levels in healthy female Saudi participants, measured in accordance with current recommended standards. Our results showed that women had significantly lower FENO levels than did men. However, when adjustments were made for body surface area, the difference in FENO levels between female and male participants was non-significant. To the best of our knowledge, the current study is the first to analyze body surface area adjustments for FENO. In our previous study, FENO levels ranged between 7.66 ppb and 46.6 ppb (mean 22.79±8.13 ppb) in adult men, levels correlated negatively with body weight (r=0.388,
Low levels of FENO in women have been previously reported, but the findings of our current study are novel because when we adjusted for the total body surface area, the absolute FENO values of women were not significantly different from those of men. This effect can be partly explained by differences in height, because the differences between male and female participants were not consistent after an adjustment was made for height. This probably points to a difference in NO production between the sexes [20], a relationship that has been attributed to the smaller airway size in females, as airway surface is one source of NO production [21]. The current findings are also consistent with the strong relationship between height with FENO that was observed in children [21]. However, with adjustments for body surface area, the differences related to male or female sex were insignificant, although the absolute levels were significantly lower in female participants. This suggests that not only the airway surface area, but also the whole body surface affects FENO production.
Jo et al. reported that the significant factors for FENO are weight, height, BMI, and atopy, while age is not a significant determinant. In their multiple linear regression analysis, sex and weight were found to have significant associations with FENO [14]. Their observations support our results, but with some differences. In our current study, weight and body surface area were the only independent factors associated with FENO levels. The effect of collinearity could have made the predictive value of other factors in our study non-significant. The different results between the studies could be also be attributable to our analyzing for the effect of body surface area, whereas the other researchers did not [14].
NO formation is already detected at birth [22,23] and is important in humans, which are under strict biological control. NO formation is a complex and energy consuming process whereby NO is produced by inducible NO synthase in the airway epithelium. This indicates that the total surface area of the airway epithelium/mucosa is important in the detection of FENO levels, and was theoretically shown to negatively correlate with the anatomic dead space volume in healthy children [24]. Therefore, it is assumed that anthropometric factors are important in evaluating FENO levels, as was previously observed for lung function parameters [19]. This was also demonstrated in linear regression models of FENO levels in many studies which had results similar to our current study [25,26].
Recently, the lambda-mu-sigma method of model adjustment for FENO levels predicted slightly lower values for both sexes without a statistically significant difference, which was attributed to the skewed distribution of the FENO levels. The adjustments of the lambda-mu-sigma model were done to assess the differences with this method. It was observed that a significant proportion of the individuals with normal levels of FENO, according to the current recommendations, were classified as having intermediate levels [27]. Our study supports the inference of an extensive systematic review that showed the effect of all significant determinants of FENO values, including age, height, atopy, smoking, weight, sex, and race [28]. The present study suggests that the absolute values obtained for FENO may not provide an exact status of the respiratory airways, and adjustments for body surface area and demographic variables should be considered along with sex, ethnicity, smoking status, and age.
Conclusions
Our results revealed that FENO levels are significantly affected by demographic variables. Women have lower FENO values than do men. However, after adjusting for body surface area, the difference becomes non-significant. Therefore, it is important to consider the factors influencing FENO values to make valid clinical interpretations. Future studies should consider racial and genetic differences. Larger sample sizes are needed to develop standard reference equations based on predetermined physiological models for the evaluation of normal FENO.
Figures
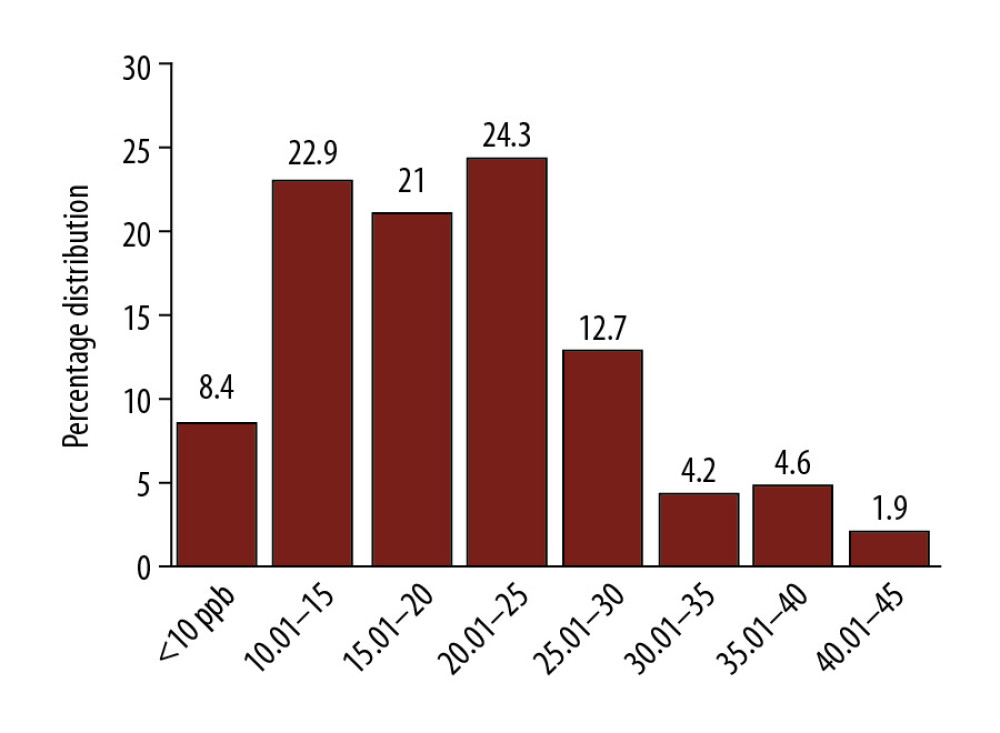 Figure 1. Ranges of fractional exhaled nitric oxide (FENO) values at different levels of distribution in females.
Figure 1. Ranges of fractional exhaled nitric oxide (FENO) values at different levels of distribution in females. 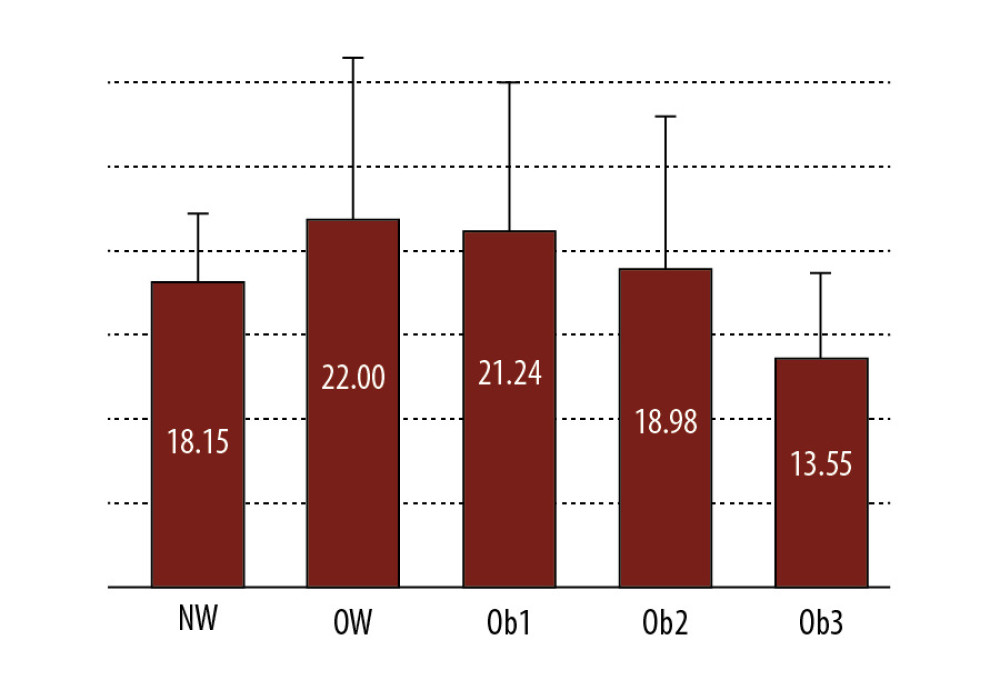 Figure 2. Distribution of fractional exhaled nitric oxide (FENO) according to different categories of body mass index (BMI). NW – normal weight; OW – overweight; Ob1, Ob2, and Ob3 – Obesity class 1, 2, and 3.
Figure 2. Distribution of fractional exhaled nitric oxide (FENO) according to different categories of body mass index (BMI). NW – normal weight; OW – overweight; Ob1, Ob2, and Ob3 – Obesity class 1, 2, and 3. 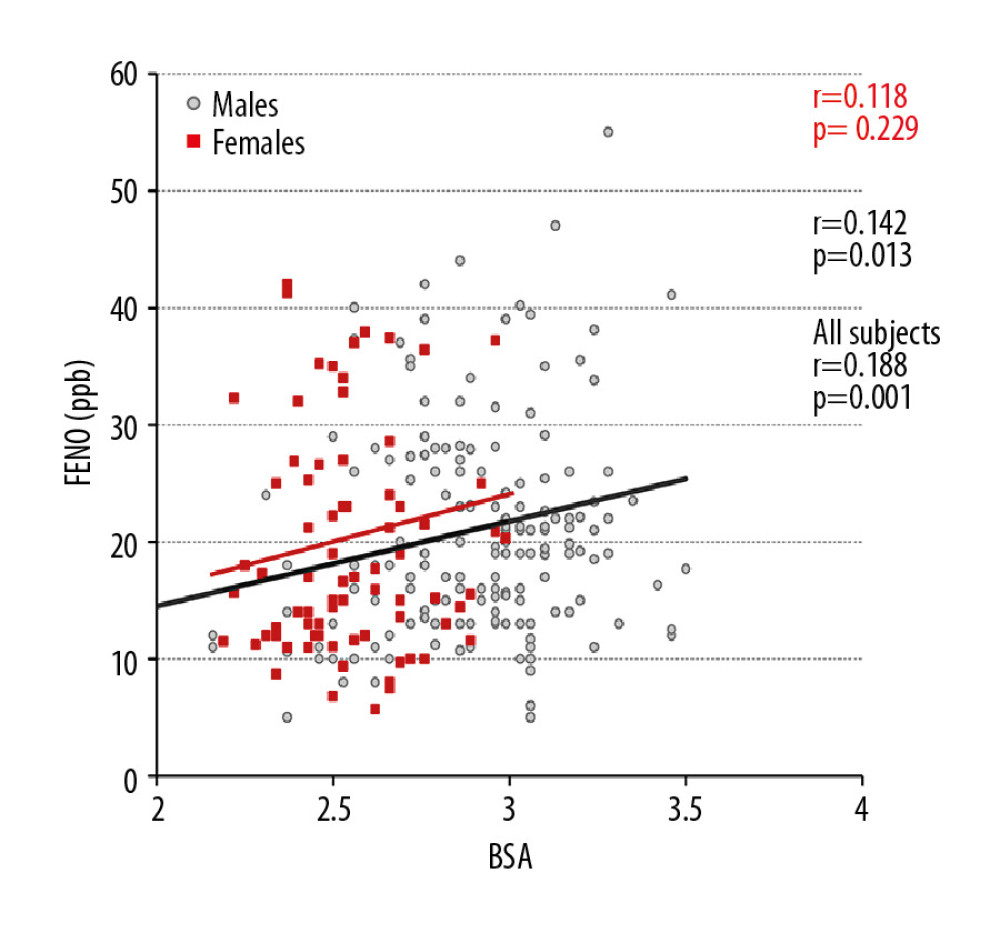 Figure 3. The relationship between fractional exhaled nitric oxide (FENO) levels and body surface area (BSA) in all participants, men and women.
Figure 3. The relationship between fractional exhaled nitric oxide (FENO) levels and body surface area (BSA) in all participants, men and women. 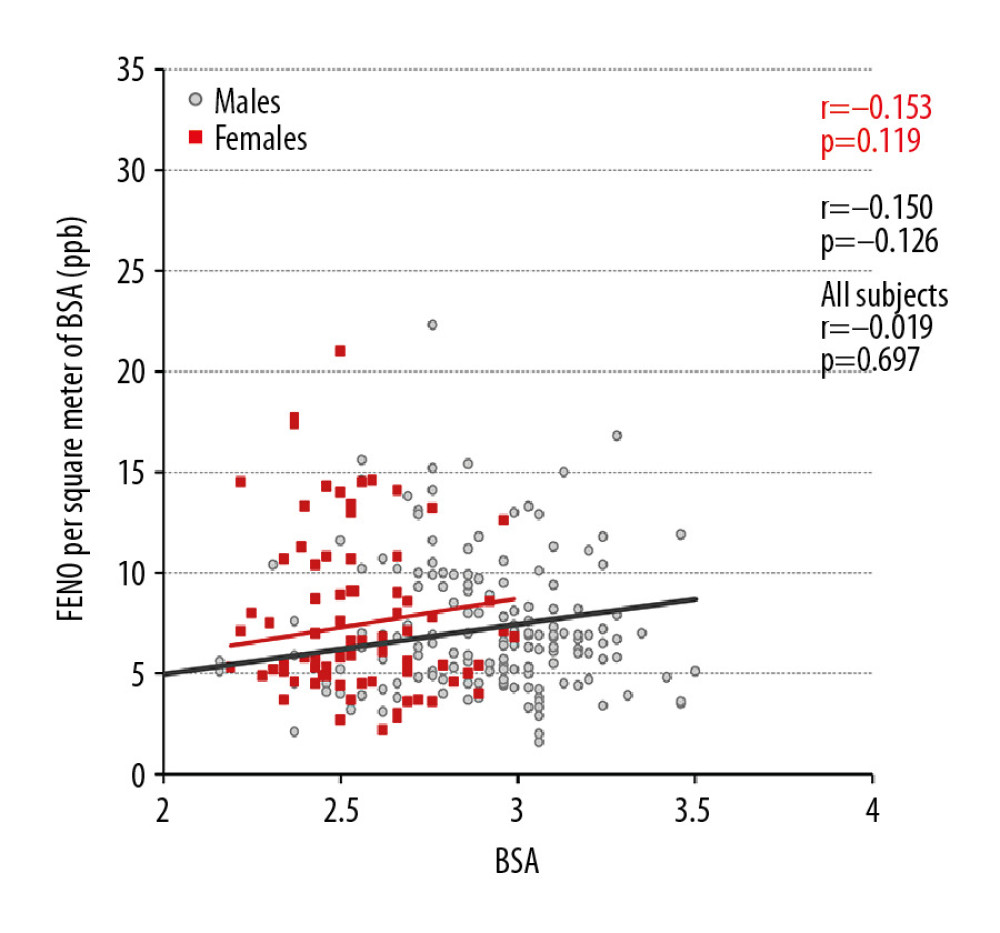 Figure 4. The relationship between adjusted fractional exhaled nitric oxide (FENO) levels and body surface area (BSA) in all participants, men and women.
Figure 4. The relationship between adjusted fractional exhaled nitric oxide (FENO) levels and body surface area (BSA) in all participants, men and women. 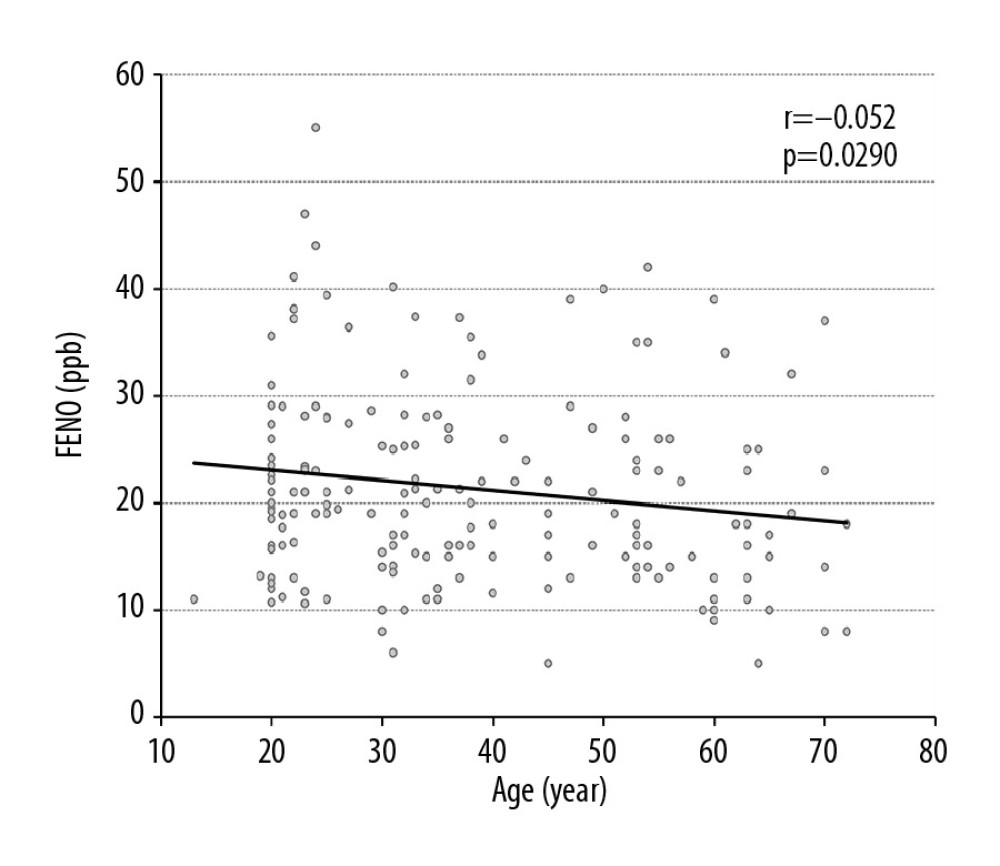 Figure 5. The relationship between fractional exhaled nitric oxide (FENO) levels and age in years in all participants.
Figure 5. The relationship between fractional exhaled nitric oxide (FENO) levels and age in years in all participants. 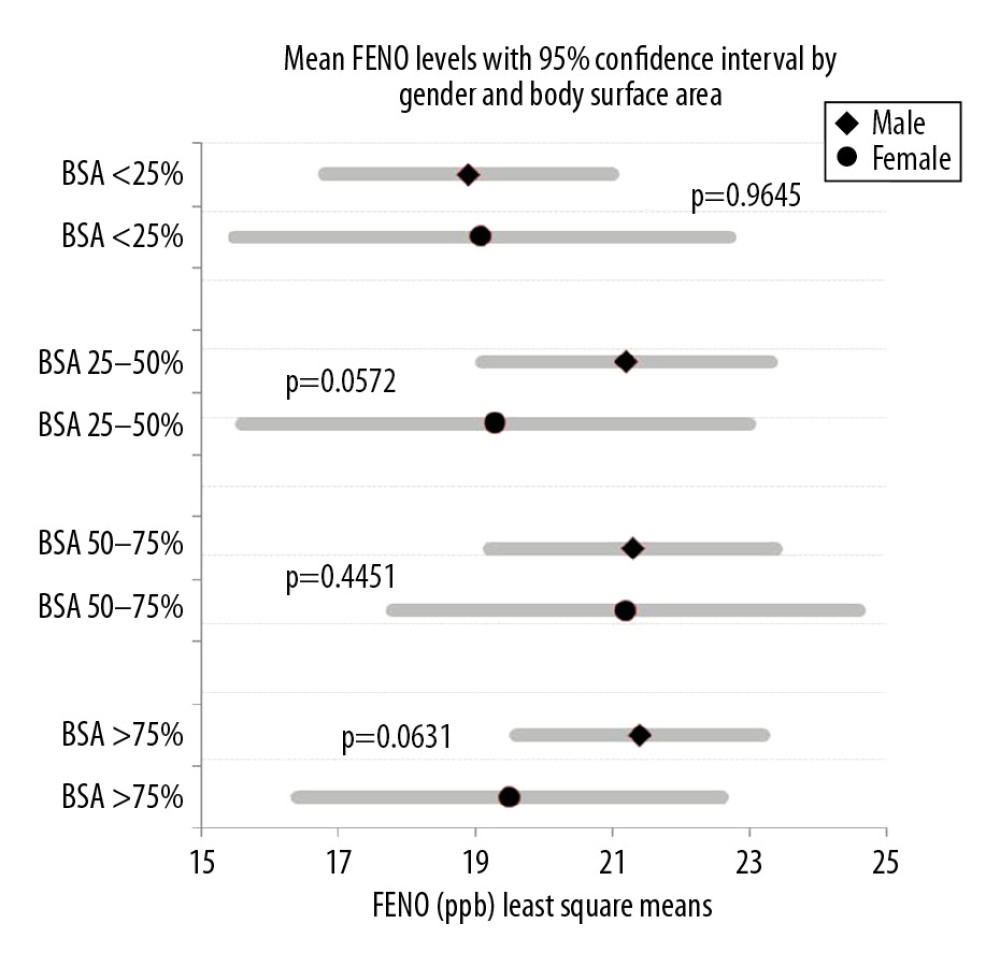 Figure 6. Comparison of mean fractional exhaled nitric oxide (FENO) level distribution between men and women according to different percentiles of body surface area (BSA).
Figure 6. Comparison of mean fractional exhaled nitric oxide (FENO) level distribution between men and women according to different percentiles of body surface area (BSA). Tables
Table 1. Clinical and demographic characteristics and FENO levels of all participants (n=412).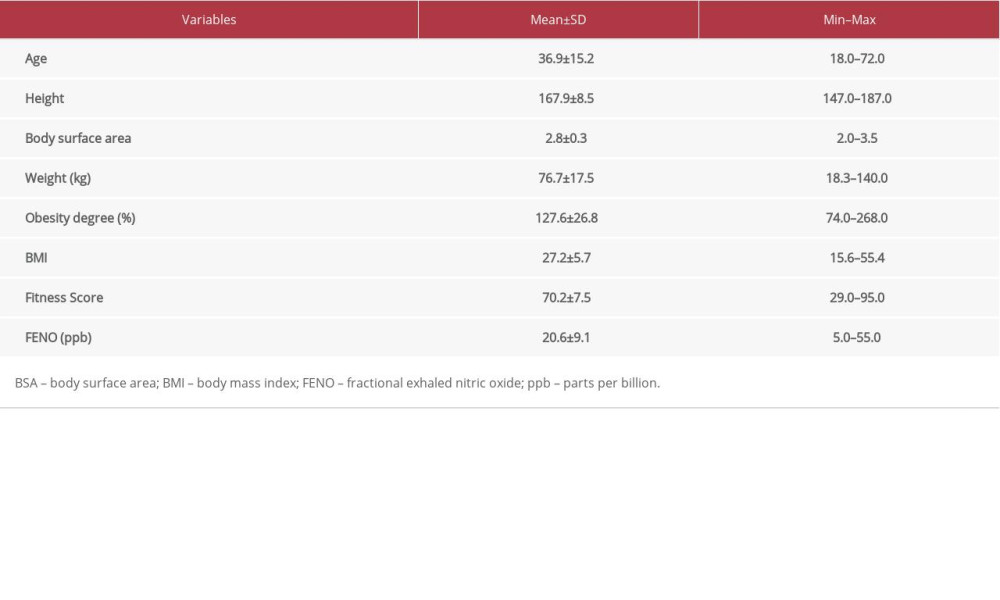 Table 2. Differences by sex in demographic characteristics, and FENO levels in all participants.
Table 2. Differences by sex in demographic characteristics, and FENO levels in all participants.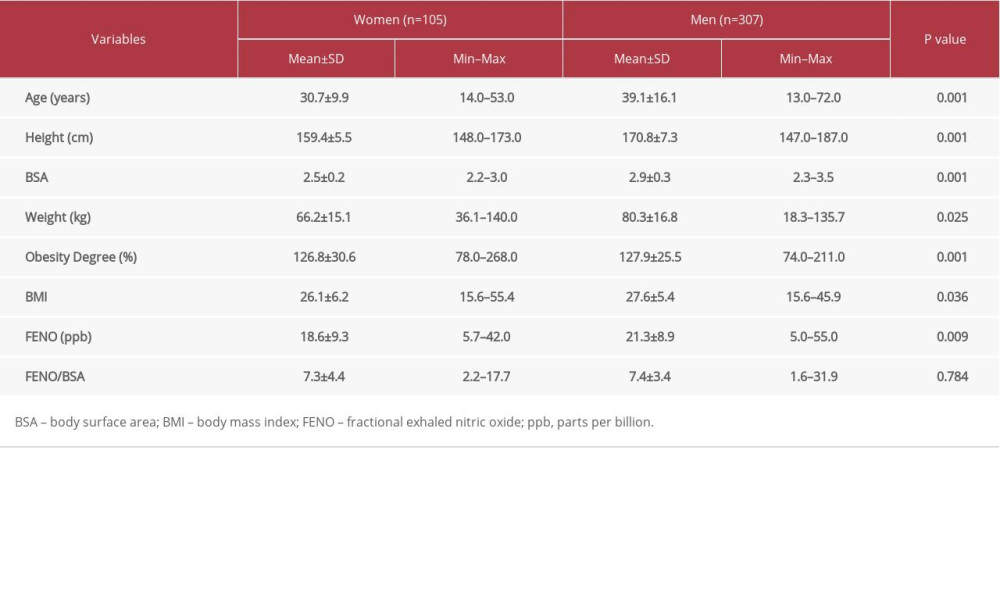 Table 3. Multiple linear regression analysis with FENO as dependent variable with demographic factors as independent variables.
Table 3. Multiple linear regression analysis with FENO as dependent variable with demographic factors as independent variables.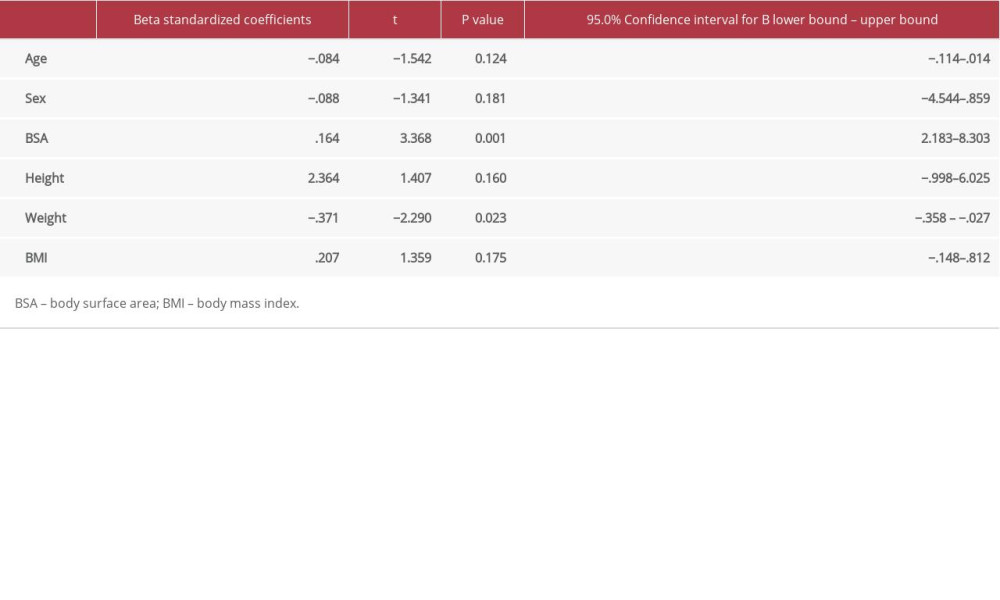
References
1. Sandrini A, Taylor DR, Thomas PS, Yates DH, Fractional exhaled nitric oxide in asthma: An update: Respirology, 2010; 15(1); 57-70
2. Mummadi SR, Hahn PY, Update on exhaled nitric oxide in clinical practice: Chest, 2016; 149(5); 1340-44
3. Gagliardo R, La Grutta S, Chanez P, Non-invasive markers of airway inflammation and remodeling in childhood asthma: Pediatr Allergy Immunol, 2009; 20(8); 780-90
4. Corren J1, Lemanske RF, Hanania NA, Lebrikizumab treatment in adults with asthma: N Engl J Med, 2011; 365(12); 1088-98
5. Kowal K, Bodzenta-Lukaszyk A, Zukowski S, Exhaled nitric oxide in evaluation of young adults with chronic cough: J Asthma, 2009; 46(7); 692-98
6. Maniscalco M, Fuschillo S, Gaudiosi C, Exhaled and nasal nitric oxide measurement in the evaluation of chronic cough: Nitric Oxide, 2019; 83; 19-23
7. Karrasch S, Linde K, Rücker G, Accuracy of FENO for diagnosing asthma: A systematic review: Thorax, 2017; 72(2); 109-16
8. Malerba M, Radaeli A, Olivini A, Exhaled nitric oxide as a biomarker in COPD and related comorbidities: Biomed Res Int, 2014; 2014 271918
9. Al-shamkhi N, Alving K, Dahlen SE, Important non-disease-related determinants of exhaled nitric oxide levels in mild asthma – results from the Swedish GA2LEN study: Clin Exp Allergy, 2016; 46(9); 1185-93
10. Bjermer L, Alving K, Diamant Z, Current evidence and future research needs for FeNO measurement in respiratory diseases: Respir Med, 2014; 108(6); 830-41
11. Syk J, Malinovschi A, Johansson G, Anti-inflammatory treatment of atopic asthma guided by exhaled nitric oxide: A randomized, controlled trial: J Allergy Clin Immunol Pract, 2013; 1(6); 639-48.e1-8
12. Olin AC, Rosengren A, Thelle DS, Height, age, and atopy are associated with fraction of exhaled nitric oxide in a large adult general population sample: Chest, 2006; 130(5); 1319-25
13. Dweik RA, Boggs PB, Erzurum SC, An official ATS clinical practice guideline: Interpretation of exhaled nitric oxide levels (FENO) for clinical applications: Am J Respir Crit Care Med, 2011; 184(5); 602-15
14. Jo EJ, Song WJ, Kim TW, Reference ranges and determinant factors for exhaled nitric oxide in a healthy Korean elderly population: Allergy Asthma Immunol Res, 2014; 6(6); 504-10
15. Habib SS, Ahmed SM, Al Drees AM, Husain A, Effect of cigarette smoking on fractional exhaled nitric oxide in Saudi medical college students: J Pak Med Assoc, 2011; 61(2); 120-23
16. Arnold RJ, Massanari M, Lee TA, Brooks E, A review of the utility and cost effectiveness of monitoring fractional exhaled nitric oxide (FeNO) in asthma management: Manag Care, 2018; 27(7); 34-41
17. Beg MF, Alzoghaibi MA, Abba AA, Habib SS, Exhaled nitric oxide in stable chronic obstructive pulmonary disease: Ann Thorac Med, 2009; 4(2); 65-70
18. Habib SS, Abba AA, Al-Zoghaibi MA, Subhan MM, Reference range values of fractional exhaled nitric oxide in healthy Arab adult males: Saudi Med J, 2009; 30(11); 1395-400
19. Torén K, Murgia N, Schiöler L, Reference values of fractional excretion of exhaled nitric oxide among non-smokers and current smokers: BMC Pulm Med, 2017; 17(1); 118
20. Jilma B, Kastner J, Mensik C, Sex differences in concentrations of exhaled nitric oxide and plasma nitrate: Life Sci, 1996; 58(6); 469-76
21. Malmberg LP, Petäys T, Haahtela T, Exhaled nitric oxide in healthy nonatopic school-age children: Determinants and height-adjusted reference values: Pediatr Pulmonol, 2006; 41(7); 635-42
22. Mallol J, Aguirre V, Córdova P, Fraction of exhaled nitric oxide in healthy Chilean schoolchildren aged 8–15 years: Allergol Immunopathol (Madr), 2015; 43(6); 528-32
23. Baraldi E, Azzolin NM, Cracco A, Zacchello F, Reference values of exhaled nitric oxide for healthy children 6–15 years old: Pediatr Pulmonol, 1999; 27(1); 54-58
24. Pedroletti C, Högman M, Meriläinen P, Nitric oxide airway diffusing capacity and mucosal concentration in asthmatic schoolchildren: Pediatr Res, 2003; 54(4); 496-501
25. Torén K, Murgia N, Schiöler L, Reference values of fractional excretion of exhaled nitric oxide among non-smokers and current smokers: BMC Pulm Med, 2017; 17(1); 118
26. Levesque MC, Hauswirth DW, Mervin-Blake S, Determinants of exhaled nitric oxide levels in healthy, nonsmoking African American adults: J Allergy Clin Immunol, 2008; 121(2); 396-402.e3
27. Jacinto T, Amaral R, Malinovschi A, Exhaled NO reference limits in a large population-based sample using the Lambda-Mu-Sigma method: J Appl Physiol (1985), 2018; 125(5); 1620-26
28. Jacinto T, Alving K, Correia R, Setting reference values for exhaled nitric oxide: A systematic review: Clin Respir J, 2013; 7(2); 113-20
Figures
 Figure 1. Ranges of fractional exhaled nitric oxide (FENO) values at different levels of distribution in females.
Figure 1. Ranges of fractional exhaled nitric oxide (FENO) values at different levels of distribution in females. Figure 2. Distribution of fractional exhaled nitric oxide (FENO) according to different categories of body mass index (BMI). NW – normal weight; OW – overweight; Ob1, Ob2, and Ob3 – Obesity class 1, 2, and 3.
Figure 2. Distribution of fractional exhaled nitric oxide (FENO) according to different categories of body mass index (BMI). NW – normal weight; OW – overweight; Ob1, Ob2, and Ob3 – Obesity class 1, 2, and 3. Figure 3. The relationship between fractional exhaled nitric oxide (FENO) levels and body surface area (BSA) in all participants, men and women.
Figure 3. The relationship between fractional exhaled nitric oxide (FENO) levels and body surface area (BSA) in all participants, men and women. Figure 4. The relationship between adjusted fractional exhaled nitric oxide (FENO) levels and body surface area (BSA) in all participants, men and women.
Figure 4. The relationship between adjusted fractional exhaled nitric oxide (FENO) levels and body surface area (BSA) in all participants, men and women. Figure 5. The relationship between fractional exhaled nitric oxide (FENO) levels and age in years in all participants.
Figure 5. The relationship between fractional exhaled nitric oxide (FENO) levels and age in years in all participants. Figure 6. Comparison of mean fractional exhaled nitric oxide (FENO) level distribution between men and women according to different percentiles of body surface area (BSA).
Figure 6. Comparison of mean fractional exhaled nitric oxide (FENO) level distribution between men and women according to different percentiles of body surface area (BSA). Tables
 Table 1. Clinical and demographic characteristics and FENO levels of all participants (n=412).
Table 1. Clinical and demographic characteristics and FENO levels of all participants (n=412). Table 2. Differences by sex in demographic characteristics, and FENO levels in all participants.
Table 2. Differences by sex in demographic characteristics, and FENO levels in all participants. Table 3. Multiple linear regression analysis with FENO as dependent variable with demographic factors as independent variables.
Table 3. Multiple linear regression analysis with FENO as dependent variable with demographic factors as independent variables. Table 1. Clinical and demographic characteristics and FENO levels of all participants (n=412).
Table 1. Clinical and demographic characteristics and FENO levels of all participants (n=412). Table 2. Differences by sex in demographic characteristics, and FENO levels in all participants.
Table 2. Differences by sex in demographic characteristics, and FENO levels in all participants. Table 3. Multiple linear regression analysis with FENO as dependent variable with demographic factors as independent variables.
Table 3. Multiple linear regression analysis with FENO as dependent variable with demographic factors as independent variables. Most Viewed Current Articles
15 Jun 2022 : Clinical Research
Evaluation of Apical Leakage After Root Canal Obturation with Glass Ionomer, Resin, and Zinc Oxide Eugenol ...DOI :10.12659/MSMBR.936675
Med Sci Monit Basic Res 2022; 28:e936675
07 Jul 2022 : Laboratory Research
Cytotoxicity, Apoptosis, Migration Inhibition, and Autophagy-Induced by Crude Ricin from Ricinus communis S...DOI :10.12659/MSMBR.936683
Med Sci Monit Basic Res 2022; 28:e936683
01 Jun 2022 : Laboratory Research
Comparison of Sealing Abilities Among Zinc Oxide Eugenol Root-Canal Filling Cement, Antibacterial Biocerami...DOI :10.12659/MSMBR.936319
Med Sci Monit Basic Res 2022; 28:e936319
08 Dec 2022 : Original article
Use of Estimated Glomerular Filtration Rate and Urine Albumin-to-Creatinine Ratio Based on KDIGO 2012 Guide...DOI :10.12659/MSMBR.938176
Med Sci Monit Basic Res 2022; 28:e938176








