15 February 2021: Human Study
Relationship Between Composition of Fatty Acid in Platelet Phospholipid Membrane and Markers of Oxidative Stress in Healthy Men and Men After a Myocardial Infarction
Inga Bikulčienė1ABCDEF*, Neda Garjonytė1CDF, Vytautas Žėkas1B, Rėda Matuzevičienė1CD, Živilė Žymantienė2B, Aldona Baublytė3CG, Vaiva Hendrixson1E, Dovilė Karčiauskaitė1ADG, Algirdas Utkus4DG, Arvydas Kaminskas1ADGDOI: 10.12659/MSMBR.929634
Med Sci Monit Basic Res 2021; 27:e929634
Abstract
BACKGROUND: Oxidative stress (OS) is known to be extremely damaging for phospholipids in cell membranes, especially their polyunsaturated fatty acids (PUFAs). OS is known to be associated with increased platelet activation and thrombosis, which lead to cardiovascular lesions. The aim of this study was to investigate how changes in the composition of fatty acids (FAs) in the platelet phospholipid membrane correlate with OS in healthy men and in men who have experienced a myocardial infarction (post-MI men).
MATERIAL AND METHODS: FA methyl esters from the platelet phospholipid membrane of 79 apparently healthy and 20 post-MI men were identified using gas chromatography/mass spectrometry. Malondialdehyde (MDA) was measured in the blood serum using high-performance liquid chromatography, and platelet-white blood cell aggregates (PWAs) were analysed based on whole-blood flow cytometry. The composition of platelet membrane FAs was compared to MDA concentration (µg/l) and the percentage of PWA formation between healthy men and individuals who had suffered a myocardial infarction (MI).
RESULTS: Statistically, post-MI patients had a significantly higher concentration of blood serum MDA than those in the control group (p=0.000). The level of PUFAs was also higher in the platelet phospholipid membrane of post-MI patients than in healthy individuals (p=0.016). However, the percentage of PWA formation was lower in patients compared with the control group (p<0.05).
CONCLUSIONS: A higher level of blood serum MDA concentration due to OS stimulates platelets to incorporate more PUFAs into the phospholipid membrane, thereby affecting platelet activation. This may lead the individual to develop cardiovascular diseases in the future.
Keywords: Cardiovascular Diseases, Platelet Activation, Blood Platelets, Fatty Acids, Phospholipids
Background
Cardiovascular diseases (CVDs) are the principal global cause of death [1]; each year CVDs cause 3.9 million deaths in Europe [2]. According to the WHO, the most important behavioural risk factors associated with heart disease are an unhealthy diet, physical inactivity, tobacco use, and abuse of alcohol [1,3]. The risk factors mentioned above, together with an increase in caloric intake over the past 100 years, have contributed to an intensified generation and accumulation of reactive oxygen species (ROS), leading to the development of oxidative stress (OS) in cells [4].
Phospholipids in cell membranes, especially their polyunsaturated fatty acids (PUFAs), are extremely sensitive to OS and lipid peroxidation. ROS modify the lipid composition, as well as the structure and dynamics of cell membranes, through lipid peroxidation [5]. Under these conditions, the structure, activity, and physical properties of the cell itself may change; these changes can lead to alterations in the production of biologically active compounds, which in turn influences the atherogenic and prothrombotic effects in the human body [6].
Lipids account for 16–19% of dry platelet matter and include 65% phospholipids [7]. Glycolipids and phospholipids are well-known targets of damaging and potentially lethal peroxidative modification [8]. Lipid products, such as eicosanoids, derived from ω3 or ω6 PUFAs, also significantly regulate and alter the function of platelets [9]. Moreover, the induction of platelet aggregation by arachidonic acid is associated with the formation of large amounts of malondialdehyde (MDA), which is a lipid peroxidation product. Platelets are therefore acknowledged to be one of the main sources of lipid peroxidation in human blood [10].
Malondialdehyde is among the most thoroughly investigated products of lipid peroxidation. This biological marker of OS is produced from PUFAs with 2 or more methylene-interrupted double bonds. MDA exists in 2 forms – free or covalently – bound to/conjugated with proteins, nucleic acids, lipoproteins, and certain amino acids [10,11]. The measurement of MDA values can be made in a variety of biological samples: in plasma, serum, tissues, and occasionally in urine [10,12]. According to scientific data, MDA contributes to many pathological conditions and diseases [5], being associated with carcinogenic and cytotoxic effects on the cell [13–17], thrombosis, and increased platelet activity [18].
We therefore designed this study to examine the association between changes in the composition of fatty acids (FAs) in the platelet phospholipid membrane, the concentration of blood serum MDA, and the formation of platelet-white blood cell aggregates (PWAs) in healthy men and those who had an MI (post-MI patients).
Material and Methods
STUDY DESIGN AND PATIENT SELECTION:
A case-control study was carried out on a group of 20 post-MI and 79 volunteer men. It sought to establish the association between the platelet phospholipid membrane FA spectrum and markers of OS. The case group consisted of patients 40 to 60 years of age (average age 52.2±11.3 years) who had a single myocardial infarction (MI) and whose coronary angiography had confirmed coronary occlusion of more than 50% in at least 2 arteries. Coronary catheterization and coronary angioplasty were performed on all patients. At the time a blood sample was collected, the patients were under antihypertensive treatment and received aspirin and statins. In addition, the post-MI patients had been treated for myocardial infarction at least 3 months prior to their inclusion in this study.
All post-MI patients had primary arterial hypertension. Eight patients had been diagnosed with diabetes mellitus. Various systemic inflammatory diseases (such as podagra, psoriasis, or autoimmune diseases) affected 5 patients at the time of blood collection. The control group of 79 apparently healthy men (average age 36.5±10.8 years) had no prior history of any cardiac or chronic diseases, strokes, venous thromboembolisms, and were not being treated for CVDs. No female subjects were included in this study, as males are known to have an earlier onset of disease than females [19].
All individuals were enrolled at Vilnius University Hospital Santaros Clinics and filled out written consent forms to participate in this study. The research was conducted at the laboratory of the Department of Physiology, Biochemistry, Microbiology, and Laboratory Medicine of the Institute of Biomedical Sciences at the Faculty of Medicine of Vilnius University. The study protocol was approved by the Vilnius Regional Bioethics Committee (Approval No. 15820-15-807-319, No. 219/2-1093-592) and was supported by the Research Council of Lithuania (Grant No. MIP-050/2015).
DISTRIBUTION OF INDIVIDUALS AND SEQUENCE OF ASSAYS:
In the investigation of OS influence on the FA composition of the platelet phospholipid membrane of healthy (N=79) and post-MI men (N=20), blood serum MDA concentration (μg/l) was first measured. Then, all the subjects (N=99) were tested for platelet activation markers to assess the percentage of PWA (ie, formation of platelet and monocyte aggregates [PMA], platelet and neutrophil aggregates [PNA], and platelet and lymphocyte aggregates [PLYA]). Finally, the composition of platelet phospholipid membrane FA was determined both in post-MI patients and healthy volunteers (N=99) (Figure 1).
The results of assays in this study were compared between patients and the control group. The correlation between the FA spectrum of the platelet phospholipid membrane and MDA concentration in blood serum in post-MI men was also calculated.
DETERMINATION OF MDA CONCENTRATION IN BLOOD SERUM:
A method published by Khoschsorur et al, with some minor modifications, was used to measure the concentration of blood serum MDA (μg/l) [20]. The sample preparation serves for the sample purge and for the derivatization of the analyte with thiobarbituric acid (TBA) into a detectable form (ie, the MDA-TBA adduct). MDA concentration was determined using the Shimadzu Nexera X2 UHPLC system (Shimadzu). Data were collected and processed using LabSolutions software (Shimadzu).
PLATELET EXTRACTION:
To obtain platelets from all subjects, blood samples were collected in a sodium heparin vacutainer tube and centrifuged immediately at 3000 g rcf for 10 min. Then, ¾ of the plasma was removed without touching the cell and the buffy coat. The remaining portion (ie, ¼ of the plasma), rich in thrombocytes, was extracted and mixed with freezing media (Biological Industries, Israel) at a ratio of 2: 1 and frozen at −80°C [21,22].
MEASUREMENT OF PLATELET ACTIVATION:
Measurement of PWAs was performed by flow cytometric (BD FACS Canto, BD Biosciences, USA) analysis of platelet surface antigens in agonist non-stimulated EDTA anticoagulated blood not later than 10 min after blood collection. WBC subpopulations (neutrophils, monocytes, and lymphocytes) were gated on CD45 vs CD14 dot plot according to the CD45/CD14 expression: neutrophils (CD45+, CD14−, high side-scattered light), monocytes (CD45+, CD14+, mean side-scattered light), and lymphocytes (CD45++, CD14−, low side-scattered light). Platelet marker CD42a expression on these WBC subpopulations was measured and the percentage of positive population was calculated. Data analysis was carried out using BD FACS Diva software (version 6.1.2). Combination of WBC markers together with platelet markers on WBC subpopulations is considered to be characteristic for PWAs and is an indicator of the adhesion phase. The results were presented as marker expression percentages and mean of fluorescence intensity of the platelet marker expression on the studied WBC subpopulations.
EXTRACTION AND DETERMINATION OF PLATELET MEMBRANE FAS:
The lipid extraction was carried out according to Folch method [23]. The dried extract was resuspended for further thin-layer chromatography (Sil G-25 UV 254) analysis [24]. FA methyl esters of platelet membrane phospholipids were analyzed by gas chromatography/mass spectrometry (GCMS-QP2010 Ultra – Shimadzu). Data analysis was carried out using LabSolutions software (Shimadzu).
The content of each FA was calculated from the total FAs amount (100%), counting the percentage of total SFAs (C 14: 0, C 16: 0, C 18: 0), MUFAs (C 16: 1ω7, C 18: 1ω9, C 18: 1ω7, C 20: 1ω9), PUFAs (C 18: 2ω6, C 18: 3ω3, C 20: 4ω6, C 20: 5ω3, C 22: 5ω3, C 22: 6ω3), the percentage of PUFAs ω3 and PUFAs ω6, and ratios: PUFAs ω3 to PUFAs ω6, PUFAs to SFAS, C 18: 2ω6 to C 20: 4ω6, C 18: 3ω3 to C 20: 5ω3, C 20: 4ω6 to C 20: 5ω3.
STATISTICAL ANALYSIS:
Statistical analysis was performed by using Microsoft Excel 2016 and IBM SPSS software (version 26.0). The normality of distribution was tested using the Shapiro-Wilk’s W test. The Mann-Whitney U nonparametric test was used to assess differences between the groups for significance. The correlation between variables was determined by Spearman’s rank correlation coefficient.
Data are expressed in the median, minimum, maximum, and interquartile range (IQR). Differences were considered statistically significant when P<0.05.
Results
According to our study results, MDA concentration was statistically significantly lower in the control group than it was in the group of post-MI patients (p=0.000) (Figure 2).
The results of platelet activation markers (Table 1) in whole blood showed that in post-MI patients, PWA formation had reached a statistically significantly lower percentage than in healthy individuals. Additionally, the percentages of PMA, PNA, and PLYA formation separately were higher in the control group than the levels found in the whole-blood samples of post-MI patients (p=0.000; p=0.001; p=0.000).
For further analysis, 13 platelet phospholipid membrane FAs were identified in all subjects (Table 2). Our results showed that the total sums of saturated fatty acids (SFAs) and monounsaturated fatty acids (MUFAs) separately did not differ when we compared healthy individuals to post-MI patients (median 69.34, IQR 21.40 vs median 70.86, IQR 21.49, p=0.547 and median 13.91, IQR 9.84 vs median 15.8, IQR 7.99, p=0.469). However, statistically significantly less C 20: 1ω9 was found in patients than in the control group (median 1.38, IQR 0.91 vs median 3.09, IQR 3.46, p=0.000).
The level of PUFAs was statistically significantly higher in the platelet phospholipid membranes of post-MI patients than in healthy individuals (median 16.78, IQR 10.37 vs median 10.99, IQR 10.27, p=0.016) (Figure 3). Moreover, post-MI men had a higher ratio of PUFAs to SFAs than the control group of healthy men (median 0.25, IQR 0.21 vs median 0.15, IQR 0.18, p=0.037).
Assessing the total sum of ω6 PUFAs, we observed that post-MI men had statistically significantly more ω6 PUFAs in their platelet phospholipid membrane than the healthy men (median 10.81, IQR 8.01 vs median 7.44, IQR 9.02, p=0.022). The levels of C 18: 2ω6 and C 20: 4ω6 separately were also higher in post-MI patients compared to the control group (median 8.45, IQR 6.05 vs median 6.25, IQR 7.87, p=0.045 and median 2.02, IQR 2.31 vs median 0.9, IQR 1.46, p=0.002). Furthermore, the ratio of C 18: 2ω6 to C 20: 4ω6 was found to be approximately 2-fold lower in the platelet phospholipid membrane of post-MI men compared to the control group of healthy individuals (median 3.20, IQR 4.43 vs median 6.12, IQR 7.61, p=0.014) (Figure 4).
Our results also showed that post-MI men had statistically significantly more C 20: 5ω3 and C 22: 6ω3 in their platelet phospholipid membrane than the control group (median 0.88, IQR 0.48 vs median 0.38, IQR 0.68, p=0.000 and median 1.12, IQR 0.88 vs median 0.62, IQR 1.02, p=0.005). In addition, the ratio of C 18: 3ω3 to C 20: 5ω3 was 2 times lower in patients with chronic coronary heart disease compared to healthy individuals (median 1.76, IQR 1.47 vs median 3.65, IQR 6.22, p=0.017) (Figure 5).
According to the results of the Spearman test, a moderate positive correlation between C 18: 0 and MDA concentration (r=0.466), as well as a moderate inverse correlation between ω3 and MDA concentration (r=−0.453), were observed after comparing the platelet phospholipid membrane FA spectrum to blood serum MDA concentrations in post-MI patients (Table 3). The differences, however, were not statistically significant (p=0.060; p=0.068).
Discussion
According to our data, the concentrations of MDA in the blood serum were approximately 2 times higher in post-MI patients than in heathy volunteers. This result could be explained by an intensified peroxidation of lipids observed in patients with chronic coronary heart disease due to the disrupted balance between the depletion of antioxidants and/or accumulation of ROS in the cell. MDA has been widely used as one of the most popular, convenient, and reliable biomarkers for lipid peroxidation of ω3 and ω6 PUFAs. It is also used as a marker for OS in clinical situations [8] such as obesity, type II diabetes [4], CVDs [6], thrombosis, and increased platelet activity [18]. MDA covalent adducts of amino groups, including the ɛ-amine of lysine, are also formed on platelet activation and are increased in diseases associated with platelet activation [25].
Recent literature supports that platelet activation itself can generate MDA via radical catalysed lipid peroxidation (eg, through activation of the enzyme nicotinamide adenine dinucleotide phosphate oxidase [NADPH oxidase] and inhibition of the expression of genes encoding antioxidant enzymes [26]) in addition to that generated via cyclooxygenase-1 [18]. Activated platelets also more intensely form aggregates with monocytes and are involved in the development of arterial and venous thrombosis. Furthermore, OS can increase platelet activity by reducing the bioavailability of nitric oxide, which is known to inhibit platelet aggregation, monocyte adhesion, vascular smooth muscle cell proliferation and migration, and further progression of arterial thrombosis [27,28].
As mentioned above, platelets are one of the major culprits in the pathogenesis of CVDs [29–31]. Circulating PMAs have been shown to be elevated in patients with peripheral artery disease [32], CVD [33], unstable angina [34], and acute myocardial infarction [35]. Circulating T lymphocyte activation is also present in ischemic heart disease, especially in patients who had acute myocardial infarction [36]. PNAs are also more likely to be related to pathological developments associated with coronary artery abnormalities [37]. The data we gathered from determining whole-blood platelet activation markers in post-MI patients showed the opposite, however. The percentages of PWA formation (ie, PMAs, PNAs, and PLYAs separately) were statistically significantly 2 times lower in post-MI patients than in the control group (p<0.05). This is probably due to the low-dose (75 mg per day) consumption of aspirin, as all patients in our study were receiving antiplatelet therapy. On the other hand, Allen et al found that PMAs did not significantly differ with low-dose aspirin. The researchers also found that clopidogrel was able to decrease circulating PMAs and was particularly effective in reducing cardiovascular events compared to aspirin [29]. Nevertheless, these observations constantly demonstrate the necessity for further studies, since human monocytes display a range of heterogeneity, having at least 3 subtypes [38]. Each subtype is hypothesized to have a unique function, and the association between monocyte subtype and PMA is relatively unexplored [29].
We identified and analyzed the composition of the platelet phospholipid membrane FAs of the participants in this study to add another dimension to the analysis. The results showed that there were no statistically significant differences associated with SFAs between patients and the control group as well as MUFAs between patients and the control group. However, SFAs did account for the highest percentage of all FAs in the platelet phospholipid membrane of the individuals in this study. For both groups, C 16: 0 was the main FA of the platelet membrane. According to recent scientific data, higher levels of SFAs are detected in those cell membranes that are closely related to signalling mechanisms [39,40]. C 14: 0 and C 16: 0 can covalently modify proteins associated with signal transmission (eg, N-myristoylated proteins [41]) and are thought to take part in the regulation of transcription factors that modify lipid metabolism: cholesterol, FAs, and triacylglycerol biosynthesis; lipoprotein assembly, secretion and clearance (eg, the hepatocyte nuclear factor-4); and inflammation (eg, the nuclear factor kappa-light-chain-enhancer of activated B cells) [42,43]. Moreover, the proportion of SFAs may be higher than that of PUFAs in plasma, platelets, and erythrocyte lipids, since these FAs are selectively incorporated in the stable sn-1 position of glycerophospholipids [44].
Our results also showed that C 18: 1ω9 accounted for the highest percentage of FAs compared to other MUFAs in both groups. The same tendency was observed in other scientific studies [40,45–47]. However, in our case, the difference was not statistically significant: this result could be explained in several ways. First, the lack or absence of ω3 and ω6 PUFAs: this may intensify C 18: 1ω9 synthesis, as it is the precursor of other ω9 PUFAs required for cell membranes,[40] but not for the synthesis of biologically active compounds [48,49]. Second, C 18: 1ω9 FA is the most prevalent and is found in high amounts in many oils such as olive, peanut, palm, canola/rapeseed, and sunflower [50]. The well-known Mediterranean diet, rich in olive oil, reduces the incidence of CVDs, Parkinson disease, Alzheimer disease, and cancer [51]. This diet also results in the downregulation of circulating inflammatory biomarkers [52] and OS. Thus, we assume that a diet high in C 18: 1ω9 or/and an intensified production of C 18: 1ω9 MUFA might have elevated the percentage of this earlier-mentioned FA in the platelet phospholipid membrane of all our study subjects.
After separately comparing PUFAs and the ratio of PUFAs to SFAs, the results of our present study showed that they were both higher in the platelet phospholipid membrane in post-MI patients than in the control group. The increased level of PUFAs in these patients, along with a higher blood serum MDA concentration, could be explained as a platelet response that occurs to prepare for future activation. Increased oxidation stimulates the platelets to synthesise and incorporate more PUFAs in the platelet phospholipid membrane, consequently leading to modifications in platelet function [44,53].
In vivo biosynthesis of long-chain PUFAs requires the precursors FAs C 18: 2ω6 and C 18: 3ω3, when a series of desaturase and elongase enzymes catalyse the conversion, or they can be directly obtained from the diet [54]; for example, the major dietary sources of C 18: 2ω6 are vegetable oils, nuts, seeds, meats, and eggs [55], while the dietary sources of C 18: 3ω3 are linseed oil, kiwifruit oil, chia seed oil, canola (rapeseed) oil, soybean, purslane, and walnuts [56]. In our present study, post-MI patients had a statistically significantly higher level of C 18: 2ω6 compared to healthy individuals. The percentage of ω6 PUFAs was also found to be higher in post-MI patients. It is well-known that omega FAs are inflammation-modulating agents, which can stimulate or suppress the synthesis of pro- and/or anti-inflammatory cell signalling molecules. For instance, C 18: 2ω6 and C 18: 3ω6 are able to indirectly provoke the synthesis of ROS superoxide, a pro-inflammatory mediator, mainly by activating p47 and NADPH oxidase enzyme complex before increasing the expression of vascular cell adhesion molecule-1 (VCAM-1) [56,57]. VCAM-1, according to a recent study, helps regulate inflammation-associated vascular adhesion and the transendothelial migration of leukocytes [58].
Another ω6 FA that is also claimed to be one of the most recognizable potent bioactive lipid mediators is C 20: 4ω6. This FA directly impacts inflammation as it can in vitro enhance the ability of endothelial cells to bind monocytes, thus facilitating the pro-inflammatory process [59]. Furthermore, in vivo, C 20: 4ω6 plays a role in the enzymatic production of pro-inflammatory prostaglandins (PGs), thromboxanes (TXs), and leukotrienes (LTs), which are thought to be mediators and regulators of inflammatory responses [50]. Our results also corroborate the aforementioned C 20: 4ω6-induced pro-inflammatory process. Individuals with chronic coronary heart disease had an approximately 2-fold higher level of C 20: 4ω6 in the platelet phospholipid membrane than the control group of this study. Furthermore, the ratio of C 18: 2ω6 to C 20: 4ω6 was also 2 times lower in post-MI patients. A higher percentage of ω6 PUFAs in the platelet phospholipid membrane of patients with chronic coronary heart disease may therefore lead to a higher amount of pro-inflammatory biologically active compounds synthesised from ω6 PUFAs. Moreover, a more intensive conversion of C 18: 2ω6 to C 20: 4ω6 with a higher concentration of blood serum MDA in post-MI patients reaffirm our hypothesis of platelet preparation for the synthesis of pro-inflammatory eicosanoids leading to further platelet activation.
The effect of ω3 PUFAs on the cardiovascular system is opposite that of ω6. An increased consumption of dietary ω3 has a cardioprotective effect via a reduction in blood pressure and serum/plasma triacylglycerol levels, an antithrombotic effect, an anti-inflammatory effect, an increase in heart rate variability, and secondary prevention of CVD [28,60]. Moreover, long-chain ω3 PUFA (eg, C 22: 6ω3) can modulate the gene expression of the enzyme or enzymes that are involved in the formation and metabolism of plasma homocysteine, an independent CVD risk factor [61]. According to published literature, the aforementioned protective effect of ω3 PUFAs comes from reducing the availability C 20: 4ω6 for producing lipid mediators through the cyclooxygenase and lipoxygenase pathways – including 4-series LT, 2-series PG, and TX – while increasing the production of 5-series LT, and 3-series PG, and TX. 3-series C 20: 5ω3-derived eicosanoids are thought to be less potent than C 20: 4ω6-derived eicosanoids, thus contributing to anti-inflammatory effects, as well as anti-aggregatory and vasodilatory effects [62]. In addition, both C 20: 5ω3 and C 22: 6ω3 undergo a series of reactions involving cyclooxygenase-2 in the presence of aspirin and 5-lipoxygenase, leading to a novel class of lipid mediators known as E-series resolvins (Rv) from C 20: 5ω3 and D-series Rv and neuroprotectin D1 from C 22: 6ω3, which are involved in the resolution of inflammation, accordingly reducing the expression of VCAM-1, interleukin-8, macrophage inflammatory protein-1b, and tumour necrosis factor-α by endothelial cells and reducing leucocyte transmigration through the endothelium [62].
According to our study results, the levels of C 20: 5ω3 and C 22: 6ω3 PUFAs were higher in the platelet phospholipid membrane of post-MI patients than in the control group. In addition, the ratio of C 18: 3ω3 to C 20: 5ω3 was 2 times lower in post-MI subjects. Such results reveal that increased oxidation stimulates platelets to synthesise more ω3 PUFAs from the essential FA C 18: 3ω3. The conversion of C 18: 3ω3 to C 20: 5ω3 is also more intense, leading to an increased production of biologically active compounds and platelet activation. Moreover, a higher percentage of C 20: 5ω3 and C 22: 6ω3 PUFAs may additionally act as a protective response and/or adaptation for constant OS, causing the reduction of platelet activation in the future. Platelets with higher levels of C 22: 6ω3 and C 20: 5ω3 are less able to bind fibrinogen, secrete their granule contents, and generate thrombin on their surface [63].
However, the present study found no statistically significant correlations between the platelet phospholipid membrane FA spectrum and blood serum MDA concentration in post-MI men. This result could possibly be explained by some limitations that our study had. First, the case group of our research was too small. The number of patients with markedly higher blood serum MDA concentration should at least be doubled so that we could confirm the observable effect of oxidation. Although we did not focus on any specific concentrations of eicosanoids/docosanoids, an assessment of the level of their alteration in both groups would benefit the analysis, since these biologically active compounds have a significant impact on the pathogenesis and progression of CVDs.
Conclusions
The results of our study suggest that OS causes an alteration in the FA spectrum of the platelet phospholipid membrane, particularly by stimulating the incorporation of PUFAs, which are well-known to be involved in the synthesis of biologically active compounds. Because these substances also participate in the inflammation-modulating process, together with OS, they can also directly affect platelet activation in whole blood, leading to future developments and progressions of CVDs. However, further studies are needed to more fully evaluate how platelet phospholipid membranes respond to constant OS in both healthy individuals and patients with cardiovascular lesions.
Figures
 Figure 1. The distribution of study population and sequence of assays.
Figure 1. The distribution of study population and sequence of assays. 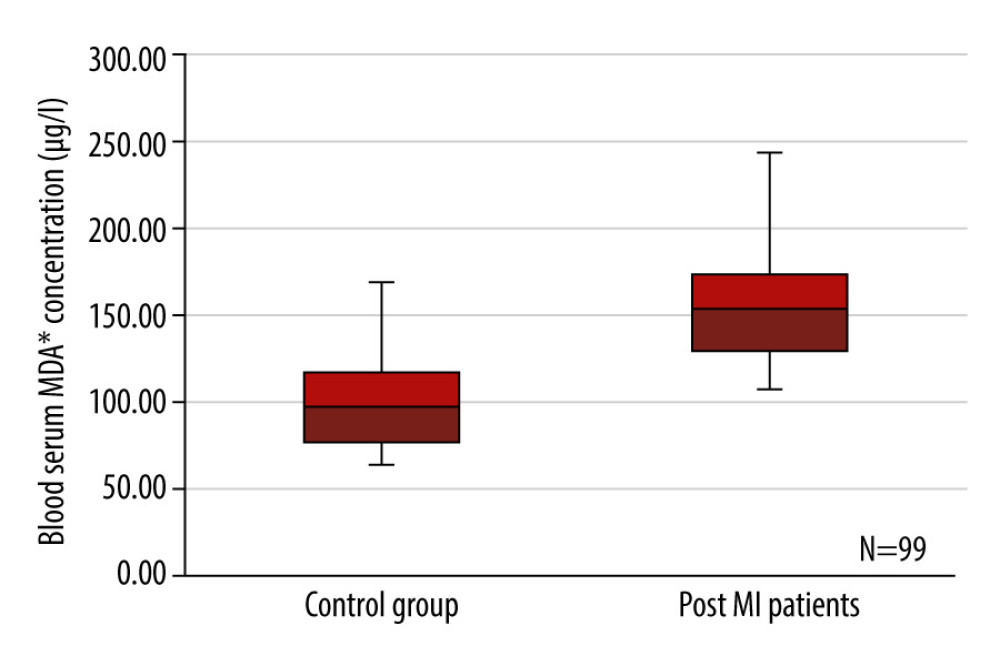 Figure 2. Box plots representing a comparison of blood serum malondialdehyde concentration (μg/l) between the control group of healthy individuals and the post-myocardial infarction patients. P=0.000, n=99. MDA – malondialdehyde.
Figure 2. Box plots representing a comparison of blood serum malondialdehyde concentration (μg/l) between the control group of healthy individuals and the post-myocardial infarction patients. P=0.000, n=99. MDA – malondialdehyde. 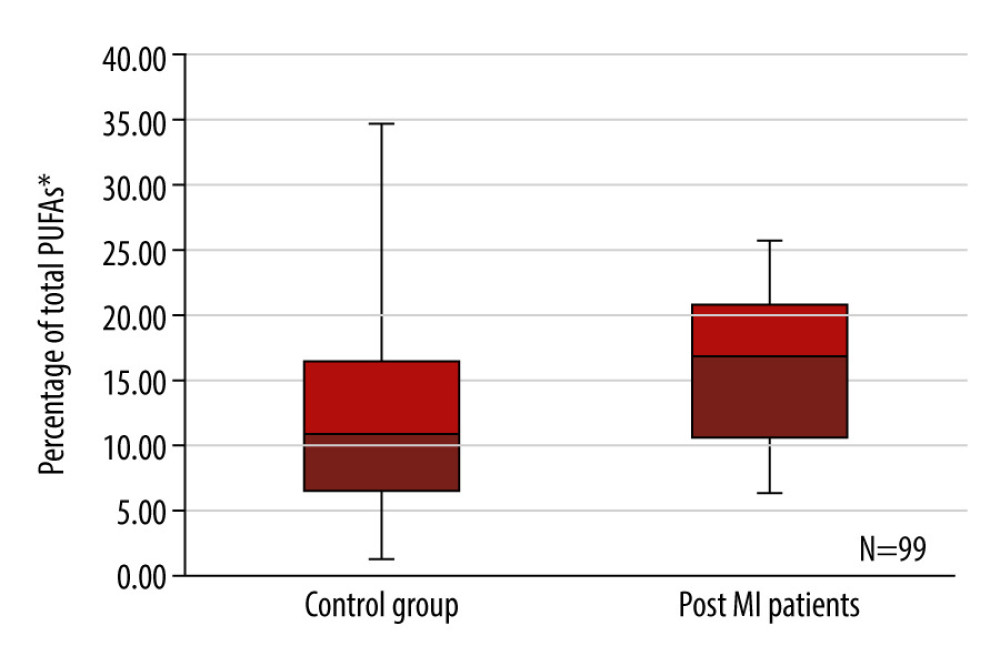 Figure 3. Box plots representing a comparison of polyunsaturated fatty acid percentages between the control group of healthy individuals and post-myocardial infarction patients. P=0.016, n=99. PUFAs – polyunsaturated fatty acids.
Figure 3. Box plots representing a comparison of polyunsaturated fatty acid percentages between the control group of healthy individuals and post-myocardial infarction patients. P=0.016, n=99. PUFAs – polyunsaturated fatty acids. 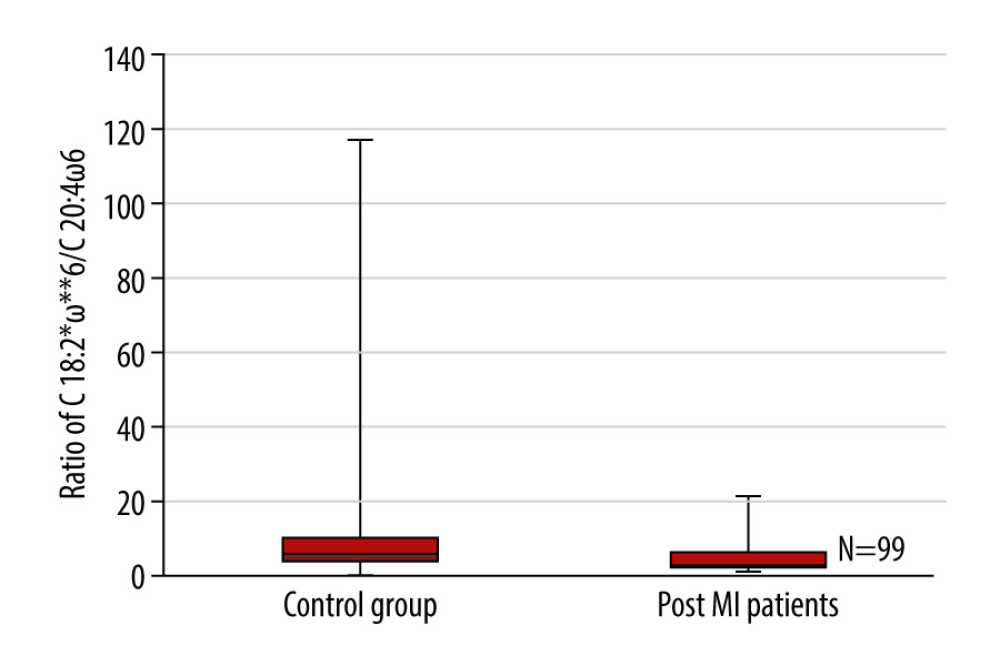 Figure 4. Box plots representing a comparison of the ratio of C 18: 2ω6 to C 20: 4ω6 between the control group of healthy individuals and the post-myocardial infarction patients. P=0.014, n=99. * Number of carbon atoms and double bonds; ** position of a double bond between carbon atoms.
Figure 4. Box plots representing a comparison of the ratio of C 18: 2ω6 to C 20: 4ω6 between the control group of healthy individuals and the post-myocardial infarction patients. P=0.014, n=99. * Number of carbon atoms and double bonds; ** position of a double bond between carbon atoms. 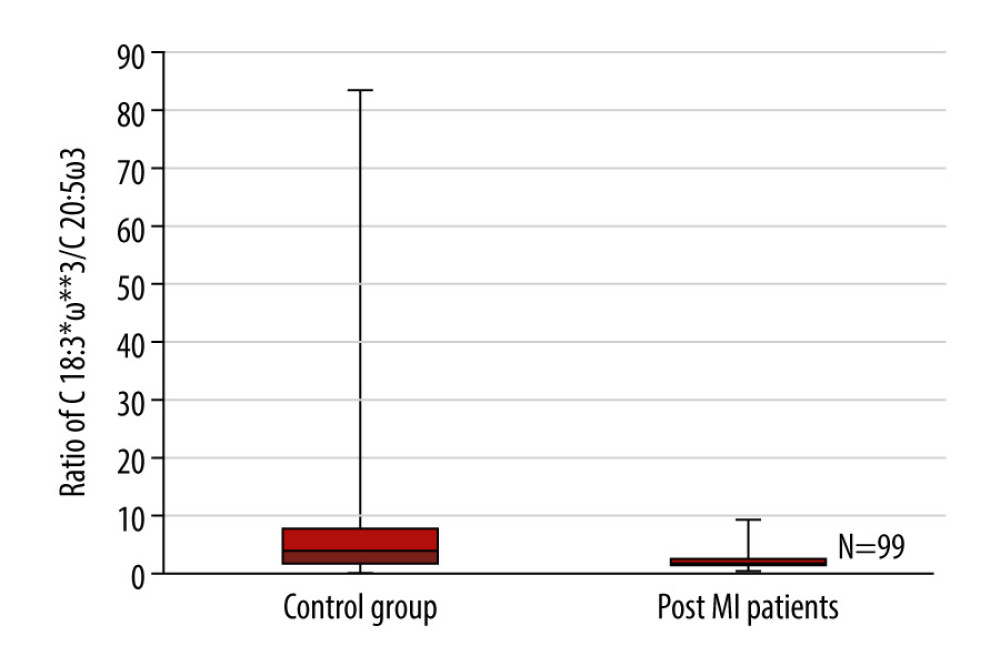 Figure 5. Box plots representing a comparison of the ratio of C 18: 3ω3 to C 20: 5ω3 between the control group of healthy individuals and the post-myocardial infarction patients. P=0.017, n=99. * Number of carbon atoms and double bonds; ** position of a double bond between carbon atoms.
Figure 5. Box plots representing a comparison of the ratio of C 18: 3ω3 to C 20: 5ω3 between the control group of healthy individuals and the post-myocardial infarction patients. P=0.017, n=99. * Number of carbon atoms and double bonds; ** position of a double bond between carbon atoms. Tables
Table 1. A comparison of platelet-white blood cell aggregate formation percentages in whole blood between post-MI patients and the control group of healthy individuals.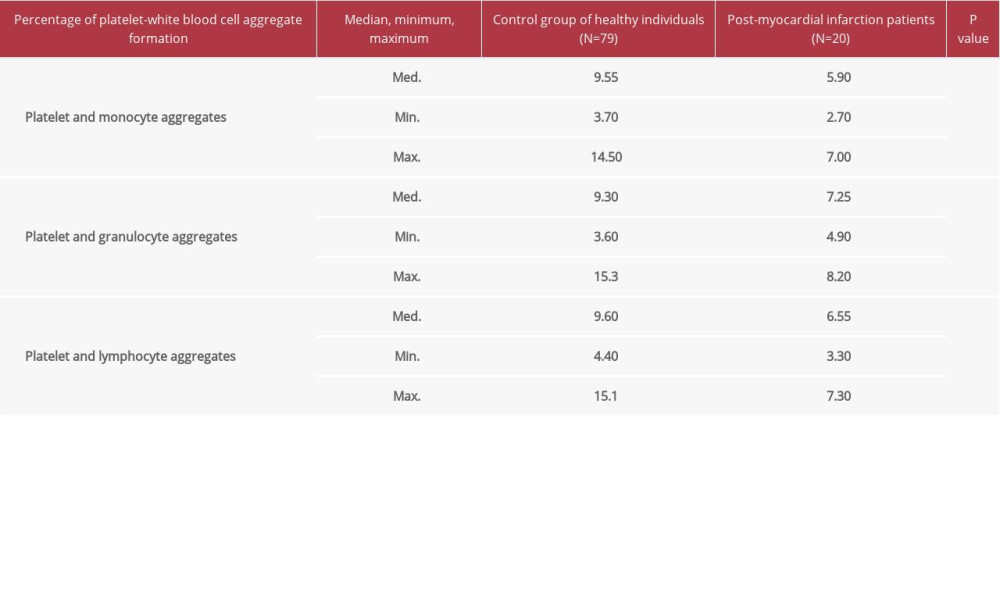 Table 2. A comparison of the composition of fatty acids in the phospholipid membrane of platelets between post-myocardial infarction patients and the control group of healthy individuals.
Table 2. A comparison of the composition of fatty acids in the phospholipid membrane of platelets between post-myocardial infarction patients and the control group of healthy individuals.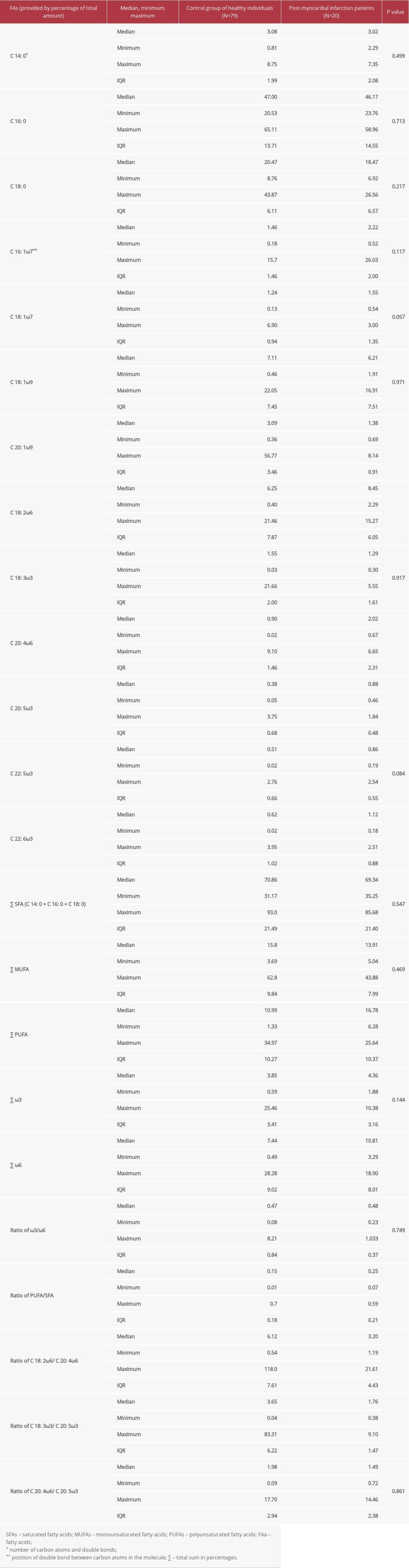 Table 3. The correlation of the fatty acid spectrum in the phospholipid membrane of platelets with blood serum malondialdehyde concentration in post-myocardial infarction patients.
Table 3. The correlation of the fatty acid spectrum in the phospholipid membrane of platelets with blood serum malondialdehyde concentration in post-myocardial infarction patients.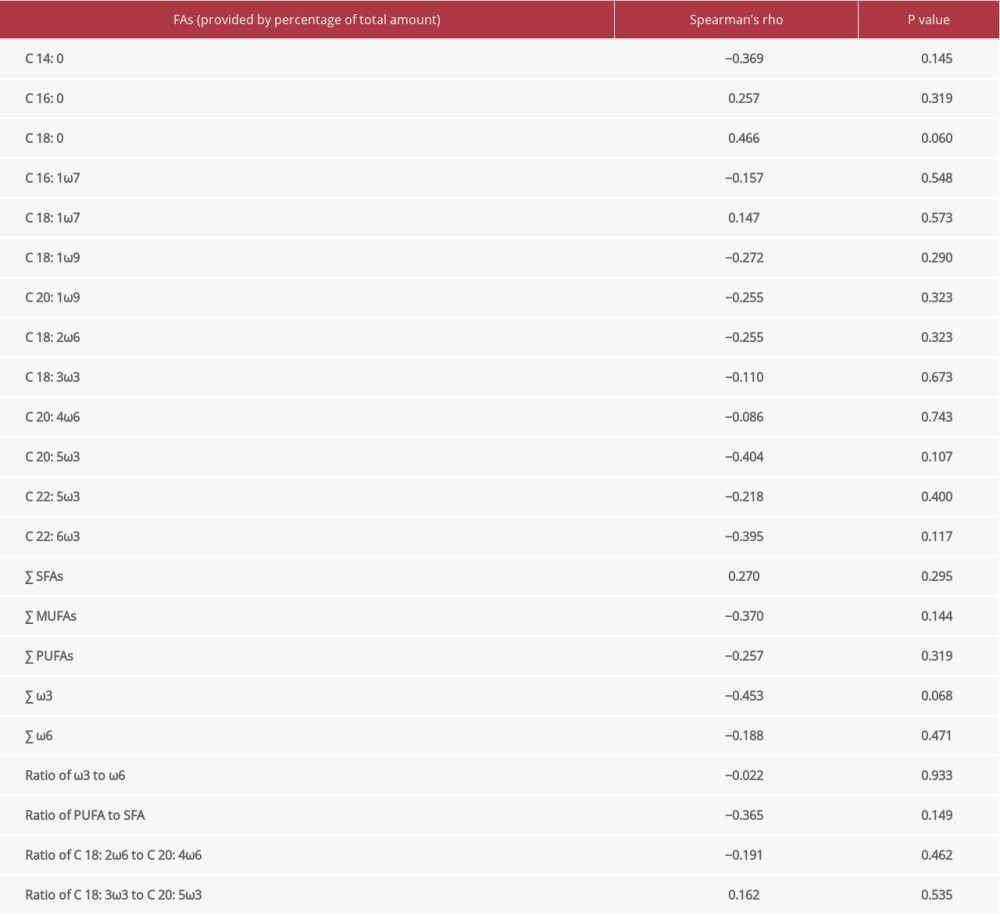
References
1. WHO, Cardiovascular diseases, 2017, WHO World Health Organization https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds)
2. Wilkins E, Wilson L, Wickramasinghe K, European Cardiovascular Disease Statistics 2017: European Heart Network http://www.ehnheart.org/images/CVD-statistics-report-August-2017.pdf
3. Lesa K, Siddikey AT, The impact of heart disease risk factors on the age of 30 to 80 years old patients – residing in and around Khulna district of Bangladesh: Diabetes Metab Synd, 2019; 13(5); 3011-15
4. Görlach A, Dimova EY, Petry A, Reactive oxygen species, nutrition, hypoxia and diseases: Problems solved?: Redox Biol, 2015; 6; 372-85
5. Birben E, Sahiner UM, Sackesen C, Oxidative stress and antioxidant defense: World Allerg Organ J, 2012; 5(1); 9-19
6. Vichova T, Motovska Z, Oxidative stress: Predictive marker for coronary artery disease: Exp Clin Cardiol, 2013; 18(2); e88-91
7. Dolegowska B, Lubkowska A, De Girolamo L, Platelet lipidomic: J Biol Regul Homeost Agents, 2012; 26(2 Suppl 1); 23S-33S
8. Ayala A, Muñoz MF, Argüelles S, Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal: Oxid Med Cell Longev, 2014; 2014 360438
9. Yeung J, Hawley M, Holinstat M, The expansive role of oxylipins on platelet biology: J Mol Med, 2017; 95(6); 575-88
10. Tsikas D, Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: Analytical and biological challenges: Anal Biochem, 2017; 524; 13-30
11. Rio D, Stewart AJ, Pellegrini N, A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress: Nutr Metab Cardiovasc Dis, 2005; 15(4); 316-28
12. Agarwal R, Chase SD, Rapid, fluorimetric-liquid chromatographic determination of malondialdehyde in biological samples: J Chromatogr B, 2002; 775; 121-26
13. Cheng J, Wang F, Yu DF, The cytotoxic mechanism of malondialdehyde and protective effect of carnosine via protein cross-linking/mitochondrial dysfunction/reactive oxygen species/MAPK pathway in neurons: Eur J Pharmacol, 2011; 650(1); 184-94
14. Esterbauer H, Schaur RJ, Zollner H, Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes: Free Radic Biol Med, 1991; 11(1); 81-128
15. Lakshminarayana R, Sathish UV, Dharmesh SM, Baskaran V, Antioxidant and cytotoxic effect of oxidized lutein in human cervical carcinoma cells (HeLa): Food Chem Toxicol, 2010; 48(7); 1811-16
16. Kangari P, Farahany TZ, Golchin A, Enzymatic antioxidant and lipid peroxidation evaluation in the newly diagnosed breast cancer patients in Iran: Asian Pac J Cancer Prev, 2018; 19(12); 3511-15
17. Negre-Salvayre A, Auge N, Ayala V, Pathological aspects of lipid peroxidation: Free Radic Res, 2010; 44(10); 1125-71
18. Violi F, Pignatelli P, Platelet oxidative stress and thrombosis: Thromb Res, 2012; 129(3); 378-81
19. Fairweather DL, Sex differences in inflammation during atherosclerosis: Clin Med Insights Cardiol, 2014; 8(Suppl 3); 49-59
20. Khoschsorur GA, Winklhofer-Roob BM, Rabl H, Evaluation of a sensitive HPLC method for the determination of Malondialdehyde, and application of the method to different biological materials: Chromatographia, 2000; 52(3–4); 181-84
21. Sánchez M, Delgado D, Sánchez P, Combination of intra-articular and intraosseous injections of platelet rich plasma for severe knee osteoarthritis: A pilot study: BioMed Res Int, 2016; 2016 4868613
22. Dhurat R, Sukesh MS, Principles and methods of preparation of platelet-rich plasma: A review and author’s perspective: J Cutan Aesthet Surg, 2014; 7(4); 189-97
23. Folch J, Lees M, Stanley G, A simple method for the isolation and purification of total lipids from animal tissue: J Biol Chem, 1957; 226; 497-99
24. Touchstone JC, Thin-layer chromatographic procedures for lipid separation: J Chromatogr B: Biomed Sci Apls, 1995; 671(1-2); 169-95
25. Zagol-Ikapite I, Sosa RI, Oram D, Modification of platelet proteins by malondialdehyde: Prevention by dicarbonyl scavengers: J Lip Res, 2015; 56(11); 2196-95
26. Peña-Orihuela P, Camargo A, Rangel-Zuñiga OA, Antioxidant system response is modified by dietary fat in adipose tissue of metabolic syndrome patients: J Nutr Biochem, 2013; 24(10); 1717-23
27. Fuentes E, Palomo I, Role of oxidative stress on platelet hyperreactivity during aging: Life Sci, 2016; 148; 17-23
28. Ander BP, Dupasquier CMC, Prociuk MA, Pierce GN, Polyunsaturated fatty acids and their effects on cardiovascular disease: Exp Clin Cardiol, 2003; 8(4); 164-72
29. Allen N, Barrett TJ, Guo Y, Circulating monocyte-platelet aggregates are a robust marker of platelet activity in cardiovascular disease: Atherosclerosis, 2019; 282; 1-18
30. Loguinova M, Pinegina N, Vagida M, Monocytes of different subsets in complexes with platelets in patients with myocardial infarction: Thromb Haemost, 2018; 118(11); 1969-81
31. Blann AD, Draper Z, Platelet activation as a marker of heart attack: Clinica Chimica Acta, 2011; 412; 841-42
32. Dopheide JF, Rubrech J, Trumpp A, Leukocyte-platelet aggregates – a phenotypic characterization of different stages of peripheral arterial disease: Platelets, 2016; 27(7); 658-67
33. Furman MI, Benoit SE, Barnard MR, Increased platelet reactivity and circulating monocyte-platelet aggregates in patients with stable coronary artery disease: J Am Coll Cardiol, 1998; 31(2); 352-58
34. Zeng S, Zhou X, Ge L, Monocyte subsets and monocyte-platelet aggregates in patients with unstable angina: J Thromb Thrombolysis, 2014; 38; 439-46
35. Furman MI, Barnard MR, Krueger LA, Circulating monocyte-platelet aggregates are an early marker of acute myocardial infarction: J Am Coll Cardiol, 2001; 38(4); 1002-6
36. Czyz A, Kołacz E, Angerer D, Zawilska K, Expression of activation antigens on lymphocyte surface and circulating platelet-leukocyte aggregates in ischaemic heart disease: Kardiol Pol, 2005; 62(3); 189-200 discussion 201
37. Ueno K, Nomura Y, Morita Y, Circulating platelet-neutrophil aggregates play a significant role in Kawasaki disease: Circ J, 2015; 79; 1349-56
38. Ziegler-Heitbrock L, Ancuta P, Crowe S, Nomenclature of monocytes and dendritic cells in blood: Blood, 2010; 116(16); e74-80
39. Escribá PV, Busquets X, Inokuchi J, Membrane lipid therapy: Modulation of the cell membrane composition and structure as a molecular base for drug discovery and new disease treatment: Prog Lip Res, 2015; 59; 38-53
40. Papackova Z, Cahova M, Fatty acid signaling: The new function of intracellular lipases: Int J Mol Sci, 2015; 16; 3831-55
41. Hayashi N, Titani K, N-myristoylated proteins, key components in intracellular signal transduction systems enabling rapid and flexible cell responses: Proc Jpn Acad Ser B Phys Biol Sci, 2010; 86(5); 494-508
42. Calder PC, Functional roles of fatty acids and their effects on human health: JPEN J Parenter Enteral Nutr, 2015; 39(1 Suppl); 18S-32S
43. Rustan A, Drevon C, Fatty acids: Structures and properties: Encyclopedia of Life Sciences Nature Publishing London, 2005; 1-7
44. Risé P, Eligini S, Ghezzi S, Fatty acid composition of plasma, blood cells and whole blood: Relevance for the assessment of the fatty acid status in humans: Prostaglandins Leukot Essent Fatty Acids, 2007; 76(6); 363-69
45. Sánchez-Rodríguez P, Rodríguez MC, Sánchez-Yagüe J, Identification of potential erythrocyte phospholipid fatty acid biomarkers of advanced lung adenocarcinoma, squamous cell lung carcinoma, and small cell lung cancer: Tumor Biol, 2015; 36; 5687-98
46. Qiu JF, Zhang KL, Zhang XJ, Abnormalities in plasma phospholipid fatty acid profiles of patients with hepatocellular carcinoma: Lipids, 2015; 50; 977-85
47. Castro J, Hernández-Hernández A, Rodríguez MC, Llanillo M, Sánchez-Yagüe J, Comparison of changes in erythrocyte and platelet fatty acid composition and protein oxidation in advanced non-small cell lung cancer: Cancer Invest, 2006; 24(4); 339-45
48. Wiktorowska-Owczarek A, Berezińska M, Nowak JZ, PUFAs: Structures, metabolism and functions: Adv Clin Exp Med, 2015; 24(6); 931-41
49. López-Miranda J, Pérez-Jiménez F, Ros E, Olive oil and health: Summary of the II international conference on olive oil and health consensus report, Jaén and Córdoba (Spain) 2008: Nutr Metab Cardiovasc Dis, 2010; 20(4); 284-94
50. Kenar JA, Moser BR, List GR, Naturally occurring fatty acids: Source, chemistry, and uses: Fatty Acids, 2017; 23-82
51. Sofi F, Cesari F, Abbate R, Adherence to Mediterranean diet and health status: Meta-analysis: BMJ, 2008; 337; a1344
52. Urpi-Sarda M, Casas R, Chiva-Blanch G, Virgin olive oil and nuts as key foods of the Mediterranean diet effects on inflammatory biomarkers related to atherosclerosis: Pharmacol Res, 2012; 65(6); 577-83
53. Haggag MESES, Elsanhoty RM, Ramadan MF, Impact of dietary oils and fats on lipid peroxidation in liver and blood of albino rats: Asian Pac J Trop Biomed, 2014; 4(1); 52-58
54. Li D, Zhang H, Hsu-Hage BH-H, The influence of fish, meat and polyunsaturated fat intakes on platelet phospholipid polyunsaturated fatty acids in male Melbourne Chinese and Caucasian: Eur J Clin Nutr, 2001; 55; 1036-42
55. Whelan J, Fritsche K, Linoleic Acid: Adv Nutr, 2013; 4(3); 311-12
56. Johnson M, Bradford C, Omega-3, Omega-6 and Omega-9 fatty acids: Implications for cardiovascular and other diseases: J Glycomics Lipidomics, 2014; 4; 123
57. Hatanaka E, Dermargos A, Hirata AE, Oleic, linoleic and linolenic acids increase ros production by fibroblasts via NADPH oxidase activation: PLoS One, 2013; 8(4); e58626
58. Kong DH, Kim YK, Kim MR, Emerging roles of vascular cell adhesion molecule-1 (VCAM-1) in immunological disorders and cancer: Int J Mol Sci, 2018; 19(4); 1057
59. Grenon SM, Aguado-Zuniga J, Hatton JP, Effects of fatty acids on endothelial cells: Inflammation and monocyte adhesion: J Surg Res, 2012; 177; e35-43
60. Li D, Yu XM, Xie HB, Platelet phospholipid n-3 PUFA negatively associated with plasma homocysteine in middle-aged and geriatric hyperlipaemia patients: Prostaglandins Leukot Essent Fatty Acids, 2007; 76(5); 293-97
61. Ganguly P, Alam SF, Role of homocysteine in the development of cardiovascular disease: Nutr J, 2015; 14; 6
62. Cottin S, Sanders T, Hall W, The differential effects of EPA and DHA on cardiovascular risk factors: Proc Nutr Soc, 2011; 70(2); 215-31
63. Larson MK, Shearer GC, Ashmore JH, Omega-3 fatty acids modulate collagen signaling in human platelets: Prostaglandins Leukot Essent Fatty Acids, 2011; 84(3–4); 93-98
Figures
 Figure 1. The distribution of study population and sequence of assays.
Figure 1. The distribution of study population and sequence of assays. Figure 2. Box plots representing a comparison of blood serum malondialdehyde concentration (μg/l) between the control group of healthy individuals and the post-myocardial infarction patients. P=0.000, n=99. MDA – malondialdehyde.
Figure 2. Box plots representing a comparison of blood serum malondialdehyde concentration (μg/l) between the control group of healthy individuals and the post-myocardial infarction patients. P=0.000, n=99. MDA – malondialdehyde. Figure 3. Box plots representing a comparison of polyunsaturated fatty acid percentages between the control group of healthy individuals and post-myocardial infarction patients. P=0.016, n=99. PUFAs – polyunsaturated fatty acids.
Figure 3. Box plots representing a comparison of polyunsaturated fatty acid percentages between the control group of healthy individuals and post-myocardial infarction patients. P=0.016, n=99. PUFAs – polyunsaturated fatty acids. Figure 4. Box plots representing a comparison of the ratio of C 18: 2ω6 to C 20: 4ω6 between the control group of healthy individuals and the post-myocardial infarction patients. P=0.014, n=99. * Number of carbon atoms and double bonds; ** position of a double bond between carbon atoms.
Figure 4. Box plots representing a comparison of the ratio of C 18: 2ω6 to C 20: 4ω6 between the control group of healthy individuals and the post-myocardial infarction patients. P=0.014, n=99. * Number of carbon atoms and double bonds; ** position of a double bond between carbon atoms. Figure 5. Box plots representing a comparison of the ratio of C 18: 3ω3 to C 20: 5ω3 between the control group of healthy individuals and the post-myocardial infarction patients. P=0.017, n=99. * Number of carbon atoms and double bonds; ** position of a double bond between carbon atoms.
Figure 5. Box plots representing a comparison of the ratio of C 18: 3ω3 to C 20: 5ω3 between the control group of healthy individuals and the post-myocardial infarction patients. P=0.017, n=99. * Number of carbon atoms and double bonds; ** position of a double bond between carbon atoms. Tables
 Table 1. A comparison of platelet-white blood cell aggregate formation percentages in whole blood between post-MI patients and the control group of healthy individuals.
Table 1. A comparison of platelet-white blood cell aggregate formation percentages in whole blood between post-MI patients and the control group of healthy individuals. Table 2. A comparison of the composition of fatty acids in the phospholipid membrane of platelets between post-myocardial infarction patients and the control group of healthy individuals.
Table 2. A comparison of the composition of fatty acids in the phospholipid membrane of platelets between post-myocardial infarction patients and the control group of healthy individuals. Table 3. The correlation of the fatty acid spectrum in the phospholipid membrane of platelets with blood serum malondialdehyde concentration in post-myocardial infarction patients.
Table 3. The correlation of the fatty acid spectrum in the phospholipid membrane of platelets with blood serum malondialdehyde concentration in post-myocardial infarction patients. Table 1. A comparison of platelet-white blood cell aggregate formation percentages in whole blood between post-MI patients and the control group of healthy individuals.
Table 1. A comparison of platelet-white blood cell aggregate formation percentages in whole blood between post-MI patients and the control group of healthy individuals. Table 2. A comparison of the composition of fatty acids in the phospholipid membrane of platelets between post-myocardial infarction patients and the control group of healthy individuals.
Table 2. A comparison of the composition of fatty acids in the phospholipid membrane of platelets between post-myocardial infarction patients and the control group of healthy individuals. Table 3. The correlation of the fatty acid spectrum in the phospholipid membrane of platelets with blood serum malondialdehyde concentration in post-myocardial infarction patients.
Table 3. The correlation of the fatty acid spectrum in the phospholipid membrane of platelets with blood serum malondialdehyde concentration in post-myocardial infarction patients. Most Viewed Current Articles
15 Jun 2022 : Clinical Research
Evaluation of Apical Leakage After Root Canal Obturation with Glass Ionomer, Resin, and Zinc Oxide Eugenol ...DOI :10.12659/MSMBR.936675
Med Sci Monit Basic Res 2022; 28:e936675
07 Jul 2022 : Laboratory Research
Cytotoxicity, Apoptosis, Migration Inhibition, and Autophagy-Induced by Crude Ricin from Ricinus communis S...DOI :10.12659/MSMBR.936683
Med Sci Monit Basic Res 2022; 28:e936683
01 Jun 2022 : Laboratory Research
Comparison of Sealing Abilities Among Zinc Oxide Eugenol Root-Canal Filling Cement, Antibacterial Biocerami...DOI :10.12659/MSMBR.936319
Med Sci Monit Basic Res 2022; 28:e936319
08 Dec 2022 : Original article
Use of Estimated Glomerular Filtration Rate and Urine Albumin-to-Creatinine Ratio Based on KDIGO 2012 Guide...DOI :10.12659/MSMBR.938176
Med Sci Monit Basic Res 2022; 28:e938176








