27 July 2021: Laboratory Research
Disinfection of Infected Artificial Dental Periapical Lesions with Diode Laser: An In Vitro Study
Agron Bytyqi1BDE, Xhevdet Aliu2AD, Merita Barani2BDF, David Stubljar3CDE, Tomislav Jukic4CDE, Andrej Starc5CDE, Sokol Krasniqi6ABDE*DOI: 10.12659/MSMBR.932492
Med Sci Monit Basic Res 2021; 27:e932492
Abstract
BACKGROUND: Periapical lesions are primarily caused by infections in the root canals. The objective of this study was to assess the antibacterial effectiveness of diode laser during root canal treatment in artificial models of infected periapical lesions.
MATERIAL AND METHODS: One hundred twenty-two extracted premolar single-rooted teeth were inserted into methyl methacrylate artificial models of periapical lesions, and bacterial solutions of Enterococcus faecalis (ATCC 29212) and Streptococcus mitis (ATCC 49456) were then applied to the models. The respective diameters of lesions in the artificial models represented 3 different subgroups based on lesion size. The laser protocol used for endodontic disinfection had a power output of 1.5 W and a wavelength of 810 nm. The impact on cell viability was evaluated by flow cytometry.
RESULTS: Disinfection with laser did not differ between microorganisms (P=0.137), and laser irradiation with a longer duration had better disinfecting action for both microorganisms (P<0.001). Compared with larger lesions, smaller lesions had a higher percentage of dead cells for both microorganisms (P<0.001). The percentage of dead cells in the treatment groups was significantly higher than in the control group (P<0.001).
CONCLUSIONS: Laser treatment had a poor, almost negligible effect on elimination of bacterial cells in large periapical lesions. Application of a laser might serve as an adjuvant method to standard irrigation with sodium hypochlorite.
Keywords: Enterococcus faecalis, Lasers, Periapical Diseases, Streptococcus mitis, Root Canal Therapy, Anti-Bacterial Agents, Dental Pulp Cavity, Disinfection, Lasers, Semiconductor, Mouth Diseases
Background
Most cases of apical periodontitis are caused by dental intraradicular infection, and endodontic treatment consists of removing the infectious substances. Even with proper root canal cleansing and filling, periapical periodontitis can persist in asymptomatic form, leading to postendodontic periapical lesions. Chronic inflammatory periapical lesions are the most common pathology found in relation to the alveolar bones of the jaw. From a histological point of view, these lesions can be classified as chronic periapical periodontitis (periapical granuloma), radicular cysts, scar tissue, or abscesses. The most common type is periapical granuloma, which consists of a mass of chronically inflamed tissue in which isolated epithelial nests are present. A radicular cyst is characterized by the presence of a cavity that is partially or completely lined with epithelium. It is generally accepted that periradicular lesions cannot be differentially diagnosed as radicular or apical granulomas based on X-ray images alone [1].
If the infection is not eliminated, the periapical lesion remains, which is considered therapy failure. Persistent periapical periodontitis presents an asymptomatic clinical picture due to the complex root canal system, with smaller canals, canal branching, and anastomoses that cannot be accessed, cleaned, or filled with conventional techniques [1]. Thus, apical lesions such as granulomas, abscesses, or cysts, are major consequences of ongoing infection in the root canals. These cystic lesions may undergo asymptomatic evolution and become large. They may be discovered during a routine X-ray imaging or owing to acute pain, but the diagnosis can only be confirmed with a surgical biopsy of the lesion. Cysts can occur in 6% to 55% of infections. As the size of a lesion increases, the size of a radicular cyst does as well [2–7]. Treatment is mainly based on an endodontic approach or surgical excision of cyst walls by apicoectomy. These 2 well-established procedures are the criterion standards for management of these lesions. The optimal treatment should eliminate not only periapical cysts, but also the etiological factors from the canal system. Therefore, a successful outcome of treatment comprises not only disinfection of the root canal but also the prevention of recurring infections [8]. Traditionally, mechanical instrumentation is followed by irrigation with disinfecting solutions and intracanal medications. The disadvantage of mechanical instrumentation is that some areas are left intact [9]. Despite disinfecting solutions being applied to these areas, irrigation is inadequate owing to the design of the application needle, which causes the tip of the needle to have the highest flow rate [10]. Lasers have shown greater effectiveness, but diode laser treatment of periapical lesions has not yet been assessed [11].
Diode lasers are a modern approach to disinfection of the root canal. Laser light can extend into areas that are unreachable by mechanical instrumentation or even irrigation. These lasers have high power and generate heat that destroys infecting bacteria. Some studies showed that diode lasers have great antimicrobial effectiveness [12,13], but others found them to be less effective than irrigation with sodium hypochlorite (NaOCl) [14,15]. Our objective was to assess the antibacterial effectiveness of diode laser during root canal treatment in artificial models of periapical lesions and against
Material and Methods
PREPARATION OF TEETH AND ARTIFICIAL PERIAPICAL LESIONS:
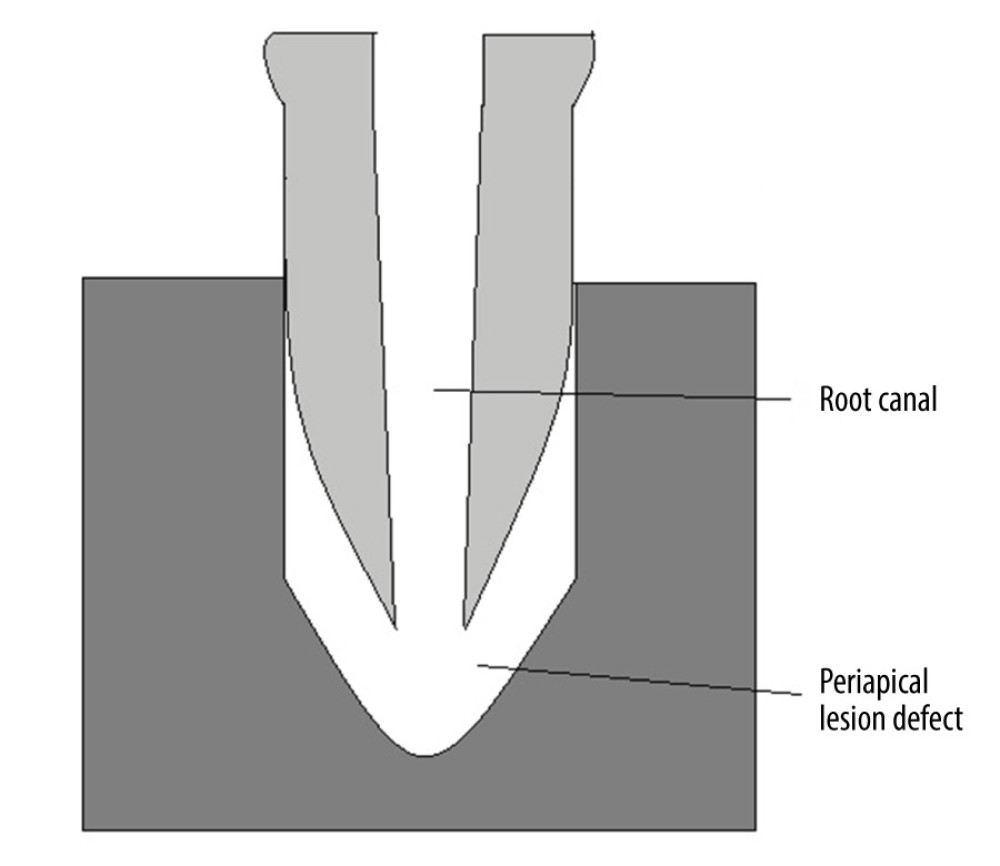
A total of 122 premolar single-rooted teeth were used in creating models of artificial periapical lesions made from methyl methacrylate. The crown of each tooth was removed using a cutting saw. A circular entrance into the 15-mm-long root canal was obtained, which was then enlarged using a Protaper file (#35, Maillefer Instruments, Switzerland).
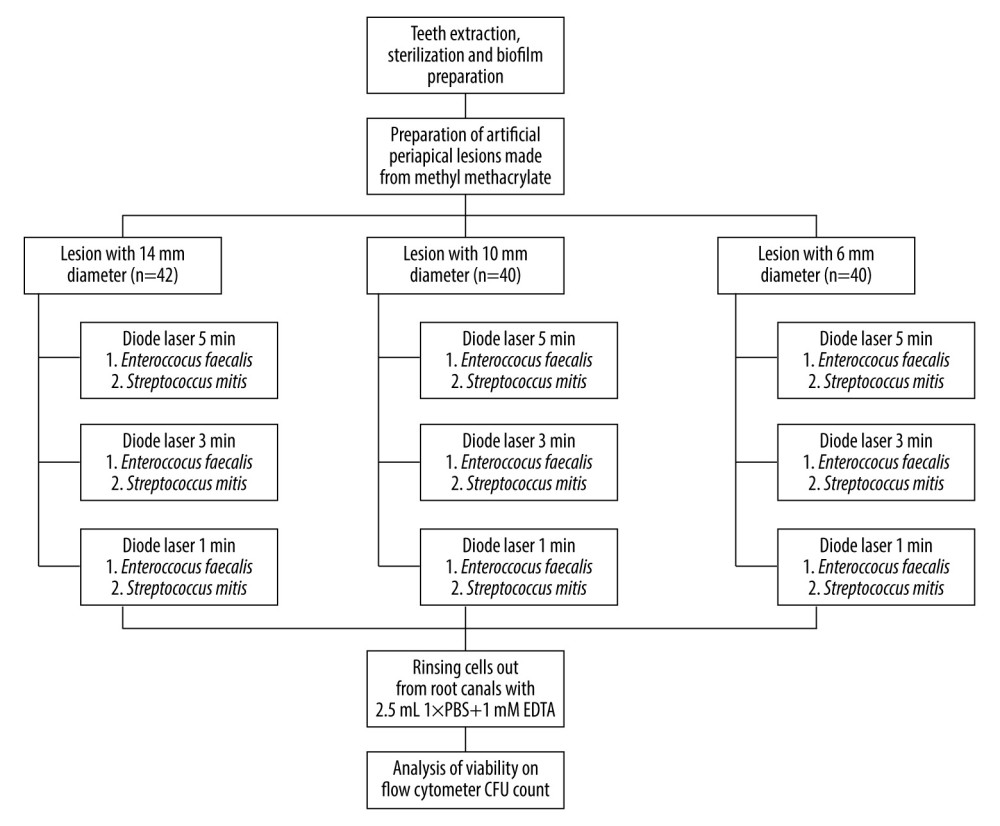
Plastic models of periapical lesions of 3 different spherical dimensions were created from methyl methacrylate. The largest lesions were 14 mm in diameter (n=42), the medium-sized lesions were 10 mm in diameter (n=40), and the smallest lesions were 6 mm in diameter (n=40). The surfaces of the lesion walls were debrided using 17% EDTA.
The models were sterilized and rinsed again with sterile 17% EDTA. The rinse solution was incubated on blood agar plates for 24 h at 37°C to confirm sterility. Sterilized teeth were then inserted into the plastic models to create an artificial model of periapical lesions under the apical part of teeth (Figure 1).
BACTERIAL BIOFILM GROWTH:
The 122 lesion models were divided into 2 groups of 61 specimens each. One group was inoculated with
For both models, the specimens were divided into 3 groups based on the diameter of the periapical lesion: the smallest lesion of 6 mm (n=20), medium size lesion of 10 mm (n=20), and the largest lesion of 14 mm (n=21). These groups were further separated into subgroups according to the duration of laser irradiation, including control samples without laser treatment and those treated for 1 (n=6), 3 (n=6), or 5 min (n=6).
The remaining 14 specimens (7 each for the E. faecalis and S. mitis models) were included as positive controls and were not treated with diode laser irradiation. Each model included 3 lesions of 14 mm, 2 of 10 mm, and 2 of 6 mm. The experimental workflow is presented in Figure 2.
DIODE LASER APPLICATION AND IRRADIATION:
Fotona XD-2 (Fotona, Ljubljana, Slovenia) diode laser with an optical fiber of 200-μm diameter was applied in our study. A power output of 1.5 W/cm2 and wavelength of 810 nm were used. The endodontic program was selected, and the optical fiber probe was completely inserted into the root canal to irradiate the apical site of the periapical lesion. Among treated samples, irradiation lasted for 1, 3, or 5 min.
MEASUREMENT OF BACTERIAL VIABILITY:
Damaged bacterial cells were obtained by rinsing the specimens with 2.5 mL of 1× phosphate-buffered saline (PBS) and 1 mM EDTA (pH 8.3). The viability of cells was assessed by flow cytometer and BD Cell Viability Kit (Becton Dickinson Biosciences, USA). Growth of bacterial biofilm was also confirmed for the samples that did not undergo laser irradiation.
A total of 500 μL of rinse solution was used to determine cell viability. At the same time, an additional 100 μL was inoculated on blood agar and incubated for 24 h at 37°C in aerobic conditions. Colony-forming units (CFU) were counted. More than 300 CFUs indicated successful bacterial growth.
STATISTICAL ANALYSIS:
SPSS Statistics 21 (IBM, New York, USA) software was used to perform statistical analyses.
Two-way analysis of variance was conducted with the percentage of dead microbial cells as the dependent variable and the microorganisms, laser treatment, and dimension of the periapical lesions as independent variables. Post hoc Tukey test was used to detect differences based on the duration of laser irradiations. In addition, the following interactions between factors were evaluated: organism×dimension of the lesion, organism×duration of irradiation, dimension of the lesion×duration of irradiation, and organism×dimension of lesion×duration of irradiation. Statistical significance for all tests was set at
Results
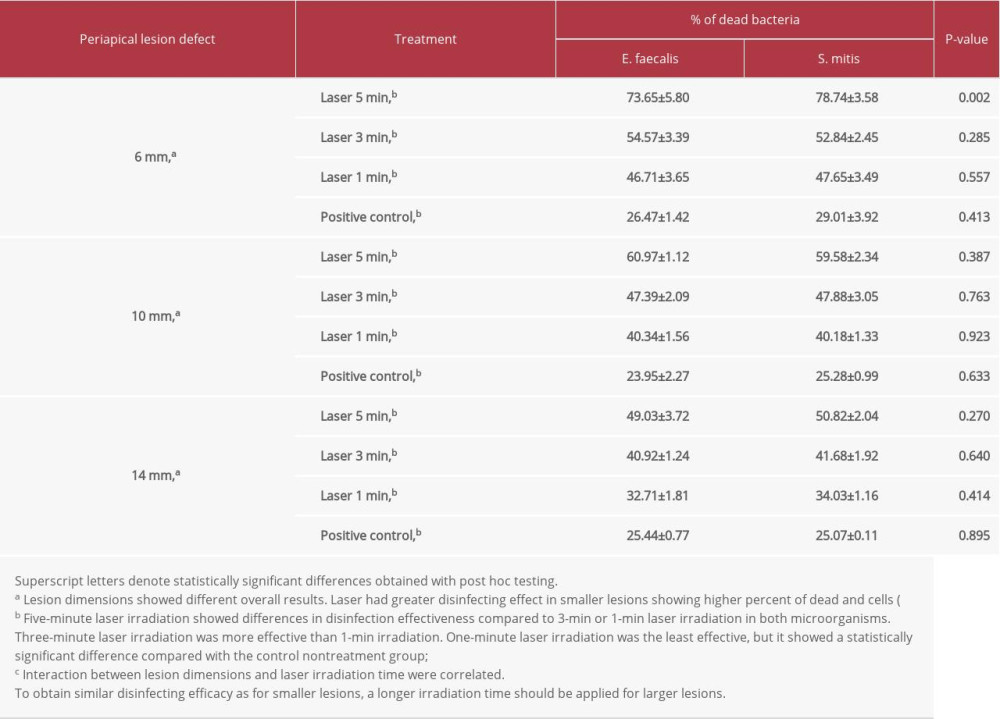
Table 1 presents the average values for each microorganism. Overall statistical analysis showed no differences in the percentage of dead cells between
Comparisons of overall durations of laser irradiation showed statistically significant differences for both
Lesions with a smaller diameter (6 mm) had a higher percentage of dead cells compared with the larger lesions for both
The percentages of dead cells varied between lesions with different dimensions, with irradiation having a greater effect in smaller lesions, which showed higher percentages of dead cells (
Discussion
Apical cysts are the product, not the cause of apical lesions, and they can delay but not prevent the development of periapical lesions after nonsurgical root canal therapy [5]. Their treatment is mainly based on endodontic treatment or surgical excision of cysts by apicoectomy. The outcome of root canal treatment is based on an efficient disinfection of the root canal system and prevention of reinfection. Traditionally, this is accomplished by a combination of mechanical instruments, using disinfecting solution for irrigation and placement of intracanal medication between treatments. After using mechanical instrumentation, large areas of the root canal are left intact, regardless of the rotary or manual techniques. It has generally been accepted that simple surgical treatment with proper infection control can lead to healing. When this method of treatment is not successful in resolving periradicular pathologies, additional treatment options should be considered. Surgical treatment of persistent extensive periradicular lesions most commonly involves apical resection. New technologies such as laser irradiation have increased the success of treatments [16,17]. The mechanism of action of lasers is based on generating heat that damages bacterial cells. Recently, laser systems have been increasingly used as an alternative method for antimicrobial therapy in treating infected dental canals, periapical lesions, and cysts. They have very good effectiveness in destroying bacteria and treating damaged tissue in very short periods of time. Owing to these good results, we investigated whether diode laser radiation without any additional disinfectants is effective in disinfecting infected canals and periapical lesions in which periapical cysts are located and thus could be applied in clinics. In our study, a high-power diode laser (810 nm, 1.5 W/cm2) was tested for antibacterial activity in artificially prepared lesion defects under single-rooted teeth.
The laser heat that was generated impaired the majority of bacterial cells in periapical lesions. Moreover, no difference in efficacy was observed between microorganisms, which were phenotypically and genotypically very similar. Irradiation of 5 min destroyed the majority of the bacteria (62.13%), while 3-min irradiation had an average of 47.55% and 1-min irradiation impaired 40.27% of
A significant difference in effectiveness was observed between periapical lesions of different dimensions (
Most previous studies have evaluated the effectiveness of different lasers on planktonic cells, so the effects on bacterial biofilm are less known [25–29]. Bergmans et al [30] reported a significant decline (99.7%) in
Nevertheless, lasers might still be used in endodontic treatment because their mechanism of action with heat showed bacterial destruction. Substrates (dentin) around bacteria absorb the light and heat from the environment around bacteria, which results in the death of bacterial cells. Another possible mechanism of action is that the laser light directly damages bacterial cells [21]. According to Kouchi et al [33] bacteria penetrate the periluminal dentin up to a depth of 1.1 mm. Meanwhile, chemical disinfectants have a limited ability to penetrate, going no deeper than 0.13 mm according to Berutti et al [34]. Consequently, dentin with a thickness over 1 mm enables a safe zone for bacteria and may lead to failure of the treatment [20,29]. This possibility accords with our results for lesions with a greater diameter having a lower percentage of damaged cells. Lesions of 10 and 14 mm were too large for the heat produced to be effective.
Conclusions
In conclusion, our study indicated that diode laser irradiation with the generation of heat is not the most optimal solution for antibacterial effectiveness. Large periapical lesions should be disinfected through a combination of adjuvant treatment with diode laser irradiation and a standard method of irrigation with NaOCl. Nevertheless, for small periapical lesions, the usability and accessibility of laser beam could be effective.
References
1. Garcia CC, Sempere FV, Diago MP, Bowen EM, The post-endodontic periapical lesion: Histologic and etiopathogenic aspects: Med Oral Patol Oral Cir Bucal, 2007; 12(8); E585-90
2. Paque F, Laib A, Gautschi H, Zehnder M, Hard-tissue debris accumulation analysis by high-resolution computed tomography scans: J Endod, 2009; 35; 1044-47
3. Ten Cate AR, The epithelial cell rests of Malassez and the genesis of the dental cyst: Oral Surg Oral Med Oral Pathol, 1972; 34; 956-64
4. Ramchandran Nair PN, Pajrola G, Schroeder HE, Types and incidence of human periapical lesions obtained with extracted teeth: Oral Surg Oral Med Oral Pathol Oral Radiol Endod, 1996; 81; 93-102
5. Natkin E, Oswald RJ, Carnes LI, The relationship of lesion size to diagnosis. Incidence and treatment of periapical cysts and granulomas: Oral Surg Oral Med Oral Pathol, 1984; 57; 82-94
6. Lin LM, Domenico R, Jarshen L, Rosenberg PA, Nonsurgical root canal therapy of large cyst-like inflammatory periapical lesions and inflammatory apical cysts: J Endod, 2009; 35; 607-15
7. Marquis VL, Dao T, Farzaneh M, Treatment outcome in endodontics: The Toronto study – phase III: Initial treatment: J Endod, 2006; 32; 299-306
8. Rossi A, Silva LAB, Leonardo MR, Effect of rotary or manual instrumentation, with or without a calcium hydroxide 1% chlorhexidine intracanal dressing, on the healing of experimentally induced chronic periapical lesions: Oral Surg Oral Med Oral Pathol Oral Radiol Endod, 2005; 99; 628-36
9. Paque F, Balmer M, Attin T, Peters OA, Preparation of oval-shaped root canals in mandibular molars using nickel-titanium rotary instrumentation: A micro-computed tomography study: J Endod, 2010; 36; 703-7
10. Bago I, Plečko V, Gabrić Pandurić D, Antimicrobial efficacy of a high-power diode laser, photo-activated disinfection, conventional and sonic activated irrigation during root canal treatment: Int Endod J, 2013; 46; 339-47
11. Kafas P, Kalfas S, Carbonization of a radicular cyst using fiber-optic diode laser: A case report: Cases J, 2008; 1; 113
12. Gutknecht N, van Gogswaardt D, Conrads G, Diode laser radiation and its bactericidal effect in root canal wall dentin: J Clin Laser Med Surg, 2000; 18; 57-60
13. Gutknecht N, Franzen R, Schippers M, Lampert F, Bactericidal effect of a 980-nm diode laser in the root canal wall dentin of bovine teeth: J Clin Laser Med Surg, 2004; 22; 9-13
14. Piccolomini R, D’Arcangelo C, D’Ercole S, Bacteriologic evaluation of the effect of Nd: YAG laser irradiation in experimental infected root canals: J Endod, 2002; 28; 276-78
15. Gerek M, Asci S, Yaylali DI, Ex vivo evaluation of antibacterial effects of Nd: YAG and diode lasers in root canals: Biotechnol Biotechnolog Equipment, 2010; 24; 2031-34
16. Demidova TN, Hamblin MR, Photodynamic therapy targeted to pathogens: Int J Immunopathol Pharmacol, 2004; 17; 245-54
17. Rahimi S, Shani S, Gholizadeh S: Photomed Laser Surg, 2012; 30; 637-41
18. Asnaashari M, Safavi N, Disinfection of contaminated canals by different laser wavelengths, while performing root canal therapy: J Lasers Med Sci, 2013; 4; 8-16
19. Aziz A, Chandler NP, Hauman CH, Infection of apical dentin and root-end cavity disinfection: J Endod, 2012; 38; 1387-90
20. Meire MA, Coenye T, Nelis HJ, De Moor JG: Int Endod J, 2012; 45; 482-91
21. Schoop U, Kluger W, Moritz A, Bactericidal effect of different laser systems in the deep layers of dentin: Lasers Surg Med, 2004; 35; 111-16
22. Gojkov-Vukelic M, Hadzic S, Dedic A, Application of a diode laser in the reduction of targeted periodontal pathogens: Acta Inform Med, 2013; 21; 237-40
23. Nagayoshi M, Nishihara T, Nakashima K: ISRN Dent, 2011; 870364
24. Ricatto LG, Conrado LA, Turssi CP, Comparative evaluation of photodynamic therapy using LASER or light emitting diode on cariogenic bacteria: An in vitro study: Eur J Dent, 2014; 8; 509-14
25. Klinke T, Klimm W, Gutknecht N, Antibacterial effects of Nd: YAG laser irradiation within root canal dentin: J Clin Laser Med Surg, 1997; 15; 29-31
26. Rooney J, Midda M, Leeming J, A laboratory investigation of the bactericidal effect of Nd: YAG laser: Br Dent J, 1994; 176; 61-64
27. Yasuda Y, Kawamorita T, Yamaguchi H, Saito T, Bactericidal effect of Nd: YAG and Er: YAG lasers in experimentally infected curved root canals: Photomed Laser Surg, 2010; 28; S75-78
28. Yavari HR, Rahimi S, Shahi S: Photomed Laser Surg, 2010; 28(Suppl 1); S91-96
29. Nunes MR, Mello I, Franco GC: Photomed Laser Surg, 2011; 29; 803-8
30. Bergmans L, Moisiadis P, Teughels W, Bacterial effect of Nd: YAG laser irradiation of some endodontic pathogens ex vivo: Int Endod J, 2006; 39; 547-57
31. Folwaczny M, Mehl A, Jordan C, Hickel R, Antibacterial effects of pulsed Nd: YAG laser radiation at different energy settings in root canals: J Endod, 2002; 28; 24-29
32. Pirnat S, Lukac M, Ihan A, Study of the direct bactericidal effect of Nd: YAG and diode laser parameters used in endodontics on pigmented and nonpigmented bacteria: Lasers Med Sci, 2011; 26; 755-61
33. Kouchi Y, Ninomiya J, Yasuda H: J Dent Res, 1980; 59; 2038-46
34. Berutti E, Marini R, Angeretti A, Penetration ability of different irrigants into dentinal tubules: J Endod, 1997; 23; 725-27
Figures
Most Viewed Current Articles
15 Jun 2022 : Clinical Research
Evaluation of Apical Leakage After Root Canal Obturation with Glass Ionomer, Resin, and Zinc Oxide Eugenol ...DOI :10.12659/MSMBR.936675
Med Sci Monit Basic Res 2022; 28:e936675
07 Jul 2022 : Laboratory Research
Cytotoxicity, Apoptosis, Migration Inhibition, and Autophagy-Induced by Crude Ricin from Ricinus communis S...DOI :10.12659/MSMBR.936683
Med Sci Monit Basic Res 2022; 28:e936683
01 Jun 2022 : Laboratory Research
Comparison of Sealing Abilities Among Zinc Oxide Eugenol Root-Canal Filling Cement, Antibacterial Biocerami...DOI :10.12659/MSMBR.936319
Med Sci Monit Basic Res 2022; 28:e936319
08 Dec 2022 : Original article
Use of Estimated Glomerular Filtration Rate and Urine Albumin-to-Creatinine Ratio Based on KDIGO 2012 Guide...DOI :10.12659/MSMBR.938176
Med Sci Monit Basic Res 2022; 28:e938176