19 January 2021: Human Study
Examining the Beneficial Aspects of Nutritional Guidance Using Estimated Daily Salt Intake in Cancer Patients with Ischemic Heart Disease
Takashi Aoyama1ABCDEF*, Takuya Oyakawa2B, Akifuimi Notsu3C, Emi Oiyama4B, Masao Hashimoto4B, Reiko Suzuki5E, Kei Iida6BDOI: 10.12659/MSMBR.927719
Med Sci Monit Basic Res 2021; 27:e927719
Abstract
BACKGROUND: The outcomes associated with nutritional guidance for patients with ischemic heart disease undergoing cancer treatment have not been explored. We examined the effects of nutritional guidance using estimated daily salt intake in cancer patients with ischemic heart disease.
MATERIAL AND METHODS: We examined the data from physical examinations and laboratory assessments of 27 patients with suspected excessive salt intake who underwent coronary angiography for the first time and received nutritional guidance on their next visit to the Department of Cardiology of Shizuoka Cancer Center between May 2018 and March 2020. Salinity measurement was not used in the nutritional guidance method, but the patients were instructed to reduce consumption of salt-containing foods. We compared the frequency of the estimated daily salt intake with the frequency of categories requiring salt control (food, cooking, and table salts).
RESULTS: The median age of the participants was 74 (range, 63–86) years. The estimated daily salt intake and the rate of change in the triglyceride level were negatively correlated (r=–0.61, P<0.01). The estimated daily salt intake was reduced in 16 cases; there was a relative decrease in the frequency of food intake among categories requiring salt control compared with that in the nonimproved cases (P<0.01). No difference was found between the cancer stage and the affected site of the digestive system in either group (P=0.64, P=0.39).
CONCLUSIONS: Nutritional guidance on dietary habits without using salinity measurement was beneficial in preventing ischemic heart disease and food intake reduction in cancer patients.
Keywords: Cancer Care Facilities, Coronary Angiography, Nutrition Assessment, Aged, 80 and over, Feeding Behavior, Myocardial Ischemia, Neoplasms, Sodium Chloride, Dietary
Background
Heart storage disease has the second highest mortality rate after cancer [1]. The prevalence of cancer increases with age [2], but the epidemiological statistics for heart disease are not clear [3]. Medical guidelines indicate a daily salt intake of 3.8 g due to the role of salt intake in the development of this illness [4–6]. Although there is evidence that a reduction in dietary salt results in nutritional deficiency and loss of appetite [7], there has been no discussion concerning the effects on triglycerides (TGs) [8], which reflect the oral intake quantity of nutrients. No significant effects have been reported for the effects of sodium and the Dietary Approaches to Stop Hypertension (DASH) diet on serum lipids [9]. However, the adverse effects of high TG levels on the heart are well known [10]. In recent years, guidelines for hypertension have been presented [11], but there has been no report focusing on the actual dietary habits of patients. The methodologies and outcomes of nutritional guidance for patients with ischemic heart disease undergoing cancer treatment have not been explored. This could be because there are many ready-made food options in cities, latent variations in salt content that cannot be aggregated, and several available resources on the salt content of foods. Furthermore, because there are no evaluation tools, it is likely that the salinity calculation method of each patient and dietitian would not be consistent. In addition, salinity measurement is probably infeasible in cancer patients with suspected ischemic heart disease who have adverse events [12] and a psychological burden [13] due to cancer treatment. The key to the therapeutic effect may lie in the subjective assessment of each patient [14]. According to the National Nutrition Survey, salt intake is the highest for men and women in their 60s [15]. The estimation of daily salt intake [16] can be easily evaluated from voluntary urine using salt intake evaluation methods [11], and it has been shown to be useful for hypertensive patients. Although this phenomenon has been reported, it has not been discussed as a nutritional guidance for patients with heart disease who are undergoing cancer treatment and its outcomes have not been considered. The purpose of this study was to explore the benefit of voluntary urine (estimated daily salt intake) in the nutritional guidance regarding the dietary habits of cancer patients undergoing coronary angiography (CAG).
Material and Methods
PARTICIPANTS:
We conducted a retrospective subset analysis. The study was approved by the Institutional Review Board (IRB) of the Shizuoka Cancer Center (SCC) (SCCIRB approval number: 30-J78) and performed in accordance with the principles of the Declaration of Helsinki. Patient consent was obtained using the in-hospital bulletin board of the SCCIRB for a retrospective study.
We enrolled 27 patients who underwent CAG for the first time and accepted nutritional guidance during the study period from May 2018 to March 2020. Patients were excluded if they received cancer treatment during the evaluation period, had renal cancer, or were judged to be ineligible for this clinical study by the attending physician.
NUTRITIONAL GUIDANCE:
The baseline evaluation (T1) was the morning before CAG or the day of CAG (day of admission), and the follow-up evaluation (T2), in which nutritional guidance was implemented, was performed the morning on the day of the next department’s medical examination. Figure 1 shows a patient interview sheet filled by medical staff during nutritional guidance.
The following variables were evaluated from T1 to T2. Estimated daily salt intake [16] was calculated from voluntary urine (from the second urine after waking for the day and onward, once, >10 mL), as follows:
where Na is the urine sodium concentration (mEq/L), and Cr is the urine creatinine concentration (mg/dL).
Body composition (skeletal muscle mass, fat mass, body water, and phase angle) was evaluated using the body weight and bioelectrical impedance analysis (In Body S20®), which examined fasting and used bioelectrical impedance analysis, respectively [17,18]. Blood biochemical values (total cholesterol [TC], TGs, high-density lipoprotein cholesterol [HDL-C]) were evaluated to examine fasting.
The rate of change of these variables from T1 to T2 was calculated, and the relationship with estimated daily salt intake was investigated. We also investigated the relationship between the estimated daily salt intake and changes in the skeletal muscle mass, and the relationship between the cancer stage in T2 and the rate of change in skeletal muscle mass from T1 to T2.
The method for nutritional guidance method was as follows. The categories listed below requiring salt control [19] (food, cooking, and table salt) were subjectively evaluated by the patients at T1 based on their dietary habits and patterns. Guidance for healthy food substitutions was provided, and each category was reevaluated at T2.
Food salt included salted products (e.g., pickles, meat/fish processed food, dried goods, bread, and noodles) for which the T1 alternatives were raw vegetables, boiled vegetables, tofu, egg dishes, rice, unsalted noodles (e.g., soba), and white fish. At the T2 evaluation, the frequency of use improved by >80% compared with T1. The substitution of ready-made products was not assessed.
The cooking salt category included commercial soup with added salt, consumed multiple times a day. The T1 alternative instruction was natural or commercial dashi without added salt, consumed once a day. The T2 evaluation assessed whether the alternative guidance had been adopted.
The table salt category included use of a soy sauce dispenser (frequent use) and ready-made products. The T1 alternative instruction was to use a push-type soy sauce dispenser, how to read the amount of salinity of ready-made products (400 mg of sodium corresponds to approximately 1.0 g of salt), and preparation of homemade lunches that provide balanced nutrition with limited cooking salt. The T2 evaluation assessed adoption of alternative guidance.
Through dietary records and interviews, the frequency of use of the categories requiring salt control in eating habits were investigated during the past 3 days in all patients. Patients were divided into 2 groups, with and without reduced estimated daily salt intake at T2 (improved and nonimproved groups, respectively), and intake was compared with the frequency of use at T1. The cancer stage at T2 of both groups was also compared based on the cancer site (digestive and nondigestive systems).
STATISTICAL ANALYSIS:
All variables were expressed as medians. The comparison of study variables was performed using
Results
The participants included 20 men and 7 women. Their median age was 74 (range, 63–86) years. The body mass index at T1 was 24.1 (range, 20.3–32.4) kg/m2. The median period from T1 to T2 was 35 (range, 7–122) days, and all patients were diagnosed as having ischemic heart disease at T1 after undergoing CAG (24 cases of angina pectoris, 2 of coronary artery sclerosis, and 1 of myocardial infarction).
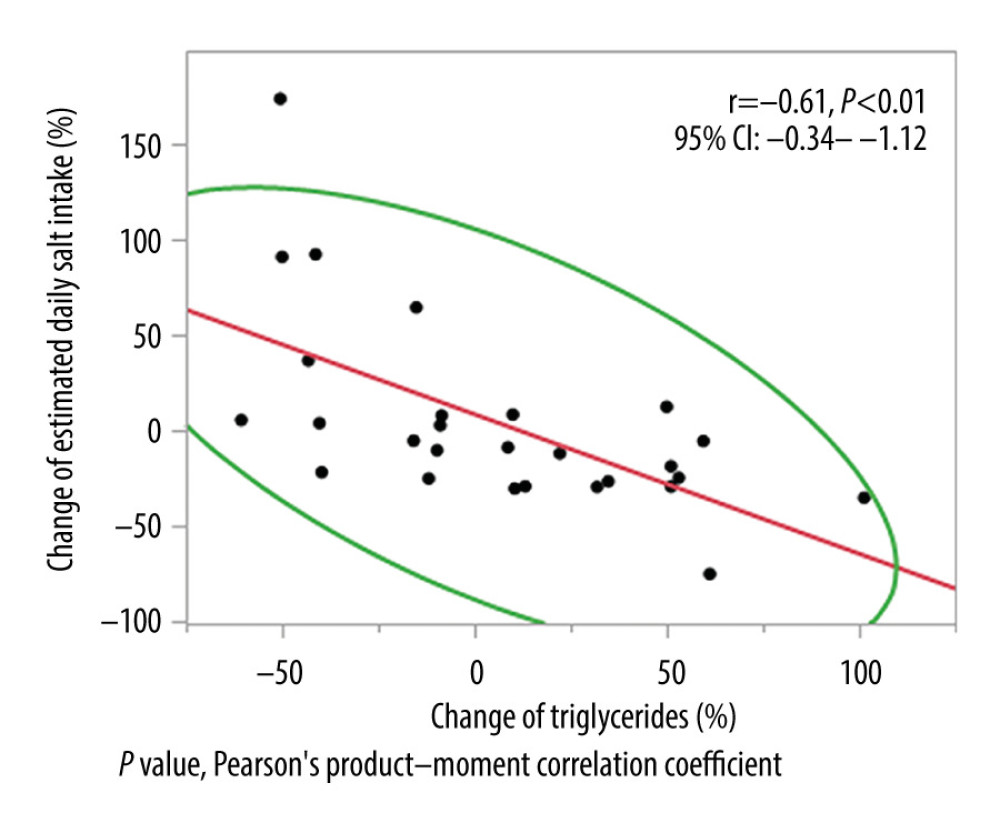
There was no difference in the estimated daily salt intake, body composition, and biochemical test values (Table 1) between T1 and T2. The change in the estimated daily salt intake from T1 to T2 and the rate of change of TGs were negatively correlated (r=−0.61,
In 16 of the 27 cases, the estimated daily salt intake improved at T2 following nutritional guidance at T1. A significant difference was found in the rate of TG change and the estimated daily salt intake (Table 2). Compared with the nonimproved group (11 cases), the frequency of consuming foods, such as salted food, bread and noodles etc., decreased by >80% per day in the improved group (16 cases,
Discussion
We tested blood biochemical values related to nutrient metabolism and found that TGs were negatively correlated with the estimated daily salt intake (r=−0.61,
However, 2 crucial questions arise. Does the taste of salt in foods reflect salt intake? Is a salty taste equal to the salt content? The estimated daily salt intake at T1 was 9.7 g. This value was comparable to the salt intake of people in their 70s of the same generation (9.8 and 11.1 g/d in women and man participants, respectively) from the National Health and Nutrition Survey [15]. This finding indicated that the global intake of salt is high and suggested the need to support the treatment [24–26]. From T1 to T2, there was a relative decrease in the frequency of foods that the patients consumed, which resulted in improved estimated daily salt intake (
Skeletal muscle mass and bioelectrical impedance analysis [27] were expected to affect nutritional metabolism. However, our results did not change during the course of the study (Table 1) and did not correlate with the rate of change in the estimated daily salt intake (r=0.32,
This research aimed to support cancer patients who wanted to be treated without bias from the viewpoint of a registered dietitian. It proposed a new nutritional guidance method that can be widely applied even to patients without cancer. However, while we discussed the topic of ischemic heart disease and cancer, the effects in the context of salinity and nutrition aspects (e.g., saturated fatty acids, potassium) and exercise are not clear.
This study had some limitations. It was based on dietary habit adherence [6], evaluated through subjective evaluation of individual patients, and had a small sample size; however, it had a scientific guidance effect. This suggested that it was a suitable method of nutritional intervention for cancer patients [30–34]. Furthermore, this study involved a disease with a low prevalence, we did not include a comparison group of patients without cancer, and the outcome could not be traced back.
Conclusions
This study suggested that estimation of daily salt intake and nutritional guidance using dietary habits for cancer patients with ischemic heart disease had beneficial aspects. Considering the burden on cancer patients, nutritional guidance based on adherence (i.e., reduction of salty food consumption frequency to <80% per day) that does not require salt measurement may be sufficient.
References
1. Ministry of Health, Labour and Welfare: Vital statistics https://www.mhlw.go.jp/english/database/db-hw/outline/index.html
2. Center for Cancer Control and Information Services, National Cancer Center: Cancer statistics in Japan https://ganjoho.jp/en/professional/statistics/table_download.html
3. Ministry of Health, Labour and Welfare: Patient survey https://www.mhlw.go.jp/english/database/db-hss/sps_2014.html
4. Strazzullo P, D’Elia L, Kandala NB, Cappuccio FP, Salt intake, stroke, and cardiovascular disease: Meta-analysis of prospective studies: BMJ, 2009; 339; b4567
5. Taylor RS, Ashton KE, Moxham T, Reduced dietary salt for the prevention of cardiovascular disease: Cochrane Database Syst Rev, 2011; 7; CD009217
6. American Society of Nephrology: ASN statement in support of US dietary guidelines for Americans, 2010 http://www.newswise.com/articles/asn-statement-in-support-of-us-dietary-guidelines-for-americans-2010
7. Alderman MH, Lamport B, Moderate sodium restriction: Do the benefits justify the hazards?: Am J Hypertens, 1990; 3; 499-504
8. Mensink RP, Katan MB, Effect of dietary fatty acids on serum lipids and lipoproteins: A meta-analysis of 27 trials: Arterioscler Thromb, 1992; 12; 911-19
9. Harsha DW, Sacks FM, Obarzanek E, Effect of dietary sodium intake on blood lipids results from the DASH – sodium trial: Hypertension, 2004; 43; 393-98
10. Goldberg IJ, Fat in the blood, fat in the artery, fat in the heart: Triglyceride in physiology and disease: Arterioscler Thromb Vasc Biol, 2018; 38; 700-6
11. The Japanese Society of Hypertension: Guidelines for the Management of Hypertension, 2019 https://www.jpnsh.jp/index_e.html
12. Japan Clinical Oncology Group: Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0, 2017 http://www.jcog.jp/en/
13. Taniyama T, Shimizu C, Kakimoto M, The preferences for survival or quality of life in the treatments for breast cancer patients: A comparison between patients and healthcare-providers: Palliat Care Res, 2014; 9; 101-9
14. Sabaté E, World Health Organization, Noncommunicable Diseases and Mental Health Cluster: Adherence to long-term therapies: Policy for action: meeting report June 4–5, 2001 https://apps.who.int/iris/handle/10665/66984
15. Ministry of Health, Labour and Welfare: National Health and Nutrition Survey https://www.nibiohn.go.jp/eiken/english/research/project_nhns.html
16. Tanaka T, Okamura T, Miura K, A simple method to estimate populational 24-h urinary sodium and potassium excretion using a casual urine specimen: J Hum Hypertens, 2002; 16; 97-103
17. Shafer KJ, Siders WA, Johnson LK, Lukaski HC, Validity of segmental multiple-frequency bioelectrical impedance analysis to estimate body composition of adults across a range of body mass index: Nutrition, 2009; 25; 25-32
18. Cunningham JJ, Body composition as a determinant of energy expenditure: A synthetic review and a proposed general prediction equation: Am J Clin Nutr, 1991; 54; 963-69
19. Stevens SS, On the theory of scales of measurement: Science, 1946; 103; 677-80
20. Yahagi N, Shimano H, Microarray analyses of SREBP-1 target genes: Understanding lipid metabolism with microarrays and other omic approaches, 2005; 237-48, Boca Raton, FL, CRC Press
21. Iso H, Naito Y, Sato S, Serum triglycerides and risk of coronary heart disease among Japanese men and women: Am J Epidemiol, 2001; 153; 490-99
22. Nadeem S, Sandhu MS, Ricketts SL, Triglyceride-mediated pathways and coronary disease: collaborative analysis of 101 studies: Lancet, 2010; 375; 1634-39
23. Warburg O, On the origin of cancer cells: Science, 1956; 123; 309-14
24. Powles J, Fahimi S, Micha R, Global, regional and national sodium intakes in 1990 and 2010: A systematic analysis of 24 h urinary sodium excretion and dietary surveys worldwide: BMJ Open, 2013; 3; e003733
25. Zamorano JL, Lancellotti P, Rodriguez Muñoz D, 2016 ESC position paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for practice guidelines: The task force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC): Eur Heart J, 2016; 37; 2768-801
26. Ganatra S, Hayek SS, Cardio-oncology for GenNext: A missing piece of the training puzzle: J Am Coll Cardiol, 2018; 71; 2977-81
27. Aoyama T, Yoshitsugu K, Fukaya M, Benefit of reducing body weight loss with a nutritional support pathway in patients undergoing allogeneic hematopoietic stem cell transplantation: Med Sci Monit Basic Res, 2019; 25; 179-87
28. Kawaguchi K, Furukawa S, Mitekura H, Usefulness of estimated creatinine clearance method: Jpn J Med Tech, 2008; 57; 1392-96
29. Burton ME, Shaw LM, Schentag JJ: Applied pharmacokinetics & pharmacodynamics: Principles of therapeutic drug monitoring, 2006; 188, Baltimore, Lippincott Williams & Wilkins
30. Baldwin C, Spiro A, Ahern R, Emery PW, Oral nutritional interventions in malnourished patients with cancer: A systematic review and meta-analysis: J Natl Cancer Inst, 2012; 104; 371-85
31. Aoyama T, Imataki O, Mori K, Nutritional risk in allogeneic stem cell transplantation: Rationale for a tailored nutritional pathway: Ann Hematol, 2017; 96; 617-25
32. Aoyama T, 2018; 1-76, Shizuoka, Japan, Laboratory of Clinical Nutrition and Management, Graduate School of Integrated Pharmaceutical and Nutritional Sciences, University of Shizuoka [in Japanese], http://id,nii,ac,jp/1417/00004696/
33. Aoyama T, Imataki O, Arai H, Comparison of nutrition-related adverse events and clinical outcomes between ICE (Ifosfamide, Carboplatin, and Etoposide) and MCEC (Ranimustine, Carboplatin, Etoposide, and Cyclophosphamide) therapies as pretreatment for autologous peripheral blood stem cell transplantation in patients with malignant lymphoma: Med Sci Monit Basic Res, 2018; 24; 31-39
34. Aoyama T, Yamada A, Akiyama K, Informative aspects of eating rate in allogeneic hematopoietic stem cell transplantation using patients’ nutritional pathway: Journal of Hematopoietic Cell Transplantation, 2020; 9; 83-92
Figures
Tables
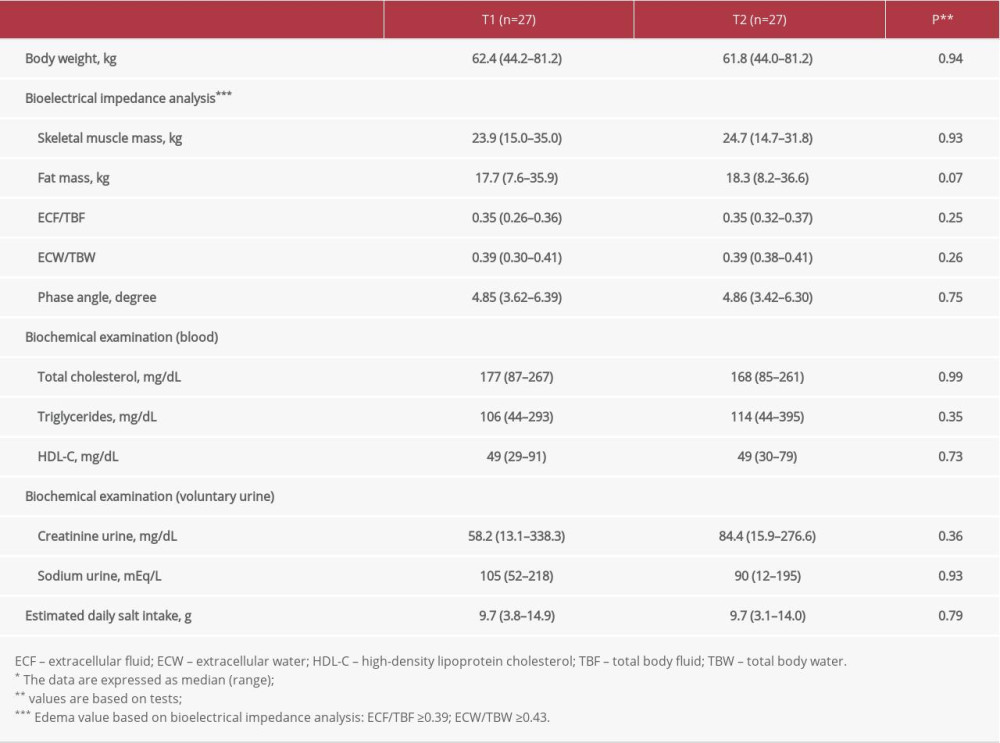 Table 1. Comparison of the parameters*.
Table 1. Comparison of the parameters*.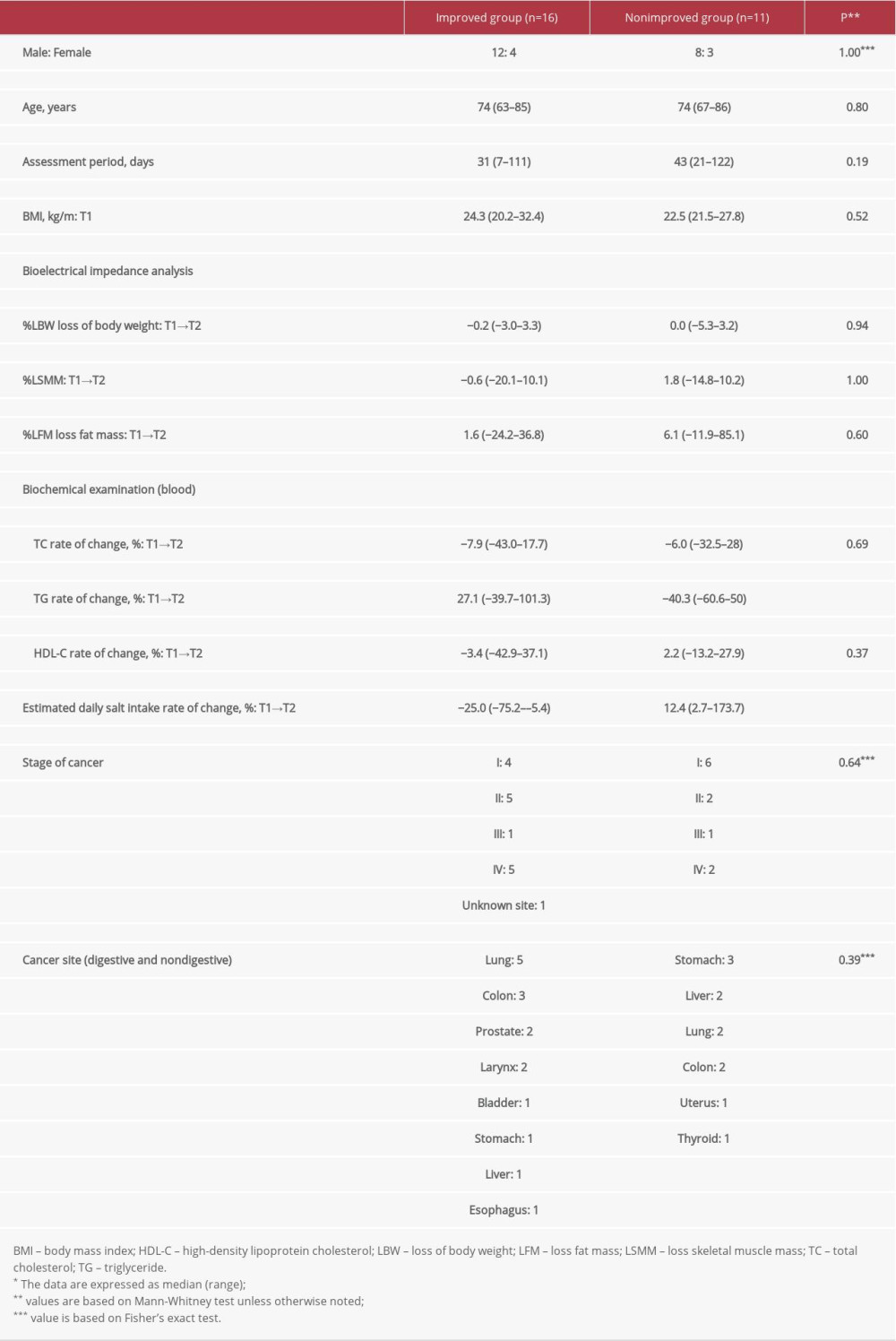 Table 2. Comparison between the improved group and the nonimproved group for estimated daily salt intake*.
Table 2. Comparison between the improved group and the nonimproved group for estimated daily salt intake*.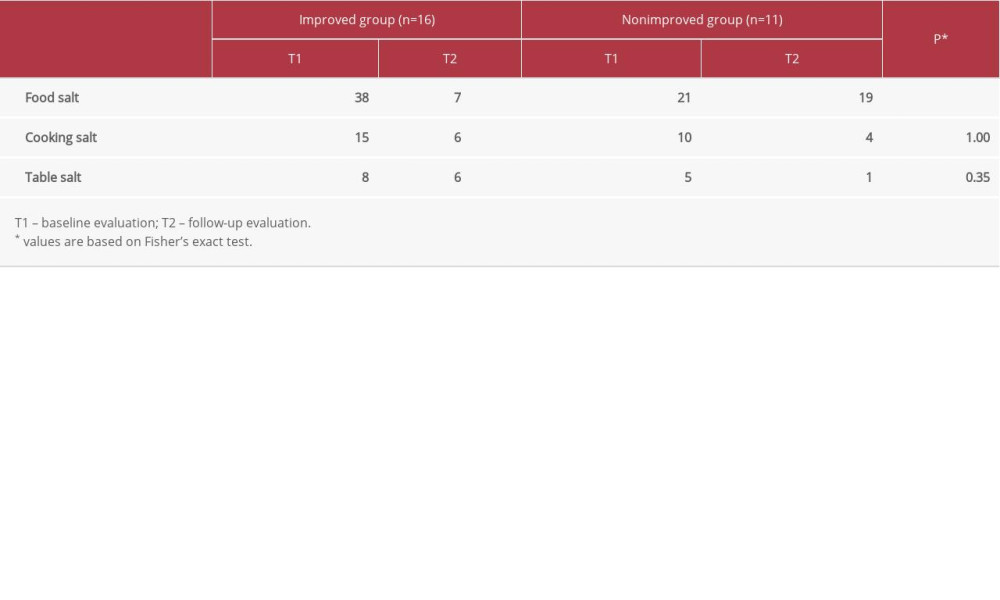 Table 3. Frequency ratio of categories requiring salt control in improved and nonimproved groups of daily estimated salt intake.
Table 3. Frequency ratio of categories requiring salt control in improved and nonimproved groups of daily estimated salt intake. Table 1. Comparison of the parameters*.
Table 1. Comparison of the parameters*. Table 2. Comparison between the improved group and the nonimproved group for estimated daily salt intake*.
Table 2. Comparison between the improved group and the nonimproved group for estimated daily salt intake*. Table 3. Frequency ratio of categories requiring salt control in improved and nonimproved groups of daily estimated salt intake.
Table 3. Frequency ratio of categories requiring salt control in improved and nonimproved groups of daily estimated salt intake. Most Viewed Current Articles
15 Jun 2022 : Clinical Research
Evaluation of Apical Leakage After Root Canal Obturation with Glass Ionomer, Resin, and Zinc Oxide Eugenol ...DOI :10.12659/MSMBR.936675
Med Sci Monit Basic Res 2022; 28:e936675
07 Jul 2022 : Laboratory Research
Cytotoxicity, Apoptosis, Migration Inhibition, and Autophagy-Induced by Crude Ricin from Ricinus communis S...DOI :10.12659/MSMBR.936683
Med Sci Monit Basic Res 2022; 28:e936683
01 Jun 2022 : Laboratory Research
Comparison of Sealing Abilities Among Zinc Oxide Eugenol Root-Canal Filling Cement, Antibacterial Biocerami...DOI :10.12659/MSMBR.936319
Med Sci Monit Basic Res 2022; 28:e936319
08 Dec 2022 : Original article
Use of Estimated Glomerular Filtration Rate and Urine Albumin-to-Creatinine Ratio Based on KDIGO 2012 Guide...DOI :10.12659/MSMBR.938176
Med Sci Monit Basic Res 2022; 28:e938176