17 January 2022: Human Study
Anodal Transcranial Direct Current Stimulation (tDCS) Over the Primary Motor Cortex (M1) Enhances Motor Response Inhibition and Visual Recognition Memory
Shahid BashirDOI: 10.12659/MSMBR.934180
Med Sci Monit Basic Res 2022; 28:e934180
Abstract
BACKGROUND: Transcranial direct current stimulation (tDCS) is a non-invasive brain modulatory technique that can boost cognitive processes and alter performance in cognitive tasks. The effect of anodal-tDCS on cognitive functions following a single session has been repeatedly reported. However, results are varied, mixed, and confounded by several factors, including differences in tDCS current intensity and sham conditions used. Therefore, we assessed the effect of a single session of anodal-tDCS over the primary motor cortex (M1) on cognitive functions, particularly response inhibitory control and visual recognition memory, in healthy adults.
MATERIAL AND METHODS: Thirty healthy male subjects participated in a randomized, double-blind, sham-controlled tDCS study (2 mA, for 20 min). In the sham stimulation, subjects received the same intensity (2 mA, for 30 s, ramped up, then ramped down). We assessed the cognitive functions response inhibitory control and visual recognition memory using the stop signal task (SST) and pattern recognition memory (PRM) task before and after the stimulation.
RESULTS: There was a statistically significant difference in cognitive function as assessed by the stop signal task go trial (SST) (P=0.019) and SST correct response time on go trials (P=0.04) after real stimulation only. There was no significant effect observed in sham condition.
CONCLUSIONS: The findings suggest that anodal-tDCS applied over M1 improves inhibitory control and visual recognition memory compared to sham stimulation. Thus, application of anodal-tDCS to the M1, as an integral node in inhibitory motor control, might be a promising adjuvant therapeutic intervention for modulation of motor response inhibition.
Keywords: Memory, Motor Cortex, Transcranial Direct Current Stimulation, Adult, Cognition, Humans, Male, Reaction Time
Background
Transcranial direct current stimulation (tDCS) is a non-invasive and painless form of brain stimulation technique that modulates spontaneous cortical activity, in which anodal stimulation enhances cortical excitability through neural depolarization and cathodal stimulation reduces cortical excitability through neural hyperpolarization [1,2]. Following stimulation, changes in N-methyl-D-aspartate (NMDA) receptor efficacy have been observed, as well as altered cortical functions that persist for a certain time [3], which is thought to be associated with long-term potentiation and plasticity in depression [1,4–6].
Evidence from various fields of research shows the critical role of the human right inferior frontal cortex (rIFC) [7] and pre-supplementary motor areas (preSMA) in motor response inhibition via the cortico-subthalamo-pallidal hyper-direct pathway, in which both foci IFC and preSMA project to the subthalamic nucleus (STN) in a hyper-direct pathway for modulating inhibitory control [8–12]. A combined magnetoencephalography (MEG) and transcranial magnetic stimulation (TMS) study has shown parallel activation of the inferior frontal cortex and pre-supplementary motor area during response inhibition [13]. A recent functional brain imaging study, using the context-cuing stop signal paradigm, has revealed a widely distributed common and discrete involvement of motor response inhibition in the fronto-parietal network, engaging a right-lateralized network that extends to subcortical structures [14]. This indicates that inhibitory motor control is mediated by parallel neural network recruiting a wide spread of cortical and subcortical brain regions [9,15,16].
Response inhibitory control, which is the ability to suppress or withhold unwanted or inappropriate motor responses, maintains flexible and goal-oriented behavior and is one of the most intriguing aspects of the behavior [17]. Abundant evidence shows that tDCS of the primary motor cortex (M1) enhances cognitive functions and motor learning in healthy subjects and in patients with neurological diseases [18–20].
Although it is well established that the neural substrates of voluntary inhibition reside within a right-lateralized frontal cortical-basal ganglia-thalamic network, comprising 2 main foci, the preSMA an IFG [12,21–24], it is highly likely that these regions exert top-down control that influences motor cortical areas crucially involved in mediating movement preparation and execution processes such as primary motor (M1) and premotor (PM) cortices [25–28].
M1, as a hub brain region, receives and integrates multiple types of inputs from a wide range of cortical and subcortical regions [29–31]. M1 is thought to be an integral cortical site of the response inhibition process, in which movement preparation and suppression are mediated by M1 intrinsic motor programming circuits, given its role in coding descending motor commands [32,33].
Various studies have shown that, depending on the behavioral inhibition paradigms used, one or more of the inhibitory control functions can be activated at different time points and are associated with increase of primary motor cortex activity [25–27]. Event-related potential results from electrocorticographic (ECoG) recordings in patients with intractable epilepsy, while performing the stop signal task, have revealed that successful movement inhibiting-specific event-related potential components reside in M1 and the premotor cortex [34]. Consistent with the above evidence, seminal work investigating the dynamic changes of cortical activity during response inhibition obtained from intracranial electrophysiological (ECoG) recordings from patients with refractory epilepsy showed a high-gamma increase and a beta decrease related to movement preparation and initiation and a beta rebound and absence of high-gamma changes in successfully inhibited responses [25]. In line with these findings, other studies, using transcranial magnetic stimulation, have shown inhibitory control activity within M1, suggesting GABA (B)-receptor-mediated intracortical inhibition as a possible indicator of stopping ongoing movements [34]. Similarly, a novel study using paired-pulse TMS to examine the modulation of M1 inhibitory networks during response inhibition tasks showed that M1 has a significant role in engaging proactive and reactive processes during motor response suppression when preceded by informative or uninformative cues. This study confirmed the role of M1 intracortical inhibition as a potential mechanism within M1 that might support both proactive and reactive processes [28]. Therefore, M1 has a crucial role in shaping and coding descending motor output, and it has modulatory effects during response inhibition [32,37,38]. These modulatory effects on descending motor commands that modify behavior are thought to be mediated by intracortical inhibitory networks within M1 [28,39]. Based on these aforementioned studies, we hypothesized that the anodal stimulation over dominant M1 can enhance inhibition performance as assessed by the stop signal task (SST).
It is postulated that primary motor cortex is a key node in mediating inhibitory control through its dense intracortical connectivity and its projections to the subcortical structures, particularly basal ganglia nuclei (ie, putamen) [37,38]. Thus, this study assessed the influence of tDCS on the M1 and its effect on cognitive functions domains of response inhibitory control and visual recognition memory employing the SST and pattern recognition memory task (PRM), respectively, by using the Cambridge neuropsychological test automated battery (CANTAB).
Material and Methods
SUBJECTS:
Thirty healthy subjects (30 males; mean±standard deviation, 24.3±5.03 years; age range, 21–24 years) participated in this study. All participants were right-handed and naive to brain stimulation. None of the subjects was taking medications or had a history of neurological or psychiatric disorders. We explained the study to and obtained written informed consent from all participants. All procedures were carried out in accordance with the Declaration of Helsinki, approved by the Ethics Committee of King Saud University. Prior to the first session, all participants were evaluated for contraindications to tDCS [40], and the potential adverse effects of tDCS were explained to all subjects [41]. We aborted any experimental session immediately if the subject was not in a suitable condition.
DESIGN:
This was a randomized, double-blind, sham-controlled study with uncontrolled quota sampling, in which the the sample population was divided into 2 subgroups; one group received real stimulation (anodal-tDCS) and the other group received sham stimulation (sham-tDCS).
TDCS:
Participants were seated in a comfortable chair. The StarStim NE non-invasive wireless t-DCS neurostimulator (NE Neuroelectrics®, Barcelona, Spain) was employed to deliver the DC current. This device includes a wireless neoprene cap that is designed based on the International 10–20 system, which was positioned on the participants’ heads, with the central CZ electrode position located at the vertex. Specific (Pi electrodes, Neuroelectrics®), small Ag/AgCl gelled electrodes, with a surface contact area of 3.14 cm2, were located at the left M1 at C3 (anode) and at the contralateral supraorbital area (AF8; cathode). Electrodes were connected to a control device, which was wirelessly connected to a computer with NIC software (version 1.2, Neuroelectrics®). Anodal stimulation was delivered at an intensity of 2-mA for 20 min, while sham stimulation was delivered with the same stimulation intensity that ramped up then ramped down over a 30-s window at the beginning and end of the stimulation period.
COGNITIVE FUNCTION:
CANTAB research suite software (version 6. 0.37, Cambridge Cognition, Cambridge, United Kingdom) was used to conduct neuropsychological testing 2 times before and after tDCS stimulation. The tests from the battery needed 25–30 min to finish all tasks. Following the manufacturer’s instructions, participants were asked to sit comfortably on a chair and use the index finger of their dominant hand to press the response button.
STOP SIGNAL TEST (SST):
The SST measured the response inhibition (impulse control), where subjects had to respond to an arrow stimulus by choosing 1 of 2 options according to the direction in which the arrow points. Subjects have to inhibit that response during an audio cue. Thus, this test consists of 2 sections. In the first section the subjects were shown a stimulus on the screen of an arrow pointing left or right and were asked to press the button according to the direction of the arrow. Subjects had a rehearsal run, where 1 block offered 16 trials of this task. In the second section subjects were instructed to keep pressing the buttons when they see the arrows, as before, but if they heard an auditory cue, they must suppress their response and refrain from pressing the button. This test covers direction error and measures the rate of successful stops, response time on Go trials, and stop signal reaction time. A detailed description of the task procedure and outcome assessed is available on the CANTAB website (version 6. 0.37, Cambridge Cognition, Cambridge, United Kingdom).
PATTERN RECOGNITION MEMORY:
Pattern recognition memory (PRM) is a 2-choice, forced discrimination paradigm to examine visual pattern recognition memory. In the task, a series of visual patterns, which are difficult to be labeled verbally, are shown in the center of a screen. In the recognition phase subjects were required to choose between a pattern they have already seen and a new pattern. For both tasks, all subjects during the real and sham stimulations were performing the Piano Tiles game to control variability.
OUTCOME MEASURES IN THE STOP SIGNAL TEST AND IN THE PATTERN RECOGNITION MEMORY TEST:
Data analysis was conducted using SPSS version 21.0 (IBM Corporation, Armonk, NY, USA) for Windows (Microsoft Corporation, Redmond, WA, USA). We assessed 5 main outcome measures of the stop signal task (SST): direction errors, the proportion of successful stops, RT on GO trials, SSD (50%), and SSRT. A 2-tailed
Results
DEMOGRAPHIC CHARACTERISTICS:
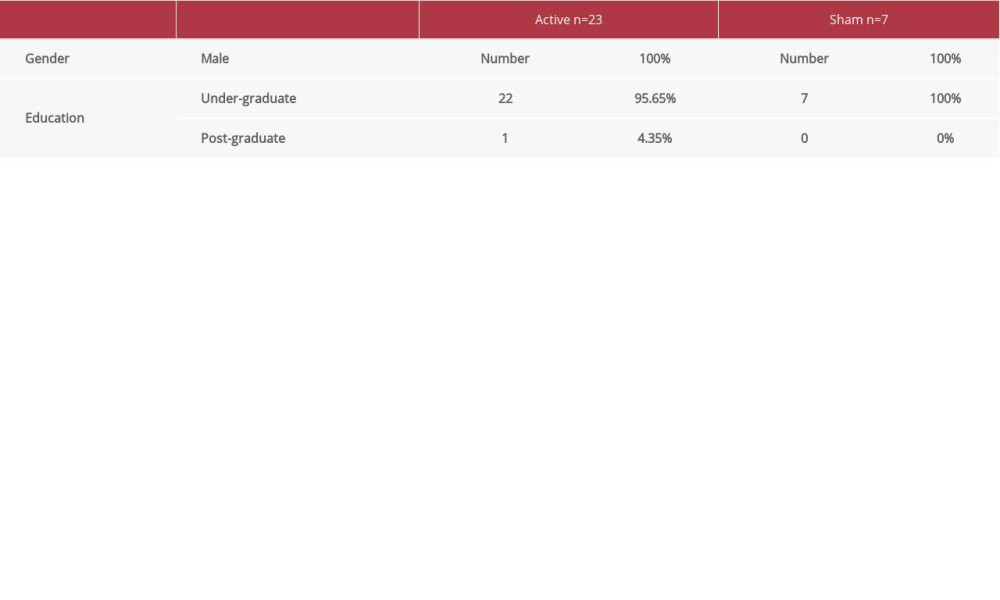
There were 23 participants (21.8±3.7 years) in the real stimulation group and 7 participants (21.2±0.4 years) in the sham stimulation group. There was no statistically significant difference in age between the 2 groups (P>0.05). Demographic data of the subjects are summarized in Table 1.
CANTAB:
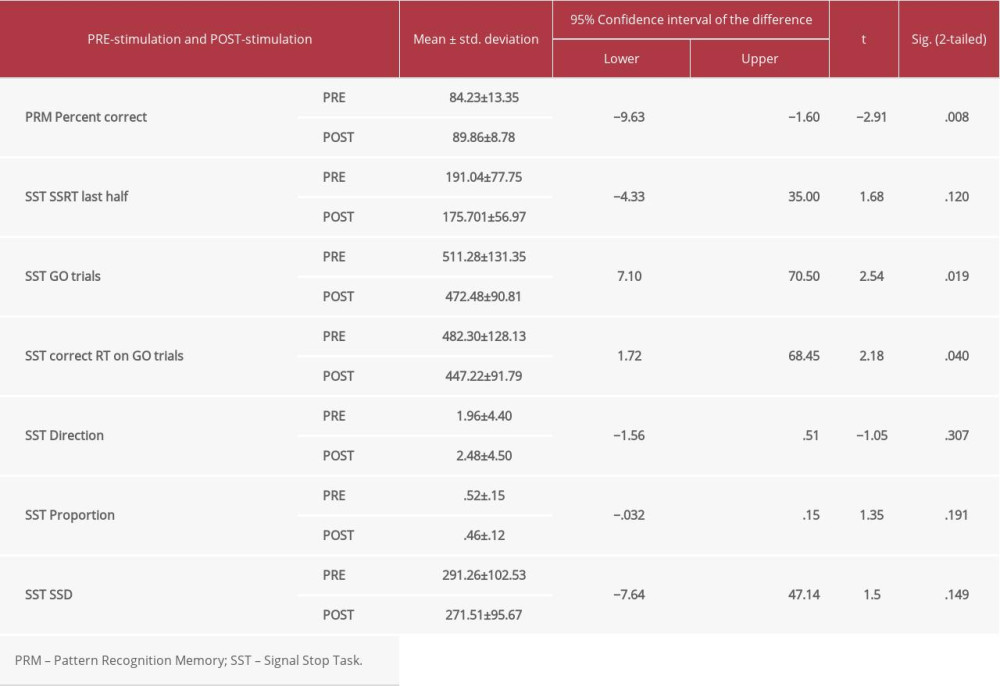
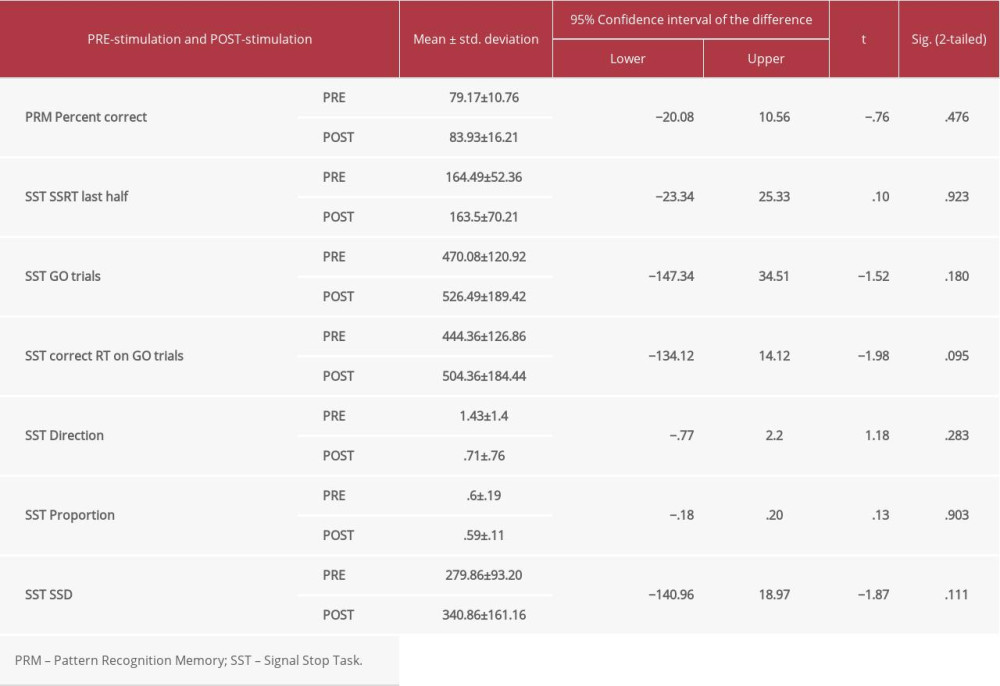
The group who received real stimulation was compared with the group who received sham stimulation. In the real stimulation group, there was a statistically significant difference (post- vs pre-stimulation) in cognitive function in terms of pattern recognition memory (PRM) (P=0.008), for stop signal task (SST) (go trials [SST GO trials] [P=0.019]), and for SST correct response time (RT in MS) on Go Trials (P=0.04) following real stimulation only (Table 2). However, there were no statistically significant differences in the sham stimulation group for any cognitive performances (Table 3).
Discussion
The primary aim of this work was to assess the potential efficacy for further proof-of-principle studies to show the effects of tDCS over M1 on cognition, particularly in inhibitory motor control and visual recognition memory domains in healthy subjects.
The main findings of this study were a significant improvement of stopping an initiated motor response during performance of the SST following anodal-tDCS of the M1, but not under the sham stimulation. We found a statistically significant decrease in SSRT following anodal-tDCS compared to the sham condition. This reveals that anodal-tDCS over M1 enhances SST performance as indicated by speeding up of SSRT, suggesting that response inhibition is tied to the neural processing of the M1. There were no significant changes in the processing times of go trials under either real or sham conditions. One inference drawn from these findings is that improvement in the stop process might be attributed to enhanced inhibitory control via the effect of direct stimulation following delivery of tDCS to the M1, whereby anodal stimulation modulated and reduced the non-stopped response rates on the SST task [41,42]. Another possible explanation is that anodal-tDCS over M1 resulted in exertion of excitatory signals to modulate motor output, and help to bias the competition between the motor responses [32,43]. It is evident that the connectivity between M1 and the input subcortical nuclei within the basal ganglia and subthalamic nucleus have a vital role in facilitating and augmenting the selectivity of desired motor output, while inhibiting or supressing unwanted movement [37,38]. Hence, stimulating M1 would enhance this motor inhibitory function that is necessary for behavioral flexibility. Our findings are consistent with several studies that have reported that M1 is a critical node in controlling and mediating the process of motor response execution and inhibition [43–45].
With regard to the visual pattern recognition memory task, a significant enhancement was detected following the delivery of anodal-tDCS over M1 compared with sham stimulation. One possible explanation for this is that such effects might be attributed to the remote effects of stimulating primary motor cortex using tDCS, which resulted in influencing prefrontal regions [46] that have significant role in memory, such as the premotor cortex, SMA [47,48], DLPFC [49], and cerebellum [50]. Although we cannot conclusively state that the enhancement of PRM observed here resulted from distant effects of stimulating M1 by anodal-tDCS, the extensive functional intercortical connectivity between M1 and other prefrontal areas makes this an acceptable claim to investigate explicitly in future studies.
Administering anodal-tDCS to M1 resulted in improvement of the stopping process in inhibitory control and memory enhancement in the PRM. Evidence indicates functional links between M1 and the fronto-striatal-thalamic network activated by behavioral inhibition [9,15,16]. Thus, application of anodal-tDCS to the M1, as an integral node in inhibitory motor control, is a promising adjuvant therapeutic intervention for modulation of motor response inhibition.
Some limitations should be taken into account when interpreting the findings of the current study. The design of the study was one of its inherent limitations, in addition to lack of examining another brain region to compare with M1. No female participants were recruited in this study, which might have affected the findings. Moreover, only a single session of tDCS was employed to enhance the cognitive functions of volitional behavior control and memory. Combining neurophysiological techniques (ie, EEG) or brain stimulation modalities such as transcranial magnetic stimulation (TMS) with administration of tDCS in multiple sessions would elucidate the underlying mechanisms of inhibitory functioning within M1 and investigate tDCS potential therapeutic effects for inhibitory control deficits. Combining TMS and functional imaging is also another promising approach in assessing the contributions of motor cortex in response inhibition and memory domains.
It is important to point out that the sample size of the sham group was very small, and the sham condition for cognitive performance showed no significant response, as in previous research [51–55]. The data included in this study cannot unambiguously support this conclusion. Follow-up research including more subjects in the sham group for both the right and left M1 will help investigate the specific role of the M1 in cognitive assessment of related motor tasks. Although we cannot exclude that selection bias may be present in our sample (eg, people volunteered to participate in our study), we cannot identify any plausible reason how these factors would explain a decreased response time in active condition only. Nonetheless, replication with other samples will be useful to increase confidence in the observed results.
Conclusions
Findings of the present study have clinical implications in enhancing inhibitory functioning in neurodevelopmental disorders such as Tourette syndrome and attention deficit hyperactivity disorder in which deficient volitional control is exhibited. Further studies with larger sample sizes with longitudinal design and multiple sessions of non-invasive brains stimulation techniques would shed light on the role of M1 in multiple cognitive function domains such as inhibitory control, attention, and memory, and would help explore the relationship between intracortical inhibition and behavioral inhibition within the M1 neural population.
References
1. Nitsche MA, Paulus W, Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation: J Physiol, 2000; 527; 633-39
2. Priori A, Brain polarization in humans: A reappraisal of an old tool for prolonged non-invasive modulation of brain excitability: Clin Neurophysiol, 2003; 114(4); 589-95
3. Stagg CJ, Best JG, Stephenson MC, Polarity-sensitive modulation of cortical neurotransmitters by transcranial stimulation: J Neurosci, 2009; 29(16); 5202-6
4. Nitsche MA, Schauenburg A, Lang N, Facilitation of implicit motor learning by weak transcranial direct current stimulation of the primary motor cortex in the human: J Cogn Neurosci, 2003; 15; 619-26
5. Nitsche MA, Jaussi W, Liebetanz D, Consolidation of human motor cortical neuroplasticity by D-cycloserine: Neuropsychopharmacology, 2004; 29; 1573-88
6. Wiethoff S, Hamada M, Rothwell JC, Variability in response to transcranial direct current stimulation of the motor cortex: Brain Stimul, 2014; 7(3); 468-75
7. Aron AR, Robbins TW, Poldrac RA, Inhibition and the right inferior frontal cortex: Trends Cogn Sci, 2004; 8(4); 170-77
8. Hatanaka N, Tokuno H, Nambu A, Takada M, Transdural doppler ultrasonography monitors cerebral blood flow changes in relation to motor tasks: Cereb Cortex, 2009; 19(4); 820-31
9. Aron AR, Poldrack RA, Cortical and subcortical contributions to Stop signal response inhibition: role of the subthalamic nucleus: J Neurosci, 2006; 26(9); 2424-33
10. Wiecki TV, Frank MJ, A computational model of inhibitory control in frontal cortex and basal ganglia: Psychol Rev, 2013; 120(2); 329-55
11. Rae CL, Hughes LE, Weaver C, Selection and stopping in voluntary action: A meta-analysis and combined fMRI study: Neuroimage 1, 2014; 86(100); 381-91
12. Aron AR, Robbins TW, Poldrack RA, Inhibition and the right inferior frontal cortex: One decade on: Trends Cogn Sci, 2014; 18(4); 177-85
13. Allen C, Singh KD, Verbruggen F, Chambers CD, Evidence for parallel activation of the pre-supplementary motor area and inferior frontal cortex during response inhibition: A combined MEG and TMS study: R Soc Open Sci, 2018; 5(2); 171369
14. Maizey L, Evans CJ, Muhlert N, Cortical and subcortical functional specificity associated with response inhibition: Neuroimage, 2020; 220; 117110
15. Aron AR, The neural basis of inhibition in cognitive control: Neuroscientist, 2007; 13(3); 214-28
16. Neubert F, Mars RB, Buch ER, Cortical and sub-cortical interactions during action reprogramming and their related white matter pathways: Proc Natl Acad Sci USA, 2010; 107; 13240-45
17. Wessel JR, Aron AR, It’s not too late: the onset of the front central P3 indexes successful response inhibition in the stop-signal paradigm: Psychophysiology, 2015; 52(4); 472-80
18. Bastani A, Jaberzadeh S, Does anodal transcranial direct current stimulation enhance excitability of the motor cortex and motor function in healthy individuals and subjects with stroke: A systematic review and meta-analysis: Clin Neurophysiol, 2012; 123(4); 644-57
19. Bastani A, Jaberzadeh S, A higher number of TMS-elicited MEP from a combined hotspot improves intra- and inter-session reliability of the upper limb muscles in healthy individuals: PLoS One, 2012; 7(10); e47582 [Erratum in: PLoS One. 2013;8(9)]
20. Brunoni AR, Nitsche MA, Bolognini N, Clinical research with transcranial direct current stimulation (tDCS): Challenges and future directions: Brain Stimul, 2012; 5(3); 175-95
21. Jahanshahi M, Obeso I, Rothwell JC, Obeso JA, A fronto-striato-subthalamic-pallidal network for goal-directed and habitual inhibition: Nat Rev Neurosci, 2015; 16(12); 719-32
22. Aron AR, From reactive to proactive and selective control: Developing a richer model for stopping inappropriate responses: Biol Psychiatry, 2011; 69(12); e55-68
23. Chikazoe J, Localizing performance of go/no-go tasks to prefrontal cortical subregions: Curr Opin Psychiatry, 2010; 23; 267-72
24. Lee HW, Lu MS, Chen CY, Roles of the pre-SMA and rIFG in conditional stopping revealed by transcranial magnetic stimulation: Behav Brain Res, 2016; 296; 459-67
25. Fonken YM, Rieger JW, Tzvi E, Frontal and motor cortex contributions to response inhibition: Evidence from electrocorticography: J Neurophysiol, 2016; 115(4); 2224-36
26. Duque J, Greenhouse I, Labruna L, Ivry RB, Physiological markers of motor inhibition during human behavior: Trends Neurosci, 2017; 40(4); 219-36
27. Ficarella SC, Battelli L, Motor preparation for action inhibition: A review of single pulse TMS studies using the Go/NoGo paradigm: Front Psychol, 2019; 10; 340
28. Cirillo J, Cowie MJ, MacDonald HJ, Byblow WD, Response inhibition activates distinct motor cortical inhibitory processes: J Neurophysiol, 2018; 119(3); 877-86
29. Muakkassa KF, Strick PL, Frontal lobe inputs to primate motor cortex: Evidence for four somatotopically organized “premotor” areas: Brain Res, 1979; 177; 176-82
30. He SQ, Dum RP, Strick PL, Topographic organization of corticospinal projections from the frontal lobe: Motor areas on the medial surface of the hemisphere: J Neurosci, 1995; 15; 3284-306
31. Dum RP, Strick PL, Frontal lobe inputs to the digit representations of the motor areas on the lateral surface of the hemisphere: J Neurosci, 2005; 25; 1375-86
32. Stinear CM, Coxon JP, Byblow WD, Primary motor cortex and movement prevention: Where Stop meets Go: Neurosci Biobehav Rev, 2009; 33(5); 662-73
33. Bhattacharjee S, Kashyap R, Abualait T, The role of primary motor cortex: More than movement execution: J Mot Behav, 2021; 53(2); 258-74
34. Mattia M, Spadacenta S, Pavone L, Stop-event-related potentials from intracranial electrodes reveal a key role of premotor and motor cortices in stopping ongoing movements: Front Neuroeng, 2012; 5; 12
35. Coxon JP, Stinear CM, Byblow WD, Intracortical inhibition during volitional inhibition of prepared action: J Neurophysiol, 2006; 95; 3371-83
36. van den Wildenberg WP, Burle B, Mechanisms and dynamics of cortical motor inhibition in the stop-signal paradigm: A TMS study: J Cogn Neurosci, 2010; 22; 225-39
37. Alexander GE, DeLong MR, Strick PL, Parallel organization of functionally segregated circuits linking basal ganglia and cortex: Annu Rev Neurosci, 1986; 9; 357-81
38. Nambu A, Tokuno H, Takada M, Functional significance of the cortico-subthalamo-pallidal ‘hyperdirect’ pathway: Neurosci Res, 2002; 43(2); 111-17
39. Ziemann U, Siebner HR, Inter-subject and inter-session variability of plasticity induction by non-invasive brain stimulation: Boon or bane?: Brain Stimul, 2015; 8(3); 662-63
40. Bikson M, Grossman P, Thomas C, Safety of transcranial direct current stimulation: Evidence based update 2016: Brain Stimul, 2016; 9(5); 641-61
41. Poreisz C, Boros K, Antal A, Paulus W, Safety aspects of transcranial direct current stimulation concerning healthy subjects and patients: Brain Res Bull, 2007; 72(4–6); 208-14
42. Jacobson L, Javitt DC, Lavidor M, Activation of inhibition: Diminishing impulsive behavior by direct current stimulation over the inferior frontal gyrus: J Cogn Neurosci, 2011; 23(11); 3380-87
43. Hsu TY, Tseng LY, Yu JX, Modulating inhibitory control with direct current stimulation of the superior medial frontal cortex: Neuroimage, 2011; 56(4); 2249-57
44. Chikazoe J, Jimura K, Hirose S, Preparation to inhibit a response complements response inhibition during performance of a stop-signal task: J Neurosci, 2009; 29(50); 15870-77
45. Badry R, Mima T, Aso T, Suppression of human cortico-motoneuronal excitability during the stop-signal task: Clin Neurophysiol, 2009; 120(9); 1717-23
46. Lefaucheur JP, André-Obadia N, Antal A, Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS): Clin Neurophysiol, 2014; 125(11); 2150-206
47. Chein JM, Fiez JA, Dissociation of verbal working memory system components using a delayed serial recall task: Cereb Cortex, 2001; 11(11); 1003-14
48. Paulesu E, Frith CD, Frackowiak RS, The neural correlates of the verbal component of working memory: Nature, 1993; 362(6418); 342-45
49. Bashir S, Al-Hussain F, Hamza A, Cognitive function assessment during 2 mA transcranial direct current stimulation in DLPFC in healthy volunteers: Physiol Rep, 2019; 7(20); e14264
50. Marvel CL, Desmond JE, Functional topography of the cerebellum in verbal working memory: Neuropsychol Rev, 2010; 20(3); 271-79
Most Viewed Current Articles
15 Jun 2022 : Clinical Research
Evaluation of Apical Leakage After Root Canal Obturation with Glass Ionomer, Resin, and Zinc Oxide Eugenol ...DOI :10.12659/MSMBR.936675
Med Sci Monit Basic Res 2022; 28:e936675
07 Jul 2022 : Laboratory Research
Cytotoxicity, Apoptosis, Migration Inhibition, and Autophagy-Induced by Crude Ricin from Ricinus communis S...DOI :10.12659/MSMBR.936683
Med Sci Monit Basic Res 2022; 28:e936683
01 Jun 2022 : Laboratory Research
Comparison of Sealing Abilities Among Zinc Oxide Eugenol Root-Canal Filling Cement, Antibacterial Biocerami...DOI :10.12659/MSMBR.936319
Med Sci Monit Basic Res 2022; 28:e936319
08 Dec 2022 : Original article
Use of Estimated Glomerular Filtration Rate and Urine Albumin-to-Creatinine Ratio Based on KDIGO 2012 Guide...DOI :10.12659/MSMBR.938176
Med Sci Monit Basic Res 2022; 28:e938176